Influence of Molecular Weight of Polysaccharides from Laminaria japonica to LJP-Based Hydrogels: Anti-Inflammatory Activity in the Wound Healing Process
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
2.2. Molecular Weights
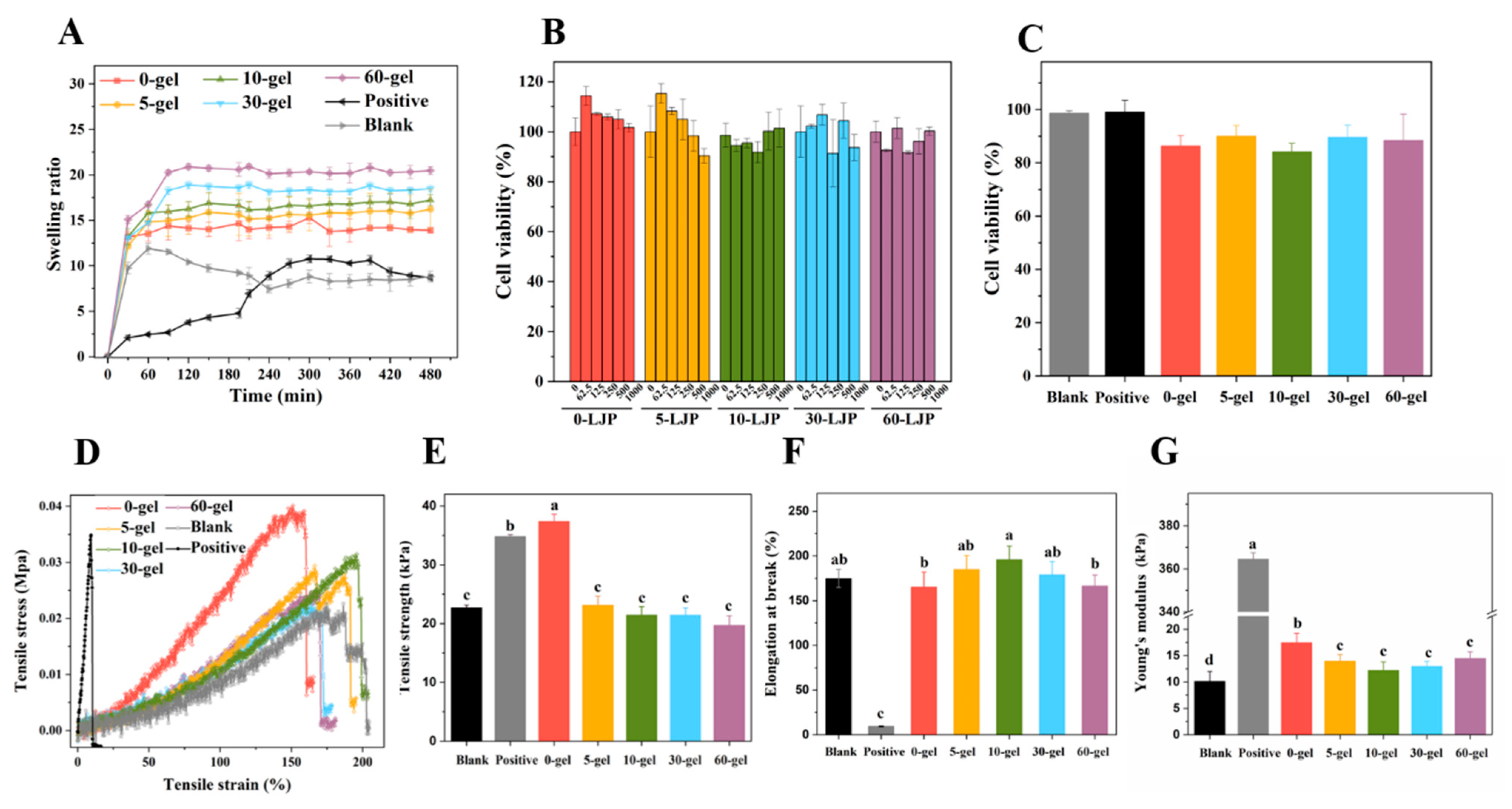
2.3. Swelling Properties
2.4. Mechanical Properties
2.5. Cell Cytotoxicity
2.6. Construction of Cell Inflammation Model Analysis
2.7. Effects of LJP and LJP-Gels on Proinflammatory Cytokines and Related Genes Expression
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation and Degradation of LJP
4.3. Characterization of LJP
4.3.1. Chemical Composition
4.3.2. Molecular Weight
4.4. Preparation of LJP-Based Hydrogels
4.5. Group of Experiments
4.6. Swelling Properties
4.7. Mechanical Properties
4.8. Cell Culture
4.8.1. Pretreatments of LJP and LJP-Gels
4.8.2. Cell Cytotoxicity in HaCaT
4.8.3. Construction of Cell Inflammation Model
4.8.4. Inflammatory Cytokines Measurement
4.8.5. qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar]
- Tang, Y.D.; Lan, X.Z.; Liang, C.F.; Zhong, Z.X.; Xie, R.T.; Zhou, Y.; Miao, X.M.; Wang, H.; Wang, W.L. Honey loaded alginate/ PVA nanofibrous membrane as potential bioactive wound dressing. Carbohyd. Polym. 2019, 219, 113–120. [Google Scholar]
- Xing, L.; Ma, Y.; Tan, H.P.; Yuan, G.L.; Li, S.K.; Li, J.L.; Jia, Y.; Zhou, T.L.; Niu, X.H.; Hu, X.H. Alginate membrane dressing toughened by chitosan floccule to load antibacterial drugs for wound healing. Polym. Test. 2019, 79, 106039. [Google Scholar]
- Ma, X.L.; Bian, Q.; Hu, J.Y.; Gao, J.Q. Stem from nature: Bioinspired adhesive formulations for wound healing. J. Control. Release 2022, 345, 292–305. [Google Scholar] [PubMed]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar]
- Lin, Z.F.; Wu, T.T.; Wang, W.S.; Li, B.L.; Wang, M.; Chen, L.L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar]
- Kang, J.I.; Park, K.M.; Park, K.D. Oxygen-generating alginate hydrogels as a bioactive acellular matrix for facilitating wound healing. J. Ind. Eng. Chem. 2019, 69, 397–404. [Google Scholar]
- Jiang, F.; Ding, Y.; Tian, Y.; Yang, R.; Quan, M.; Tong, Z.; Zhang, X.; Luo, D.; Chi, Z.; Liu, C. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar]
- Deng, H.; Yu, Z.; Chen, S.; Fei, L.; Sha, Q.; Zhou, N.; Chen, Z.; Xu, C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020, 230, 115565. [Google Scholar]
- Shyna, S.; Krishna, A.S.; Nair, P.D.; Thomas, L.V. A nonadherent chitosan-polyvinyl alcohol absorbent wound dressing prepared via controlled freeze-dry technology. Int. J. Biol. Macromol. 2020, 150, 129–140. [Google Scholar]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mat. Sci. Eng. C-Mater. 2020, 110, 110619. [Google Scholar] [CrossRef] [PubMed]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf. B Biointerfaces 2018, 172, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Golafshan, N.; Rezahasani, R.; Tarkesh, E.M.; Kharaziha, M.; Khorasani, S.N. Nanohybrid hydrogels of laponite: PVA-Alginate as a potential wound healing material. Carbohydr. Polym. 2017, 176, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Aierken, A.; Zhao, L.; Lin, Z.S.; Jiang, J.J.; Li, B.L.; Wang, J.Y.; Hua, J.L.; Tu, Q. HUC-MSCs lyophilized powder loaded polysaccharide ulvan driven functional hydrogel for chronic diabetic wound healing. Carbohyd. Polym. 2022, 288, 119404. [Google Scholar] [CrossRef]
