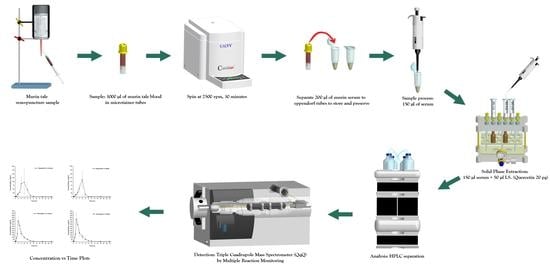
A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture
Abstract
Share and Cite
Araujo-León, J.A.; Ortiz-Andrade, R.; Hernández-Baltazar, E.; Hernández-Núñez, E.; Rivera-Leyva, J.C.; Yáñez-Pérez, V.; Vazquez-Garcia, P.; Cicero-Sarmiento, C.G.; Sánchez-Salgado, J.C.; Segura-Campos, M.R. A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules 2022, 27, 391. https://doi.org/10.3390/molecules27020391
Araujo-León JA, Ortiz-Andrade R, Hernández-Baltazar E, Hernández-Núñez E, Rivera-Leyva JC, Yáñez-Pérez V, Vazquez-Garcia P, Cicero-Sarmiento CG, Sánchez-Salgado JC, Segura-Campos MR. A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules. 2022; 27(2):391. https://doi.org/10.3390/molecules27020391
Chicago/Turabian StyleAraujo-León, Jesús Alfredo, Rolffy Ortiz-Andrade, Efrén Hernández-Baltazar, Emanuel Hernández-Núñez, Julio César Rivera-Leyva, Víctor Yáñez-Pérez, Priscila Vazquez-Garcia, Carla Georgina Cicero-Sarmiento, Juan Carlos Sánchez-Salgado, and Maira Rubí Segura-Campos. 2022. "A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture" Molecules 27, no. 2: 391. https://doi.org/10.3390/molecules27020391
APA StyleAraujo-León, J. A., Ortiz-Andrade, R., Hernández-Baltazar, E., Hernández-Núñez, E., Rivera-Leyva, J. C., Yáñez-Pérez, V., Vazquez-Garcia, P., Cicero-Sarmiento, C. G., Sánchez-Salgado, J. C., & Segura-Campos, M. R. (2022). A Pharmacokinetic Study of Mix-160 by LC-MS/MS: Oral Bioavailability of a Dosage Form of Citroflavonoids Mixture. Molecules, 27(2), 391. https://doi.org/10.3390/molecules27020391