Organic Thermoelectric Materials as the Waste Heat Remedy
Abstract
:1. Introduction
2. Organic Small-Molecular Thermoelectric Materials
2.1. Electron-Conductive Materials
| Material | Dopant | Cond. Type | HOMO [eV] | LUMO [eV] | σ [S/cm] | α [μV/K] | σα2 [μW/(mK2)] | ZT | Ref. |
|---|---|---|---|---|---|---|---|---|---|
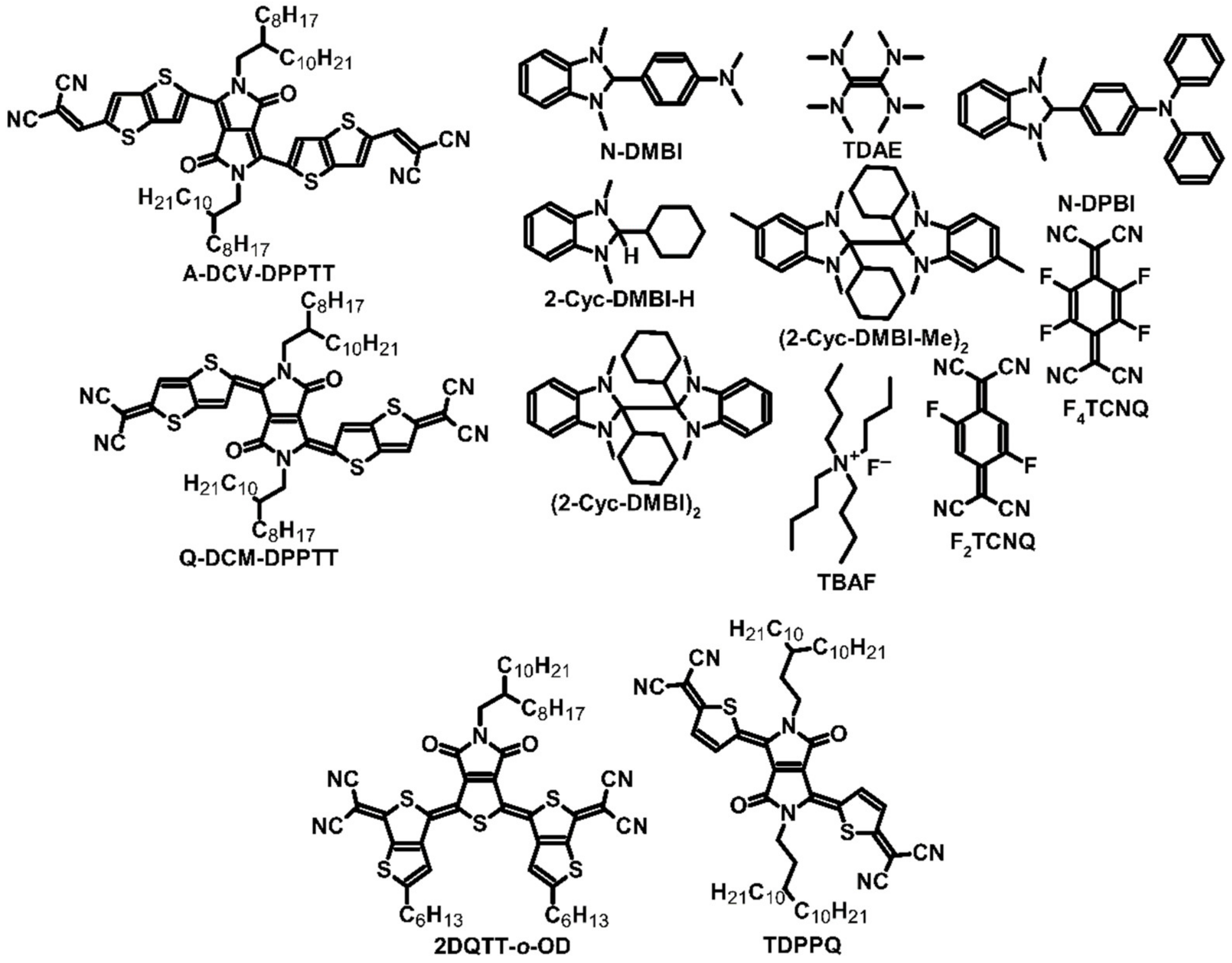
| A-DCV-DPPTT | N-DMBI | N | −5.7 | −3.9 | 3.1 (a) 4.9 (b) | −568 (a)−665 (b) | 95 (a) 217 (b) | 0.11 (a) 0.23 (b) | [24] |
| Q-DCM-DPPTT | N-DMBI | N | −6.0 | −4.5 | 0.11 (a) 0.24 (b) | −383 (a) −420 (b) | 1.7 (a) 4.2 (b) | [24] | |
| 2DQTT-o-OD | 2-Cyc-DMBI-H | N | −4.68 | 0.18 | 387 | 2.7 | [27] | ||
| 2DQTT-o-OD | (2-Cyc-DMBI-Me)2 | N | −4.68 | 1.1 (c) | ~550 (c) | 17.2 (a) 33.3 (c) | 0.02 (c) | [27] | |
| 2DQTT-o-OD | (2-Cyc-DMBI)2 | N | −4.68 | 0.43 | 409 | 7.2 | [27] | ||
| TDPPQ | Bi (2 nm) | N | 3.3 (d) | −585 (d) | 113 (d) | [28] | |||
| PDA | N | −6.47 | −3.92 | ~600 | [29] | ||||
| PDI | N | −6.11 | −3.58 | ~650 | [29] | ||||
| C8PDI | N | ~4000 | [29] | ||||||
| DNTT | −5.19 | −1.82 | ~3.6 × 104 | [29] | |||||
| C10DNTT | ~1.2 × 105 | ~0.5 | [29] | ||||||
| C8BTBT | ~7 × 104 | [29] | |||||||
| Fullerene C60 | N | ~−2 × 105 | ~0.02 | [29] | |||||
| PTEG-1 | N-DMBI | N | 2.05 | −248 | 16.7 | [30] | |||
| PCBMNDI-CN | N-DPBI | N | −6.00 −6.66 | −4.00 −4.60 | ~0.03 | −1400 | 5 | [31,32] | |
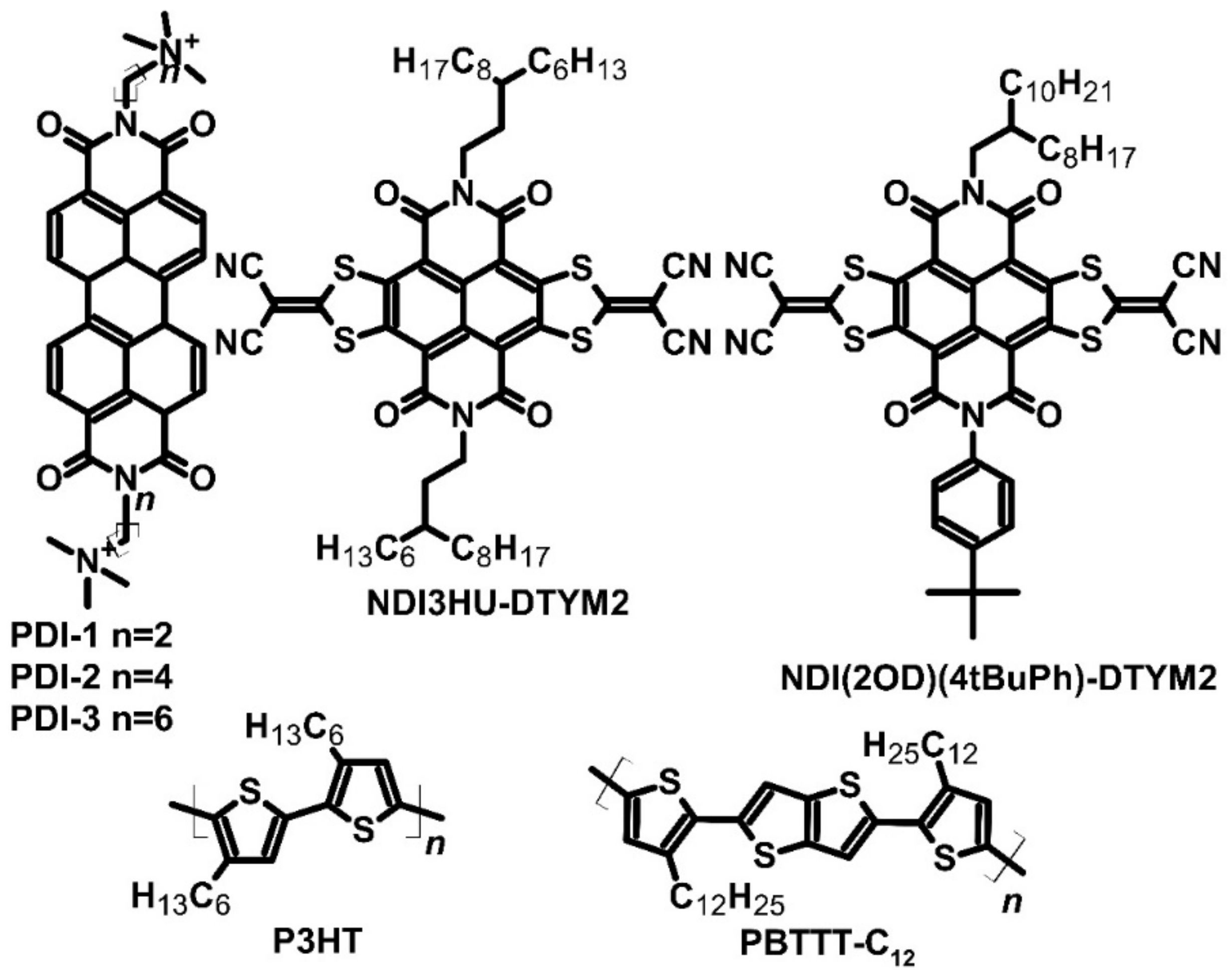
| PDI-1, n = 2 | N | −4.00 | ~1 × 10−3 | ~−200 | [33] | ||||
| PDI-2, n = 4 | N | −4.00 | ~0.01 | ~−200 | [33] | ||||
| PDI-3, n = 6 | N | −4.00 | ~0.5 | ~−200 | 1.4 | [33] | |||
| NDI3HU-DTYM2 | N | 0.4 | −250 | 2.5 | [34] | ||||
| NDI(2OD)(4tBuPh)-DTYM2 | N | 0.1 | −187 | 0.35 | [34] | ||||
| Au-BP-Au | N | −6.9 | [35] | ||||||
| Au-BDNC-Au | N | −13.3 | [36] | ||||||
| Au-BDCN-Au | N | −11.5 | [36] | ||||||
| Au-Sc3N@C80-Au | ±20 | [37] |
2.2. Hole-Conductive Materials
3. Organic Polymer Thermoelectric Materials
3.1. Electron-Conductive Materials
3.2. Hole-Conductive Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harrop, P.; Raghu, D. Thermoelectric Energy Harvesting and Sensing 2020–2030. IDTechEx: Boston, MA, USA, 2020. [Google Scholar]
- Velmre, E. Thomas Johann Seebeck (1770–1831). Proc. Est. Acad. Sci. Eng. 2007, 13, 276–282. [Google Scholar]
- Sommerfeld, A. Zur Quantentheorie der Spektrallinien. Ann. Phys. 1916, 356, 1–94. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.I.; Kroon, R.; Müller, C. Doping and processing of organic semiconductors for plastic thermoelectrics. In Handbook of Organic Materials for Electronic and Photonic Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–449. [Google Scholar]
- Oersted, H.C. Notiz von neuen electrisch—magnetischen Versuchen des Herrn Seebeck in Berlin. Ann. Phys. 1823, 73, 430–432. [Google Scholar] [CrossRef] [Green Version]
- Peltier, J.C.A. Nouvelles Expériences sur la Caloricité des courans électriques. Ann. Chim. Phys. 1834, 56, 371–386. [Google Scholar]
- Webster, J.G.; Gurevich, Y.G.; Velazquez-Perez, J.E. Peltier Effect in Semiconductors. In Wiley Encyclopedia of Electrical and Electronics Engineering; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 1–21. [Google Scholar]
