The Role of Nanoengineered Biochar Activated with Fe for Sulfanilamide Removal from Soils and Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Biochar
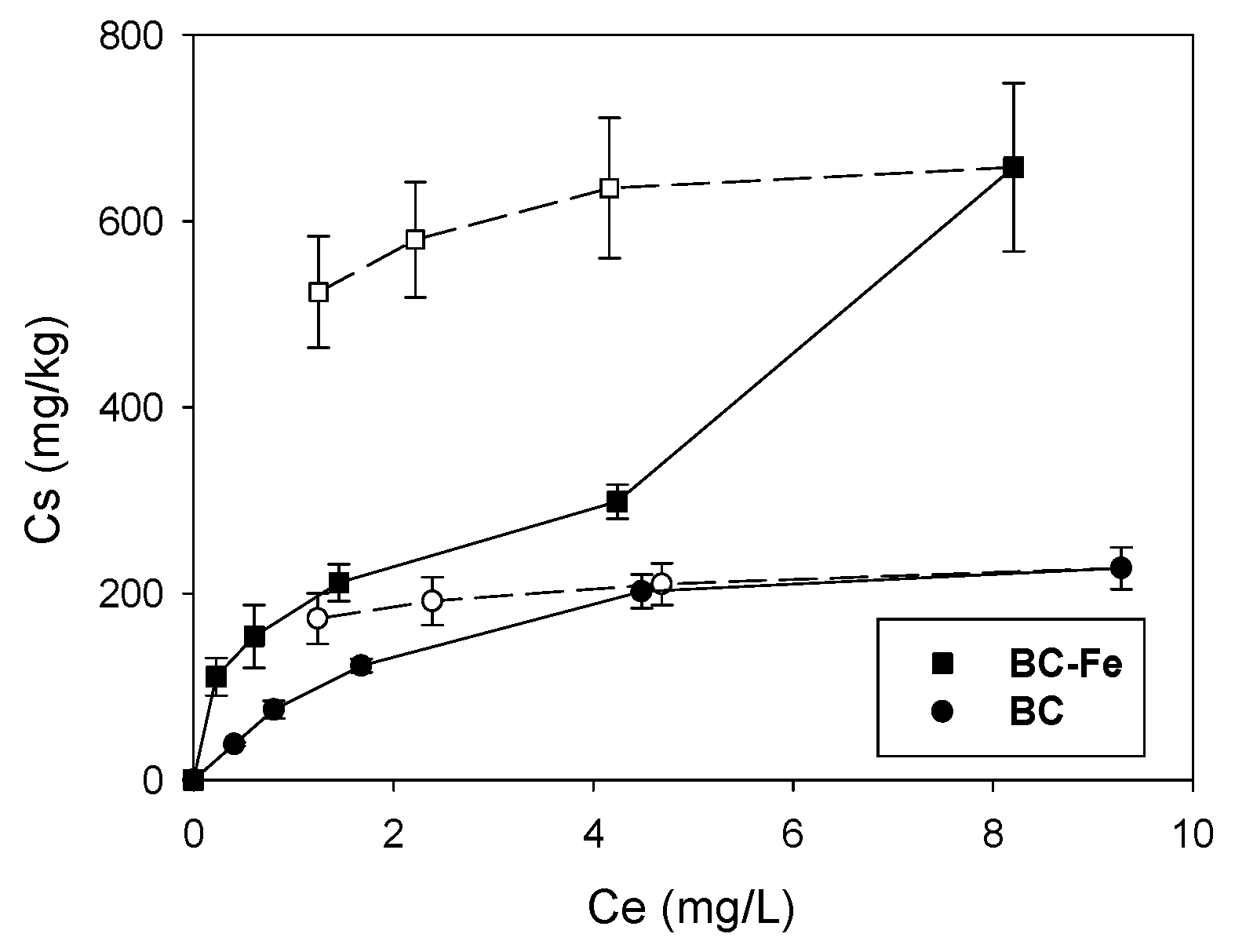
2.2. Sorption-Desorption Isotherms of SFA on Biochar
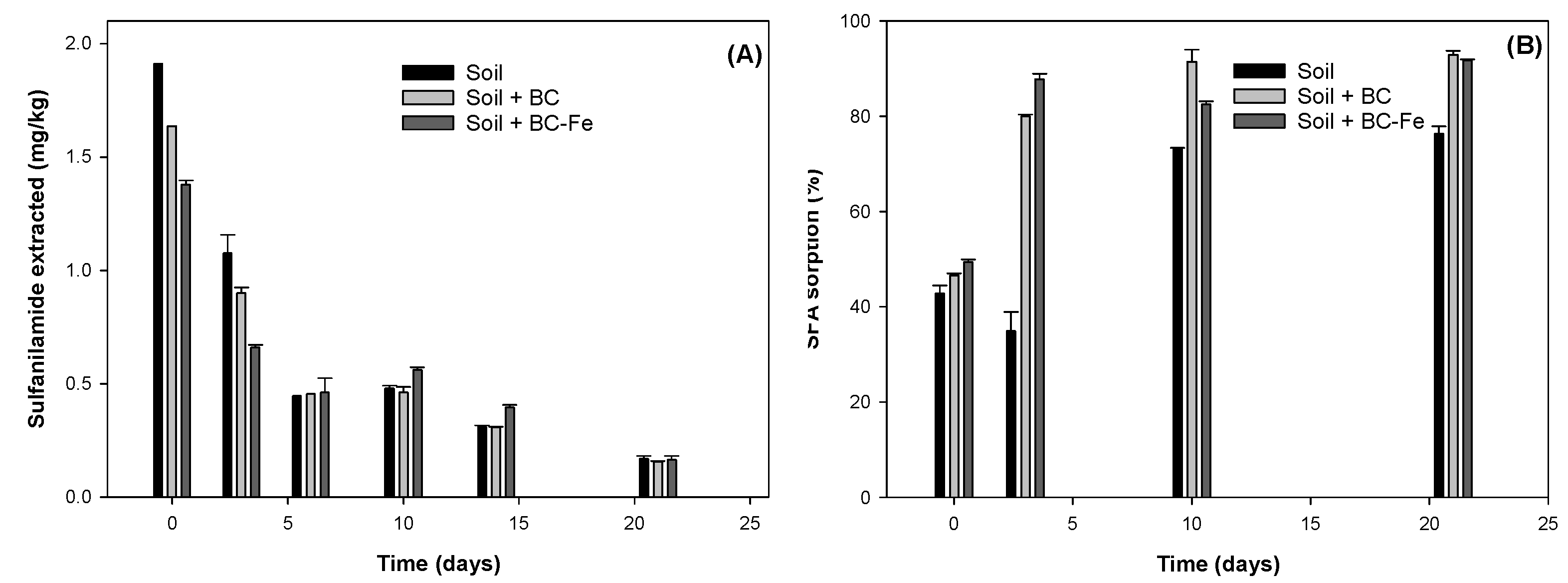
2.3. Dissipation Studies of SFA on Biochar
2.4. SFA Leaching Studies
3. Materials and Methods
3.1. Antibiotic, Soil, and Biochar
3.2. Sorption Experiments of SFA on Biochar
3.3. Dissipation Kinetic of SFA in Soil and Treated Soil
3.4. Leaching of SFA in Soil and Treated Soil
3.5. Sulfanilamide Analysis
3.6. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhang, H.; Shen, G.; Yuan, Z.; Zhang, W. Degradation kinetics and mechanism of sulfadiazine and sulfamethoxazole in an agricultural soil system with manure application. Sci. Total Environ. 2017, 607–608, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, B.; Zhang, T. Bacteria That Make a Meal of Sulfonamide Antibiotics: Blind Spots and Emerging Opportunities. Environ. Sci. Technol. 2018, 52, 3854–3868. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Arévalo, R.; García-Delgado, C.; Mayans, B.; Antón-Herrero, R.; Cuevas, J.; Segura, M.; Eymar, E. Sulfonamides in Tomato from Commercial Greenhouses Irrigated with Reclaimed Wastewater: Uptake, Translocation and Food Safety. Agronomy 2021, 11, 1016. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Adsorption/desorption and transport of sulfadiazine, sulfachloropyridazine, and sulfamethazine, in acid agricultural soils. Chemosphere 2019, 234, 978–986. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Jurado, A.; Margareto, A.; Pujades, E.; Vázquez-Suñé, E.; Diaz-Cruz, M.S. Fate and risk assessment of sulfonamides and metabolites in urban groundwater. Environ. Pollut. 2020, 267, 115480. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Zheng, X.; Xiao, D.; Yang, Y.; Zhang, Z.; Ai, B.; Sheng, Z. Adsorption of Sulfonamides in Aqueous Solution on Reusable Coconut-Shell Biochar Modified by Alkaline Activation and Magnetization. Front. Chem. 2022, 9, 814647. [Google Scholar] [CrossRef] [PubMed]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2020, 405, 126926. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. Z. Pflanzenernähr. Bodenk. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Stoob, K.; Singer, H.P.; Mueller, S.R.; Schwarzenbach, R.P.; Stamm, C.H. Dissipation and Transport of Veterinary Sulfonamide Antibiotics after Manure Application to Grassland in a Small Catchment. Environ. Sci. Technol. 2007, 41, 7349–7355. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Petri, M.S.; Höper, H.; Hamscher, G. Long-term monitoring of sulfonamides and tetracyclines in manure amended soils and leachate samples—A follow-up study. Heliyon 2020, 6, e04656. [Google Scholar] [CrossRef]
- García-Delgado, C.; Eymar, E.; Camacho-Arévalo, R.; Petruccioli, M.; Crognale, S.; D’Annibale, A. Degradation of tetracyclines and sulfonamides by stevensite- and biochar-immobilized laccase systems and impact on residual antibiotic activity. J. Chem. Technol. Biotechnol. 2018, 93, 3394–3409. [Google Scholar] [CrossRef]
- Luo, B.; Huang, G.; Yao, Y.; An, C.; Zhang, P.; Zhao, K. Investigation into the influencing factors and adsorption characteristics in the removal of sulfonamide antibiotics by carbonaceous materials. J. Clean. Prod. 2021, 319, 128692. [Google Scholar] [CrossRef]
- Novak, J.; Sigua, G.; Watts, D.; Cantrell, K.; Shumaker, P.; Szogi, A.; Johnson, M.G.; Spokas, K. Biochars impact on water infiltration and water quality through a compacted subsoil layer. Chemosphere 2016, 142, 160–167. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, G.; Wang, R.; Guo, Y.; Guo, F.; Wu, J. Preparation of Bamboo-Based Hierarchical Porous Carbon Modulated by FeCl3 towards Efficient Copper Adsorption. Molecules 2021, 26, 6014. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Q.; Jiang, X.-Y.; Yu, J.-G. A Novel Low-Cost Bio-Sorbent Prepared from Crisp Persimmon Peel by Low-Temperature Pyrolysis for Adsorption of Organic Dyes. Molecules 2022, 27, 5160. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2019, 711, 134645. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Pan, M. Biochar Adsorption of Antibiotics and its Implications to Remediation of Contaminated Soil. Water Air Soil Pollut. 2020, 231, 221. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lü, J.; Li, J.; Li, Y. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2018, 274, 353–361. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass- Convers. Biorefinery 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Yadav, V.; Khare, P.; Deshmukh, Y.; Shanker, K.; Nigam, N.; Karak, T. Performance of biochar derived from Cymbopogon winterianus waste at two temperatures on soil properties and growth of Bacopa monneri. Commun. Soil Sci. Plant Anal. 2018, 49, 2741–2764. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.; Muñoz, M.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Cabrera, A.; Cox, L.; Spokas, K.A.; Celis, R.; Hermosín, M.C.; Cornejo, J.; Koskinen, W.C. Comparative Sorption and Leaching Study of the Herbicides Fluometuron and 4-Chloro-2-methylphenoxyacetic Acid (MCPA) in a Soil Amended with Biochars and Other Sorbents. J. Agric. Food Chem. 2011, 59, 12550–12560. [Google Scholar] [CrossRef] [Green Version]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Cox, L. Dynamic Effect of Fresh and Aged Biochar on the Behavior of the Herbicide Mesotrione in Soils. J. Agric. Food Chem. 2019, 67, 9450–9459. [Google Scholar] [CrossRef]
- Xie, J.; Lin, R.; Liang, Z.; Zhao, Z.; Yang, C.; Cui, F. Effect of cations on the enhanced adsorption of cationic dye in Fe3O4-loaded biochar and mechanism. J. Environ. Chem. Eng. 2021, 9, 105744. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Shahid, M.; Song, H.; Kwon, E.E.; Rinklebe, J. Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: A review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Bai, S.; Jin, C.; Zhu, S.; Ma, F.; Wang, L.; Wen, Q. Coating magnetite alters the mechanisms and site energy for sulfonamide antibiotic sorption on biochar. J. Hazard. Mater. 