Abstract
A new liquid—liquid extraction system for molybdenum(VI) was studied. It contains 4-nitrocatechol (4NC) as a complexing chromogenic reagent and benzalkonium chloride (BZC) as a source of heavy cations (BZ+), which are prone to form chloroform-extractable ion-association complexes. The optimum conditions for the determination of trace molybdenum(VI) were found: concentrations of 4NC and BZC (7.5 × 10−4 mol dm−3 and 1.9 × 10−4 mol dm−3, respectively), acidity (3.75 × 10−2 mol dm−3 H2SO4), extraction time (3 min), and wavelength (439 nm). The molar absorptivity, limit of detection, and linear working range were 5.5 × 104 dm3 mol−1 cm−1, 5.6 ng cm−3, and 18.6–3100 μg cm−3, respectively. The effect of foreign ions was examined, and the developed procedure was applied to the analysis of synthetic mixtures and real samples (potable waters and steels). The composition of the extracted complex was 1:1:2 (Mo:4NC:BZ). Three possible structures of its anionic part [MoVI(4NC)O2(OH)2]2− were discussed based on optimizations at the B3LYP/3-21G level of theory, and simulated UV/Vis absorption spectra were obtained with the TD Hamiltonian.
1. Introduction
Molybdenum is a second-row transition metal that belongs to group six and occupies position 42 in the periodic table. It is a silvery-white refractory metal with a high thermal and electrical conductivity, low vapor pressure, low coefficient of thermal expansion, and good alloyability with ferrous and nonferrous metals. Its compounds are important for a number of industries, but the majority of molybdenum produced (over 250,000 tons in 2018 [1] is used in steels and alloys [2,3]. Molybdenum’s role in such materials is to improve the hardness, strength, ductility, and resistance to shock, fatigue, and creep, especially at elevated temperatures.
