In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt
Abstract
:1. Introduction
2. Results and Discussion
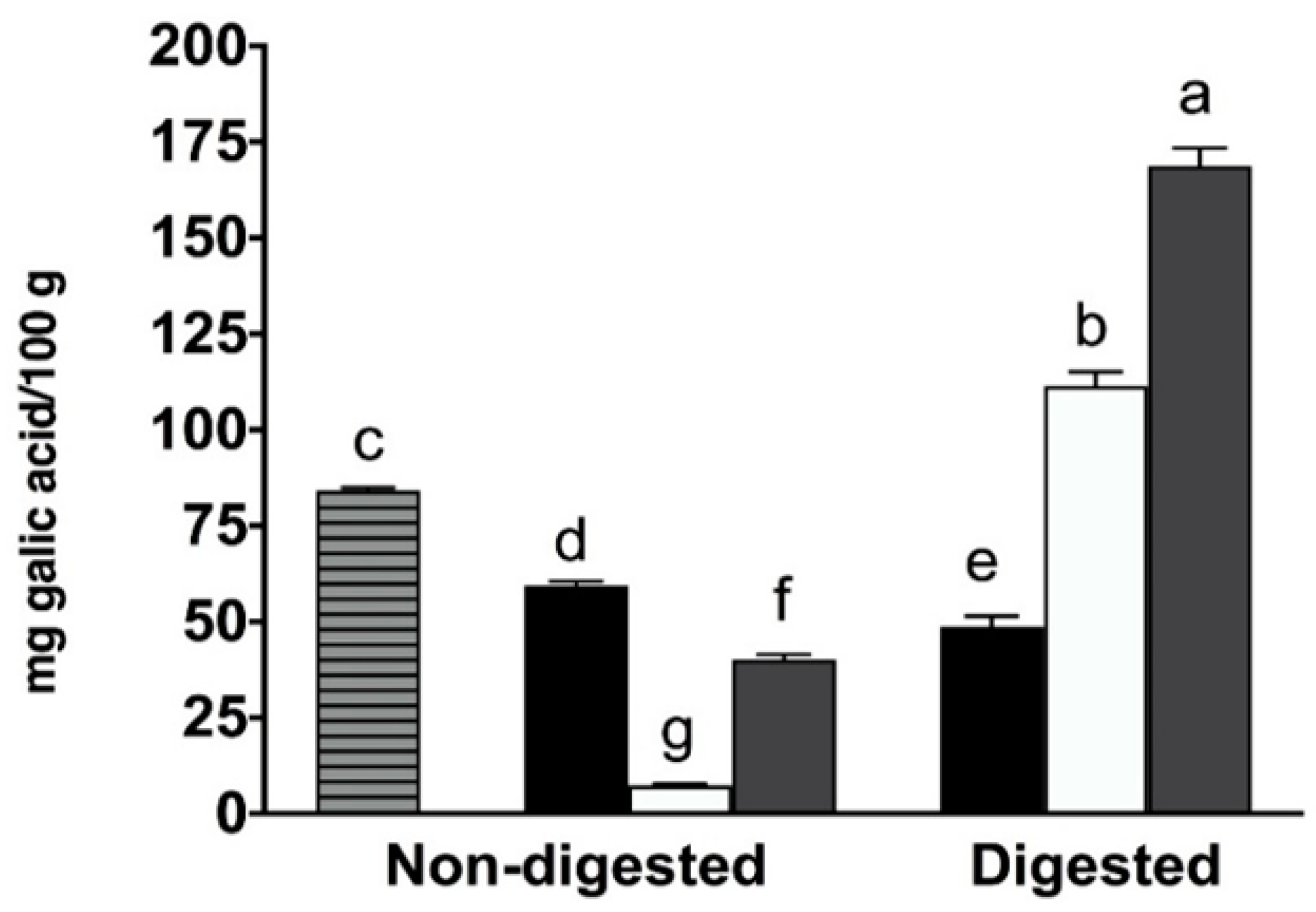
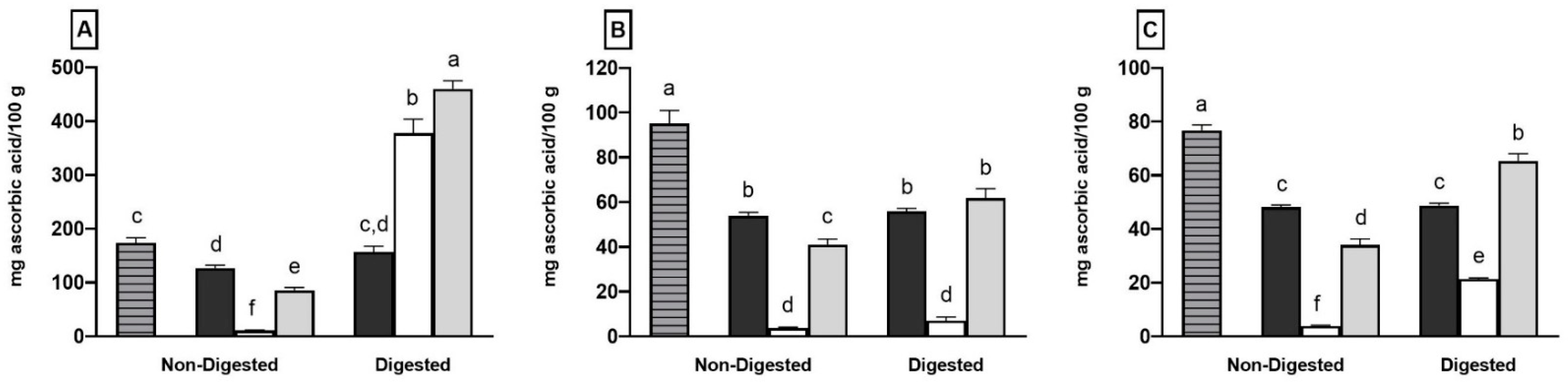
2.1. Total Phenolic Content, Individual Phenolic Compounds and Antioxidant Properties of Coffee, Fermented Coffee, Plain Yogurt and Coffee-Fortified Yogurt
2.2. Effect of In Vitro Digestion on Bioaccessibility of Total and Individual Phenolic Compounds and Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Yogurts and Coffee Samples
3.3. In Vitro Gastro-Intestinal Digestion
3.4. Samples Preparation
3.5. Determination of Total Phenolic Compounds
3.6. Antioxidant Activity Analysis
3.7. Determination of Protein Hydrolysis
3.8. Identification of Individual Phenolic Compounds by Mass Spectrometry
3.9. Bioaccessibility Index
3.10. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nicoletti, M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012, 63, 2–6. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Lévy, C.; Martin, N.; Souchon, I. Influence of proteins on the perception of flavored stirred yogurts. J. Dairy Sci. 2006, 89, 922–933. [Google Scholar] [CrossRef] [Green Version]
- Mediza Romero, M.L.; von Staszewski, M.; Martínez, M.J. The effect of green tea polyphenols addition on the physicochemical, microbiological and bioactive characteristics of yogurt. Br. Food J. 2021, 123, 2380–2397. [Google Scholar] [CrossRef]
- Rutella, G.S.; Tagliazucchi, D.; Solieri, L. Survival and bioactivities of selected probiotic lactobacilli in yogurt fermentation and cold storage: New insights for developing a bi-functional dairy food. Food Microbiol. 2016, 60, 54–61. [Google Scholar] [CrossRef] [Green Version]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giosuè, A.; Calabrese, I.; Vitale, M.; Riccardi, G.; Vaccaro, O. Consumption of Dairy Foods and Cardiovascular Disease: A Systematic Review. Nutrients 2022, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ahmed, M.; Ha, V.; Jefferson, K.; Malik, V.; Ribeiro, P.A.B.; Zuchinali, P.; Drouin-Chartier, J.P. Dairy Product Consumption and Cardiovascular Health: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2022, 13, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Tan, G.; Korel, F. Quality of flavored yogurt containing added coffee and sugar. J. Food Qual. 2007, 30, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, A.; Perna, A.; Grassi, G.; Gambacorta, E. In vitro phenols bioaccessibility and antioxidant activity of goat milk yogurt fortified with Rhus coriaria leaf powder. J. Food Sci. 2021, 86, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Tomar, O.; Akarca, G.; Çağlar, A.; İstek, Ö.; Gök, V. The effect of plant extracts on antioxidant potential, microbial and sensory attributes of stirred yoghurt. Mljekarstvo 2021, 71, 35–48. [Google Scholar] [CrossRef]