- Tchantchaleishvili, V.; Bush, B.S.; Swartz, M.F.; Day, S.W.; Massey, H.T. Plutonium-238: An ideal power source for intracorpreal ventricular assist devices? ASAIO J. 2012, 58, 550–553. [Google Scholar] [CrossRef]
- Heacock, R.L. The Voyager Spacecraft. Proc. Inst. Mech. Eng. 1980, 194, 211–224. [Google Scholar] [CrossRef]
- Strauss, R.D.T. Voyager 2 enters interstellar space. Nat. Astron. 2019, 3, 963–964. [Google Scholar] [CrossRef]
- Ioffe, A.F. Semiconductor Thermoelements and Thermo-Electric Cooling; Infosearch Limited: London, UK, 1957; pp. 3–182. [Google Scholar]
- Bennett, G. The past as prologue: A look at historical flight qualifications for space nuclear systems. IEEE Aerosp. Electron. Syst. Mag. 1992, 7, 26–36. [Google Scholar] [CrossRef] [Green Version]
- LaPorte, A.H. Design and Testing of a 150 Watt Snap 19 High Performance Generator. In Proceedings of the 8th Intersociety Energy Conversion Engineering Conference, Philadelphia, PA, USA, 13–17 August 1973; AIAA: New York, NY, USA, 1973. [Google Scholar]
- Baxter, C. Orbital performance of the SNAP-19 radioisotope fueled thermoelectric power supply. In Proceedings of the 3rd Communications Satellite Systems Conference, Los Angeles, CA, USA, 6–8 April 1970; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1970. [Google Scholar]
- Park, Y.W.; Han, W.K.; Choi, C.H.; Shirakawa, H. Metallic nature of heavily doped polyacetylene derivatives: Thermopower. Phys. Rev. B 1984, 30, 5847–5851. [Google Scholar] [CrossRef]
- LaCoe, R.; Grüner, G.; Chaikin, P. The thermoelectric power of MEM(TCNQ)2. Solid State Commun. 1980, 36, 599–601. [Google Scholar] [CrossRef]
- Park, Y.-W.; Heeger, A.J.; Druy, M.A.; MacDiarmid, A.G. Electrical transport in doped polyacetylene. J. Chem. Phys. 1980, 73, 946. [Google Scholar] [CrossRef]
- Zhao, Y.; Gschossmann, S.; Schagerl, M.; Gruener, P.; Kralovec, C. Characterization of the spatial elastoresistivity of inkjet-printed carbon nanotube thin films. Smart Mater. Struct. 2018, 27, 105009. [Google Scholar] [CrossRef]
- Park, Y.; Yoon, C.; Lee, C.; Shirakawa, H.; Suezaki, Y.; Akagi, K. Conductivity and thermoelectric power of the newly processed polyacetylene. Synth. Met. 1989, 28, D27–D34. [Google Scholar] [CrossRef]
- Dughaish, Z. Lead telluride as a thermoelectric material for thermoelectric power generation. Phys. B Condens. Matter 2002, 322, 205–223. [Google Scholar] [CrossRef]
- Heller, M.; Danielson, G. Seebeck effect in Mg2Si single crystals. J. Phys. Chem. Solids 1962, 23, 601–610. [Google Scholar] [CrossRef]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Gonzalez, M.M.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2018, 138, 100501. [Google Scholar] [CrossRef]
- Choi, J.; Gordon, M.P.; Yuan, P.; Kang, H.; Zaia, E.W.; Urban, J.J. CHAPTER 1: Introduction. In Organic Thermoelectric Materials; Royal Society of Chemistry: Cambridge, UK, 2020; Volume 2020, pp. 1–20. ISBN 9781788016230. [Google Scholar]
