In Silico Methods for Identification of Potential Active Sites of Therapeutic Targets
Abstract
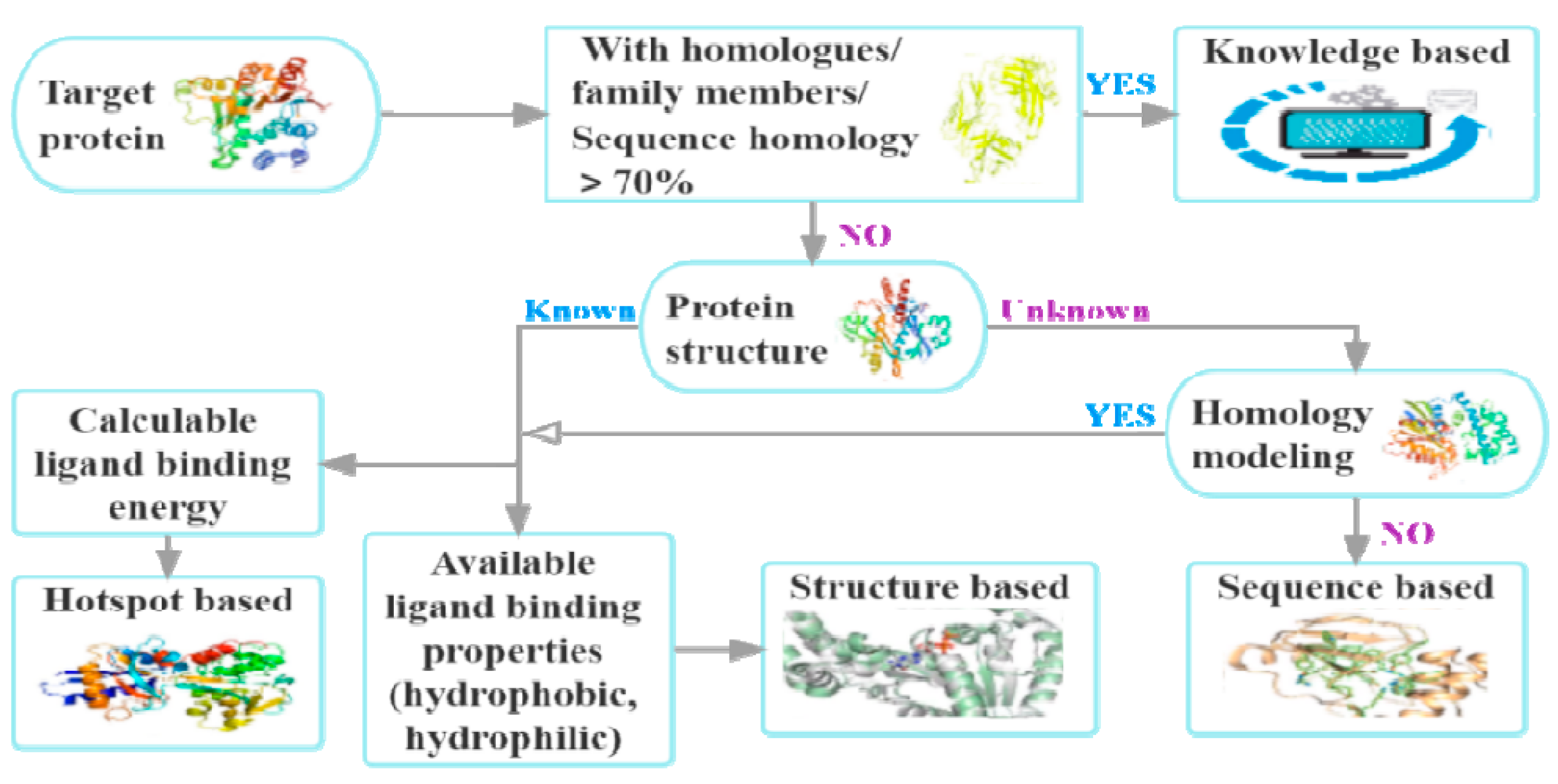
:1. Introduction
2. Methods for Target Identification
2.1. Binding Site Identification
2.1.1. Ligand-Specific Methods
2.1.2. General-Purpose Methods
Sequence-Based
Structure-Based
Consensus-Based
2.1.3. Machine Learning
2.2. Summary of Binding Site Identification Methods
2.3. Assessment of Druggability
2.3.1. Knowledge-Based
2.3.2. Sequence-Based
2.3.3. Structure-Based
2.3.4. Hotspot-Based
2.4. Summary of Druggability Evaluation Methods
2.5. Differences in Binding Site Identification and Druggability Evaluation Methods
3. Software and Tools
3.1. Binding Site Identification
3.1.1. MetaPocket 2.0
3.1.2. COACH
3.2. Binding Site Identification and Druggability Evaluation
3.2.1. PockDrug
3.2.2. FTMap
3.2.3. Sitemap
4. Databases
4.1. Resource Database
4.2. Probe Database
4.3. Benchmark Datasets
5. Application
5.1. Binding Site Identification
5.2. Binding Site Identification and Druggability Assessment
| Year | Database | Modeling Tool/Software | Tool for Model Quality Assessment | Tool | Prediction Result | Reference |
|---|---|---|---|---|---|---|
| 2016 | PDB | Swiss-Model [153] | QMEAN [154], PROCHECK [155], ProSA [156], Verify3D [157] | Fpocket | 4 binding pockets | [158] |
| 2016 | PDB | GROMACS program suite | MetaPocket | 7 binding sites | [147] | |
| 2016 | PDB | Phenix [159] | MolProbity [160] | MetaPocket 2.0 | 3 binding pockets | [161] |
| 2016 | UniProtKB [162], PDB | Molecular Operating Environment | Site Finder | 3 binding pockets | [163] | |
| 2016 | UniProt | Modeller [164] | PROCHECK, ProSA, Swiss-PDB Viewer [165] | CASTp [36], Q-SiteFinder, Sitemap | CASTp: 2 binding cavities, 11 binding residues; Q-SiteFinder: 2 binding cavities, 11 binding residues; R-Sitemap: 1 binding site region, 7 binding residues | [165] |
| 2017 | PDB | HHPred [166], RaptorX [167], (PS)2 server [168], Modeller | RAMPAGE [169], QMEAN | COACH | 2 binding sites, 17 binding residues | [148] |
| 2017 | PDB | Modeller | SAVES [170], ProSA | Sitemap | 13 binding residues | [171] |
| 2017 | NCBI, PDB | NAMD | —— | FTMap | 5 binding sites, 41 binding residues | [172] |
| 2017 | NCBI | I-TASSER | PROCHECK, ProSA, QMEANclust [173] | COACH | 1 binding site, 18 binding residues | [174] |
| 2018 | Uniprot | Swiss-Model, PRIME module of Schrödinger | ProtParam [175], PROCHECK | Sitemap | 4 binding sites | [176] |
| 2019 | PDB | Modeller | PROCHECK, ProSA | Sitemap | 4 binding cavities | [177] |
| 2019 | UniProt | Modeller | SAVES, PROCHECK, Verify3D | Sitemap | 1 binding pocket, 19 binding residues | [178] |
| 2019 | PDB | —— | —— | FTSite | 18, 29, and 40 binding residues on 3 proteins | [179] |
| 2020 | UniProt, PDB | Swiss-Model | TM-align server [180] | LISE, Sitemap | 1 consensus binding site | [181] |
| 2020 | PDB, UniProt | Modeller | ProSA, Verify3D | CPORT [182], Sitemap | 1 consensus binding site, 38 binding residues | [183] |
| 2020 | Uniprot, PDB | Modeller | PROCHECK, Verify3D, ProSA | CASTp, Sitemap, PatchDock [184] | CASTp: 10 binding residues Sitemap: 16 binding residues PatchDock: 3 binding residues | [185] |
| 2020 | PDB | PHYRE2 software [186] | PSVS server, PROCHECK, Verify3D, ProSA | Sitemap | 90 binding residues | [187] |
| 2021 | PDB, UniProt, GenBank, Pharos, PubChem | Swiss-Model | PROCHECK, ProSA, ProQ, Verify3D, PROVE, ERRAT [188] | DoGSite | 3 binding pockets | [189] |
6. Discussion
6.1. Comparison of Tools for Identification of Potential Drug Targets
6.1.1. PockDrug
- (1)
- It provides both the average druggability probability and its corresponding standard deviation [112];
- (2)
- The server accepts any structures, including X-ray, NMR, homology, or docking structures, in PDB format as input [112];
- (3)
- The PockDrug model can be used to directly score the druggability of pockets based on the results of pocket estimation methods, and, importantly, it is valid for different pocket estimation methods [112];
- (4)
- A comparison of PockDrug, Fpocket, and DoGSite in terms of the prediction sensitivity, accuracy, and MCC suggests that PockDrug performs better than Fpocket and DoGSite [112].