- Akgün, D.; Gültekin-Özgüven, M.; Yücetepe, A.; Altin, G.; Gibis, M.; Weiss, J.; Özçelik, B. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocoll. 2020, 101, 105532. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Redondo, N.; Vargas, A.E.; Teruel-Andreu, C.; Lipan, L.; Muelas, R.; Hernández-García, F.; Sendra, E.; Cano-Lamadrid, M. Evaluation of cinnammon (Cinnamomum cassia and Cinnamomum verum) enriched yoghurt during refrigerated storage. LWT 2022, 159, 113240. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; Cruz-Cansino, N.d.S.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Arias-Rico, J.; Cervantes-Elizarrarás, A. Evaluation of physicochemical, microbiological, and antioxidant properties of a drinkable yogurt added with ultrasonicated purple cactus pear (Opuntia ficus-indica) juice powder. J. Food Process. Preserv. 2021, 45, e15720. [Google Scholar] [CrossRef]
- Mittal, M.; Thakur, A.; Kaushik, R.; Chawla, P. Physicochemical properties of Ocimum sanctum enriched herbal fruit yoghurt. J. Food Process. Preserv. 2020, 44, e14976. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Helal, A.; Alnemr, T.M.; Marquez, O. Influence of Tomato Pomace Inclusion on the Chemical, Physical and Microbiological Properties of Stirred Yoghurt. Int. J. Dairy Sci. 2020, 15, 152–160. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of strawberries preparation in yoghurt: Impact on phytochemicals and milk proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Proteolytic activity, antioxidant, and α-Amylase inhibitory activity of yogurt enriched with coriander and cumin seeds. LWT 2020, 133, 109912. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingjord-Dale, M.; Papadimitriou, N.; Katsoulis, M.; Yee, C.; Dimou, N.; Gill, D.; Aune, D.; Ong, J.S.; MacGregor, S.; Elsworth, B.; et al. Coffee consumption and risk of breast cancer: A Mendelian randomization study. PLoS ONE 2021, 16, e0236904. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Seibold, P.; Chang-Claude, J.; Flesch-Janys, D.; Liu, J.; Czene, K.; Humphreys, K.; Hall, P. Coffee consumption modifies risk of estrogen-receptor negative breast cancer. Breast Cancer Res. 2011, 13, R49. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Quesada, C.; Romanos-Nanclares, A.; Navarro, A.M.; Gea, A.; Cervantes, S.; Martínez-González, M.Á.; Toledo, E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020, 59, 3461–3471. [Google Scholar] [CrossRef]