2021, 409, 125024. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Li, N.; Liu, H.; Yan, B.; Wang, Y.; Duan, X.; Hou, L.; Wang, S. Active sites decoration on sewage sludge-red mud complex biochar for persulfate activation to degrade sulfanilamide. J. Colloid Interface Sci. 2021, 608, 1983–1998. [Google Scholar] [CrossRef]
- Celis, R.; Cox, L.; Hermosín, M.C.; Cornejo, J. Sorption of Thiazafluron by Iron- and Humic Acid-Coated Montmorillonite. J. Environ. Qual. 1997, 26, 472–479. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.; Kammann, C.I.; Shepherd, J.G.; Conte, P.; Schmidt, H.-P.; Hagemann, N.; Rich, A.M.; Marjo, C.; Allen, J.; Munroe, P.; et al. Microstructural and associated chemical changes during the composting of a high temperature biochar: Mechanisms for nitrate, phosphate and other nutrient retention and release. Sci. Total Environ. 2017, 618, 1210–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouma, V.; Tziasiou, C.; Pournara, A.D.; Giokas, D.L. A novel approach to sorbent-based remediation of soil impacted by organic micropollutants and heavy metals using granular biochar amendment and magnetic separation. J. Environ. Chem. Eng. 2022, 10, 107316. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Area of Solids. J. Chem. Soc. 1960, 14, 3973–3993. [Google Scholar]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 161–222. [Google Scholar]
- Chen, Z.; Xiao, X.; Xing, B.; Chen, B. pH-dependent sorption of sulfonamide antibiotics onto biochars: Sorption mechanisms and modeling. Environ. Pollut. 2019, 248, 48–56. [Google Scholar] [CrossRef]
- di Marsico, A.; Scrano, L.; Amato, M.; Gàmiz, B.; Real, M.; Cox, L. Mucilage from seeds of chia (Salvia hispanica L.) used as soil conditioner; effects on the sorption-desorption of four herbicides in three different soils. Sci. Total Environ. 2018, 625, 531–538. [Google Scholar] [CrossRef]
- Tang, W.; Jing, F.; Laurent, Z.B.L.G.; Liu, Y.; Chen, J. High-temperature and freeze-thaw aged biochar impacts on sulfonamide sorption and mobility in soil. Chemosphere 2021, 276, 130106. [Google Scholar] [CrossRef]
