The Influence of Alkali Metals on the Doping of Poly(p-phenylene) Oligomers
Abstract
:1. Introduction
2. Results and Discussion
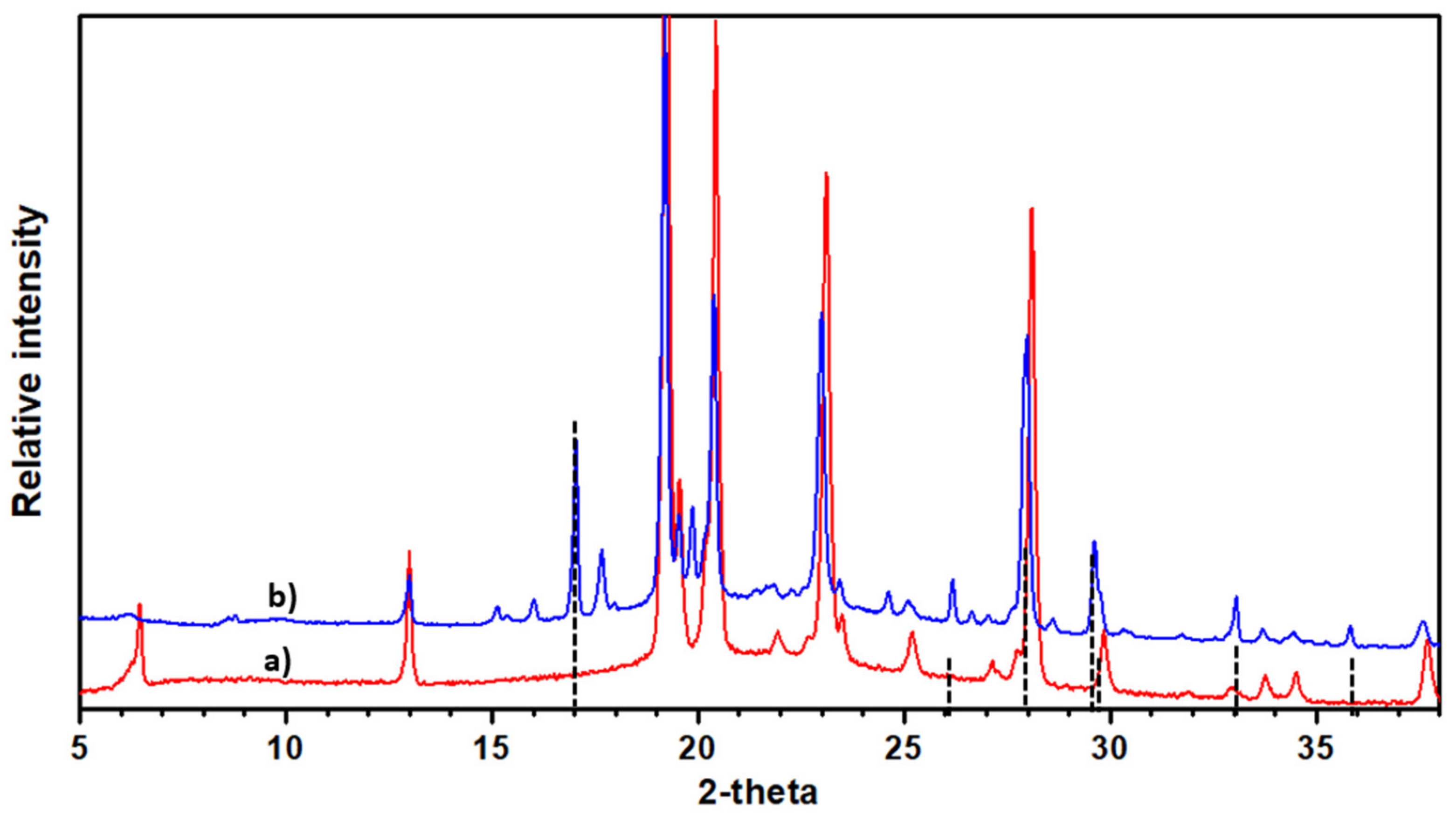
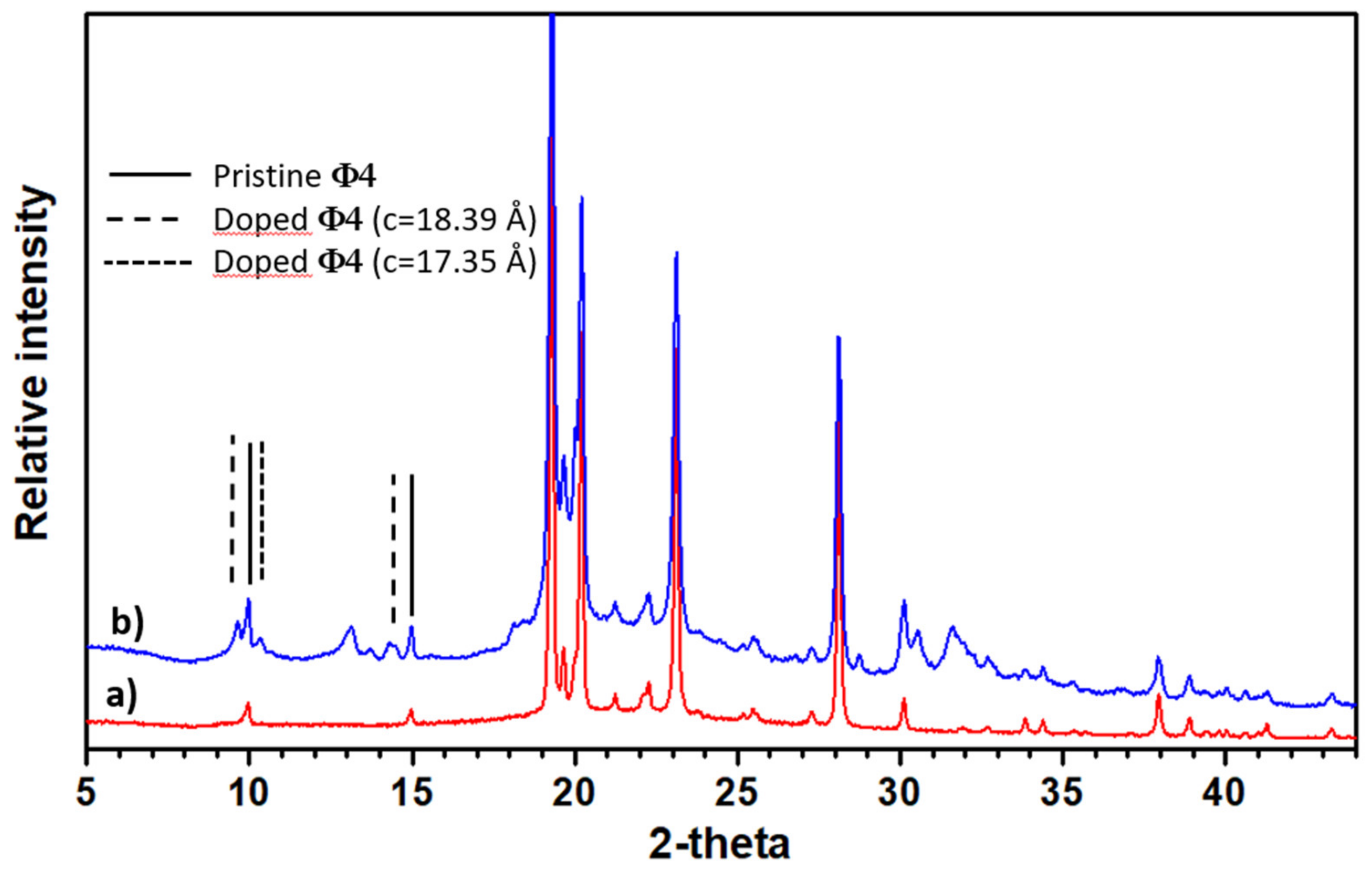
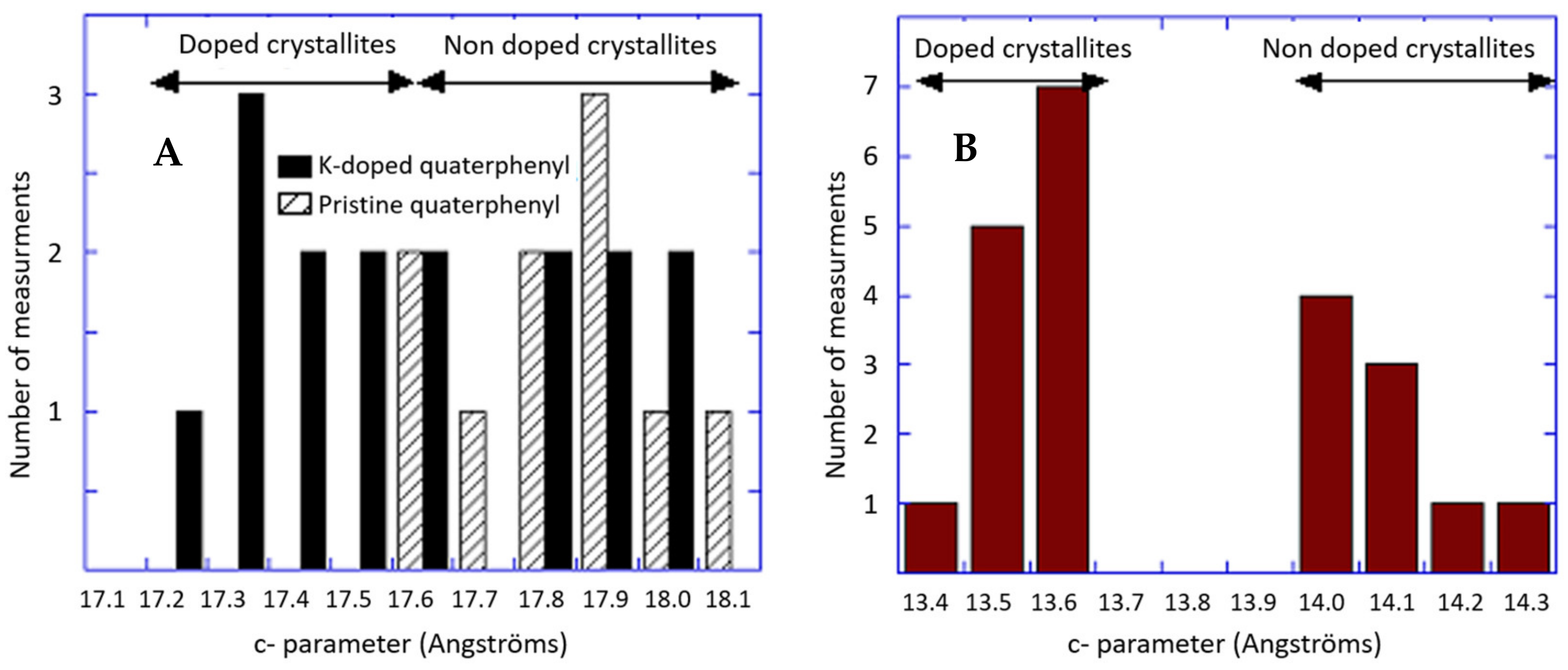
2.1. Analysis of Electron and X-ray Diffraction
2.1.1. Pristine Materials
2.1.2. Sodium-Doped Material
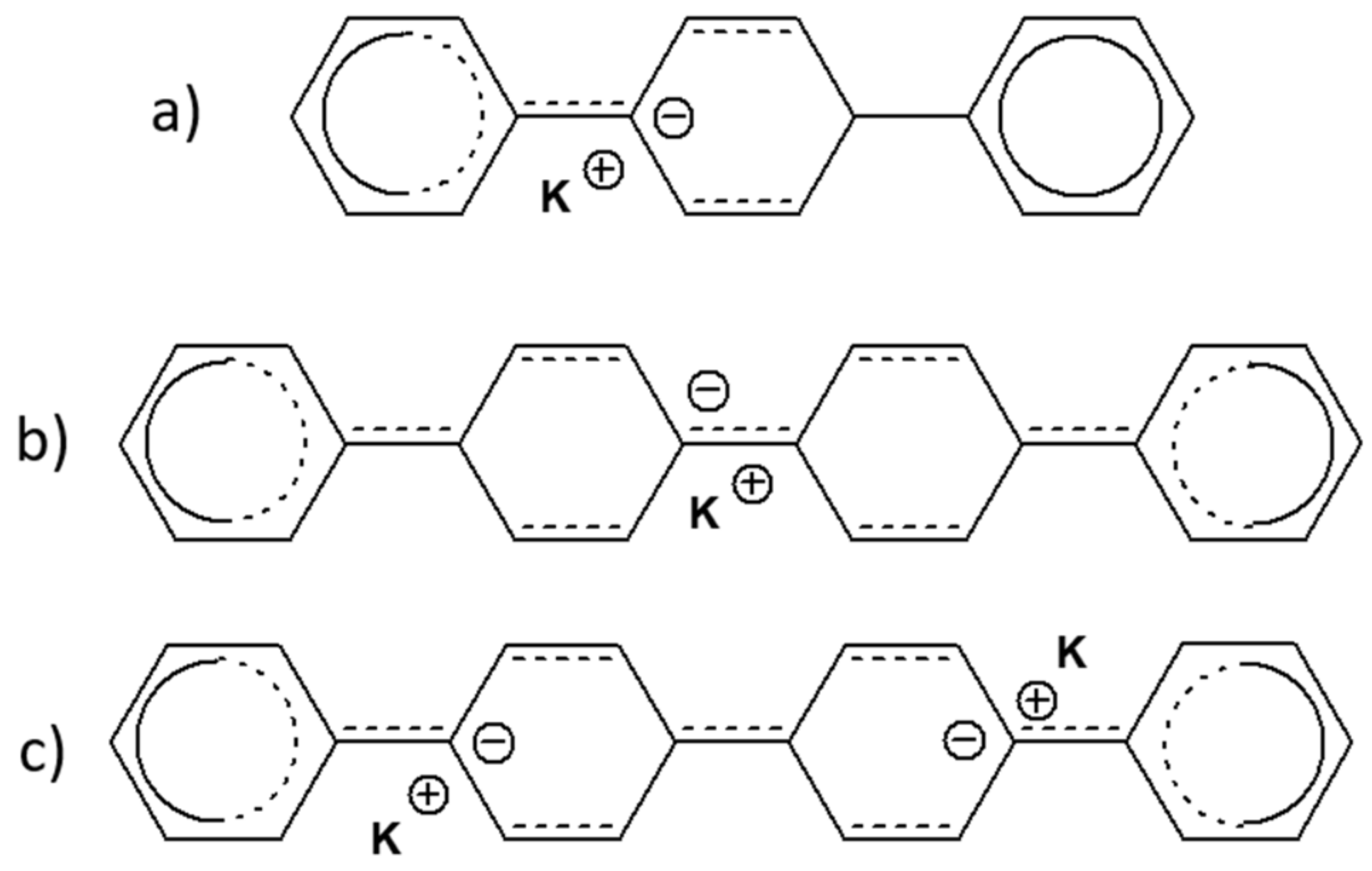
2.1.3. Potassium-Doped Materials
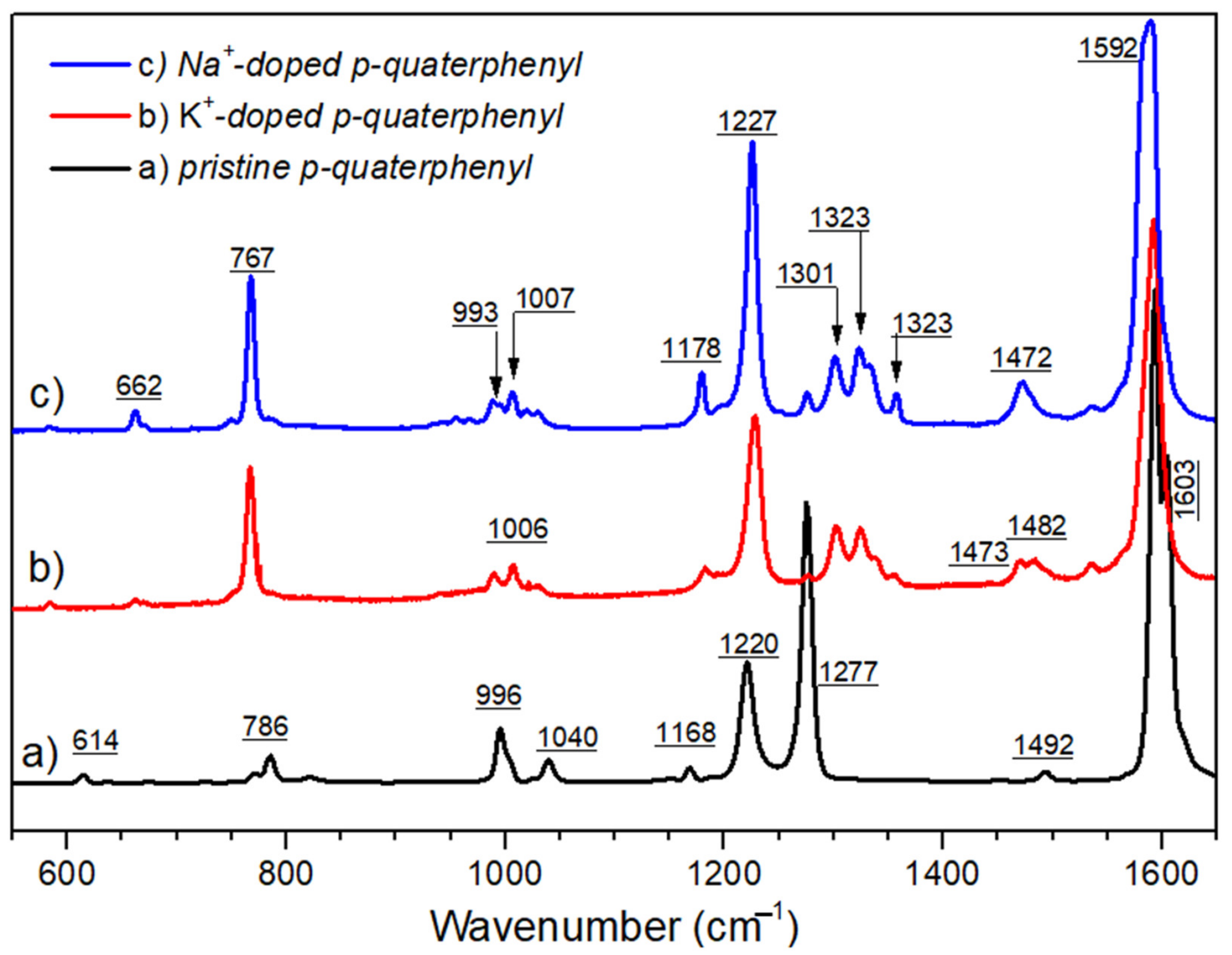
2.2. Raman Spectroscopy Analysis
- (i)
- Two strongly overlapping sharp peaks at 1595 and 1610 cm−1 that can be assigned to the aromatic C-C stretching deformations, which is consistent with the neutral state of the molecules (i.e., mono- and para-substituted benzene rings);
- (ii)
- A band at 1288 cm−1 is usually attributed to the C-C interring stretching;
- (iii)
- A weak band at ca. 1220 cm−1 together with a band at ca. 1170 cm−1 are characteristic of the C-H, when in plane bending deformations in the aromatic ring;
- (iv)
- A band that is pointed at 996 cm−1, which is always absent in para-substituted benzene rings, confirms the coexistence of mono-substituted rings.
3. Materials and Methods
3.1. Synthesis
3.2. Electron Diffraction