- Bonita, J.S.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007, 55, 187–198. [Google Scholar] [CrossRef]
- Van Dam, R.M. Coffee and type 2 diabetes: From beans to beta-cells. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 69–77. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. Nutr. 2016, 66, 87–100. [Google Scholar]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Lamothe, S.; Azimy, N.; Bazinet, L.; Couillard, C.; Britten, M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014, 5, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Damin, M.R.; Alcântara, M.R.; Nunes, A.P.; Oliveira, M.N. Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt. LWT-Food Sci. Technol. 2009, 42, 1744–1750. [Google Scholar] [CrossRef]
- Shah, N.P. Effects of milk-derived bioactives: An overview. Br. J. Nutr. 2000, 84, S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Amirdivani, S.; Baba, A.S.H. Green tea yogurt: Major phenolic compounds and microbial growth. J. Food Sci. Technol. 2015, 52, 4652–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT 2015, 63, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt Enrichment with Grape Pomace: Effect of Grape Cultivar on Physicochemical, Microbiological and Sensory Properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, T.M.; Mwafy, E.A.; Mostafa, A.M.; El-Din, H.M.F.; Mwafy, A.; Amarowicz, R.; Ozçelik, B. Spectroscopic studies of the interaction between isolated polyphenols from coffee and the milk proteins. Surf. Interfaces 2020, 20, 100558. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Gastro-pancreatic release of phenolic compounds incorporated in apolyphenols-enriched cheese-curd. LWT 2015, 60, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Z.M.R.; El Din, H.M.F.; Ali, A.A.; Nayra, S.M.; El-Messery, T.M. Interaction of some low molecular weight phenolics with milk proteins. World Appl. Sci. J. 2013, 23, 182–187. [Google Scholar] [CrossRef]