6.1.2. FTMap
- (1)
- The computerized results of FTMap are consistent with the experimental results of NMR-based screening, demonstrating the accuracy of hotspot prediction [60];
- (2)
- The probe types used in FTMap can accurately identify binding sites and provide the robustness required to eliminate false positives (e.g., sites within narrow lumens) [116];
- (3)
- The use of a detailed energy expression profile to locate probes on the surface of sampled proteins and the Fourier transform correlation approach ensures its high accuracy [60];
- (4)
- The method does not need a training dataset and thus does not depend on the quality, size, and diversity of the benchmark and validation datasets, which can minimize the potential effect of pocket predictions with different accuracies on the subsequent evaluation of druggability;
- (5)
- The method can be employed for all types of protein structures for site prediction without prior knowledge of similar structures or potential binding sites [116].
6.1.3. Sitemap
- (1)
- The performance of Sitemap for large-scale validation/test datasets is excellent with 86% and 96% accuracy, which is higher than that of Fpocket, DoGSiteScorer, and PockDrug [37];
- (2)
- Sitemap provides quantitative and graphical information about the active site, which can help guide the modification of the ligand structure. In particular, its interface can be divided into hydrophilic, hydrophobic, and neither hydrophilic nor hydrophobic regions [37]. As an example, this can help us to determine whether there is space to accommodate hydrophobic regions with larger hydrophobic groups to help design better ligands with stronger binding affinity. Modifying the ligand’s physical properties to improve potency can facilitate the subsequent molecular docking or virtual screening in drug design [37];
- (3)
- The structures of most proteins used in drug prediction are currently unknown; thus, homology modeling is required. The Prime module of the Schrödinger software package allows homology modeling, providing convenience through the use of the same software [37];
- (4)
- Prediction by Sitemap is more accurate for enzyme sites than for receptor sites [7].
6.2. Comparison of PockDrug, FTMap, and Sitemap
6.3. Recommended Methods for Identification of Potential Target Binding Sites
6.4. Previous Reviews of Binding Site Identification and Druggability Assessment
6.4.1. Binding Site Identification
6.4.2. Druggability Assessment
6.4.3. Comparison between Previous Reviews and This Review
6.5. Potential and Improvement of Methods for Identification of Potential Drug Targets
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leelananda, S.P.; Lindert, S. Computational methods in drug discovery. Beilstein J. Org. Chem. 2016, 12, 2694–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Yang, N.; Zhan, X.; Liao, J.; Mai, S.; Huang, Z. In Silico Methods for Identification of Potential Therapeutic Targets. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Egner, U.; Hillig, R.C. A structural biology view of target drugability. Expert Opin. Drug Discov. 2008, 3, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Nisius, B.; Sha, F.; Gohlke, H. Structure-based computational analysis of protein binding sites for function and druggability prediction. J. Biotechnol. 2012, 159, 123–134. [Google Scholar] [CrossRef]
- Capra, J.A.; Laskowski, R.A.; Thornton, J.M.; Singh, M.; Funkhouser, T.A. Predicting Protein Ligand Binding Sites by Combining Evolutionary Sequence Conservation and 3D Structure. PLoS Comput. Biol. 2009, 5, e1000585. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T.A. Identifying and Characterizing Binding Sites and Assessing Druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Gassmann, O.; Hinder, M. Changing R&D models in research-based pharmaceutical companies. J. Transl. Med. 2016, 14, 105. [Google Scholar]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Cole, C.; Barber, J.D.; Barton, G.J. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008, 36 (Suppl. 2), W197–W201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Brenk, R. To Hit or Not to Hit, That Is the Question—Genome-wide Structure-Based Druggability Predictions for Pseudomonas aeruginosa Proteins. PLoS ONE 2015, 10, e0137279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyaanamoorthy, S.; Chen, Y.-P.P. Structure-based drug design to augment hit discovery. Drug Discov. Today 2011, 16, 831–839. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zhang, Y.; Yu, D.-J. ATPbind: Accurate Protein–ATP Binding Site Prediction by Combining Sequence-Profiling and Structure-Based Comparisons. J. Chem. Inf. Model. 2018, 58, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Babor, M.; Gerzon, S.; Raveh, B.; Sobolev, V.; Edelman, M. Prediction of transition metal-binding sites from apo protein structures. Proteins Struct. Funct. Bioinform. 2008, 70, 208–217. [Google Scholar] [CrossRef]
- Brylinski, M.; Skolnick, J. FINDSITE-metal: Integrating evolutionary information and machine learning for structure-based metal-binding site prediction at the proteome level. Proteins Struct. Funct. Bioinform. 2011, 79, 735–751. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Dong, Q.; Yang, J.; Zhang, Y. Recognizing metal and acid radical ion-binding sites by integrating ab initio modeling with template-based transferals. Bioinformatics 2016, 32, 3260–3269. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Zhang, Z.; Lin, B.; Schroeder, M.; Huang, B. MetaDBSite: A meta approach to improve protein DNA-binding sites prediction. BMC Syst. Biol. 2011, 5, S7. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Guo, J.; Liu, H.D.; Xie, J.M.; Sun, X. Sequence-Based Prediction of DNA-Binding Residues in Proteins with Conservation and Correlation Information. IEEE/ACM Trans. Comput. Biol. Bioinform. 2012, 9, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Zhang, M.; Yang, X.; Shen, H.B.; Yu, D.J. Predicting Protein-DNA Binding Residues by Weightedly Combining Sequence-Based Features and Boosting Multiple SVMs. IEEE/ACM Trans. Comput. Biol. Bioinform. 2017, 14, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kurgan, L. DRNApred, fast sequence-based method that accurately predicts and discriminates DNA-and RNA-binding residues. Nucleic Acids Res. 2017, 45, e84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.J.; Hu, J.; Yang, J.; Shen, H.B.; Tang, J.; Yang, J.Y. Designing Template-Free Predictor for Targeting Protein-Ligand Binding Sites with Classifier Ensemble and Spatial Clustering. IEEE/ACM Trans. Comput. Biol. Bioinform. 2013, 10, 994–1008. [Google Scholar] [CrossRef]
- Hu, J.; He, X.; Yu, D.-J.; Yang, X.-B.; Yang, J.-Y.; Shen, H.-B. A New Supervised Over-Sampling Algorithm with Application to Protein-Nucleotide Binding Residue Prediction. PLoS ONE 2014, 9, e107676. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Yan, W.-X.; Yang, J.-Y.; Shen, H.-B.; Yu, D.-J. KNN-based dynamic query-driven sample rescaling strategy for class imbalance learning. Neurocomputing 2016, 191, 363–373. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Z.; Zhang, E.; He, F.; Ma, Z.; Wang, H. MPLs-Pred: Predicting Membrane Protein-Ligand Binding Sites Using Hybrid Sequence-Based Features and Ligand-Specific Models. Int. J. Mol. Sci. 2019, 20, 3120. [Google Scholar] [CrossRef] [Green Version]
- Capra, J.A.; Singh, M. Predicting functionally important residues from sequence conservation. Bioinformatics 2007, 23, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Roy, A.; Zhang, Y. Protein–ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment. Bioinformatics 2013, 29, 2588–2595. [Google Scholar] [CrossRef] [Green Version]