- Huang, D.; Yao, H.; Cui, Y.; Zou, Y.; Zhang, F.; Wang, C.; Shen, H.; Jin, W.; Zhu, J.; Diao, Y.; et al. Conjugated-Backbone Effect of Organic Small Molecules for n-Type Thermoelectric Materials with ZT over 0.2. J. Am. Chem. Soc. 2017, 139, 13013–13023. [Google Scholar] [CrossRef]
- Chen, S.-G.; Branz, H.M.; Eaton, S.S.; Taylor, P.C.; Cormier, R.A.; Gregg, B.A. Substitutional n-Type Doping of an Organic Semiconductor Investigated by Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. B 2004, 108, 17329–17336. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Mi, B.X.; Xu, G.Z.; Wan, Y.Q.; Gong, M.L.; Cheah, K.W.; Chen, C.H. An organic p-type dopant with high thermal stability for an organic semiconductor. Chem. Commun. 2007, 1, 117–119. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, D.; Zhang, C.; Zou, Y.; Di, C.-A.; Zhu, X.; Zhu, D. Efficient Solution-Processed n-Type Small-Molecule Thermoelectric Materials Achieved by Precisely Regulating Energy Level of Organic Dopants. ACS Appl. Mater. Interfaces 2017, 9, 28795–28801. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, C.; Zou, Y.; Shen, X.; Zang, Y.; Shen, H.; Gao, X.; Yi, Y.; Xu, W.; Di, C.-A.; et al. Bismuth Interfacial Doping of Organic Small Molecules for High Performance n-type Thermoelectric Materials. Angew. Chem. Int. Ed. 2016, 55, 10672–10675. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Abe, R.; Fujiwara, F.; Nakagawa, M.; Takahashi, K.; Kuzuhara, D.; Yamada, H.; Yakiyama, Y.; Sakurai, H.; Yamamoto, T.; et al. Universality of the giant Seebeck effect in organic small molecules. Mater. Chem. Front. 2018, 2, 1276–1283. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Portale, G.; Koopmans, M.; Brink, G.T.; Hummelen, J.C.; Koster, L.J.A. N-Type Organic Thermoelectrics: Improved Power Factor by Tailoring Host-Dopant Miscibility. Adv. Mater. 2017, 29, 1701641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, G.; Li, Z.; Wang, E.; Kemerink, M. High Seebeck Coefficient and Power Factor in n-Type Organic Thermoelectrics. Adv. Electron. Mater. 2017, 4, 1700501. [Google Scholar] [CrossRef] [Green Version]
- Zuo, G.; Abdalla, H.; Kemerink, M. Conjugated Polymer Blends for Organic Thermoelectrics. Adv. Electron. Mater. 2019, 5, 1800821. [Google Scholar] [CrossRef] [Green Version]
- Russ, B.; Robb, M.J.; Brunetti, F.G.; Miller, P.L.; Perry, E.E.; Patel, S.N.; Ho, V.; Chang, W.B.; Urban, J.J.; Chabinyc, M.L.; et al. Power Factor Enhancement in Solution-Processed Organic n-Type Thermoelectrics Through Molecular Design. Adv. Mater. 2014, 26, 3473–3477. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zang, Y.; Huang, D.; Di, C.-A.; Gao, X.; Sirringhaus, H.; Zhu, D. Modulated Thermoelectric Properties of Organic Semiconductors Using Field-Effect Transistors. Adv. Funct. Mater. 2015, 25, 3004–3012. [Google Scholar] [CrossRef]
- Cui, L.; Miao, R.; Wang, K.; Thompson, D.; Zotti, L.A.; Cuevas, J.C.; Meyhofer, E.; Reddy, P. Peltier cooling in molecular junctions. Nat. Nanotechnol. 2017, 13, 122–127. [Google Scholar] [CrossRef]
- Zotti, L.A.; Bürkle, M.; Pauly, F.; Lee, W.; Kim, K.; Jeong, W.; Asai, Y.; Reddy, P.; Cuevas, J.C. Heat dissipation and its relation to thermopower in single-molecule junctions. New J. Phys. 2014, 16, 015004. [Google Scholar] [CrossRef]