- Teixidó, M.; Pignatello, J.J.; Beltrán, J.L.; Granados, M.; Peccia, J. Speciation of the Ionizable Antibiotic Sulfamethazine on Black Carbon (Biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef]
- Bai, S.; Zhu, S.; Jin, C.; Sun, Z.; Wang, L.; Wen, Q.; Ma, F. Sorption mechanisms of antibiotic sulfamethazine (SMT) on magnetite-coated biochar: pH-dependence and redox transformation. Chemosphere 2020, 268, 128805. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules; Hall, C., Ed.; Springer: Dordrecht, The Netherlands, 1975. [Google Scholar]
- Srivastav, G.; Yadav, B.; Yadav, R.K. Vibrational spectra and molecular structure of sulfanilamide: IR and Low temperature Raman studies and DFT investigation of monomeric and dimeric forms. Vib. Spectrosc. 2020, 112, 103199. [Google Scholar] [CrossRef]
- Gámiz, B.; Cox, L.; Hermosín, M.C.; Spokas, K.; Celis, R. Assessing the Effect of Organoclays and Biochar on the Fate of Abscisic Acid in Soil. J. Agric. Food Chem. 2016, 65, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gámiz, B.; Facenda, G.; Celis, R. Nanoengineered Sorbents To Increase the Persistence of the Allelochemical Carvone in the Rhizosphere. J. Agric. Food Chem. 2018, 67, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liping, L.; Guanghuan, C.; Jingyou, D.; Mingyang, S.; Huanyu, C.; Qiang, Y.; Xinhua, X. Mechanism of and relation between the sorption and desorption of nonylphenol on black carbon-inclusive sediment. Environ. Pollut. 2014, 190, 101–108. [Google Scholar] [CrossRef]
- Sander, M.; Pignatello, J.J. Sorption Irreversibility of 1,4-Dichlorobenzene in Two Natural Organic Matter-Rich Geosorbents. Environ. Toxicol. Chem. 2009, 28, 447–457. [Google Scholar] [CrossRef]
- Kelsey, J.W.; Kottler, B.D.; Alexander, M. Selective Chemical Extractants To Predict Bioavailability of Soil-Aged Organic Chemicals. Environ. Sci. Technol. 1996, 31, 214–217. [Google Scholar] [CrossRef]
