The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions
Abstract
:1. Introduction
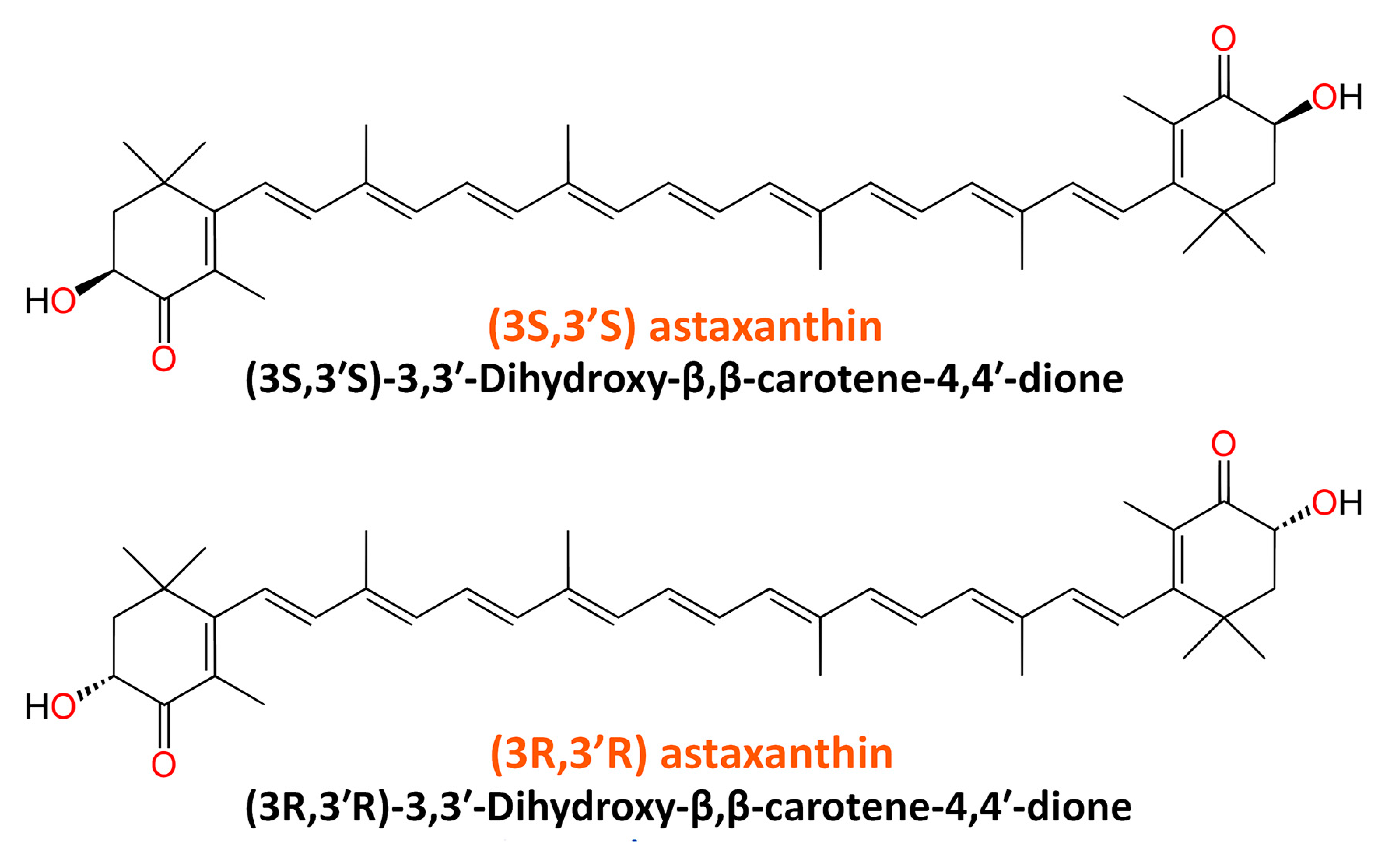
2. Structure and Sources of Astaxanthin
3. Astaxanthin in the Food Industry
4. The Role of Astaxanthin in Managing Oxidative Stress
5. The Role of Astaxanthin in the Aging Process
5.1. The Role of Astaxanthin in Skin Aging
5.2. The Role of Astaxanthin in the Brain Aging
6. Other Pharmacological Activities of Astaxanthin
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diederich, M. Natural products target the hallmarks of chronic diseases. Biochem. Pharmacol. 2020, 173, 113828. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Mukherjee, P. Chapter 20—Phyto-Pharmaceuticals, Nutraceuticals and Their Evaluation. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 707–722. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Shanaida, M.; Ongenae, A.; Lysiuk, R.; Dosa, M.D.; Tsal, O.; Piscopo, S.; Chirumbolo, S.; Bjorklund, G. Calanus oil in the treatment of obesity-related low-grade inflammation, insulin resistance, and atherosclerosis. Appl. Microbiol. Biotechnol. 2020, 104, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.N.; Roda, A.; Gouveia, L.s.F.; Fernández, N.; Bronze, M.R.r.; Matias, A.A. Astaxanthin extraction from marine crustacean waste streams: An integrate approach between microwaves and supercritical fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the neuroprotective role of astaxanthin: New perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Martins, N.; Chirumbolo, S.; Goh, B.H.; Smetanina, K.; Lysiuk, R. Brief Challenges on Medicinal Plants: An Eye-Opening Look at Ageing-Related Disorders. Basic Clin. Pharmacol. Toxicol. 2018, 122, 539–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid. Sci. 2007, 11, 16–20. [Google Scholar]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Tuncay Tanriverdi, S.; Aydin Kose, F.; Ozer, O. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J. Cell Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wu, T.; Yang, L.; Xu, Y.; Ota, T.; Fu, Z. Protective effects of astaxanthin on a combination of D-galactose and jet lag-induced aging model in mice. Endocr. J. 2018, 65, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Z.; Ali, A.S.; Campbell, M.D.; Kilroy, K.; Shankland, E.G.; Roshanravan, B.; Marcinek, D.J.; Conley, K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle 2018, 9, 826–833. [Google Scholar] [CrossRef]
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D.J. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. Physiol. Rep. 2021, 9, e14887. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef]
- Bahbah, E.I.; Ghozy, S.; Attia, M.S.; Negida, A.; Emran, T.B.; Mitra, S.; Albadrani, G.M.; Abdel-Daim, M.M.; Uddin, M.S.; Simal-Gandara, J. Molecular mechanisms of astaxanthin as a potential neurotherapeutic agent. Mar. Drugs 2021, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a putative geroprotector: Molecular basis and focus on brain aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52, 382–392. [Google Scholar]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Failla, M.L. Bioaccessibility and intestinal cell uptake of astaxanthin from salmon and commercial supplements. Food Res. Int. 2017, 99, 936–943. [Google Scholar] [CrossRef]
- Dufossé, L. Current and potential natural pigments from microorganisms (bacteria, yeasts, fungi, microalgae). In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2016; pp. 337–354. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, N.F.; Allergens, F.; Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Qi, X. The Putative Role of Astaxanthin in Neuroinflammation Modulation: Mechanisms and Therapeutic Potential. Front. Pharmacol. 2022, 13, 916653. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y. Recent Advances and the Mechanism of Astaxanthin in Ophthalmological Diseases. J. Ophthalmol. 2022, 2022, 8071406. [Google Scholar] [CrossRef]