- Gehring, P.; Thijssen, J.M.; Van Der Zant, H.S.J. Single-molecule quantum-transport phenomena in break junctions. Nat. Rev. Phys. 2019, 1, 381–396. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Liu, J. Controlling PCBM aggregation in P3HT/PCBM film by a selective solvent vapor annealing. Chin. Sci. Bull. 2013, 58, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Shinano, T.; Miyazaki, Y.; Kajitani, T. Thermoelectric properties of iodine doped pentacene thin films. Phys. Status Solidi Curr. Top. Solid State Phys. 2010, 8, 592–594. [Google Scholar] [CrossRef]
- Ito, M.; Okamoto, N.; Abe, R.; Kojima, H.; Matsubara, R.; Yamashita, I.; Nakamura, M. Enhancement of thermoelectric properties of carbon nanotube composites by inserting biomolecules at nanotube junctions. Appl. Phys. Express 2014, 7, 065102. [Google Scholar] [CrossRef]
- Ito, M.; Koizumi, T.; Kojima, H.; Saito, T.; Nakamura, M. From materials to device design of a thermoelectric fabric for wearable energy harvesters. J. Mater. Chem. A 2017, 5, 12068–12072. [Google Scholar] [CrossRef]
- Meyers, T.; Vidor, F.F.; Brassat, K.; Lindner, J.K.; Hilleringmann, U. Low-voltage DNTT-based thin-film transistors and inverters for flexible electronics. Microelectron. Eng. 2017, 174, 35–39. [Google Scholar] [CrossRef]
- Ohmura, T.; Morimasa, Y.; Suginome, M. Organocatalytic Diboration Involving “Reductive Addition” of a Boron–Boron σ-Bond to 4,4′-Bipyridine. J. Am. Chem. Soc. 2015, 137, 2852–2855. [Google Scholar] [CrossRef] [PubMed]
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The Most Widely Used Ligand. A Review of Molecules Comprising at Least Two 2,2‘-Bipyridine Units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef]
- Ledwon, P.; Ovsiannikova, D.; Jarosz, T.; Gogoc, S.; Nitschke, P.; Domagala, W. Insight into the properties and redox states of n-dopable conjugated polymers based on naphtalene diimide units. Electrochim. Acta 2019, 307, 525–535. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Mikhail, V.; Vagin, M.; Persson, P.O.; Andreasen, J.W.; Thiel, W.; Berggren, M.; Crispin, X.; Fazzi, D.; et al. Thermoelectric Properties of Solution-Processed n-Doped Ladder-Type Conducting Polymers. Adv. Mater. 2016, 28, 10764–10771. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Erdmann, T.; Wang, G.; Fazzi, D.; Lappan, U.; Puttisong, Y.; Chen, Z.; Berggren, M.; Crispin, X.; et al. A Chemically Doped Naphthalenediimide-Bithiazole Polymer for n-Type Organic Thermoelectrics. Adv. Mater. 2018, 30, 1801898. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ye, G.; Van Der Zee, B.; Dong, J.; Qiu, X.; Liu, Y.; Portale, G.; Chiechi, R.C.; Koster, L.J.A. N-Type Organic Thermoelectrics of Donor-Acceptor Copolymers: Improved Power Factor by Molecular Tailoring of the Density of States. Adv. Mater. 2018, 30, e1804290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, D.; Giovannitti, A.; Sun, H.; Biskup, T.; Hofmann, A.; Koopmans, M.; Cendra, C.; Weber, S.; Koster, L.J.A.; Olsson, E.; et al. Enhanced n-Doping Efficiency of a Naphthalenediimide-Based Copolymer through Polar Side Chains for Organic Thermoelectrics. ACS Energy Lett. 2018, 3, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Ku, S.-Y.; Economou, N.J.; Jang, W.; Wang, D.H. Selective Doping of Conjugated Block Copolymer for Organic Thermoelectric Applications. Nanoscale Microscale Thermophys. Eng. 2019, 23, 222–234. [Google Scholar] [CrossRef]