- Khan, M.I.; Alam Cheema, S.; Shen, C.; Zhang, C.; Tang, X.; Shi, J.; Chen, X.; Park, J.; Chen, Y. Assessment of phenanthrene bioavailability in aged and unaged soils by mild extraction. Environ. Monit. Assess. 2011, 184, 549–559. [Google Scholar] [CrossRef]
- Accinelli, C.; Koskinen, W.C.; Becker, J.M.; Sadowsky, M.J. Environmental Fate of Two Sulfonamide Antimicrobial Agents in Soil. J. Agric. Food Chem. 2007, 55, 2677–2682. [Google Scholar] [CrossRef]
- Sukul, P.; Spiteller, M. Sulfonamides in the Environment as Veterinary Drugs. In Reviews of Environmental Contamination and Toxicology; Garelick, H., Jones, H., Eds.; Springer: New York, NY, USA, 2006; pp. 67–102. ISBN 9780387302379. [Google Scholar]
- Mohatt, J.L.; Hu, L.; Finneran, K.T.; Strathmann, T.J. Microbially Mediated Abiotic Transformation of the Antimicrobial Agent Sulfamethoxazole under Iron-Reducing Soil Conditions. Environ. Sci. Technol. 2011, 45, 4793–4801. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.-E.; Ok, Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; López-Cabeza, R.; Velarde, P.; Spokas, K.A.; Cox, L. Biochar changes the bioavailability and bioefficacy of the allelochemical coumarin in agricultural soils. Pest Manag. Sci. 2020, 77, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Celis, R.; Real, M.; Hermosin, M.C.; Cornejo, J. Sorption and leaching behaviour of polar aromatic acids in agricultural soils by batch and column leaching tests. Eur. J. Soil Sci. 2004, 56, 287–297. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Brown, C.D. Factors Influencing Degradation of Pesticides in Soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Enantioselective Degradation of Metalaxyl in Soils: Chiral Preference Changes with Soil pH. Environ. Sci. Technol. 2003, 37, 2668–2674. [Google Scholar] [CrossRef]
- Gámiz, B.; Hermosín, M.C.; Celis, R. Sorption, persistence and leaching of abscisic acid in agricultural soils: An enantiomer-selective study. Geoderma 2016, 269, 112–118. [Google Scholar] [CrossRef]
- Ma, L.; Southwick, L.M.; Willis, G.H.; Selim, H.M. Hysteretic Characteristics of Atrazine Adsorption-Desorption by a Sharkey Soil. Weed Sci. 1993, 41, 627–633. [Google Scholar] [CrossRef]