- Patil, A.D.; Kasabe, P.J.; Dandge, P.B. Pharmaceutical and nutraceutical potential of natural bioactive pigment: Astaxanthin. Nat. Prod. Bioprospect 2022, 12, 1–27. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Toscano, A.; Vega Fernández, T.; Musco, L.; Brunet, C.; Sansone, C.; Cirino, P. Food modulation controls astaxanthin accumulation in eggs of the sea urchin Arbacia lixula. Mar. Drugs 2018, 16, 186. [Google Scholar] [CrossRef]
- Azizan, A.; Ahamad Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite profiling of the microalgal diatom Chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef] [Green Version]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-h.; Wei, D.; Lim, P.-E. Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: Selected phytohormones as positive stimulators. Bioresour. Technol. 2020, 295, 122242. [Google Scholar] [CrossRef] [PubMed]
- Stoklosa, R.J.; Johnston, D.B.; Nghiem, N.P. Phaffia rhodozyma cultivation on structural and non-structural sugars from sweet sorghum for astaxanthin generation. Process Biochem. 2019, 83, 9–17. [Google Scholar] [CrossRef]
- García-Estrada, C.; Kosalková, K.; Sánchez-Orejas, I.-C. Extraction and Analysis of Carotenes and Xanthophylls Produced by Xanthophyllomyces dendrorhous. In Microbial Carotenoids; Springer: Berlin/Heidelberg, Germany, 2018; pp. 283–295. [Google Scholar]
- Wan, X.; Zhou, X.-R.; Moncalian, G.; Su, L.; Chen, W.-C.; Zhu, H.-Z.; Chen, D.; Gong, Y.-M.; Huang, F.-H.; Deng, Q.-C. Reprogramming microorganisms for the biosynthesis of astaxanthin via metabolic engineering. Prog. Lipid. Res. 2021, 81, 101083. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M.A.; Laishevtcev, A.; Plygun, S.; Heydari, M. Xanthophyll: Health benefits and therapeutic insights. Life Sci. 2020, 240, 117104. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takaki, A.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth. Biol. 2019, 8, 2701–2709. [Google Scholar] [CrossRef]
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- de la Fuente, J.L.; Rodríguez-Sáiz, M.; Schleissner, C.; Díez, B.; Peiro, E.; Barredo, J.L. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J. Biotechnol. 2010, 148, 144–146. [Google Scholar] [CrossRef]
- Libkind, D.; Ruffini, A.; van Broock, M.; Alves, L.; Sampaio, J.P. Biogeography, host specificity, and molecular phylogeny of the basidiomycetous yeast Phaffia rhodozyma and its sexual form, Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 1120–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, M.; Varavadekar, J.; Vora, M.; Sethia, P.; Reddy, H.; Rangaswamy, V. Astaxanthin: Current Advances in Metabolic Engineering of the Carotenoid. In High Value Fermentation Products: Human Health; Wiley: Hoboken, NJ, USA, 2019; Volume 1, pp. 381–399. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Int. Food Res. J. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, J.; Lv, W.; Lu, W.; Pei, D.; Lv, Y.; Wang, W.; Zhang, M.; Ding, R.; Lv, M. Optimized extraction of astaxanthin from shrimp shells treated by biological enzyme and its separation and purification using macroporous resin. Food Chem. 2021, 363, 130369. [Google Scholar] [CrossRef] [PubMed]
- Ausich, R.L. Commercial opportunities for carotenoid production by biotechnology. Pure Appl. Chem. 1997, 69, 2169–2174. [Google Scholar] [CrossRef] [Green Version]