- Toti, D.; Viet Hung, L.; Tortosa, V.; Brandi, V.; Polticelli, F. LIBRA-WA: A web application for ligand binding site detection and protein function recognition. Bioinformatics 2018, 34, 878–880. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, Q.; Liu, M.; Zhu, L.; Wu, D.; Cao, Z.; Zhu, R. bSiteFinder, an improved protein-binding sites prediction server based on structural alignment: More accurate and less time-consuming. J. Cheminform. 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Dey, F.; Cliff Zhang, Q.; Petrey, D.; Honig, B. Toward a “Structural BLAST”: Using structural relationships to infer function. Protein Sci. 2013, 22, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinstein, W.; Brylinski, M. eFindSite: Enhanced Fingerprint-Based Virtual Screening Against Predicted Ligand Binding Sites in Protein Models. Mol. Inform. 2014, 33, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Yang, J.; Zhang, Y. COFACTOR: An accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res. 2012, 40, W471–W477. [Google Scholar] [CrossRef] [Green Version]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tufféry, P. fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38 (Suppl. 2), W582–W589. [Google Scholar] [CrossRef] [Green Version]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 2003, 31, 3352–3355. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T. New Method for Fast and Accurate Binding-site Identification and Analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Z.; Bian, Y.; Wei, Z. A novel protein descriptor for the prediction of drug binding sites. BMC Bioinform. 2019, 20, 478. [Google Scholar] [CrossRef]
- Hernandez, M.; Ghersi, D.; Sanchez, R. SITEHOUND-web: A server for ligand binding site identification in protein structures. Nucleic Acids Res. 2009, 37 (Suppl. 2), W413–W416. [Google Scholar] [CrossRef]
- Ngan, C.-H.; Hall, D.R.; Zerbe, B.; Grove, L.E.; Kozakov, D.; Vajda, S. FTSite: High accuracy detection of ligand binding sites on unbound protein structures. Bioinformatics 2012, 28, 286–287. [Google Scholar] [CrossRef] [Green Version]
- Huang, B. MetaPocket: A Meta Approach to Improve Protein Ligand Binding Site Prediction. OMICS A J. Integr. Biol. 2009, 13, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Lin, B.; Schroeder, M.; Huang, B. Identification of cavities on protein surface using multiple computational approaches for drug binding site prediction. Bioinformatics 2011, 27, 2083–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivák, R.; Hoksza, D. P2Rank: Machine learning based tool for rapid and accurate prediction of ligand binding sites from protein structure. J. Cheminform. 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-Generation Machine Learning for Biological Networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakal, A.; McKay, C.; Tanner, J.J.; Cheng, J. Artificial intelligence in the prediction of protein-ligand interactions: Recent advances and future directions. Brief. Bioinform. 2022, 23, bbab476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, Y.; Zhang, L. Exploring the computational methods for protein-ligand binding site prediction. Comput. Struct. Biotechnol. J. 2020, 18, 417–426. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Zhao, Y. SXGBsite: Prediction of Protein–Ligand Binding Sites Using Sequence Information and Extreme Gradient Boosting. Genes 2019, 10, 965. [Google Scholar] [CrossRef] [Green Version]
- Seco, J.; Luque, F.J.; Barril, X. Binding Site Detection and Druggability Index from First Principles. J. Med. Chem. 2009, 52, 2363–2371. [Google Scholar] [CrossRef]
- Fauman, E.B.; Rai, B.K.; Huang, E.S. Structure-based druggability assessment—Identifying suitable targets for small molecule therapeutics. Curr. Opin. Chem. Biol. 2011, 15, 463–468. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Huth, J.R.; Fesik, S.W. Druggability Indices for Protein Targets Derived from NMR-Based Screening Data. J. Med. Chem. 2005, 48, 2518–2525. [Google Scholar] [CrossRef]
- Yuan, Y.; Pei, J.; Lai, L. Binding Site Detection and Druggability Prediction of Protein Targets for Structure-Based Drug Design. Curr. Pharm. Des. 2012, 19, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Barril, X. Druggability predictions: Methods, limitations, and applications. WIREs Comput. Mol. Sci. 2013, 3, 327–338. [Google Scholar] [CrossRef]
- Froes, T.Q.; Baldini, R.L.; Vajda, S.; Castilho, M.S. Structure-based Druggability Assessment of Anti-virulence Targets from Pseudomonas aeruginosa. Curr. Protein Pept. Sci. 2019, 20, 1189–1203. [Google Scholar] [CrossRef]
- Finan, C.; Gaulton, A.; Kruger, F.A.; Lumbers, R.T.; Shah, T.; Engmann, J.; Galver, L.; Kelley, R.; Karlsson, A.; Santos, R.; et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 2017, 9, eaag1166. [Google Scholar] [CrossRef]
- Kandoi, G.; Acencio, M.L.; Lemke, N. Prediction of Druggable Proteins Using Machine Learning and Systems Biology: A Mini-Review. Front. Physiol. 2015, 6, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, P.J.; Huth, J.R.; Tse, C. Predicting protein druggability. Drug Discov. Today 2005, 10, 1675–1682. [Google Scholar] [CrossRef]
- DeLano, W.L. Unraveling hot spots in binding interfaces: Progress and challenges. Curr. Opin. Struct. Biol. 2002, 12, 14–20. [Google Scholar] [CrossRef]
- Vajda, S.; Guarnieri, F. Characterization of protein-ligand interaction sites using experimental and computational methods. Curr. Opin. Drug Discov. Dev. 2006, 9, 354–362. [Google Scholar]
- Brenke, R.; Kozakov, D.; Chuang, G.Y.; Beglov, D.; Hall, D.; Landon, M.R.; Mattos, C.; Vajda, S. Fragment-based identification of druggable ‘hot spots’ of proteins using Fourier domain correlation techniques. Bioinformatics 2009, 25, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Mattos, C.; Ringe, D. Locating and characterizing binding sites on proteins. Nat. Biotechnol. 1996, 14, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Tayara, H.; Zou, Q.; Chong, K.T. TS-m6A-DL: Tissue-specific identification of N6-methyladenosine sites using a universal deep learning model. Comput. Struct. Biotechnol. J. 2021, 19, 4619–4625. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Mishra, G.; Raghava, G.P.S. SAMbinder: A Web Server for Predicting S-Adenosyl-L-Methionine Binding Residues of a Protein from Its Amino Acid Sequence. Front. Pharmacol. 2020, 10, 1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, J.S.; Mishra, N.K.; Raghava, G.P.S. Identification of ATP binding residues of a protein from its primary sequence. BMC Bioinform. 2009, 10, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Hou, J.; Shi, X.; Yang, H.; Birchler, J.A.; Cheng, J. DeepGRN: Prediction of transcription factor binding site across cell-types using attention-based deep neural networks. BMC Bioinform. 2021, 22, 38. [Google Scholar] [CrossRef]
- Chen, K.; Mizianty, M.J.; Kurgan, L. ATPsite: Sequence-based prediction of ATP-binding residues. Proteome Sci. 2011, 9, S4. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Mizianty, M.J.; Kurgan, L. Prediction and analysis of nucleotide-binding residues using sequence and sequence-derived structural descriptors. Bioinformatics 2012, 28, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Khamis, A.M.; Motwalli, O.; Oliva, R.; Jankovic, B.R.; Medvedeva, Y.A.; Ashoor, H.; Essack, M.; Gao, X.; Bajic, V.B. A novel method for improved accuracy of transcription factor binding site prediction. Nucleic Acids Res. 2018, 46, e72. [Google Scholar] [CrossRef] [Green Version]
- Le, N.-Q.-K.; Ou, Y.-Y. Incorporating efficient radial basis function networks and significant amino acid pairs for predicting GTP binding sites in transport proteins. BMC Bioinform. 2016, 17, 501. [Google Scholar] [CrossRef] [Green Version]