3.3. X-ray Diffraction
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chauhan, A.K.; Jha, P.; Aswal, D.K.; Yakhmi, J.V. Organic Devices: Fabrication, Applications, and Challenges. J. Electron. Mater. 2022, 51, 447–485. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Dubois, M.; Naji, A.; Billaud, D. Electrochemical Insertion of Alkaline Ions into Polyparaphenylene: Effect of the Crystalline Structure of the Host Material. Electrochim. Acta 2001, 46, 4301–4307. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent Advances in Conjugated Polymers for Light Emitting Devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Chaubey, A.; Singh, S.P. Prospects of Conducting Polymers in Biosensors. Anal. Chim. Acta 2006, 578, 59–74. [Google Scholar] [CrossRef]
- Pinto, N.; Nicola, C.D.; Trapananti, A.; Minicucci, M.; Cicco, A.D.; Marcelli, A.; Bianconi, A.; Marchetti, F.; Pettinari, C.; Perali, A. Potassium-Doped Para-Terphenyl: Structure, Electrical Transport Properties and Possible Signatures of a Superconducting Transition. Condens. Matter 2020, 5, 78. [Google Scholar] [CrossRef]
- Yan, J.; Zhong, G.; Wang, R.; Zhang, K.; Lin, H.; Chen, X. Superconductivity and Phase Stability of Potassium-Intercalated P-Quaterphenyl. J. Phys. Chem. Lett. 2019, 10, 40–47. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, X.; Wang, R.; Han, J.; Zhang, C.; Chen, X.; Lin, H. Structural and Bonding Characteristics of Potassium-Doped p - Terphenyl Superconductors. J. Phys. Chem. C 2018, 122, 3801–3808. [Google Scholar] [CrossRef]
- Fung, M.K.; Lai, S.L.; Bao, S.N.; Lee, C.S.; Lee, S.T.; Wu, W.W.; Inbasekaran, M.; O’Brien, J.J. Interface between Poly (9,9-Dioctylfluorene) and Alkali Metals: Cesium, Potassium, Sodium, and Lithium. J. Vac. Sci. Technol. A 2002, 20, 911. [Google Scholar] [CrossRef]
- Uysal, Ş.; Kurşunlu, A.N. The Synthesis and Characterization of Star Shaped Metal Complexes of Triazine Cored Schiff Bases: Their Thermal Decompositions and Magnetic Moment Values. J. Inorg. Organomet. Polym. Mater. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Maltas, E.; Kursunlu, A.N.; Arslan, G.; Ozmen, M. A New BODIPY/Nanoparticle/Ni Affinity System for Binding of Cytochrome C. Appl. Surf. Sci. 2015, 349, 811–816. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Ozmen, M.; Guler, E. Novel Magnetite Nanoparticle Based on BODIPY as Fluorescent Hybrid Material for Ag(I) Detection in Aqueous Medium. Talanta 2016, 153, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Froyer, G.; Billaud, D. X-Ray Diffraction Characterization of Parasexiphenylene Intercalated Electrochemically with Sodium. Solid State Commun. 2002, 122, 613–618. [Google Scholar] [CrossRef]
- Dubois, M.; Froyer, G.; Billaud, D. Electrochemical Impedance Spectroscopy and Electron Spin Resonance Characterization of the Conductive State of Parasexiphenylene Electrochemically Intercalated with Sodium. Spectrochim. Acta Part A 2004, 60, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Delugeard, Y.; Desuche, J.; Baudour, J.L. Structural Transition in Polyphenyls. II.* The Crystal Structure of the High-Temperature Phase of Quaterphenyl. Acta Cryst. 1976, B32, 702–705. [Google Scholar] [CrossRef] [Green Version]
- Baudour, J.L.; Toupet, L.; Délugeard, Y.; Ghémid, S. Transitions de Phase Structurales Dans Les Polyphényles. IX. Affinements Des Structures Du p-Terphényle Hydrogénéà 200 K (Diffraction Des Rayons X) Eu Du Biphényle Deutéréà 40 K (Diffraction Des Neutrons). Acta Crystallogr. Sect. C 1986, 42, 1211–1217. [Google Scholar] [CrossRef]
- Paris, M.; Péres, L.O.; Chauvet, O.; Froyer, G. Solid-State NMR Study of Na versus K Doping of Para-Phenylene Oligomers. J. Phys. Chem. B 2006, 110, 743–747. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Furukawa, Y.; Tasumi, M. Dependencies of the Raman Spectra of P-Oligophenyls on the Chain Length and the Excitation Wavelength. Spectrochim. Acta Part A Mol. Spectrosc. 1993, 49A, 731–737. [Google Scholar] [CrossRef]
- Honda, K.; Furukawa, Y. Conformational Analysis of P-Terphenyl by Vibrational Spectroscopy and Density Functional Theory Calculations. J. Mol. Struct. 2005, 735–736, 11–19. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.J. Chain Length Effects of p -Oligophenyls with Comparison of Benzene by Raman Scattering. AIP Adv. 2018, 8, 025004. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, R.S.; Chen, X.J. Vibrational Properties of P-Terphenyl. J. Phys. Chem. A 2018, 122, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 1907296, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, T.; Kloc, C.; Sun, L.; Gurzadyan, G.G. Singlet Fission, Poleron Generation and Intersystem Crossing in Hexaphenyl Film. Molecules 2022, 27, 5067. [Google Scholar] [CrossRef]
- Dubois, M.; Froyer, G.; Louarn, G.; Billaud, D. Raman Spectroelectrochemical Study of Sodium Intercalation into Poly ( p-Phenylene ). Spectrochim. Acta Part A 2003, 59, 1849–1856. [Google Scholar] [CrossRef]
- Péres, L.O.; Spiesser, M.; Froyer, G. Reduction of P-Terphenyl, p-Quaterphenyl and p-Sexiphenyl Using Alkali Metal in Liquid Ammonia: Process and Characterization of the Reduced Compounds. Synth. Met. 2005, 155, 450–454. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Rodríguez-Carvajal, J.; Canales-Vázquez, J.; Laligant, Y.; Lacorre, P.; Palacín, M.R. Microstructural Characterisation of Battery Materials Using Powder Diffraction Data: DIFFaX, FAULTS and SH-FullProf Approaches. J. Power Sources 2007, 174, 414–420. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Patterns Analysis. Mater. Sci. Forum 2001, 378, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Werner, P.E.; Eriksson, L.; Westdahl, M. TREOR, a Semi-Exhaustive Trial-and-Error Powder Indexing Program for All Symmetries. J. Appl. Cryst. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Boultif, A.; Loue, D. Powder Pattern Indexing with the Dichotomy Method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Leinenweber, K. Welcome to the Crystal Cracker Program Page. Available online: http://multianvil.asu.edu/Crystal_Cracker/CrystalCracker.html (accessed on 21 October 2022).