- Schlitz, R.A.; Brunetti, F.G.; Glaudell, A.M.; Miller, P.L.; Brady, M.A.; Takacs, C.J.; Hawker, C.J.; Chabinyc, M.L. Solubility-Limited Extrinsic n-Type Doping of a High Electron Mobility Polymer for Thermoelectric Applications. Adv. Mater. 2014, 26, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Madan, D.; Cheng, Y.; Zhou, J.; Li, H.; Thon, S.M.; Bragg, A.E.; DeCoster, M.E.; Hopkins, P.E.; Katz, H.E. High Conductivity and Electron-Transfer Validation in an n-Type Fluoride-Anion-Doped Polymer for Thermoelectrics in Air. Adv. Mater. 2017, 29, 1606928. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Jin, W.-L.; Wang, J.; Ding, Y.-F.; Nong, S.; Shi, K.; Lu, Y.; Dai, Y.-Z.; Zhuang, F.-D.; Lei, T.; et al. Enhancing the n-Type Conductivity and Thermoelectric Performance of Donor-Acceptor Copolymers through Donor Engineering. Adv. Mater. 2018, 30, e1802850. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Di, C.-A.; Xu, W.; Zhu, D. Advances in n-Type Organic Thermoelectric Materials and Devices. Adv. Electron. Mater. 2019, 5, 1800825. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, P.; Di, C.-A.; Jiao, F.; Xu, W.; Qiu, D.; Zhu, D. Organic Thermoelectric Materials and Devices Based on p- and n-Type Poly(metal 1,1,2,2-ethenetetrathiolate)s. Adv. Mater. 2012, 24, 932–937. [Google Scholar] [CrossRef]
- Jin, W.; Liu, L.; Yang, T.; Shen, H.; Zhu, J.; Xu, W.; Li, S.; Li, Q.; Chi, L.; Di, C.-A.; et al. Exploring Peltier effect in organic thermoelectric films. Nat. Commun. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Alam, M.M.; Jenekhe, S.A. Efficient Solar Cells from Layered Nanostructures of Donor and Acceptor Conjugated Polymers. Chem. Mater. 2004, 16, 4647–4656. [Google Scholar] [CrossRef]
- Sun, H.; DiMagno, S.G. Anhydrous Tetrabutylammonium Fluoride. J. Am. Chem. Soc. 2005, 127, 2050–2051. [Google Scholar] [CrossRef]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef]
- Kaburagi, Y.; Kishi, Y. Operationally Simple and Efficient Workup Procedure for TBAF-Mediated Desilylation: Application to Halichondrin Synthesis. Org. Lett. 2007, 9, 723–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: Synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Müller, C. Thermoelectric plastics: From design to synthesis, processing and structure–property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aïch, R.B.; Blouin, N.; Bouchard, A.; Leclerc, M. Electrical and Thermoelectric Properties of Poly(2,7-Carbazole) Derivatives. Chem. Mater. 2009, 21, 751–757. [Google Scholar] [CrossRef]
- Feng-Xing, J.; Jing-Kun, X.; Bao-Yang, L.; Yu, X.; Rong-Jin, H.; Lai-Feng, L. Thermoelectric Performance of Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate). Chin. Phys. Lett. 2008, 25, 2202–2205. [Google Scholar] [CrossRef]