- Le, N.-Q.-K.; Ou, Y.-Y. Prediction of FAD binding sites in electron transport proteins according to efficient radial basis function networks and significant amino acid pairs. BMC Bioinform. 2016, 17, 298. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Q.; Liu, Z.; Shen, H.B.; Yu, D.J. TargetM6A: Identifying N6-Methyladenosine Sites from RNA Sequences via Position-Specific Nucleotide Propensities and a Support Vector Machine. IEEE Trans. NanoBiosci. 2016, 15, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, Y. Fast decoding cell type–specific transcription factor binding landscape at single-nucleotide resolution. Genome Res. 2021, 31, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Quang, D.; Guan, Y. Anchor: Trans-cell type prediction of transcription factor binding sites. Genome Res. 2019, 29, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Panwar, B.; Gupta, S.; Raghava, G.P.S. Prediction of vitamin interacting residues in a vitamin binding protein using evolutionary information. BMC Bioinform. 2013, 14, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quang, D.; Xie, X. FactorNet: A deep learning framework for predicting cell type specific transcription factor binding from nucleotide-resolution sequential data. Methods 2019, 166, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y. RBinds: A user-friendly server for RNA binding site prediction. Comput. Struct. Biotechnol. J. 2020, 18, 3762–3765. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.-Q.; Pan, X.; Shen, H.-B. GraphBind: Protein structural context embedded rules learned by hierarchical graph neural networks for recognizing nucleic-acid-binding residues. Nucleic Acids Res. 2021, 49, e51. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, Z.; Ma, Z.; Li, X.; Wong, K.-C. iCircRBP-DHN: Identification of circRNA-RBP interaction sites using deep hierarchical network. Brief. Bioinform. 2021, 22, bbaa274. [Google Scholar] [CrossRef]
- Yu, D.-J.; Hu, J.; Huang, Y.; Shen, H.-B.; Qi, Y.; Tang, Z.-M.; Yang, J.-Y. TargetATPsite: A template-free method for ATP-binding sites prediction with residue evolution image sparse representation and classifier ensemble. J. Comput. Chem. 2013, 34, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chai, H.; Gao, B.; Yang, G.; Ma, Z. HEMEsPred: Structure-Based Ligand-Specific Heme Binding Residues Prediction by Using Fast-Adaptive Ensemble Learning Scheme. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 147–156. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Q.; Gui, L.; Xu, R.; Long, Y.; Wang, H. MTTFsite: Cross-cell type TF binding site prediction by using multi-task learning. Bioinformatics 2019, 35, 5067–5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipanahi, B.; Delong, A.; Weirauch, M.T.; Frey, B.J. Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.; Mangone, I.; Ferrè, F.; Helmer-Citterich, M.; Ausiello, G. webPDBinder: A server for the identification of ligand binding sites on protein structures. Nucleic Acids Res. 2013, 41, W308–W313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Dong, Q.; Hong, D.; Wang, X. Predicting protein-ligand binding residues with deep convolutional neural networks. BMC Bioinform. 2019, 20, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, A.D.; Bitencourt-Ferreira, G.; de Azevedo Jr, W.F. Taba: A Tool to Analyze the Binding Affinity. J. Comput. Chem. 2020, 41, 69–73. [Google Scholar] [CrossRef]
- Heo, L.; Shin, W.-H.; Lee, M.S.; Seok, C. GalaxySite: Ligand-binding-site prediction by using molecular docking. Nucleic Acids Res. 2014, 42, W210–W214. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017, 33, 3036–3042. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, J.; Škalič, M.; Martínez-Rosell, G.; De Fabritiis, G. KDEEP: Protein–Ligand Absolute Binding Affinity Prediction via 3D-Convolutional Neural Networks. J. Chem. Inf. Model. 2018, 58, 287–296. [Google Scholar] [CrossRef]
- Kandel, J.; Tayara, H.; Chong, K.T. PUResNet: Prediction of protein-ligand binding sites using deep residual neural network. J. Cheminform. 2021, 13, 65. [Google Scholar] [CrossRef]
- Lee, I.; Keum, J.; Nam, H. DeepConv-DTI: Prediction of drug-target interactions via deep learning with convolution on protein sequences. PLoS Comput. Biol. 2019, 15, e1007129. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Yoo, S.; Sanchez, R. SiteComp: A server for ligand binding site analysis in protein structures. Bioinformatics 2012, 28, 1172–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Grimm, M.; Dai, W.-t.; Hou, M.-c.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, H.; Özgür, A.; Ozkirimli, E. DeepDTA: Deep drug–target binding affinity prediction. Bioinformatics 2018, 34, i821–i829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, L.; Govindaraj, R.G.; Lemoine, J.M.; Wu, H.-C.; Brylinski, M. DeepDrug3D: Classification of ligand-binding pockets in proteins with a convolutional neural network. PLoS Comput. Biol. 2019, 15, e1006718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, D.B.; Tetchner, S.J.; McGuffin, L.J. FunFOLD: An improved automated method for the prediction of ligand binding residues using 3D models of proteins. BMC Bioinform. 2011, 12, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Garcia, R.; Sorzano CO, S.; Carazo, J.M.; Segura, J. BIPSPI: A method for the prediction of partner-specific protein–protein interfaces. Bioinformatics 2019, 35, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Viet Hung, L.; Caprari, S.; Bizai, M.; Toti, D.; Polticelli, F. LIBRA: LIgand Binding site Recognition Application. Bioinformatics 2015, 31, 4020–4022. [Google Scholar] [CrossRef]
- Wu, Q.; Peng, Z.; Zhang, Y.; Yang, J. COACH-D: Improved protein–ligand binding sites prediction with refined ligand-binding poses through molecular docking. Nucleic Acids Res. 2018, 46, W438–W442. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.-R.; Liu, C.-K.; Hsiao, F.-C.; Yao, A.; Hwang, M.-J. LISE: A server using ligand-interacting and site-enriched protein triangles for prediction of ligand-binding sites. Nucleic Acids Res. 2013, 41, W292–W296. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Fan, J.; Mu, Y. OnionNet: A Multiple-Layer Intermolecular-Contact-Based Convolutional Neural Network for Protein–Ligand Binding Affinity Prediction. ACS Omega 2019, 4, 15956–15965. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Pisabarro, M.T. MSPocket: An orientation-independent algorithm for the detection of ligand binding pockets. Bioinformatics 2011, 27, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Xiong, Y.; Kihara, D. Large-scale binding ligand prediction by improved patch-based method Patch-Surfer2.0. Bioinformatics 2015, 31, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Schroeder, M. LIGSITEcsc: Predicting ligand binding sites using the Connolly surface and degree of conservation. BMC Struct. Biol. 2006, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Brady, G.P., Jr.; Stouten, P.F. Fast prediction and visualization of protein binding pockets with PASS. J. Comput.-Aided Mol. Des. 2000, 14, 383–401. [Google Scholar] [CrossRef]
- Laurie, A.T.; Jackson, R.M. Q-SiteFinder: An energy-based method for the prediction of protein-ligand binding sites. Bioinformatics 2005, 21, 1908–1916. [Google Scholar] [CrossRef]
- Laskowski, R.A. SURFNET: A program for visualizing molecular surfaces, cavities, and intermolecular interactions. J. Mol. Graph. 1995, 13, 323–330. [Google Scholar] [CrossRef]
- Kawabata, T. Detection of multiscale pockets on protein surfaces using mathematical morphology. Proteins 2010, 78, 1195–1211. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Y.; Tanaka, I.; Yao, M. Roll: A new algorithm for the detection of protein pockets and cavities with a rolling probe sphere. Bioinformatics 2010, 26, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Brylinski, M.; Skolnick, J. A threading-based method (FINDSITE) for ligand-binding site prediction and functional annotation. Proc. Natl. Acad. Sci. USA 2008, 105, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, H.A.; Borrel, A.; Geneix, C.; Petitjean, M.; Regad, L.; Camproux, A.-C. PockDrug-Server: A new web server for predicting pocket druggability on holo and apo proteins. Nucleic Acids Res. 2015, 43, W436–W442. [Google Scholar] [CrossRef] [PubMed]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngan, C.H.; Bohnuud, T.; Mottarella, S.E.; Beglov, D.; Villar, E.A.; Hall, D.R.; Kozakov, D.; Vajda, S. FTMAP: Extended protein mapping with user-selected probe molecules. Nucleic Acids Res. 2012, 40, W271–W275. [Google Scholar] [CrossRef]
- Volkamer, A.; Kuhn, D.; Rippmann, F.; Rarey, M. DoGSiteScorer: A web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics 2012, 28, 2074–2075. [Google Scholar] [CrossRef] [Green Version]
- Kozakov, D.; Grove, L.E.; Hall, D.R.; Bohnuud, T.; Mottarella, S.E.; Luo, L.; Xia, B.; Beglov, D.; Vajda, S. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 2015, 10, 733–755. [Google Scholar] [CrossRef] [Green Version]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Costanzo, L.D.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer TA, P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef]