- Calo, P.; Velazquez, J.B.; Sieiro, C.; Blanco, P.; Longo, E.; Villa, T.G. Analysis of astaxanthin and other carotenoids from several Phaffia rhodozyma mutants. J. Agric. Food Chem. 1995, 43, 1396–1399. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Kinetics for the reversible isomerization reaction of trans-astaxanthin. Food Chem. 2001, 73, 131–137. [Google Scholar] [CrossRef]
- Torres-Haro, A.; Verdín, J.; Kirchmayr, M.R.; Arellano-Plaza, M. Metabolic engineering for high yield synthesis of astaxanthin in Xanthophyllomyces dendrorhous. Microb. Cell Fact 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Diksha Kumari, A.; Panwar, A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J. Basic Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef]
- Kowsalya, K.; Vidya, N.; Vijayalakshmi, V.; Arun, M. Super nutritive marine astaxanthin, an effectual dietary carotenoid for neurodegenerative diseases. Int. Res. J. Multidiscipl. Tech. Maple Tree J. 2019, 1, 115–124. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid Med. Cell Longev. 2019, 2019, 31814873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- McNulty, H.; Jacob, R.F.; Mason, R.P. Biologic activity of carotenoids related to distinct membrane physicochemical interactions. Am. J. Cardiol. 2008, 101, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Hernández Blanco, I.; Hoffmann, T.; Martin, H.D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.-Z.; Jiang, S.; Wu, J.-J.; Zhou, X.-R.; Liu, P.-Y.; Huang, F.-H.; Wan, X. Production of High Levels of 3 S, 3′ S-Astaxanthin in Yarrowia lipolytica via Iterative Metabolic Engineering. J. Agric. Food Chem. 2022, 70, 2673–2683. [Google Scholar] [CrossRef]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Luo, Q.; Bian, C.; Tao, M.; Huang, Y.; Zheng, Y.; Lv, Y.; Li, J.; Wang, C.; You, X.; Jia, B. Genome and transcriptome sequencing of the astaxanthin-producing green microalga, Haematococcus pluvialis. Genome Biol. Evol. 2019, 11, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agrofor. Syst. 2020, 94, 1229–1234. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.; Khandual, S.; Morales-Hernandez, N.; Martínez-Bustos, F.; Vélez-Medina, J.; Nolasco-Soria, H. Fish Feed Formulation with Microalgae H. Pluvialis and A. Platensis: Effect of Extrusion Process on Stability of Astaxanthin and Antioxidant Capacity. Int. J. Food Sci. Nutr. 2020, 7, 1–8. [Google Scholar]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Rüfer, C.E.; Moeseneder, J.; Briviba, K.; Rechkemmer, G.; Bub, A. Bioavailability of astaxanthin stereoisomers from wild (Oncorhynchus spp.) and aquacultured (Salmo salar) salmon in healthy men: A randomised, double-blind study. Br. J. Nutr. 2008, 99, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Aly, H.F.; K El-Baz, F.; Ali, S.I.; Salama, A. Safety of astaxanthin-rich fraction of Haematococcus pluvialis microalgae. Egypt J. Chem. 2022, 65, 479–489. [Google Scholar] [CrossRef]
- Brown, D.R.; Gough, L.A.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. Astaxanthin in exercise metabolism, performance and recovery: A review. Front. Nutr. 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Somagond, Y.M.; Singh, S.V.; Deshpande, A.; Sheoran, P.; Chahal, V.P. Effect of dietary supplementation of astxanthin, prill fat and their combination on antioxidants and immunity status of lactating buffaloes during heat stress. Buffalo Bull 2021, 40, 451–463. [Google Scholar]