- Zheng, B.D.; Ye, J.; Yang, Y.C.; Huang, Y.Y.; Xiao, M.T. Self-healing polysaccharide-based injectable hydrogels with antibacterial activity for wound healing. Carbohyd. Polym. 2022, 275, 118770. [Google Scholar] [CrossRef]
- Chen, X.; You, L.; Ma, Y.; Zhao, Z.; Kulikouskaya, V. Influence of UV/H2O2 treatment on polysaccharides from Sargassum fusiforme: Physicochemical properties and RAW 264.7 cells responses. Food Chem. Toxicol. 2021, 153, 112246. [Google Scholar] [CrossRef]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocoll. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Y.; Cheung, P.C.; You, L.; Liao, L.; Pedisic, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef]
- Yao, W.Z.; Liu, M.Y.; Chen, X.Y.; You, L.J.; Ma, Y.X.; Hileuskaya, K. Effects of UV/H2O2 degradation and step gradient ethanol precipitation on Sargassum fusiforme polysaccharides: Physicochemical characterization and protective effects against intestinal epithelial injury. Food Res. Int. 2022, 155, 111093. [Google Scholar] [CrossRef]
- Garcia-Astrain, C.; Averous, L. Synthesis and behavior of click cross-linked alginate hydrogels: Effect of cross-linker length and functionality. Int. J. Biol. Macromol. 2019, 137, 612–619. [Google Scholar] [CrossRef]
- Li, G.Y.; Jiang, Y.C.; Li, M.Y.; Zhang, W.J.; Li, Q.; Tang, K.Y. Investigation on the tunable effect of oxidized konjac glucomannan with different molecular weight on gelatin-based composite hydrogels. Int. J. Biol. Macromol. 2021, 168, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Padol, A.M.; Draget, K.I.; Stokke, B.T. Effects of added oligoguluronate on mechanical properties of Ca—alginate—oligoguluronate hydrogels depend on chain length of the alginate. Carbohyd. Polym. 2016, 147, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, R.; Li, Y.; Li, X.; You, L.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohyd. Polym. 2020, 230, 115647. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Y.; Hu, J.; Yao, W.; You, L.; Cheung, P.C. Algal sulfated polysaccharide-based hydrogels enhance gelling properties and in vitro wound healing compared to conventional hydrogels. Algal Res. 2022, 65, 102740. [Google Scholar] [CrossRef]
- Dai, Y.; Shao, C.; Piao, Y.; Hu, H.; Lu, K.; Zhang, T.; Zhang, X.; Jia, S.; Wang, M.; Man, S. The mechanism for cleavage of three typical glucosidic bonds induced by hydroxyl free radical. Carbohydr. Polym. 2017, 178, 34–40. [Google Scholar] [CrossRef]