- Yang, J.; Roy, A.; Zhang, Y. BioLiP: A semi-manually curated database for biologically relevant ligand–protein interactions. Nucleic Acids Res. 2013, 41, D1096–D1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Fain, K.M.; Nelson, J.T.; Tse, T.; Williams, R.J. Race and ethnicity reporting for clinical trials in ClinicalTrials.gov and publications. Contemp. Clin. Trials 2021, 101, 106237. [Google Scholar] [CrossRef]
- Avram, S.; Bologa, C.G.; Holmes, J.; Bocci, G.; Wilson, T.B.; Nguyen, D.-T.; Curpan, R.; Halip, L.; Bora, A.; Yang, J.J.; et al. DrugCentral 2021 supports drug discovery and repositioning. Nucleic Acids Res. 2021, 49, D1160–D1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Lin, Y.; Wen, X.; Jorissen, R.N.; Gilson, M.K. BindingDB: A web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res. 2007, 35 (Suppl. 1), D198–D201. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Li, H.; Zhang, H.; Liu, X.; Kang, L.; Luo, X.; Zhu, W.; Chen, K.; Wang, X.; Jiang, H. PDTD: A web-accessible protein database for drug target identification. BMC Bioinform. 2008, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Harding, S.D.; Sharman, J.L.; Faccenda, E.; Southan, C.; Pawson, A.J.; Ireland, S.; Gray AJ, G.; Bruce, L.; Alexander SP, H.; Anderton, S.; et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018, 46, D1091–D1106. [Google Scholar] [CrossRef] [Green Version]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ji, Z.L.; Chen, Y.Z. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002, 30, 412–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Jaziri, F.; Parisot, N.; Abid, A.; Denonfoux, J.; Ribière, C.; Gasc, C.; Boucher, D.; Brugère, J.-F.; Mahul, A.; Hill, D.R.C.; et al. PhylOPDb: A 16S rRNA oligonucleotide probe database for prokaryotic identification. Database 2014, 2014, bau036. [Google Scholar] [CrossRef] [PubMed]
- Greuter, D.; Loy, A.; Horn, M.; Rattei, T. probeBase—An online resource for rRNA-targeted oligonucleotide probes and primers: New features 2016. Nucleic Acids Res. 2016, 44, D586–D589. [Google Scholar] [CrossRef]
- Morgan, B.S.; Sanaba, B.G.; Donlic, A.; Karloff, D.B.; Forte, J.E.; Zhang, Y.; Hargrove, A.E. R-BIND: An Interactive Database for Exploring and Developing RNA-Targeted Chemical Probes. ACS Chem. Biol. 2019, 14, 2691–2700. [Google Scholar] [CrossRef]
- Pattyn, F.; Speleman, F.; De Paepe, A.; Vandesompele, J. RTPrimerDB: The Real-Time PCR primer and probe database. Nucleic Acids Res. 2003, 31, 122–123. [Google Scholar] [CrossRef] [Green Version]
- Puvanendrampillai, D.; Mitchell, J.B. L/D Protein Ligand Database (PLD): Additional understanding of the nature and specificity of protein-ligand complexes. Bioinformatics 2003, 19, 1856–1857. [Google Scholar] [CrossRef] [Green Version]
- Glaser, F.; Rosenberg, Y.; Kessel, A.; Pupko, T.; Ben-Tal, N. The ConSurf-HSSP database: The mapping of evolutionary conservation among homologs onto PDB structures. Proteins 2005, 58, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Volkamer, A.; Griewel, A.; Grombacher, T.; Rarey, M. Analyzing the Topology of Active Sites: On the Prediction of Pockets and Subpockets. J. Chem. Inf. Model. 2010, 50, 2041–2052. [Google Scholar] [CrossRef]
- Weisel, M.; Proschak, E.; Schneider, G. PocketPicker: Analysis of ligand binding-sites with shape descriptors. Chem. Cent. J. 2007, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Glantz-Gashai, Y.; Meirson, T.; Samson, A.O. Normal Modes Expose Active Sites in Enzymes. PLoS Comput. Biol. 2016, 12, e1005293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.; Kellogg, G.E. A novel and efficient tool for locating and characterizing protein cavities and binding sites. Proteins 2010, 78, 825–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, J.-W.; Elumalai, P.; Pitti, T.; Wu, C.Y.; Tsai, K.-C.; Chang, J.-Y.; Peng, H.-P.; Yang, A.-S. Predicting Ligand Binding Sites on Protein Surfaces by 3-Dimensional Probability Density Distributions of Interacting Atoms. PLoS ONE 2016, 11, e0160315. [Google Scholar] [CrossRef]
- Wass, M.N.; Kelley, L.A.; Sternberg, M.J.E. 3DLigandSite: Predicting ligand-binding sites using similar structures. Nucleic Acids Res. 2010, 38 (Suppl. 2), W469–W473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, C.; Schneider, R.; Sander, C. The HSSP database of protein structure—Sequence alignments and family profiles. Nucleic Acids Res. 1998, 26, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Porter, C.T.; Bartlett, G.J.; Thornton, J.M. The Catalytic Site Atlas: A resource of catalytic sites and residues identified in enzymes using structural data. Nucleic Acids Res. 2004, 32 (Suppl. 1), D129–D133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.; Mu, Y.; Miao, H.; Yang, Y.; Wu, Q.; Li, J.; Ding, J.; Xu, B.; Huang, Z. The 340-cavity in neuraminidase provides new opportunities for influenza drug development: A molecular dynamics simulation study. Biochem. Biophys. Res. Commun. 2016, 470, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.M. Computational analysis, structural modeling and ligand binding site prediction of Plasmodium falciparum 1-deoxy-d-xylulose-5-phosphate synthase. Comput. Biol. Chem. 2017, 66, 1–10. [Google Scholar] [CrossRef]
- Riccio, G.; De Luca, D.; Lauritano, C. Monogalactosyldiacylglycerol and Sulfolipid Synthesis in Microalgae. Mar. Drugs 2020, 18, 237. [Google Scholar] [CrossRef]
- Du, Q.; Qian, Y.; Xue, W. Molecular Simulation of Oncostatin M and Receptor (OSM–OSMR) Interaction as a Potential Therapeutic Target for Inflammatory Bowel Disease. Front. Mol. Biosci. 2020, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Deepshikha, D.; Srivastava, P. Homology Modeling and Protein Interaction Map of CHRNA7 Neurogenesis Protein. Ann. Neurosci. 2017, 24, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniji, E.A.; Olotu, F.A.; Soliman, M.E.S. Exploring the Lapse in Druggability: Sequence Analysis, Structural Dynamics and Binding Site Characterization of K-RasG12C Variant, a Feasible Oncotherapeutics Target. Anti-Cancer Agents Med. Chem. 2018, 18, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.; Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Moss, D.S.; Thornton, J.M. Main-chain Bond Lengths and Bond Angles in Protein Structures. J. Mol. Biol. 1993, 231, 1049–1067. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35 (Suppl. 2), W407–W410. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [PubMed]
- Sebastián-Pérez, V.; Manoli, M.-T.; Pérez, D.I.; Gil, C.; Mellado, E.; Martínez, A.; Espeso, E.A.; Campillo, N.E. New applications for known drugs: Human glycogen synthase kinase 3 inhibitors as modulators of Aspergillus fumigatus growth. Eur. J. Med. Chem. 2016, 116, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66 Pt 2, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, V.B.; Arendall, W.B., III; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66 Pt 1, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Nishitani, Y.; Watanabe, S.; Hirao, Y.; Imanaka, T.; Kanai, T.; Atomi, H.; Miki, K. Crystal structure of a [NiFe] hydrogenase maturation protease HybD from Thermococcus kodakarensis KOD1. Proteins 2016, 84, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Boutet, E.; Lieberherr, D.; Tognolli, M.; Schneider, M.; Bairoch, A. UniProtKB/Swiss-Prot. Methods Mol. Biol. 2007, 406, 89–112. [Google Scholar]
- Ogindo, C.O.; Khraiwesh, M.H.; George Jr., M.; Brandy, Y.; Brandy, N.; Gugssa, A.; Ashraf, M.; Abbas, M.; Southerland, W.M.; Lee, C.M.; et al. Novel drug design for Chagas disease via targeting Trypanosoma cruzi tubulin: Homology modeling and binding pocket prediction on Trypanosoma cruzi tubulin polymerization inhibition by naphthoquinone derivatives. Bioorg. Med. Chem. 2016, 24, 3849–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar] [PubMed]