- Tolba, S.A.; Magnuson, A.D.; Sun, T.; Lei, X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020, 99, 4853–4860. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S. Influence of astaxanthin supplementation on attainment of puberty and lipid peroxidation in Sahiwal and Karan Fries (Holstein × Tharparkar) heifers during summer season. Biol. Rhythm. Res. 2020, 51, 15–28. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy-Feitosa, E.; Okuro, R.T.; Ribeiro, V.P.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasmi, A.; Chirumbolo, S.; Peana, M.; Mujawdiya, P.K.; Dadar, M.; Menzel, A.; Bjorklund, G. Biomarkers of Senescence during Aging as Possible Warnings to Use Preventive Measures. Curr. Med. Chem. 2021, 28, 1471–1488. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Ma, B.; Lu, J.; Kang, T.; Zhu, M.; Xiong, K.; Wang, J. Astaxanthin supplementation mildly reduced oxidative stress and inflammation biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Res. 2021, 99, 40–50. [Google Scholar] [CrossRef]
- Sowmya, P.R.-R.; Arathi, B.P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, H.; Lim, J.W.; Kim, H. Astaxanthin induces NADPH oxidase activation and receptorinteracting protein kinase 1mediated necroptosis in gastric cancer AGS cells. Mol. Med. Rep. 2021, 24. [Google Scholar] [CrossRef]
- Kalache, A.; de Hoogh, A.; Howlett, S.; Kennedy, B.; Eggersdorfer, M.; Marsman, D.; Shao, A.; Griffiths, J. Nutrition interventions for healthy ageing across the lifespan: A conference report. Eur. J. Nutr. 2019, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Chirumbolo, S.; Bjørklund, G.; Lysiuk, R.; Vella, A.; Lenchyk, L.; Upyr, T. Targeting cancer with phytochemicals via their fine tuning of the cell survival signaling pathways. Int. J. Mol. Sci. 2018, 19, 3568. [Google Scholar] [CrossRef] [Green Version]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Takara, T. Cognitive function improvement with astaxanthin and tocotrienol intake: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2020, 67, 307–316. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Klochkov, V.; Chalyk, N.; Pristensky, D.; Chernyshova, M.; Kyle, N.; Bashmakov, Y. Markers of hypoxia and oxidative stress in aging volunteers ingesting lycosomal formulation of dark chocolate containing astaxanthin. J. Nutr. Health Aging 2018, 22, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet Dermatol. 2020, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective effects of astaxanthin supplementation against ultraviolet-induced photoaging in hairless mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Cakir, E.; Cakir, U.; Tayman, C.; Turkmenoglu, T.T.; Gonel, A.; Turan, I.O. Favorable effects of astaxanthin on brain damage due to ischemia-reperfusion injury. Comb. Chem. High Throughput Screen 2020, 23, 214–224. [Google Scholar] [CrossRef]
- Masoudi, A.; Dargahi, L.; Abbaszadeh, F.; Pourgholami, M.H.; Asgari, A.; Manoochehri, M.; Jorjani, M. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain. Res. 2017, 329, 104–110. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people—A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.H.; Yun, S.Y.; Yu, D.S.; Lee, Y.B. Astaxanthin Protects Ultraviolet B-Induced Oxidative Stress and Apoptosis in Human Keratinocytes via Intrinsic Apoptotic Pathway. Ann. Dermatol. 2022, 34, 125. [Google Scholar] [CrossRef]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2021, 18, 169–182. [Google Scholar] [CrossRef]
- Fu, M.; Liang, X.; Zhang, X.; Yang, M.; Ye, Q.; Qi, Y.; Liu, H.; Zhang, X. Astaxanthin delays brain aging in senescence-accelerated mouse prone 10: Inducing autophagy as a potential mechanism. Nutr. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain. Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef]