- Trigueros, L.; Wojdyło, A.; Sendra, E. Antioxidant activity and protein-polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014, 62, 6417–6425. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. Antioxidant and anti-inflammatory activity of coffee brew evaluated after simulated gastrointestinal digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Vilas-Boas, A.A.; Oliveira, A.; Jesus, D.; Rodrigues, C.; Figueira, C.; Gomes, A.; Pintado, M. Chlorogenic acids composition and the impact of in vitro gastrointestinal digestion on espresso coffee from single-dose capsule. Food Res. Int. 2020, 134, 109223. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, S.; Frank, O.; Hofmann, T. Quantitative studies on the influence of the bean roasting parameters and hot water percolation on the concentrations of bitter compounds in coffee brew. J. Agric. Food Chem. 2010, 58, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Jaiswal, R.; Matei, M.F.; Kuhnert, N. Investigation of acyl migration in mono- and dicaffeoylquinic acids under aqueous basic, aqueous acidic, and dry roasting conditions. J. Agric. Food Chem. 2014, 62, 9160–9170. [Google Scholar] [CrossRef] [PubMed]
- Bekedam, E.K.; Schols, H.A.; Van Boekel, M.A.J.S.; Smit, G. Incorporation of chlorogenic acids in coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Mehta, T.; Esteban-Muñoz, A.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Effect of in vitro digestion-fermentation on green and roasted coffee bioactivity: The role of the gut microbiota. Food Chem. 2019, 279, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Cui, L.; Yang, G.; Lu, S.; Zeng, X.; He, J.; Guo, Y.; Pan, D.; Wu, Z. Antioxidant peptides derived from hydrolyzed milk proteins by Lactobacillus strains: A BIOPEP-UWM database-based analysis. Food Res. Int. 2022, 156, 111339. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Cebeci, F.; Şahin-Yesilcubuk, N. The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability. Int. J. Food Sci. Nutr. 2014, 65, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods 2014, 7, 506–516. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Antiproliferative Activity and Cell Metabolism of Hydroxycinnamic Acids in Human Colon Adenocarcinoma Cell Lines. J. Agric. Food Chem. 2019, 67, 3919–3931. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from Thai rice bran extracts. Food Chem. 2020, 329, 127157. [Google Scholar] [CrossRef] [PubMed]
- Qie, X.; Wu, Y.; Chen, Y.; Liu, C.; Zeng, M.; Qin, F.; Wang, Z.; Chen, J.; He, Z. Competitive interactions among tea catechins, proteins, and digestive enzymes modulate in vitro protein digestibility, catechin bioaccessibility, and antioxidant activity of milk tea beverage model systems. Food Res. Int. 2021, 140, 110050. [Google Scholar] [CrossRef] [PubMed]