- Ramatenki, V.; Dumpati, R.; Vadija, R.; Vellanki, S.; Potlapally, S.R.; Rondla, R.; Vuruputuri, U. Targeting the ubiquitin-conjugating enzyme E2D4 for cancer drug discovery—A structure-based approach. J. Chem. Biol. 2017, 10, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Biegert, A.; Mayer, C.; Remmert, M.; Söding, J.; Lupas, A.N. The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res. 2006, 34 (Suppl. 2), W335–W339. [Google Scholar] [CrossRef] [Green Version]
- Källberg, M.; Wang, H.; Wang, S.; Peng, J.; Wang, Z.; Lu, H.; Xu, J. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 2012, 7, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-T.; Hwang, J.-K.; Chen, C.-H.; Chu, C.-S.; Lee, C.-W.; Chen, C.-C. (PS)2: Protein structure prediction server version 3.0. Nucleic Acids Res. 2015, 43, W338–W342. [Google Scholar] [CrossRef] [Green Version]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., 3rd; de Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ,ψ and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gaur, K.; Tiwari, R.K.; Gaur, M.S. Computational interaction analysis of organophosphorus pesticides with different metabolic proteins in humans. J. Biomed. Res. 2011, 25, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Bhargavi, M.; Sivan, S.K.; Potlapally, S.R. Identification of novel anti cancer agents by applying insilico methods for inhibition of TSPO protein. Comput. Biol. Chem. 2017, 68, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Caliman, A.D.; Miao, Y.; McCammon, J.A. Mapping the allosteric sites of the A2A adenosine receptor. Chem. Biol. Drug Des. 2018, 91, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Schwede, T.; Tosatto, S.C.E. QMEANclust: Estimation of protein model quality by combining a composite scoring function with structural density information. BMC Struct. Biol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Kumari, L. Discovery of an Unexplored Protein Structural Scaffold of Serine Protease from Big Blue Octopus (Octopus cyanea): A New Prospective Lead Molecule. Curr. Drug Discov. Technol. 2017, 14, 135–140. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.-C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASy Server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Anantram, A.; Janve, M.; Degani, M.; Singhal, R.; Kundaikar, H. Homology modelling of human divalent metal transporter (DMT): Molecular docking and dynamic simulations for duodenal iron transport. J. Mol. Graph. Model. 2018, 85, 145–152. [Google Scholar] [CrossRef]
- Lanka, G.; Bathula, R.; Dasari, M.; Nakkala, S.; Bhargavi, M.; Somadi, G.; Potlapally, S.R. Structure-based identification of potential novel inhibitors targeting FAM3B (PANDER) causing type 2 diabetes mellitus through virtual screening. J. Recept. Signal Transduct. 2019, 39, 253–263. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Biswal, J.; Kanagarajan, S.; Prabhu, D.; Gogoi, P.; Prasad Kanaujia, S.; Jeyakanthan, J. Design of novel PhMTNA inhibitors, targeting neurological disorder through homology modeling, molecular docking, and dynamics approaches. J. Recept. Signal Transduct. 2019, 39, 28–38. [Google Scholar] [CrossRef]
- Sabek, J.; Martínez-Pérez, P.; García-Rupérez, J. Computational binding study of cardiac troponin I antibody towards cardiac versus skeletal troponin I. Comput. Biol. Chem. 2019, 80, 147–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Wu, Y.; Lou, L.; Xie, Z.-R. A Pilot Study of All-Computational Drug Design Protocol—From Structure Prediction to Interaction Analysis. Front. Chem. 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.J.; Bonvin, A.M. CPORT: A consensus interface predictor and its performance in prediction-driven docking with HADDOCK. PLoS ONE 2011, 6, e17695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyanga, T.A.; Tastan Bishop, Ö. Structural Characterization of Carbonic Anhydrase VIII and Effects of Missense Single Nucleotide Variations to Protein Structure and Function. Int. J. Mol. Sci. 2020, 21, 2764. [Google Scholar] [CrossRef] [PubMed]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33 (Suppl. 2), W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Veeravarapu, H.; Malkhed, V.; Mustyala, K.K.; Vadija, R.; Malikanti, R.; Vuruputuri, U.; Muthyala, M.K.K. Structure-based drug design, synthesis and screening of MmaA1 inhibitors as novel anti-TB agents. Mol. Divers. 2021, 25, 351–366. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Panwar, S.; Kothidar, A.; Tiwari, V. Rational targeting of Wzb phosphatase and Wzc kinase interaction inhibits extracellular polysaccharides synthesis and biofilm formation in Acinetobacter baumannii. Carbohydr. Res. 2020, 492, 108025. [Google Scholar] [CrossRef]
- Visegrády, B.; Than, N.G.; Kilár, F.; Sümegi, B.; Than, G.N.; Bohn, H. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13). Protein Eng. 2001, 14, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Pooja, M.; Reddy, G.J.; Hema, K.; Dodoala, S.; Koganti, B. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. Eur. J. Pharmacol. 2021, 890, 173688. [Google Scholar]
- Surekha, K.; Prabhu, D.; Richard, M.; Nachiappan, M.; Biswal, J.; Jeyakanthan, J. Investigation of vital pathogenic target orotate phosphoribosyltransferases (OPRTase) from Thermus thermophilus HB8: Phylogenetic and molecular modeling approach. Gene 2016, 583, 102–111. [Google Scholar] [CrossRef]
- Gudipati, S.; Muttineni, R.; Mankad, A.U.; Pandya, H.A.; Jasrai, Y.T. Molecular docking based screening of Noggin inhibitors. Bioinformation 2018, 14, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlowe, T.; Dementiev, A.; Figel, S.; Rivera, A.; Flavin, M.; Cance, W. High resolution crystal structure of the FAK FERM domain reveals new insights on the druggability of tyrosine 397 and the Src SH3 binding site. BMC Mol. Cell Biol. 2019, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Nyamai, D.W.; Tastan Bishop, Ö. Aminoacyl tRNA synthetases as malarial drug targets: A comparative bioinformatics study. Malar. J. 2019, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäffer, A.A.; Aravind, L.; Madden, T.L.; Shavirin, S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V.; Altschul, S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Homan, E.J.; Wiita, E.; Pedersen, K.; Almlöf, I.; Gustavsson, A.-L.; Lundbäck, T.; Helleday, T.; Warpman Berglund, U. In Silico Druggability Assessment of the NUDIX Hydrolase Protein Family as a Workflow for Target Prioritization. Front. Chem. 2020, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, X.; Wang, Y.; Han, S.; Wang, F.; Liu, X.; Xiao, F.; Hu, G. Identifying Drug Targets in Pancreatic Ductal Adenocarcinoma through Machine Learning, Analyzing Biomolecular Networks, and Structural Modeling. Front. Pharmacol. 2020, 11, 534. [Google Scholar] [CrossRef]
- Gossen, J.; Albani, S.; Hanke, A.; Joseph, B.P.; Bergh, C.; Kuzikov, M.; Costanzi, E.; Manelfi, C.; Storici, P.; Gribbon, P.; et al. A Blueprint for High Affinity SARS-CoV-2 Mpro Inhibitors from Activity-Based Compound Library Screening Guided by Analysis of Protein Dynamics. ACS Pharmacol. Transl. Sci. 2021, 4, 1079–1095. [Google Scholar] [CrossRef]
- Dos Santos Vasconcelos, C.R.; Rezende, A.M. Systematic in silico Evaluation of Leishmania spp. Proteomes for Drug Discovery. Front. Chem. 2021, 9, 607139. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Xiang, X.; Li, J.; Huang, J. Characterization of mRNA Expression and Endogenous RNA Profiles in Bladder Cancer Based on The Cancer Genome Atlas (TCGA) Database. Med. Sci. Monit. 2019, 25, 3041–3060. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Yu, B.; Zhang, X.-X.; Zhu, Y.-H. Identification of TNIK as a novel potential drug target in thyroid cancer based on protein druggability prediction. Medicine 2021, 100, e25541. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, S.; Hu, Q.; Gao, S.; Ma, X.; Zhang, W.; Shen, Y.; Chen, F.; Lai, L.; Pei, J. CavityPlus: A web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018, 46, W374–W379. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Chandra, N. PocketMatch (version 2.0): A parallel algorithm for the detection of structural similarities between protein ligand binding-sites. In Proceedings of the 2013 National Conference on Parallel Computing Technologies (PARCOMPTECH), Bangalore, India, 21–23 February 2013. [Google Scholar]