- Huang, X.; Deng, L.; Liu, F.; Zhang, Q.; Chen, G. Effect of Crystalline Microstructure Evolution on Thermoelectric Performance of PEDOT: PSS Films. Energy Mater. Adv. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Xu, S.; Hong, M.; Shi, X.-L.; Wang, Y.; Ge, L.; Bai, Y.; Wang, L.; Dargusch, M.; Zou, J.; Chen, Z.-G. High-Performance PEDOT:PSS Flexible Thermoelectric Materials and Their Devices by Triple Post-Treatments. Chem. Mater. 2019, 31, 5238–5244. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Liu, Y.; Liu, P.; Wang, Y.; Li, M.; Jiang, F.; Lan, X.; Xu, J. PEDOT:PSS/PVA/Te ternary composite fibers toward flexible thermoelectric generator. Compos. Commun. 2021, 27, 100855. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Liu, Y.; Wang, B.; Fang, L.; Qiu, J.; Zhang, K.; Wang, S. Exceptional thermoelectric properties of flexible organic−inorganic hybrids with monodispersed and periodic nanophase. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Glaudell, A.M.; Peterson, K.A.; Thomas, E.M.; O’Hara, K.A.; Lim, E.; Chabinyc, M.L. Morphology controls the thermoelectric power factor of a doped semiconducting polymer. Sci. Adv. 2017, 3, e1700434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroshige, Y.; Ookawa, M.; Toshima, N. High thermoelectric performance of poly(2,5-dimethoxyphenylenevinylene) and its derivatives. Synth. Met. 2006, 156, 1341–1347. [Google Scholar] [CrossRef]
- Toshima, N. Recent progress of organic and hybrid thermoelectric materials. Synth. Met. 2017, 225, 3–21. [Google Scholar] [CrossRef]
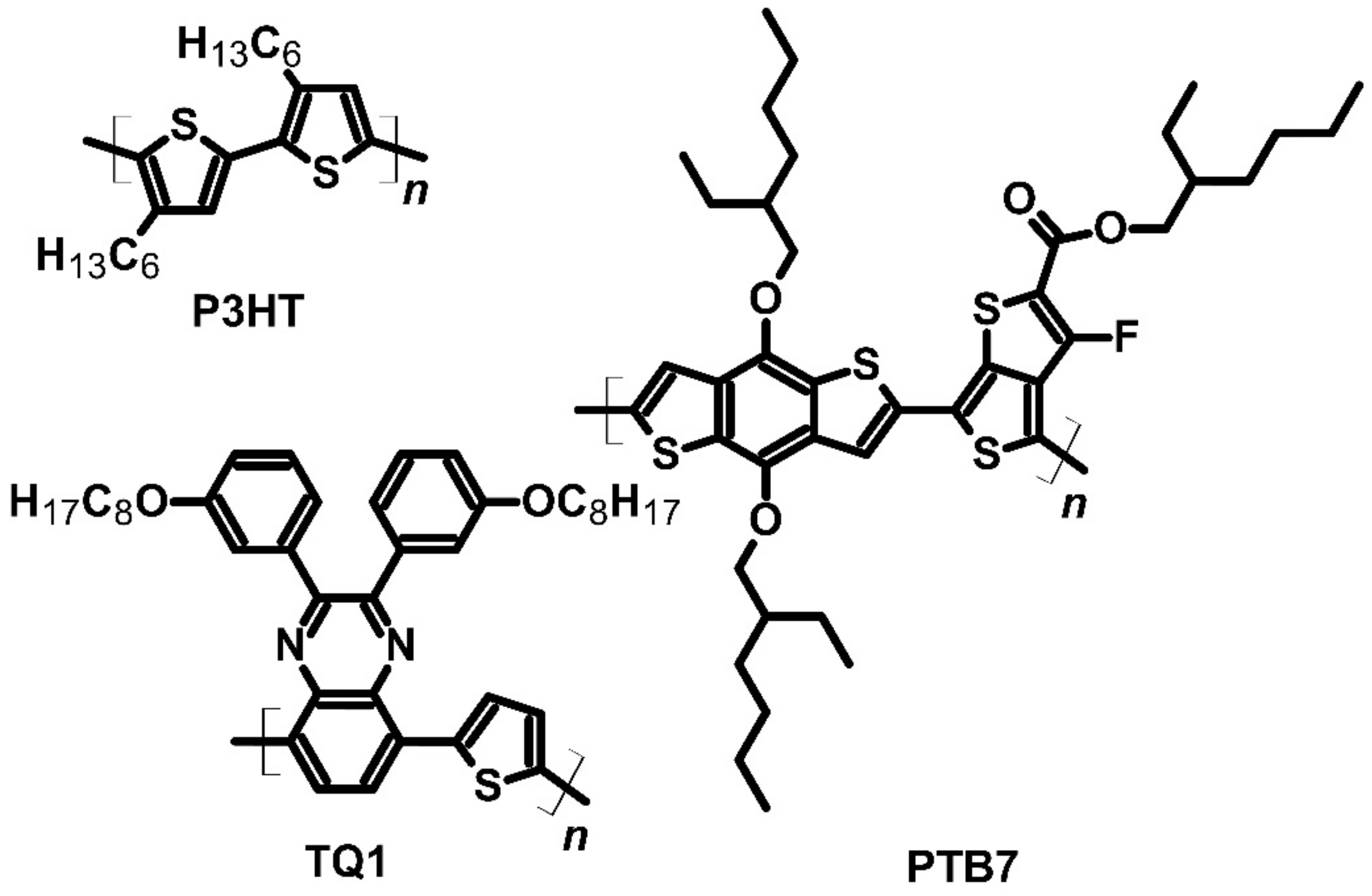
- Hynynen, J.; Järsvall, E.; Kroon, R.; Zhang, Y.; Barlow, S.; Marder, S.R.; Kemerink, M.; Lund, A.; Müller, C. Enhanced Thermoelectric Power Factor of Tensile Drawn Poly(3-hexylthiophene). ACS Macro Lett. 2018, 8, 70–76. [Google Scholar] [CrossRef] [Green Version]
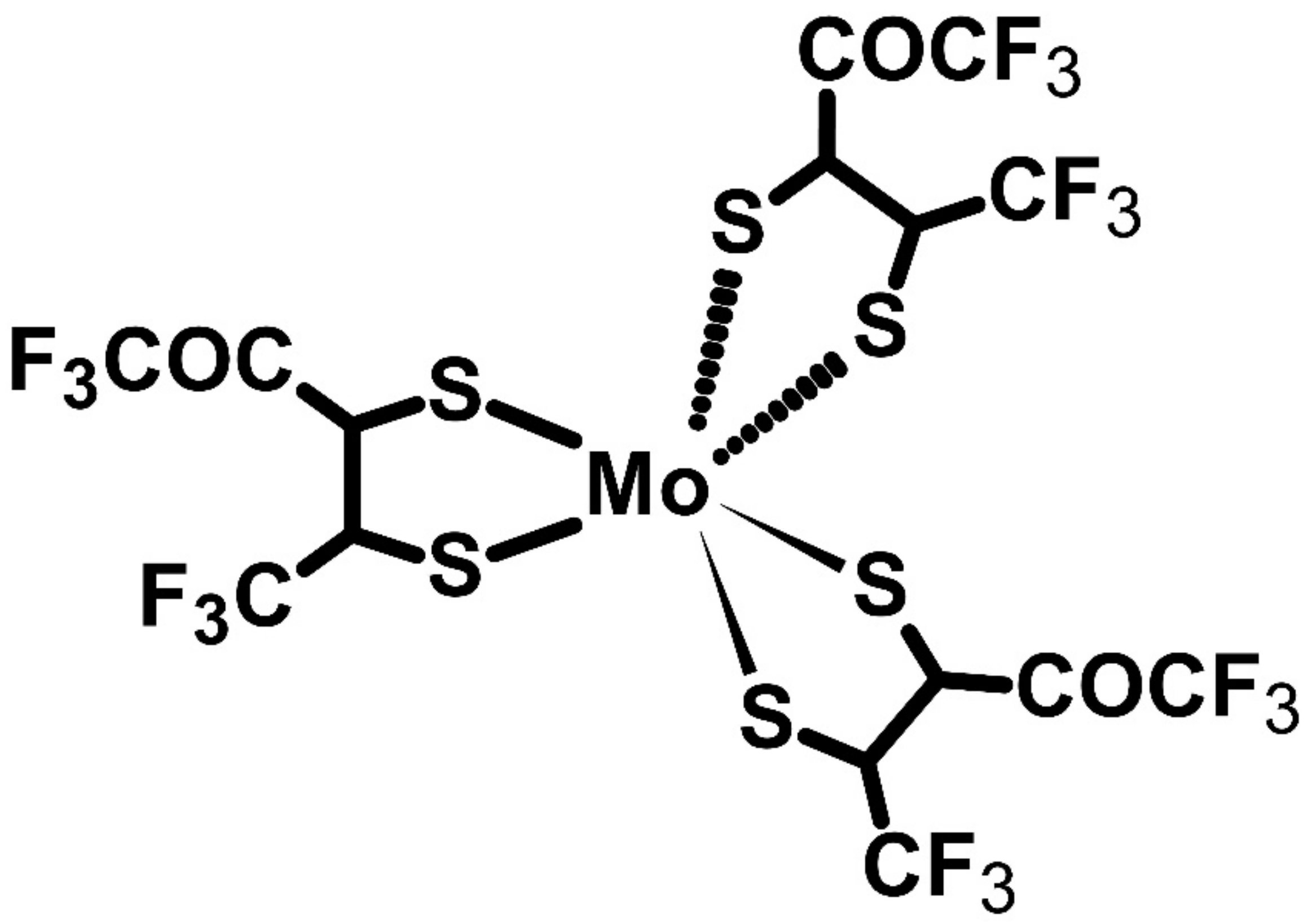
- Untilova, V.; Hynynen, J.; Hofmann, A.I.; Scheunemann, D.; Zhang, Y.; Barlow, S.; Kemerink, M.; Marder, S.R.; Biniek, L.; Müller, C.; et al. High Thermoelectric Power Factor of Poly(3-hexylthiophene) through In-Plane Alignment and Doping with a Molybdenum Dithiolene Complex. Macromolecules 2020, 53, 6314–6321. [Google Scholar] [CrossRef]
- Mai, C.-K.; Schlitz, R.A.; Su, G.M.; Spitzer, D.; Wang, X.; Fronk, S.L.; Cahill, D.G.; Chabinyc, M.L.; Bazan, G.C. Side-Chain Effects on the Conductivity, Morphology, and Thermoelectric Properties of Self-Doped Narrow-Band-Gap Conjugated Polyelectrolytes. J. Am. Chem. Soc. 2014, 136, 13478–13481. [Google Scholar] [CrossRef]
- Mai, C.-K.; Arai, T.; Liu, X.; Fronk, S.L.; Su, G.M.; Segalman, R.A.; Chabinyc, M.L.; Bazan, G.C. Electrical properties of doped conjugated polyelectrolytes with modulated density of the ionic functionalities. Chem. Commun. 2015, 51, 17607–17610. [Google Scholar] [CrossRef]
- Kjelstrup, S.; Vie, P.; Akyalcin, L.; Zefaniya, P.; Pharoah, J.; Burheim, O. The Seebeck coefficient and the Peltier effect in a polymer electrolyte membrane cell with two hydrogen electrodes. Electrochim. Acta 2013, 99, 166–175. [Google Scholar] [CrossRef]