- Chen, X.; Sun-Waterhouse, D.; Yao, W.; Li, X.; Zhao, M.; You, L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021, 365, 130524. [Google Scholar] [CrossRef]
- Ma, C.L.; Bai, J.W.; Shao, C.T.; Liu, J.W.; Zhang, Y.; Li, X.Q.; Yang, Y.; Xu, Y.Q.; Wang, L.B. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Res. Int. 2021, 143, 110281. [Google Scholar] [CrossRef]
- Xiao, J.R.; Chen, X.; Zhan, Q.P.; Zhong, L.; Hu, Q.H.; Zhao, L.Y. Effects of ultrasound on the degradation kinetics, physicochemical properties and prebiotic activity of Flammulina velutipes polysaccharide. Ultrason. Sonochem. 2022, 82, 105901. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, H.; Yang, X.; Li, L.; Qi, B.; Hu, X.; Ma, H.; Li, C.; Pan, C. Degradation of sulphated polysaccharides from Grateloupia livida and antioxidant activity of the degraded components. Int. J. Biol. Macromol. 2020, 156, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Jiang, C.M.; Chen, X.L.; He, K.W.; Hu, Y.Y. Protective effects of sinomenine against LPS-induced inflammation in piglets. Microb. Pathog. 2017, 110, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Singh, P. Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorg. Chem. 2022, 121, 105663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhu, Y.J.; Li, H.; Zhang, Y.G.; Shen, Y.Q.; Sun, T.W.; Chen, F. Preparation and enhanced mechanical properties of hybrid hydrogels comprising ultralong hydroxyapatite nanowires and sodium alginate. J. Colloid Interf. Sci. 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohyd. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E.; Ozkahraman, B.; Suloglu, A.K.; Idil, N.; Percin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Technol. 2020, 58, 101536. [Google Scholar] [CrossRef]
- Chang, S.; Zou, X.; Zhu, B.; You, L.; Zhao, Z.; Hileuskaya, K. The characteristics of polysaccharide from Gracilaria chouae and its application in food packaging with carboxymethyl cellulose and lysozyme. Food Hydrocoll. 2023, 135, 108109. [Google Scholar] [CrossRef]
- Atashgahi, M.; Ghaemi, B.; Valizadeh, A.; Moshiri, A.; Nekoofar, M.H.; Amani, A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: A bi-layer nano-biomaterial for rapid hemostasis. Int. J. Pharm. 2021, 608, 121074. [Google Scholar] [CrossRef] [PubMed]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Milan, P.B.; Amoupour, M. Using artificial neural network for design and development of PVA/chitosan/starch/heparinized nZnO hydrogels for enhanced wound healing. J. Ind. Eng. Chem. 2022, 108, 88–100. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; He, L.; Qu, Y.Z.; Ouyang, L.; Han, Y.; Xu, C.; Duan, D.Y. Platelet-Rich Plasma-Derived exosomal USP15 promotes cutaneous wound healing via deubiquitinating EIF4A1. Oxid. Med. Cell. Longev. 2021, 2021, 9674809. [Google Scholar] [CrossRef]