- Abeywickrama, T.D.; Perera, I.C. In Silico Characterization and Virtual Screening of GntR/HutC Family Transcriptional Regulator MoyR: A Potential Monooxygenase Regulator in Mycobacterium tuberculosis. Biology 2021, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Broomhead, N.K.; Soliman, M.E. Can We Rely on Computational Predictions To Correctly Identify Ligand Binding Sites on Novel Protein Drug Targets? Assessment of Binding Site Prediction Methods and a Protocol for Validation of Predicted Binding Sites. Cell Biochem. Biophys. 2017, 75, 15–23. [Google Scholar] [CrossRef]
- Feng, T.; Barakat, K. Molecular Dynamics Simulation and Prediction of Druggable Binding Sites. Comput. Drug Discov. Des. 2018, 1762, 87–103. [Google Scholar]
- Amodei, D.; Ananthanarayanan, S.; Anubhai, R.; Bai, J.; Battenberg, E.; Case, C.; Casper, J.; Catanzaro, B.; Cheng, Q.; Chen, G. Deep speech 2: End-to-end speech recognition in English and Mandarin. In Proceedings of the 33rd International Conference on Machine Learning, New York, New York, USA, 20–22 June 2016; pp. 173–182. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, G.; Chen, L.-C.; Murphy, K.P.; Yuille, A.L. Weakly- and semi-supervised learning of a deep convolutional network for semantic image segmentation. In Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015; pp. 1742–1750. [Google Scholar] [CrossRef]
- Voulodimos, A.; Doulamis, N.; Doulamis, A.; Protopapadakis, E. Deep Learning for Computer Vision: A Brief Review. Comput. Intell. Neurosci. 2018, 2018, 7068349. [Google Scholar] [CrossRef]
- Wooller, S.K.; Benstead-Hume, G.; Chen, X.; Ali, Y.; Pearl, F.M.G. Bioinformatics in translational drug discovery. Biosci. Rep. 2017, 37, BSR20160180. [Google Scholar] [CrossRef] [Green Version]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef] [PubMed]
- Agüero, F.; Al-Lazikani, B.; Aslett, M.; Berriman, M.; Buckner, F.S.; Campbell, R.K.; Carmona, S.; Carruthers, I.M.; Chan, A.W.E.; Chen, F.; et al. Genomic-scale prioritization of drug targets: The TDR Targets database. Nat. Rev. Drug Discov. 2008, 7, 900–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyrisch, S.; Helms, V. Transient pockets on protein surfaces involved in protein–protein interaction. J. Med. Chem. 2007, 50, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Strecker, C.; Meyer, B. Plasticity of the Binding Site of Renin: Optimized Selection of Protein Structures for Ensemble Docking. J. Chem. Inf. Model. 2018, 58, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Liang, H.; Nettesheim, D.; Meadows, R.P.; Harlan, J.E.; Eberstadt, M.; Yoon, H.S.; Shuker, S.B.; Chang, B.S.; Minn, A.J.; et al. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science 1997, 275, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Stank, A.; Kokh, D.B.; Fuller, J.C.; Wade, R.C. Protein Binding Pocket Dynamics. Acc. Chem. Res. 2016, 49, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Antunes, D.A.; Devaurs, D.; Kavraki, L.E. Understanding the challenges of protein flexibility in drug design. Expert Opin. Drug Discov. 2015, 10, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Melse, O.; Hecht, S.; Antes, I. DynaBiS: A hierarchical sampling algorithm to identify flexible binding sites for large ligands and peptides. Proteins 2022, 90, 18–32. [Google Scholar] [CrossRef]
- Grove, L.E.; Hall, D.R.; Beglov, D.; Vajda, S.; Kozakov, D. FTFlex: Accounting for binding site flexibility to improve fragment-based identification of druggable hot spots. Bioinformatics 2013, 29, 1218–1219. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.P.; Hajduk, P.J. Effects of conformational dynamics on predicted protein druggability. ChemMedChem 2006, 1, 70–72. [Google Scholar] [CrossRef]
- Uehara, S.; Tanaka, S. Cosolvent-Based Molecular Dynamics for Ensemble Docking: Practical Method for Generating Druggable Protein Conformations. J. Chem. Inf. Model. 2017, 57, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Verma, C.S. Straightforward Incorporation of Multiple Ligand Types into Molecular Dynamics Simulations for Efficient Binding Site Detection and Characterization. J. Chem. Theory Comput. 2020, 16, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kasam, V.; Tautermann, C.S.; Seeliger, D.; Vaidehi, N. Computational Method To Identify Druggable Binding Sites That Target Protein–Protein Interactions. J. Chem. Inf. Model. 2014, 54, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Wang, S. Hydrophobic Binding Hot Spots of Bcl-xL Protein–Protein Interfaces by Cosolvent Molecular Dynamics Simulation. ACS Med. Chem. Lett. 2011, 2, 280–284. [Google Scholar] [CrossRef] [PubMed]





| Method | Principle | Example Tool | Available | Applicable Conditions | Advantage | Disadvantage | ||
|---|---|---|---|---|---|---|---|---|
| Ligand-specific method | Interaction with different types of ligands | SXGBsite [47] | FOSS | Require specific ligand types | Accurate prediction of sites for the desired ligand type | Poor performance for non-specific ligand types | ||
| General-purpose methods | Sequence-based | Residue conservation | Concavity | FOSS | Only known sequence | Effective identification of sequence-conserved sites | Exclude physicochemical characteristics | |
| Template-based | Sequence similarity | LIBRA-WA | NA | Known protein with high homology in databases | Acceptable predictive ability for conserved sites | Poor prediction of novel sites | ||
| Structure-based | Geometry-based | Geometric characteristics | Sitemap | FOSS | Require specific geometric features | High prediction rates in large and superficially bound cystic cavities | Do not consider ligand binding energy | |
| Energy-based | Energy of interactions | FTSite | Free | Require excellent ligand binding energy | Superior performance in predicting ligand binding energy | Exclude geometric features | ||
| Consensus-based | Comprehensive assessment of the above four methods | COACH | FOSS | All feasible | Address inter-method limitations | Time consuming with huge amounts of data | ||
| Method | Principle | Applicable Conditions | Advantage | Disadvantage |
|---|---|---|---|---|
| Knowledge-based | Data searching | Known homolog or family member | Highest prediction accuracy | Strict search requirements may lead to no results |
| Sequence-based | Machine learning and linear regression | Only known sequence | Easy access to data | Low prediction accuracy with lack of dynamic analysis |
| Structure-based | Geometric and energetic criteria on 3D grids | Known structure | Focus on geometric and energy characteristics | Dataset performance affects prediction accuracy |
| Hotspot-based | Geometric and energy characteristics | Based on ligand binding energy | Exclude protein flexibility and geometric features |
| Method | Definition | Key Scoring Factor | Relationship | Purpose |
|---|---|---|---|---|
| Binding site identification | Selection of binding regions with good ligand binding ability | Site size, depth, burial properties, and ligand binding capacity | Provide site information for druggability evaluation | Design inhibitors and antagonists to target binding sites |
| Druggability assessment | Screening for binding sites with drug-like molecule binding ability | Size, enclosure, and hydrophobicity | Independent of or dependent on site prediction | Reduce the number of potential binding sites or predicted targets |
| Database | Description | Coverage | Database Type | Information Type | Extracted Date | URL | Available |
|---|---|---|---|---|---|---|---|
| UniProt | A collection of sequences and annotations | 568,002 manual annotation, 226,771,949 automated annotation | Sequence | Target | 2022/4/28 | http://www.uniprot.org/ | √ |
| Swiss-Model | A collection of homology modeling structures | 2,260,758 models, 183,354 structures | Sequence, structure | Target | 2022/8/24 | http://swissmodel.expasy.org/ | √ |
| PDB | 3D structural data for large biological molecules | 194,820 structures, 1,000,361 computational structure models | Sequence, structure, drug | Target, ligand | 2022/8/23 | https://www.rcsb.org/ | √ |
| NCBI | A search and retrieval system of sequences, including structural data and images | 33,664,932 genes, 968,236,913 proteins, 110,628,849 compounds | Sequence, structure, drug | Ligand | 2021/9/4 | https://www.ncbi.nlm.nih.gov/ | √ |