- Bergfield, J.P.; Solis, M.A.; Stafford, C.A. Giant Thermoelectric Effect from Transmission Supernodes. ACS Nano 2010, 4, 5314–5320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Miao, R.; Jiang, C.; Meyhofer, E.; Reddy, P. Perspective: Thermal and thermoelectric transport in molecular junctions. J. Chem. Phys. 2017, 146, 092201. [Google Scholar] [CrossRef] [Green Version]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Wright, D.A. Thermoelectric Properties of Bismuth Telluride and its Alloys. Nature 1958, 181, 834. [Google Scholar] [CrossRef]






| Material | Dopant | Cond. Type | HOMO [eV] | LUMO [eV] | σ [S/cm] | α [μV/K] | σα2 [μW/(mK2)] | ZT | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pentacene | I2 | P | −4.61 | −2.40 | ~50 | 40–70 | 20 | [39] | |
| Sumanene | ~3 × 104 | [29] | |||||||
| BP | −4.70 | −2.28 | ~1 × 105 (a) | ~1.5 | 5 × 10−3 | [29] | |||
| C12BP | ~8 × 104 | [29] | |||||||
| Pentacene | P | −4.61 | −2.40 | ~4.5 × 104 | [29] |
| Material | Dopant | Cond. Type | HOMO [eV] | LUMO [eV] | σ [S/cm] | α [μV/K] | σα2 [μW/(mK2)] | ZT | Ref. |
|---|---|---|---|---|---|---|---|---|---|
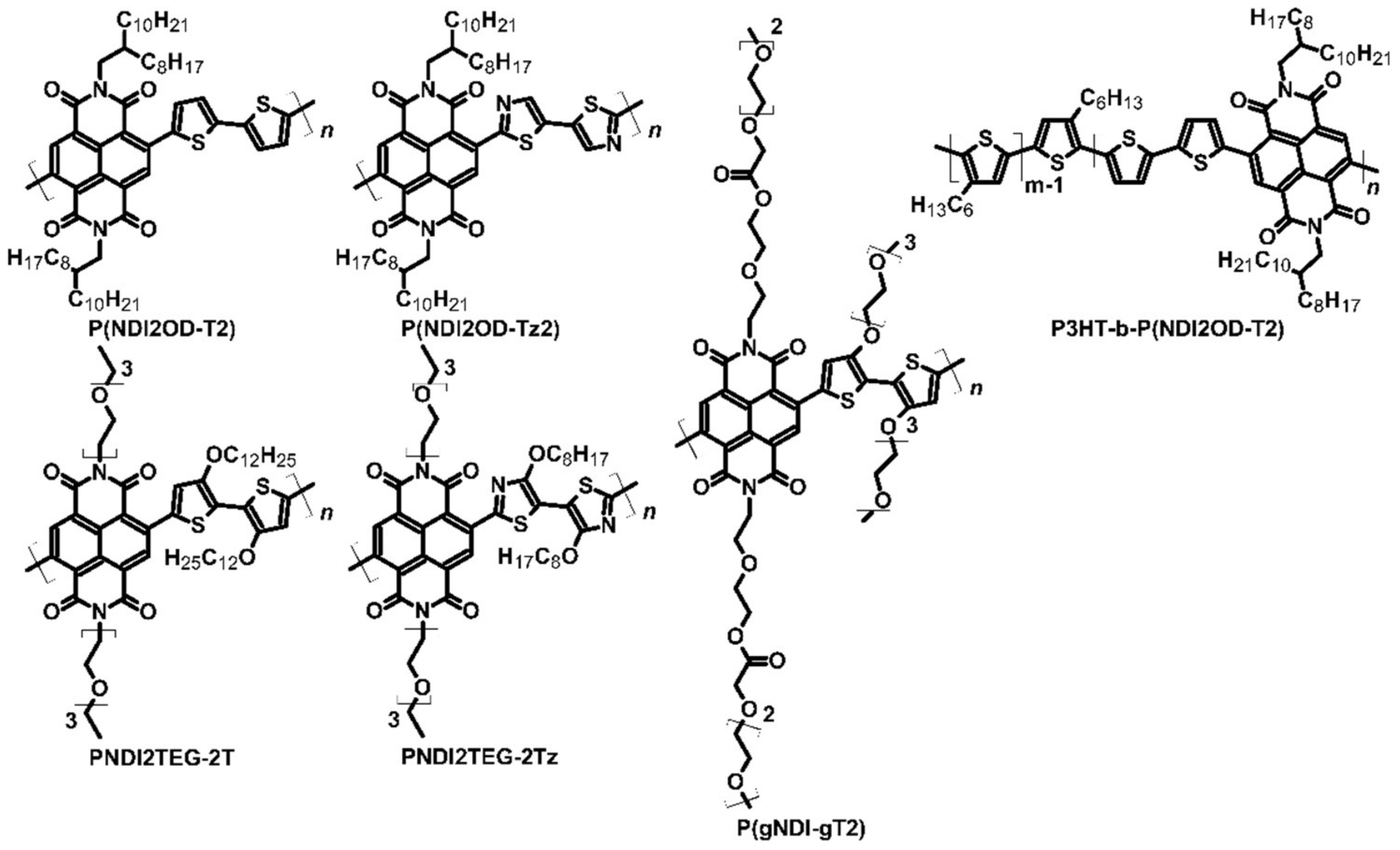
| P(NDI2OD-T2) | TDAE | N | −5.46 | −3.99 | 3 × 10−3 | −208 | 0.013 | [46] | |
| P(NDI2OD-Tz2) | TDAE | N | −5.90 | −4.10 | 0.06 | −447 | 1.5 | [47] | |
| PNDI2TEG-2Tz | N-DMBI | N | −5.56 | −4.26 | 1.8 | −159 | 4.6 | [48] | |
| P(gNDI-gT2) | N-DMBI | N | −4.83 | −4.12 | 0.1 | −200 | 0.4 | [49] | |
| P3HT-b-P(NDI2OD-T2) | N-DMBI | N | −5.20 −5.46 | −3.99 | 1.7 × 10−4 | −602 | 6.6 × 10−3 | [50] | |
| P(NDI2OD-T2) | N-DMBI | N | −5.46 | −3.99 | 8 × 10−3 | −850 | 6 × 10−7 | [51] | |
| P(NDI2OD-T2) | N-DPBI | N | −5.46 | −3.99 | 4 × 10−3 | −770 | 2 × 10−7 | [51] | |
| BBL | TDAE | N | −5.90 | −4.00 | 1 | −60 | 0.43 | [46] | |
| ClBDPPV | TBAF | N | −5.90 | −4.30 | 0.62 | −99.2 | 0.63 | 5.5 × 10−4 | [52] |
| PDPF | N-DMBI | N | −5.82 | −4.11 | 1.3 | −235 | 4.65 | [53] | |
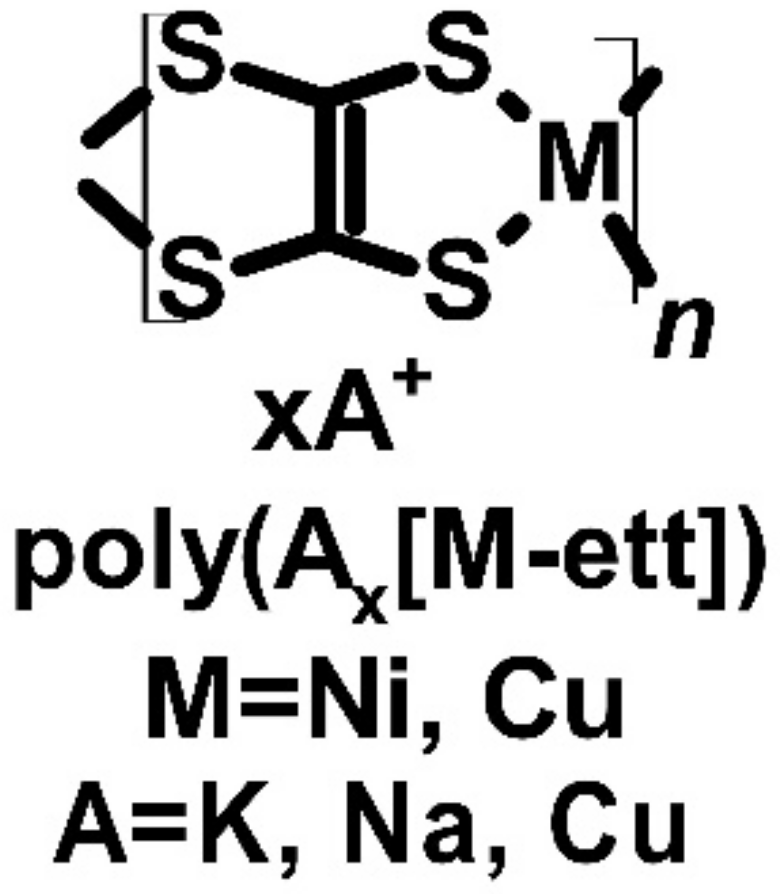
| poli(Kx[Ni-ett]) | N | 44 | −121.6 | 66 | 0.2 (a) | [54,55,56] | |||
| poli(Nax[Ni-ett]) | N | 40 | −75 | 22.5 | 0.042 (a) 0.1 (b) | [54,55,56] |
| Material | Dopant | Cond. Type | HOMO [eV] | LUMO [eV] | σ [S/cm] | α [μV/K] | σα2 [μW/(mK2)] | ZT | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PNDI2TEG-2T | N-DMBI | P | −5.39 | −4.18 | 7 × 10−4 | 57.2 | 2.3 × 10−4 | [48] | |
| P3HT-b-P(NDI2OD-T2) | F4TCNQ | P | −5.20 −5.46 | −3.99 | 1.4 × 10−3 | 196 | 5.5 × 10−3 | [50] | |
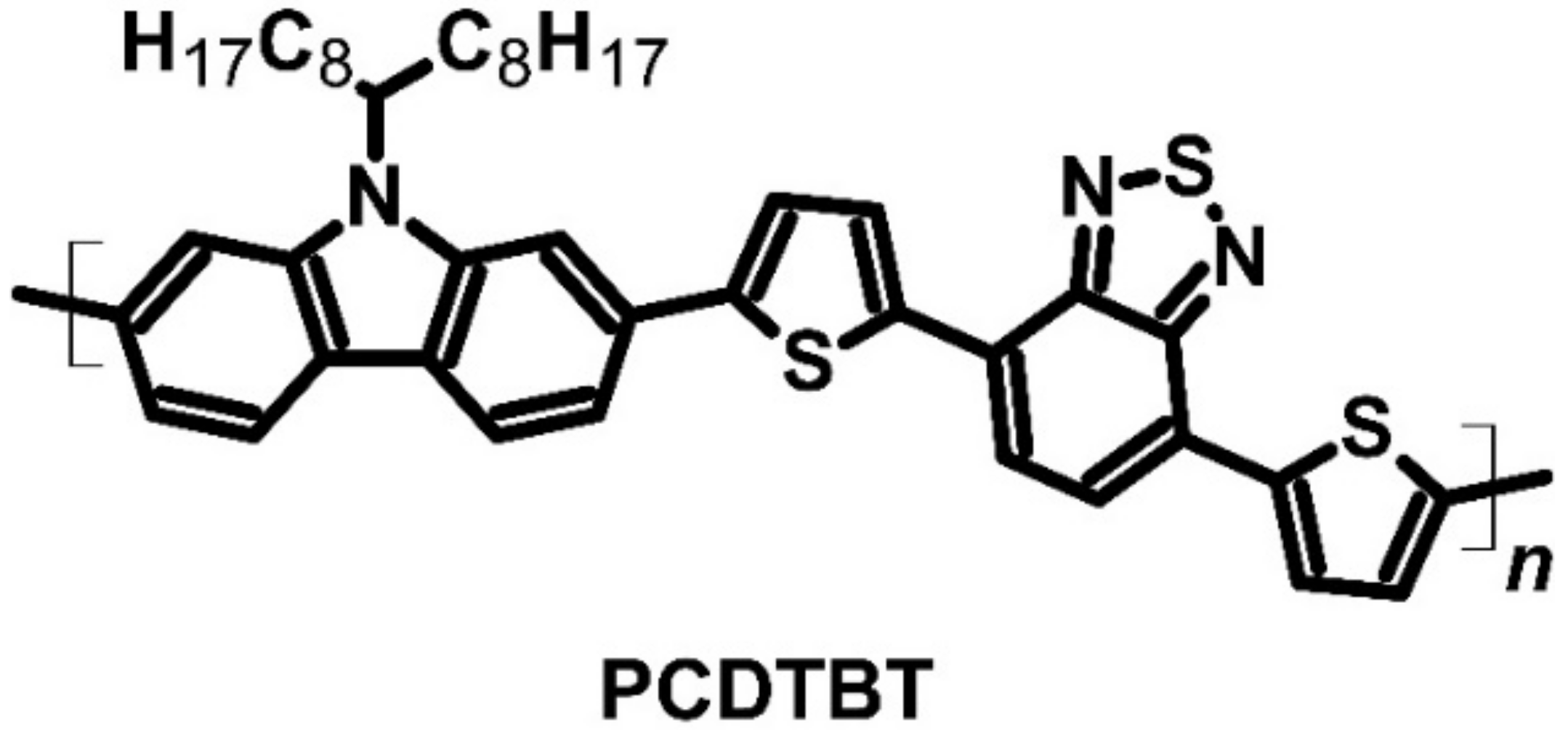
| PCDTBT | FeCl3 | P | −5.50 | −3.60 | 160 | 34 | 19 | [62,63] | |
| PEDOT:PSS | P | −5.20 | −2.40 | 9.2 (a) | 15.7 (a) | 0.23 (a) | 4 × 10−4 (a) | [64] | |
| PEDOT:PSS | 5% DMSO | P | −5.20 | −2.40 | 38.7 (b) | 12.0 (b) | 0.56 (b) | 1.9 × 10−3 (b) | [64] |
| PEDOT:PSS | 10% DMSO | P | −5.20 | −2.40 | 27.5 (c) | 13.5 (c) | 0.50 (c) | 1.35 × 10−3 (c) | [64] |
| PEDOT:PSS | 5% EG | P | −5.20 | −2.40 | 45.0 (c) | 11.8 (c) | 0.63 (c) | 1.65 × 10−3 (c) | [64] |