- Laugier, J.; Bochu, B. LMGP-Suite of Programs for the Interpretation of X-ray Experiments. Available online: http://www.inpg.fr/LMGP (accessed on 21 October 2022).



| hkl | 2θobs | 2θcalc | 2θobs−2θcalc |
|---|---|---|---|
| 002 | 9.89 | 9.91 | −0.02 |
| 202 | 15.15 | 15.114 | 0.036 |
| 011 | 15.37 | 15.363 | 0.007 |
| −103 | 16.02 | 16.018 | 0.002 |
| 012 | 17.66 | 17.622 | 0.038 |
| 301 | 17.95 | 17.917 | 0.033 |
| 302/004 | 19.85 | 19.853 | −0.003 |
| 312 | 24.63 | 24.693 | −0.063 |
| −304 | 26.58 | 26.584 | −0.004 |
| p-Terphenyl | p-Quaterphenyl | Benzene | |||||
|---|---|---|---|---|---|---|---|
| Raman Scattering (cm−1) | Raman Scattering (cm−1) | ||||||
| Un-Doped | K-Doped | Na-Doped | Un-Doped | K-Doped | Na-Doped | Wilson Notation | Assignments |
| 1608 | 1592 | 1592 | 1603 | 1592 | 1592 | 1596 (8a) 1596 (8b) | C-C stretch + C-H bend para benzene ring |
| 1590 | 1596 | ||||||
| 1472 | 1482 | 1492 | 1473 | 1472 | 1486 (19a) | C-C stretch + C-H bend (intra ring) | |
| ring stretch + deformation | |||||||
| 1276 | 1348 | 1350 | 1277 | 1340 | 1340 | C-C stretch (inter ring) | |
| 1310 | 1335 | 1301 | 1301 | ||||
| 1220 | 1223 | 1221 | 1220 | 1230 | 1227 | 1178 (9a) | Aromatic C-H bend (para benzene ring) |
| 1170 | 1170 | 1190 | 1168 | 1180 | 1178 | ||
| 1038 | 1004 | 1036 | 1040 | 1030 | 1040 | 1010 (12) | ring deformation (mono substituted ring) |
| 1004 | 989 | 995 | 996 | 992 | 993 | ||
| 992 | |||||||
| 773 | 751 | 755 | 786 | 768 | 767 | 849 (10) | ring deformation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péres, L.O.; da Rochas Rodrigues, R.; Louarn, G. The Influence of Alkali Metals on the Doping of Poly(p-phenylene) Oligomers. Molecules 2022, 27, 8699. https://doi.org/10.3390/molecules27248699
Péres LO, da Rochas Rodrigues R, Louarn G. The Influence of Alkali Metals on the Doping of Poly(p-phenylene) Oligomers. Molecules. 2022; 27(24):8699. https://doi.org/10.3390/molecules27248699
Chicago/Turabian StylePéres, Laura Oliveira, Rebeca da Rochas Rodrigues, and Guy Louarn. 2022. "The Influence of Alkali Metals on the Doping of Poly(p-phenylene) Oligomers" Molecules 27, no. 24: 8699. https://doi.org/10.3390/molecules27248699
APA StylePéres, L. O., da Rochas Rodrigues, R., & Louarn, G. (2022). The Influence of Alkali Metals on the Doping of Poly(p-phenylene) Oligomers. Molecules, 27(24), 8699. https://doi.org/10.3390/molecules27248699