- de Azevedo, T.; Bezerra, M.; Santos, M.; Souza, L.A.; Marques, C.T.; Benevides, N.; Leite, E.L. Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomed. Pharmacother. 2009, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Xu, F.; Ma, X.Y.; Guo, J.Q.; Li, C.C.; Shen, S.D.; Puglia, D.; Chen, J.W.; Xu, P.W.; Kenny, J.; et al. Highly-toughened PVA/ nanocellulose hydrogels with anti-oxidative and antibacterial properties triggered by lignin-Ag nanoparticles. Mat. Sci. Eng. C-Mater. 2021, 129, 112385. [Google Scholar] [CrossRef]
- Schonborn, K.; Willenborg, S.; Schulz, J.N.; Imhof, T.; Eming, S.A.; Quondamatteo, F.; Brinckmann, J.; Niehoff, A.; Paulsson, M.; Koch, M.; et al. Role of collagen XII in skin homeostasis and repair. Matrix Biol. 2020, 94, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.Q.; Nie, S.P.; Wang, Y.X.; Cui, S.W.; Xie, M.Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015, 79, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]



| Composition | Reducing Sugar (%) | Sulfate Group (%) | Uronic Acids (%) | Protein (%) |
|---|---|---|---|---|
| 0-LJP | 6.93 ± 0.21 c | 7.89 ± 0.11 a | 30.31 ± 0.15 e | 1.46 ± 0.03 b |
| 5-LJP | 7.85 ± 0.41 b | 6.95 ± 0.04 b | 33.83 ± 0.36 d | 0.65 ± 0.03 c |
| 10-LJP | 7.92 ± 0.35 b | 6.67 ± 0.08 c | 36.85 ± 0.21 c | 1.84 ± 0.01 a |
| 30-LJP | 8.18 ± 0.47 a | 6.50 ± 0.10 c | 43.34 ± 0.65 b | 0.72 ± 0.03 c |
| 60-LJP | 8.37 ± 0.27 a | 6.35 ± 0.02 d | 47.56 ± 0.48 a | 0.21 ± 0.02 d |
| Gene | Primers Sequences (5′-3′) | Amplicon Size (bp) | |
|---|---|---|---|
| GAPDH | Forward | CCCTCTGGAAAGCTGTGG | 220 |
| Reverse | GCTTCACCACCTTCTTGATGT | ||
| TNF-α | Forward | GCTGCACTTTGGAGTGATCG | 112 |
| Reverse | CTTGTCACTCGGGGTTCGAG | ||
| IL-6 | Forward | CTGACCCAACCACAAATGC | 162 |
| Reverse | TCTGAGGTGCCCATGCTAC | ||
| IL-1β | Forward | CTGTACCTGTCCTGCGTGTT | 199 |
| Reverse | AGACGGGCATGTTTTCTGCT | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Huang, W.; Chen, Y.; Wu, M.; Jia, R.; You, L. Influence of Molecular Weight of Polysaccharides from Laminaria japonica to LJP-Based Hydrogels: Anti-Inflammatory Activity in the Wound Healing Process. Molecules 2022, 27, 6915. https://doi.org/10.3390/molecules27206915
Chen Y, Huang W, Chen Y, Wu M, Jia R, You L. Influence of Molecular Weight of Polysaccharides from Laminaria japonica to LJP-Based Hydrogels: Anti-Inflammatory Activity in the Wound Healing Process. Molecules. 2022; 27(20):6915. https://doi.org/10.3390/molecules27206915
Chicago/Turabian StyleChen, Yifan, Weixuan Huang, Yang Chen, Minqian Wu, Ruohan Jia, and Lijun You. 2022. "Influence of Molecular Weight of Polysaccharides from Laminaria japonica to LJP-Based Hydrogels: Anti-Inflammatory Activity in the Wound Healing Process" Molecules 27, no. 20: 6915. https://doi.org/10.3390/molecules27206915
APA StyleChen, Y., Huang, W., Chen, Y., Wu, M., Jia, R., & You, L. (2022). Influence of Molecular Weight of Polysaccharides from Laminaria japonica to LJP-Based Hydrogels: Anti-Inflammatory Activity in the Wound Healing Process. Molecules, 27(20), 6915. https://doi.org/10.3390/molecules27206915