| PEDOT:PSS | 10% EG | P | −5.20 | −2.40 | 34.5 (b) | 12.3 (b) | 0.52 (b) | 1.47 × 10−3 (b) | [64] |
| PEDOT:PSS baked | 5% DMSO | P | −5.20 | −2.40 | 32.5 (b) | 12.3 (b) | 0.49 (b) | 1.4 × 10−3 (b) | [64] |
| PEDOT:PSS baked | 10% DMSO | P | −5.20 | −2.40 | 26.0 (a) | 11.6 (a) | 0.35 (a) | 8.1 × 10−4 (a) | [64] |
| PEDOT:PSS baked | 5% EG | P | −5.20 | −2.40 | 51.0 (d) | 11.6 (d) | 0.69 (d) | 1.75 × 10−3 (d) | [64] |
| PEDOT:PSS baked | 10% EG | P | −5.20 | −2.40 | 37.0 (a) | 12.0 (a) | 0.53 (a) | 1.25 × 10−3 (a) | [64] |
| PEDOT:PSS (pristine) | - | P | −5.20 | −2.40 | 1–3 | 13–15 | 0.2 | [65] | |
| PEDOT:PSS (annealed in 220 °C) | - | P | −5.20 | −2.40 | 596 | 23.3 | 32.5 | [65] | |
| PEDOT:PSSf) | DMSO | P | −5.20 | −2.40 | 880 | 72 | 469 | 0.42 | [66] |
| PEDOT:PSSf) | EG | P | −5.20 | −2.40 | 890 | 62 | 345 | 0.28 | [66] |
| PEDOT:PSS | CH3NO, H2SO4, NaBH4 | P | −5.20 | −2.40 | 1786 | 28.1 | 141 | [67] | |
| PEDOT:PSS | EG, PVA, Te-NWs | P | −5.20 | −2.40 | 382.4 | 11.3 | 8.5 | [68] | |
| PEDOT | Bi2Te3 | P | −5.20 | −2.40 | 483 | 168 | 1350 | 0.58 | [69] |
| PDPH | N-DMBI | P | −5.61 | −3.93 | 1.01 × 10−3 | 71 | 5.11 × 10−4 | [53] | |
| PBTTT-C14 | F4TCNQ | P | −5.10 | −3.10 | 3.51 (e) 220 (f) | 60 (e) 39 (f) | 1.3 (e) 32 (f) | [70] | |
| PBTTT-C14 | F2TCNQ | P | −5.10 | −3.10 | 2 × 10−3 (e)36 (f) | 755 (e) 140 (f) | 0.11 (e) 70 (f) | [70] | |
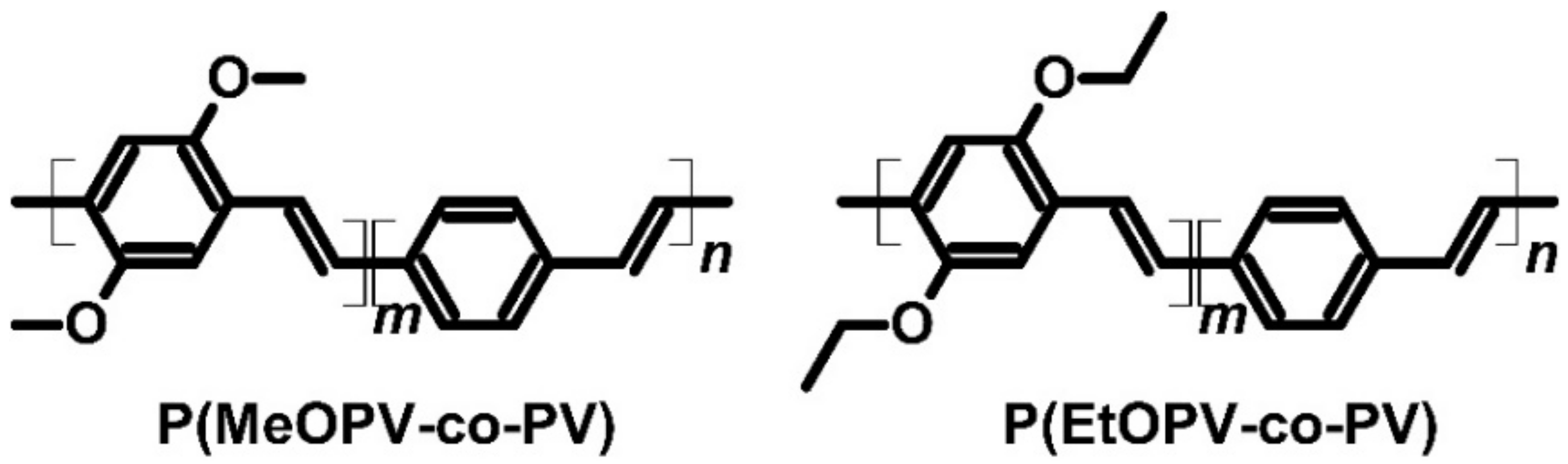
| P(MeOPV-co-PV) | I2 | P | 183 | 43.5 | 34.6 | 0.014 | [71,72] | ||
| P(EtOPV-co-PV) | I2 | P | 350 | 47 | 77.3 | 0.1 | [71,72] | ||
| P3HTPTB7 | F4TCNQ | P | −5.00 −5.15 | ~4 (f) | 1100 (h) ~130 (g) | ~7 (g) | [32] | ||
| P3HTTQ1 | F4TCNQ | P | −5.00 −5.60 | ~4 (f) | 2000 (i) ~130 (g) | ~7 (g) | [32] | ||
| P3HT | Mo(tfd-COCF3)3 | P | −5.00 | 12.7 | 112 | 16 | [73] | ||
| P3HT | Mo(tfd-COCF3)3 | P | −5.00 | 509 | 56 | 160 | [74] | ||
| CPE-Na | P | 0.16 | 165 | 0.44 | [75] | ||||
| CPE-K | P | 0.024 | 230 | 0.13 | [75] | ||||
| CPE-TBA | P | <1 × 10−4 | [75] | ||||||
| CPE-C3-Na | P | 0.22 | 195 | 0.84 | [75] | ||||
| CPE-C3-K | P | 0.048 | 200 | 0.19 | [75] | ||||
| CPE-K90 | P | 0.25 | ~230 | ~1.16 | [76] | ||||
| CPE-K80 | P | 0.44 | ~230 | 2.33 | [76] | ||||
| CPE-K70 | P | 0.30 | ~230 | ~1.66 | [76] | ||||
| poli(Cux[Cu-ett]) | P | 9.5 | 83 | 6.5 | 2 × 10−3 (j) 0.014 (k) | [54,55,56] | |||
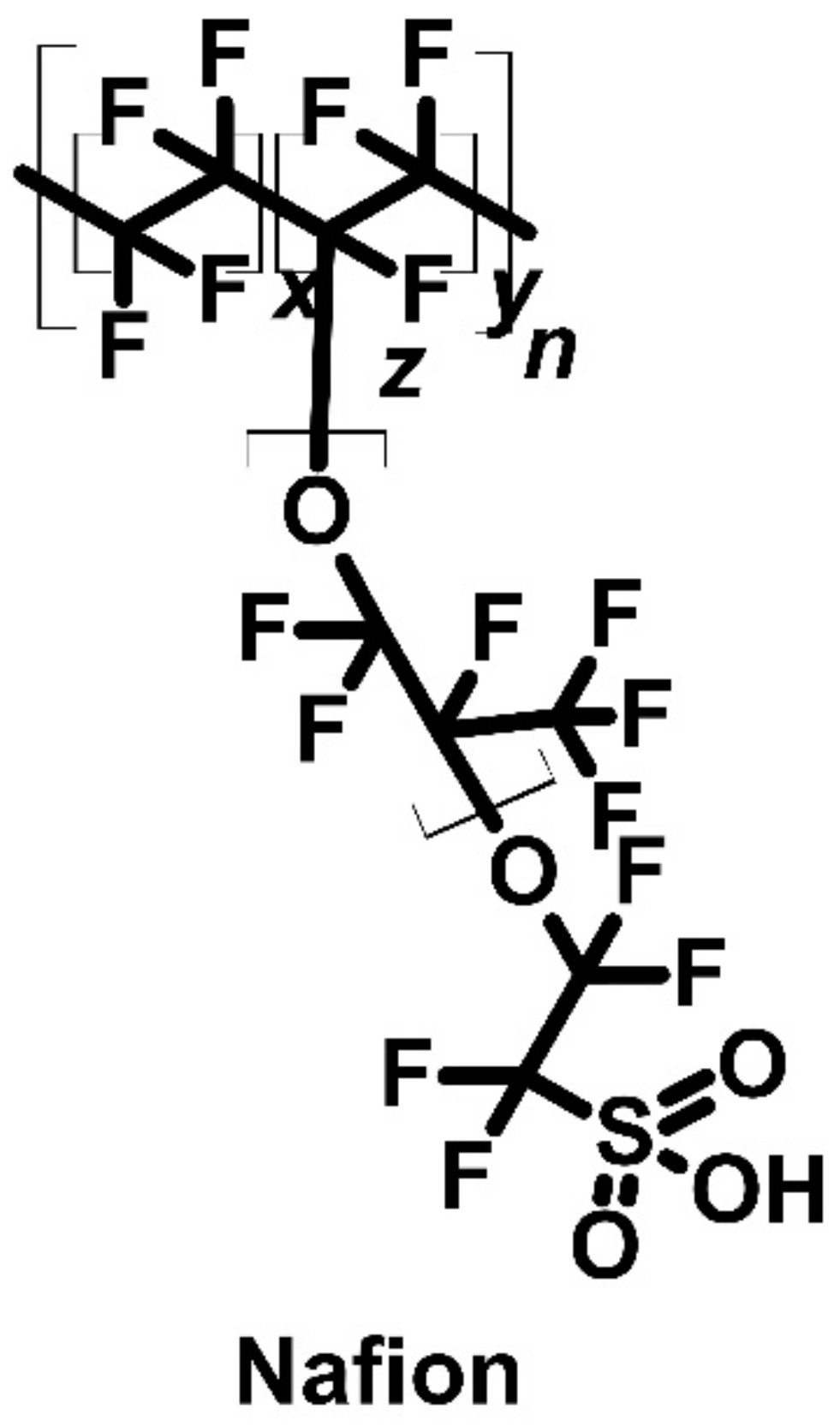
| Nafion membrane | P | 670 | [77] | ||||||
| Au-BPDT-Au | P | 13.0 | [35] | ||||||
| Au-TPDT-Au | P | 15.7 | [35] | ||||||
| Au-BDA-Au | P | 2.2 | [36] | ||||||
| Au-BDT-Au | P | 2.4 | [36] | ||||||
| Au-PPE-Au | P | 1000 | >4 | [78,79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogoc, S.; Data, P. Organic Thermoelectric Materials as the Waste Heat Remedy. Molecules 2022, 27, 1016. https://doi.org/10.3390/molecules27031016
Gogoc S, Data P. Organic Thermoelectric Materials as the Waste Heat Remedy. Molecules. 2022; 27(3):1016. https://doi.org/10.3390/molecules27031016
Chicago/Turabian StyleGogoc, Szymon, and Przemyslaw Data. 2022. "Organic Thermoelectric Materials as the Waste Heat Remedy" Molecules 27, no. 3: 1016. https://doi.org/10.3390/molecules27031016