- Li, S.; Takahara, T.; Fujino, M.; Fukuhara, Y.; Sugiyama, T.; Li, X.-K.; Takahara, S. Astaxanthin prevents ischemia-reperfusion injury of the steatotic liver in mice. PLoS ONE 2017, 12, e0187810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, C.-A. Enhanced proliferation and differentiation of mesenchymal stem cells by astaxanthin-encapsulated polymeric micelles. PLoS ONE 2019, 14, e0216755. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, L.; Qiao, X.; Xue, C.; Xu, J. Dietary astaxanthin: An excellent carotenoid with multiple health benefits. Crit. Rev. Food Sci. Nutr. 2021, 28, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tang, N.; Kord-Varkaneh, H.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105113. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chen, K.-C.; Liaw, K.-C.; Peng, C.-C.; Peng, R.Y. Astaxanthin protects PC12 cells against homocysteine-and glutamate-induced neurotoxicity. Molecules 2020, 25, 214. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [Green Version]
- Miyachi, M.; Matsuno, T.; Asano, K.; Mataga, I. Anti-inflammatory effects of astaxanthin in the human gingival keratinocyte line NDUSD-1. J. Clin. Biochem. Nutr. 2015, 56, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.-R.; Ayazi-Nasrabadi, R. Astaxanthin protective barrier and its ability to improve the health in patients with COVID-19. Iran J. Microbiol. 2021, 13, 434. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Chen, J.; Zhang, L. Effects of astaxanthin supplementation on oxidative stress. Int. J. Vitam. Nutr. Res. 2019, 90, 179–194. [Google Scholar] [CrossRef]
- Yaqoob, Z.; Arshad, M.S.; Imran, M.; Munir, H.; Qaisrani, T.B.; Khalid, W.; Asghar, Z.; Suleria, H.A.R. Mechanistic role of astaxanthin derived from shrimp against certain metabolic disorders. Food Sci. Nutr. 2022, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Bu, S.; Ma, H.; Gao, L.; Cai, Y.; Zhang, Y.; Zhou, W. Preventive and therapeutic effects of astaxanthin on depressive-like behaviors in high-fat diet and streptozotocin-treated rats. Front. Pharmacol. 2020, 10, 1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Lee, Y.; Park, Y.-K.; Shin, D.-G.; Joshi, P.; Hong, S.-H.; Alder, N.; Koo, S.I.; Lee, J.-Y. Astaxanthin attenuates the increase in mitochondrial respiration during the activation of hepatic stellate cells. J. Nutr. Biochem. 2019, 71, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Pisano, A.; Farace, C.; Giancipoli, E.; Pinna, A.; Dore, S.; Madeddu, R. A pilot study to evaluate the levels of aqueous humor trace elements in open-angle glaucoma. J. Trace Elem. Med. Biol. 2020, 61, 126560. [Google Scholar] [CrossRef] [PubMed]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Baccouche, B.; Benlarbi, M.; Barber, A.J.; Ben Chaouacha-Chekir, R. Short-term administration of astaxanthin attenuates retinal changes in diet-induced diabetic Psammomys obesus. Curr. Eye Res. 2018, 43, 1177–1189. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Shi, L.; Jiang, L.; Li, M.; Zhang, C.; Peng, H. Kidney-targeted astaxanthin natural antioxidant nanosystem for diabetic nephropathy therapy. Eur. J. Pharm. Biopharm. 2020, 156, 143–154. [Google Scholar] [CrossRef]
- Aribisala, J.O.; Nkosi, S.; Idowu, K.; Nurain, I.O.; Makolomakwa, G.M.; Shode, F.O.; Sabiu, S. Astaxanthin-Mediated Bacterial Lethality: Evidence from Oxidative Stress Contribution and Molecular Dynamics Simulation. Oxid. Med. Cell Longev. 2021, 2021, 7159652. [Google Scholar] [CrossRef]
- Rather, A.H.; Singh, S.; Choudhary, S. Antibacterial Activity of Haematococcus pluvialis Crude Astaxanthin Extract. J. Drug Deliv. Ther. 2021, 11, 28–30. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Gasmi Benahmed, A.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjorklund, G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021, 224, 108651. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Gasmi Benahmed, A.; Bjorklund, G. Micronutrients as immunomodulatory tools for COVID-19 management. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Benahmed, A.G.; Bjorklund, G. The microbiota-mediated dietary and nutritional interventions for COVID-19. Clin. Immunol. 2021, 226, 108725. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]