- Qie, X.; Cheng, Y.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Li, W.; He, Z. In vitro phenolic bioaccessibility of coffee beverages with milk and soy subjected to thermal treatment and protein–phenolic interactions. Food Chem. 2022, 375, 131644. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Kroll, J.; Riese, B. Reactions of chlorogenic acid with lysozyme: Physicochemical characterization and proteolytic digestion of the derivatives. J. Food Sci. 2000, 65, 1091–1098. [Google Scholar] [CrossRef]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef]
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of food matrix components on nutritional and functional properties of fruit-based products. Curr. Opin. Food Sci. 2018, 22, 153–159. [Google Scholar] [CrossRef]
- Lamothe, S.; Guérette, C.; Dion, F.; Sabik, H.; Britten, M. Antioxidant activity of milk and polyphenol-rich beverages during simulated gastrointestinal digestion of linseed oil emulsions. Food Res. Int. 2019, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Bellesia, A.; Conte, A. Composition and properties of peptides that survive standardised in vitro gastro-pancreatic digestion of bovine milk. Int. Dairy J. 2016, 61, 196–204. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Conte, A.; Desobry, S. Antioxidant properties of polyphenols incorporated in casein/sodium caseinate films. Int. Dairy J. 2012, 25, 10–15. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Martini, S.; Cavalchi, M.; Conte, A.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2019, 70, 335–348. [Google Scholar] [CrossRef]
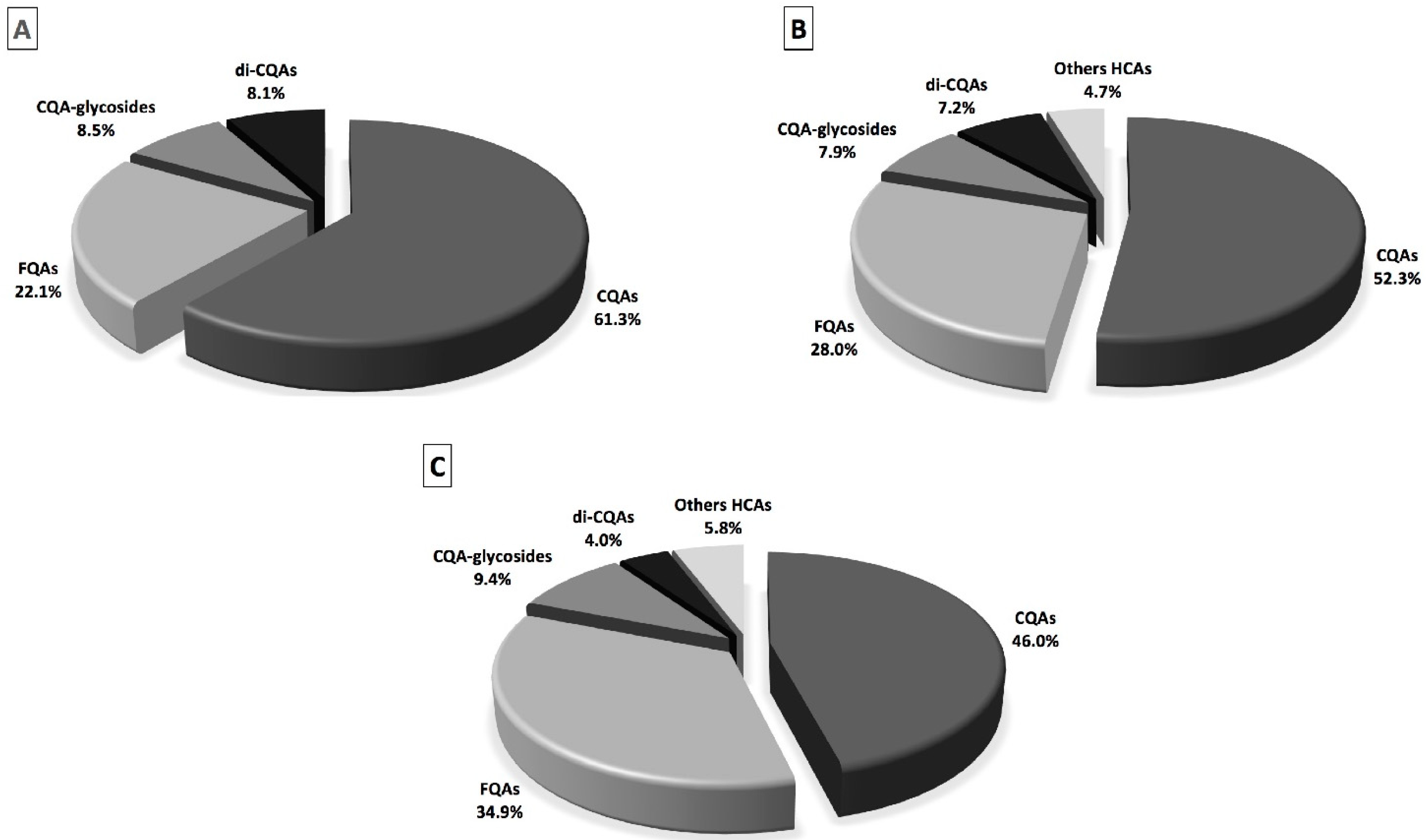

| Phenolic Compounds | Before Digestion | After Digestion | |||
|---|---|---|---|---|---|
| C | FC | CY | FC | CY | |
| Caffeoylquinic acids | |||||
| 3-O-Caffeoylquinic acid | 10.76 ± 0.14 a | 6.00 ± 0.15 b | 4.27 ± 0.09 c | 1.56 ± 0.09 d | 1.55 ± 0.03 d |
| 4-O-Caffeoylquinic acid | 1.90 ± 0.02 b | 2.53 ± 0.35 a | 1.27 ± 0.01 c | n.d. | 1.39 ± 0.01 c |
| 5-O-Caffeoylquinic acid | 10.32 ± 0.19 a | 5.15 ± 0.24 b | 3.50 ± 0.09 c | 1.77 ± 0.01 d | 0.51 ± 0.00 e |
| Total caffeoylquinic acids | 22.98 ± 0.24 a | 13.68 ± 0.45 b | 9.04 ± 0.13 c | 3.33 ± 0.10 d | 3.45 ± 0.04 d |
| BI% | 14.5 | 15.0 | |||
| Feruloylquinic acids | |||||
| 3-O-Feruloylquinic acid | 4.67 ± 0.11 a | 2.72 ± 0.01 b | 1.94 ± 0.00 c | 1.11 ± 0.01 d | 4.74 ± 0.10 a |
| 4-O-Feruloylquinic acid | 0.82 ± 0.01 d | 2.84 ± 0.22 b | 3.17 ± 0.14 a | 2.50 ± 0.01 c | 2.97 ± 0.08 ab |