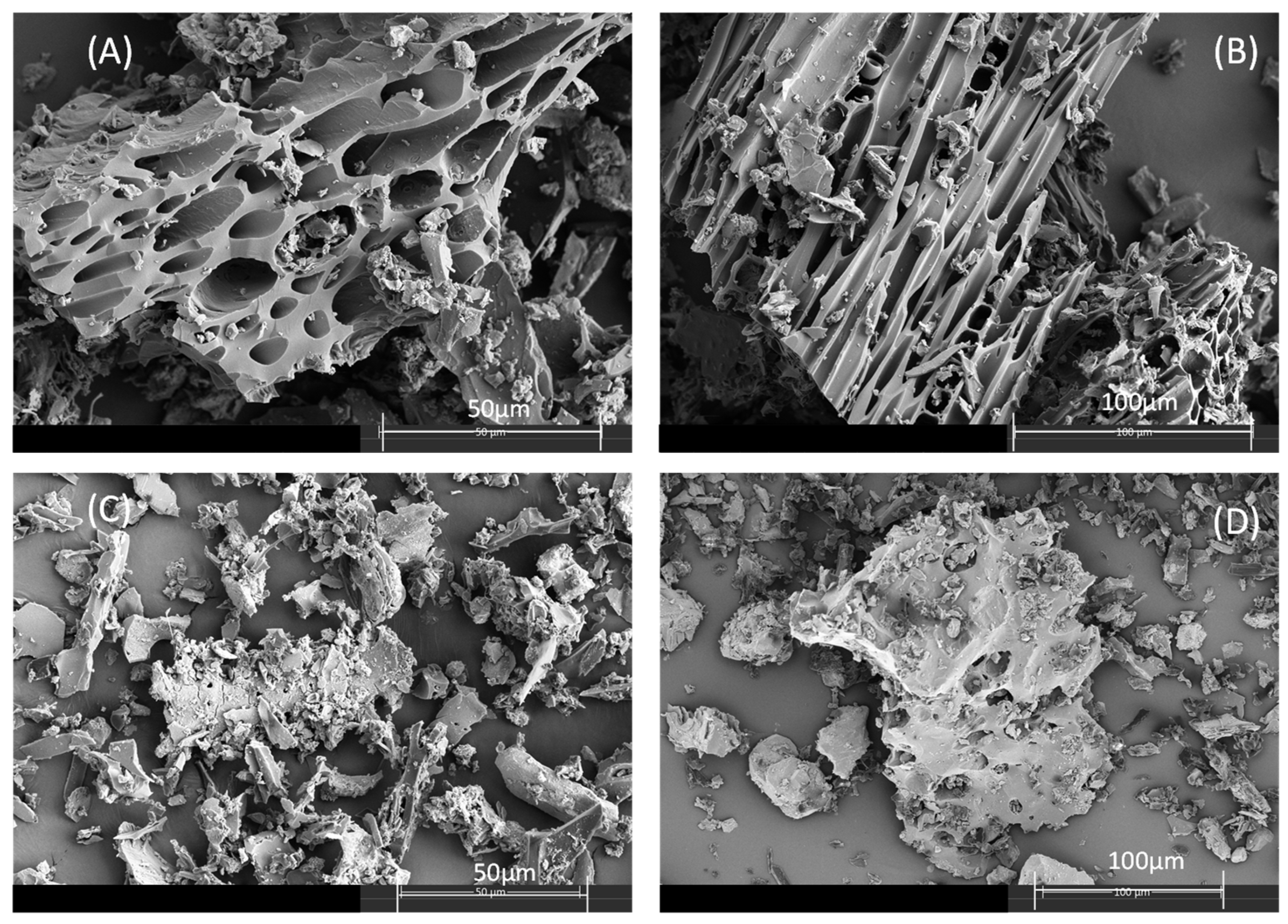
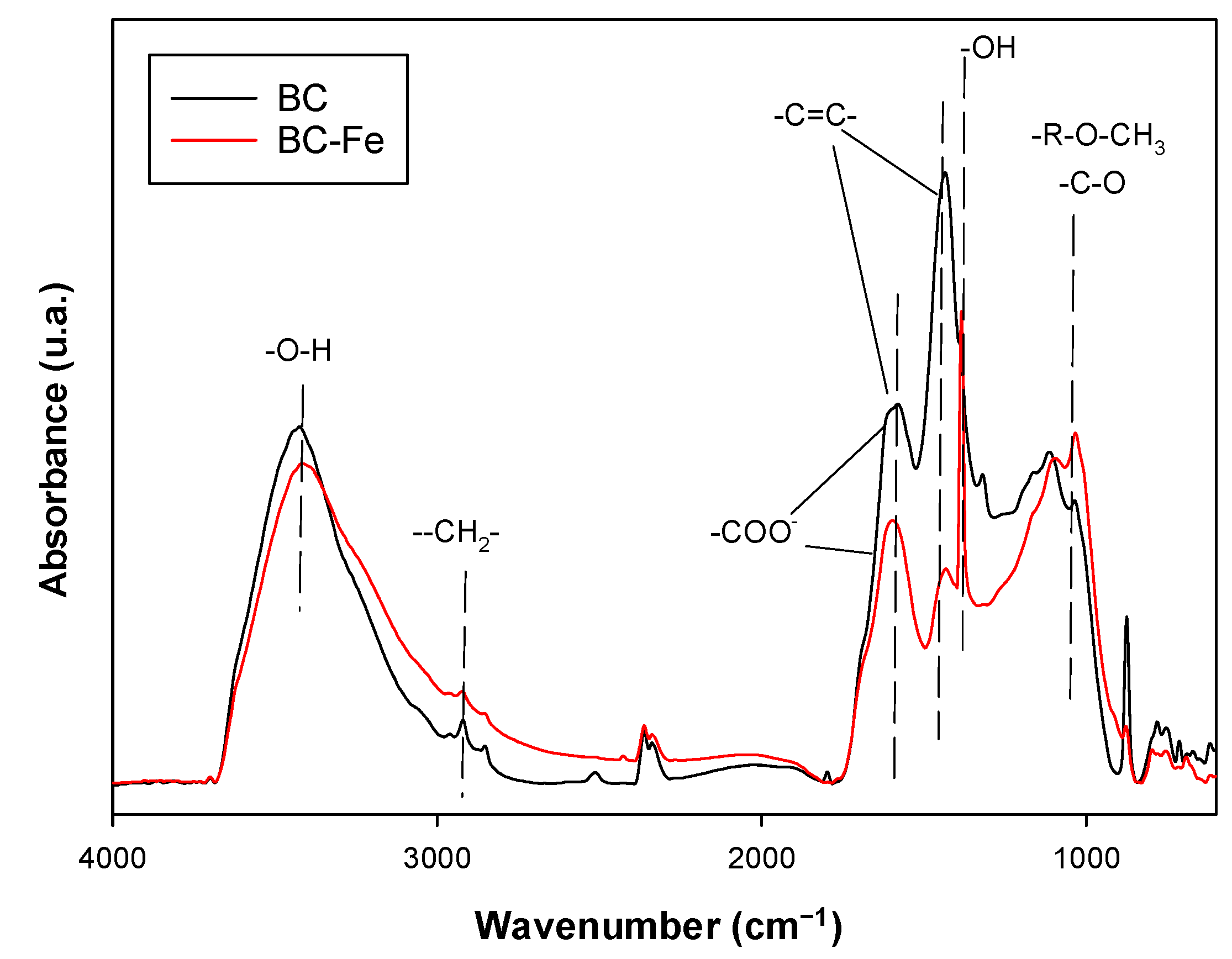

| Biochar | C (%) | H (%) | O (%) 1 | N (%) | Ash (%) 2 | Fe (g/kg) | SSA (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmeso (cm3/g) | pH 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 39.1 | 1.2 | 17.5 | 0.6 | 12 | 2.7 | 2.20 | 0.01300 | 0.00056 | 0.00727 | 7.9 |
| BC-Fe | 46.6 | 1.4 | 31.8 | 0.9 | 34 | 43.9 | 4.13 | 0.01051 | 0.00452 | 0.00320 | 5.6 |
| Treatment | Kf | Nf | R2 | Kfd | Nfd | R2 | TII 1 | Kd0.5 (L/kg) 2 |
|---|---|---|---|---|---|---|---|---|
| BC | 77 (68–87) 3 | 0.64 ± 0.07 4 | 0.953 | 169 (167–171) | 0.135 ± 0.007 | 0.996 | 0.79 | 98 |
| BC-Fe | 201 (178–228) | 0.57 ± 0.02 | 0.997 | 76.9 (68.2–86.7) | 0.122 ± 0.021 | 0.944 | 0.77 | 278 |
| k (d−1) | t1/2 (d) | R2 | |
|---|---|---|---|
| Soil | 0.172 ± 0.029 1 | 4.0 | 0.950 |
| Soil + BC | 0.153 ± 0.026 | 4.5 | 0.945 |
| Soil + BC-Fe | 0.109 ± 0.031 | 6.4 | 0.831 |
| Treatment | Cmax (mg/L) 1 | Total Leached (%) | Total Extracted (%) | Total Not Recovered (%) |
|---|---|---|---|---|
| Soil | 0.78 ± 0.03 ba | 31 ± 1 a | <1 | 69 |
| Soil + BC | 0.61 ± 0.05 a | 19 ± 1 b | 9 ± 3 | 72 |
| Soil + BC-Fe | 0.45 ± 0.11 b | 16 ± 4 b | 7 ± 2 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámiz, B.; Velarde, P.; Spokas, K.A.; Cox, L. The Role of Nanoengineered Biochar Activated with Fe for Sulfanilamide Removal from Soils and Water. Molecules 2022, 27, 7418. https://doi.org/10.3390/molecules27217418
Gámiz B, Velarde P, Spokas KA, Cox L. The Role of Nanoengineered Biochar Activated with Fe for Sulfanilamide Removal from Soils and Water. Molecules. 2022; 27(21):7418. https://doi.org/10.3390/molecules27217418
Chicago/Turabian StyleGámiz, Beatriz, Pilar Velarde, Kurt A. Spokas, and Lucía Cox. 2022. "The Role of Nanoengineered Biochar Activated with Fe for Sulfanilamide Removal from Soils and Water" Molecules 27, no. 21: 7418. https://doi.org/10.3390/molecules27217418