| Group of Organisms | Representative | References |
|---|---|---|
| Haematococcus pluvialis | [9,10] | |
| Plantae (microalgae) | Chlorella zofingiensis Chlorococcum Chromochloriszofingiensis Chlamydomonas reinhardtii Diatoms | [33,34,35] |
| Fungus (yeasts) | Xanthophyllomyces dendrorhous (Phaffia rhodozyma) Yarrowia lipolytica § Saccharomyces cerevisiae § | [36,37,38] |
| Lichene | Clodia aggregata, Concamerella fistulata, Usnea amaliae, Usnea densirostra | [39] |
| Bacteria | Corynebacterium glutamicum§ Cyanobacteria (Synechococcus sp.) Agrobacterium aurantiacum Paracoccus carotinifaciens Escherichia coli § | [38,40,41] |
| Animalia | Redfish Crustaceans (Euphausia superba, Pandalus borealis, Calanus finmarchicus, etc.) Wild salmon (Oncorhynchus species) | [4,7,26] |
| Routes of Administration | Concentrations | Experimental Model | Goals/ Health Benefits | Reference |
|---|---|---|---|---|
| Oral | 0.25 mg/kg BW/day | Buffaloes | Increase of milk production and improvement of overall health | [72] |
| Oral | 10, 20, 40, and 80 mg/kg | Broiler hens | Management of heat stress and inflammation | [73] |
| Oral | 0.25 mg/kg BW/day | Heifers | Prevention of heat stress | [54] |
| Oral | 0.01% ASX | Mice | Improvement of the oxidative status and muscle function | [18] |
| Topical | 20 J/cm2 | Mice | Prevention of photoaging caused by UV irradiation | [91,92] |
| Oral | 25 mg/kg | Rats | Protection from oxidative damage caused by cerebral ischemia-reperfusion injury | [93] |
| Transcutaneous intrathecal (i.t.) injection | 10 μL of 0.2 mM | Rats | Protection against spinal cord injury-induced neuronal loss, demyelination, and functional deficit | [94] |
| Oral | 5 mg per/day | Human | Study of the bioavailability of ASX | [68] |
| Oral | 40 mg | Human | Study of the bioavailability of ASX | [69] |
| Oral | 12 mg/day | Human | Prevention age-related cognitive decline | [88] |
| Oral | 7 mg/day | Human | Improvement of the oxidative status | [89] |
| Oral | 6 mg or 12 mg | Human | Prevention of age-related skin damage and improvement of skin conditions | [95] |
| Oral | 4 mg/day | Human | A strong antioxidant effect and facial skin rejuvenation | [46] |
| Oral | 4 mg | Human | Reduction the skin damage caused by exposure to UV rays | [96] |
| Oral | 6 or 12 mg/day | Human | Prevention of age-related dementia | [97,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. https://doi.org/10.3390/molecules27217167
Bjørklund G, Gasmi A, Lenchyk L, Shanaida M, Zafar S, Mujawdiya PK, Lysiuk R, Antonyak H, Noor S, Akram M, et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules. 2022; 27(21):7167. https://doi.org/10.3390/molecules27217167
Chicago/Turabian StyleBjørklund, Geir, Amin Gasmi, Larysa Lenchyk, Mariia Shanaida, Saba Zafar, Pavan Kumar Mujawdiya, Roman Lysiuk, Halyna Antonyak, Sadaf Noor, Muhammad Akram, and et al. 2022. "The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions" Molecules 27, no. 21: 7167. https://doi.org/10.3390/molecules27217167
APA StyleBjørklund, G., Gasmi, A., Lenchyk, L., Shanaida, M., Zafar, S., Mujawdiya, P. K., Lysiuk, R., Antonyak, H., Noor, S., Akram, M., Smetanina, K., Piscopo, S., Upyr, T., & Peana, M. (2022). The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules, 27(21), 7167. https://doi.org/10.3390/molecules27217167