| 5-O-Feruloylquinic acid | 2.79 ± 0.09 a | 1.76 ± 0.18 c | 1.74 ± 0.05 c | 2.52 ± 0.01 b | 2.81 ± 0.10 a |
| Total feruloylquinic acid | 8.28 ± 0.15 b | 7.32 ± 0.29 c | 6.85 ± 0.15 c | 6.13 ± 0.05 d | 10.52 ± 0.16 a |
| BI% | 74.0 | 127.1 | |||
| Caffeoylquinic acid-glycosides | |||||
| Caffeoylquinic acid-glycoside isomer 1 | 1.11 ± 0.03 a | 1.12 ± 0.09 a | 1.03 ± 0.02 a | n.d. | n.d. |
| Caffeoylquinic acid-glycoside isomer 2 | 0.68 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Caffeoylquinic acid-glycoside isomer 3 | 0.80 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Caffeoylquinic acid-glycoside isomer 4 | 0.60 ± 0.02 c | 0.96 ± 0.03 a | 0.81 ± 0.01 b | n.d. | n.d. |
| Total caffeoylquinic acid-glycosides | 3.19 ± 0.06 a | 2.08 ± 0.09 b | 1.84 ± 0.04 c | / | / |
| BI% | / | / | |||
| Di-caffeoylquinic acids | |||||
| 3,4-di-O-caffeoylquinic acid | 2.10 ± 0.02 a | 1.39 ± 0.00 b | n.d. | n.d. | n.d. |
| 4,5-di-O-caffeoylquinic acid | 0.50 ± 0.00 b | 0.49 ± 0.00 b | 0.78 ± 0.02 a | n.d. | n.d. |
| 3,5-di-O-caffeoylquinic acid | 0.45 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Total dicaffeoylquinic acids | 3.05 ± 0.04 a | 1.88 ± 0.00 b | 0.78 ± 0.02 c | / | / |
| BI% | / | / | |||
| Others hydroxycinnamic acids | |||||
| Caffeoylshikimic acid isomer 1 | n.d. | 0.12 ± 0.01 | n.d. | n.d. | n.d. |
| Caffeoylshikimic acid isomer 2 | n.d. | 1.10 ± 0.02 a | 0.55 ± 0.01 b | n.d. | n.d. |
| Caffeoyl hexose | n.d. | n.d. | 0.59 ± 0.01 | n.d. | n.d. |
| Total others hydroxycinnamic acids | / | 1.22 ± 0.02 a | 1.14 ± 0.05 a | / | / |
| BI% | / | / | |||
| Total hydroxycinnamic acids | 37.50 ± 0.17 a | 26.18 ± 0.54 b | 19.65 ± 0.21 c | 9.46 ± 0.11 e | 13.97 ± 0.16 d |
| BI% | 25.2 | 37.3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, A.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt. Molecules 2022, 27, 6843. https://doi.org/10.3390/molecules27206843
Helal A, Cattivelli A, Conte A, Tagliazucchi D. In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt. Molecules. 2022; 27(20):6843. https://doi.org/10.3390/molecules27206843
Chicago/Turabian StyleHelal, Ahmed, Alice Cattivelli, Angela Conte, and Davide Tagliazucchi. 2022. "In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt" Molecules 27, no. 20: 6843. https://doi.org/10.3390/molecules27206843
APA StyleHelal, A., Cattivelli, A., Conte, A., & Tagliazucchi, D. (2022). In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Coffee-Fortified Yogurt. Molecules, 27(20), 6843. https://doi.org/10.3390/molecules27206843