| BioLiP | A database for high-quality, biologically relevant ligand-protein binding interactions | 116,643 proteins, 23,492 entries with binding affinity data | Structure | Target | 2022/4/1 | http://zhanglab.ccmb.med.umich.edu/BioLiP/ | √ |
| PubChem | A database with molecules such as nucleotides, carbohydrates, lipids, and peptides | 111,889,485 compounds, 185,291 proteins | Structure, drug | Target, ligand | 2022 | https://pubchem.ncbi.nlm.nih.gov/ | √ |
| BindingDB [126] | A database of binding affinities and interactions of drug targets with small, drug-like molecules | 2,588,694 binding data | Structure, drug | Target, ligand | 2022/8/28 | https://www.bindingdb.org/bind/index.jsp | √ |
| PDTD [127] | A web-accessible protein database for in silico target identification | >830 known or potential drug targets | Structure, drug | Target | —— | http://www.dddc.ac.cn/pdtd/ | × |
| DrugCentral | An online drug information resource on active ingredients, chemical entities, etc. | 4714 drugs, 129,975 pharmaceuticals | Drug | Target | 2022/7 | http://drugcentral.org/ | √ |
| Clinicaltrials.gov | A web-based resource of clinical studies on diseases and conditions | 426,507 studies | Drug | Target | 2022/8/23 | https://clinicaltrials.gov/ct2/home/ | √ |
| DrugBank | An online database containing information on drugs and drug targets | 14,755 drug entries | Drug | Target | 2022/1/3 | https://go.drugbank.com/ | √ |
| KEGG [128] | A database resource for high-level functions and utilities of the biological system | 18,965 substances, 11,953 drugs | Drug | Target | 2022/7/1 | http://www.kegg.jp/ | √ |
| IUPHAR [129] | An expert-curated resource of pharmacological targets and substances | 3002 targets, 11,348 ligands | Drug | Target, ligand | 2022/6/9 | https://www.guidetopharmacology.org/ | √ |
| ChEMBL [130] | A database of molecules with drug-like properties, chemicals, and bioactivity | 15,072 targets, 2,331,700 compounds | Drug | Target | 2022/7/12 | https://www.ebi.ac.uk/chembl/ | √ |
| TTD [131] | A database consisting of target-interacting proteins, patented agents, and their targets | 3578 targets, 38,760 drugs | Drug | Target | 2021/11/8 | http://db.idrblab.net/ttd/ | √ |
| SwissTargetPrediction [132] | A website to estimate the most probable macromolecular targets of a small molecule | 3068 targets, 376,342 active compounds, 580,496 interactions | Drug | Ligand | 2019 | http://swisstargetprediction.ch/ | √ |
| Database | Description | Coverage | Probe Type | Species | URL | Available |
|---|---|---|---|---|---|---|
| PhylOPDb [133] | A web interface to browse 16S rRNA-targeted probes | 74,003 probes | Oligonucleotide | Bacteria and Archaea | http://g2im.u-clermont1.fr/phylopdb/ | √ |
| ProbeBase [134] | A database of rRNA-targeted oligonucleotide probes and primers | 2788 probes, 175 PCR primers | Oligonucleotide | Microorganism | http://www.probebase.net/ | √ |
| R-BIND [135] | A database with tools for probe development and information | 113 ligands | RNA | —— | https://rbind.chem.duke.edu/ | √ |
| RTPrimerDB [136] | A public database of PCR primer and probe sequence records | Probe records | Nucleotide | Human, rat, mouse, fruit fly, and zebrafish | http://www.realtimeprimerdatabase.ht.st/ | × |
| Dataset | Year | Coverage | Source Database | Applied Tool |
|---|---|---|---|---|
| Huang and Schroeder | 2006 | 48 unbound/bound structures and 210 bound structures | PLD [137], ConSurf HSSP [138], PDB | LIGSITEcsc [104], MetaPocket, MetaPocket 2.0, FTSite, Fpocket, DoGSite [139], COFACTOR, P2Rank [43], PocketPicker [140], PUResNet [89], EXPOSITE [141], VICE [142], ISMBLab-LIG [143], MSPocket [101], bSiteFinder [31], POCASA [109] |
| FINDSITE | 2008 | 901 protein–ligand complexes | PDB | FINDSITE, 3DligandSite [144], LISE |
| COACH validation set | 2013 | 500 proteins, 815 binding ligands | BioLiP | COACH |
| LigASite dataset (v7.0) | 2009 | 337 proteins with apo (unbound) structures | PDB, HSSP [145], Catalytic Site Atlas [146] | ConCavity |
| MPLs-Pred validation set | 2019 | 234 proteins | UniProt | MPLs-Pred |
| Sitemap validation set | 2009 | 538 proteins | PDB | Sitemap |
| COFACTOR validation set | 2012 | 450 non-homologous proteins | PDB | COFACTOR |
| SXGBsite validation set | 2019 | 5 nucleotides, 5 metal ions, DNA, and hemoglobin | BioLiP | SXGBsite |
| Year | Database | Modeling and Evaluation Tool | Binding Site Identification Tool | Web Server/Software for Druggability Assessment | Prediction of Druggability | Reference |
|---|---|---|---|---|---|---|
| 2016 | UniProtKB, Cluster, PDB | Prime module of Schrödinger software, PROCHECK | Sitemap | 1 druggable binding site (Dscore = 1.33) | [190] | |
| 2017 | UniProt | Prime module of Schrödinger software | Sitemap | 1 druggable binding pocket (Dscore = 1.228) | [151] | |
| 2018 | PDB | Swiss-Model | Sitemap | 5 sites (SiteScore > 1 is druggable) | [191] | |
| 2018 | PDB | Modeller, HHpred, PRIMO | SiteHound, MetaPocket 2.0, Sitemap | Sitemap | 4 of 6 sites are druggable (Dscore > 0.83) | [152] |
| 2019 | PDB | PyMOL module of Schrödinger software, Schrödinger Multiple Sequence Viewer | Sitemap | 1 druggable binding pocket | [192] | |
| 2019 | PDB, PDBind, MOAD | —— | PockDrug, FTMap | LasI protein: 6 binding sites (2 are druggable, scores of 1.0 and 0.92 ± 0.05, respectively) | [53] | |
| 2019 | NCBI, UniProt, PDB | Modeller, HHpred, PRIMO, ProSA, Verify3D, QMEN | FTMap and Sitemap | Binding sites of 3 of 4 models are druggable | [193] | |
| 2020 | PDB, NCBI | Blast [194], Modeller | FTMap | 3 of 10 binding sites are druggable | [150] | |
| 2020 | PDB | —— | DoGSite, FTMap, CryptoSite | Sitemap | NUDT1, NUDT5, NUDT7, NUDT9, NUDT12, NUDT15, NUDT17, and NUDT22 are druggable | [195] |
| 2020 | NCBI, GEO, PDB | I-TASSER, Swiss-Model | Fpocket | 14 genes are druggable | [196] | |
| 2021 | PDB | Markov state model | TRAPP and Sitemap | All pockets (except PDB ID 6WTK) are druggable | [197] | |
| 2021 | TriTrypDB, BindingDB, UniProt, PDB | Swiss-Model | Fpocket | 599 (87.9%) and 629 (88.8%) protein structures with druggable binding sites | [198] | |
| 2021 | TCGA [199], STRING [200] | —— | PockDrug | 1 of 3 predicted protein pockets is druggable | [201] | |
| 2021 | PDB, UniProt, GenBank, Pharos, PubChem | Swiss-Model, Phyre2, I-TASSER, Verify3D, PROCHECK, ProQ, ERRAT, ProSA | MetaPocket 2.0, CavityPlus [202], Pocket Match [203], ConSurf | PockDrug | All 4 binding pockets > 0.91 | [204] |
| Reference | Year | Questions/Issues Posed | Solution |
|---|---|---|---|
| [205] | 2017 | Lack of protein flexibility | Molecular dynamics simulations, molecular docking and combined thermodynamic methods |
| [206] | 2018 | Lack of protein flexibility | Cosolvent molecular dynamics simulation |
| [46] | 2020 | Unable to identify mystery sites | Protein conformation sampling techniques |
| [211] | 2017 | Insufficient accuracy of druggability methods | Identify drug targets and new uses for old drugs using a web-based approach |
| [212] | 2020 | Inadequate prediction accuracy and exclusion of protein–ligand interactions | Combine druggability and drug-likenesses |
| This review | 2022 | How to improve prediction accuracy | Ensure consistent prediction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Wang, Q.; Wu, F.; Huang, Z. In Silico Methods for Identification of Potential Active Sites of Therapeutic Targets. Molecules 2022, 27, 7103. https://doi.org/10.3390/molecules27207103
Liao J, Wang Q, Wu F, Huang Z. In Silico Methods for Identification of Potential Active Sites of Therapeutic Targets. Molecules. 2022; 27(20):7103. https://doi.org/10.3390/molecules27207103
Chicago/Turabian StyleLiao, Jianbo, Qinyu Wang, Fengxu Wu, and Zunnan Huang. 2022. "In Silico Methods for Identification of Potential Active Sites of Therapeutic Targets" Molecules 27, no. 20: 7103. https://doi.org/10.3390/molecules27207103
APA StyleLiao, J., Wang, Q., Wu, F., & Huang, Z. (2022). In Silico Methods for Identification of Potential Active Sites of Therapeutic Targets. Molecules, 27(20), 7103. https://doi.org/10.3390/molecules27207103