Molybdenum is a relatively rare element in the continental Earth’s crust (average content of 1.2 mg kg−1) [4] and fresh waters [5,6]. However, it is essential for microorganisms, plants, and animals [7]. More than 50 molybdenum-dependent enzymes are known in all kingdoms of life. Important for humans are four enzymes with a pterin-based cofactor [8]. Their synthesis and function depend on many factors, including diet [5]. According to some metabolic balance studies, the adequate molybdenum intake for healthy people (over the age of 15) is 65 μg per day [9].
Various techniques have been used for the determination of molybdenum in environmental and industrial samples, e.g., inductively coupled plasma mass spectrometry, inductively coupled plasma optical emission spectrometry, electrothermal atomic absorption spectrometry, and spectrophotometry [10,11,12,13]. Spectrophotometry is a simple, cheap, convenient, and mature analytical technique [14,15,16]. It can be easily combined with extraction methods [17,18,19,20,21,22,23] to improve analytical performance.
The aim of the present work is to develop a sensitive and selective extractive spectrophotometric procedure for the determination of molybdenum in steels and potable waters with 4-nitrocatechol (4NC) and benzalkonium chloride (BZC). An effective ligand for the formation of colored complexes that are attractive for analytical applications is 4-nitrocatechol [24,25,26,27]. Benzalkonium chloride is a mixture of alkyl dimethyl benzyl ammonium chlorides [28,29], with an average molar mass of 360 g mol−1. Recently, it has been used in our laboratory as a liquid—liquid extraction reagent for cobalt [30].
2. Results and Discussion
2.1. Liquid—Liquid Extraction—Spectrophotometric Optimization
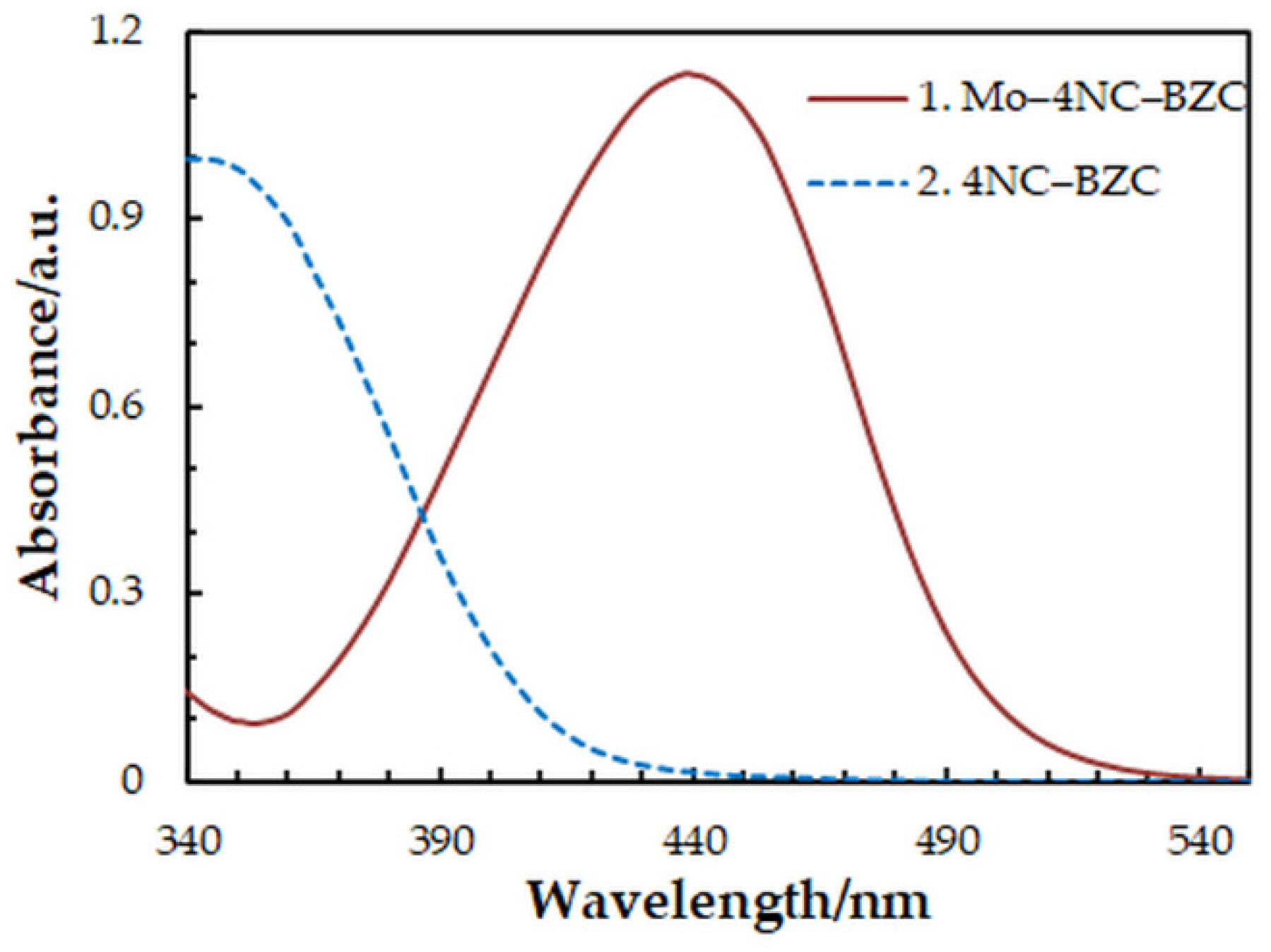
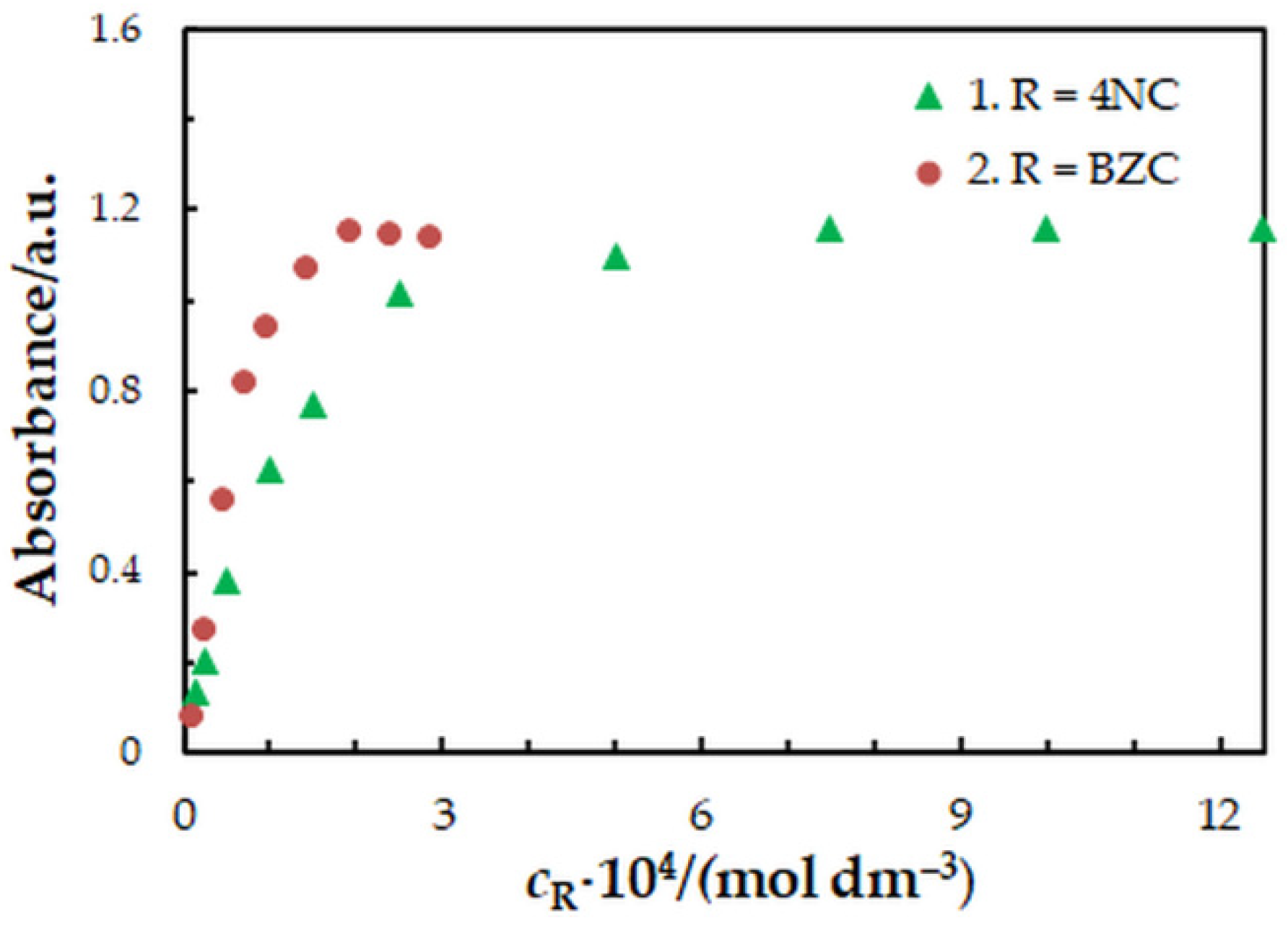
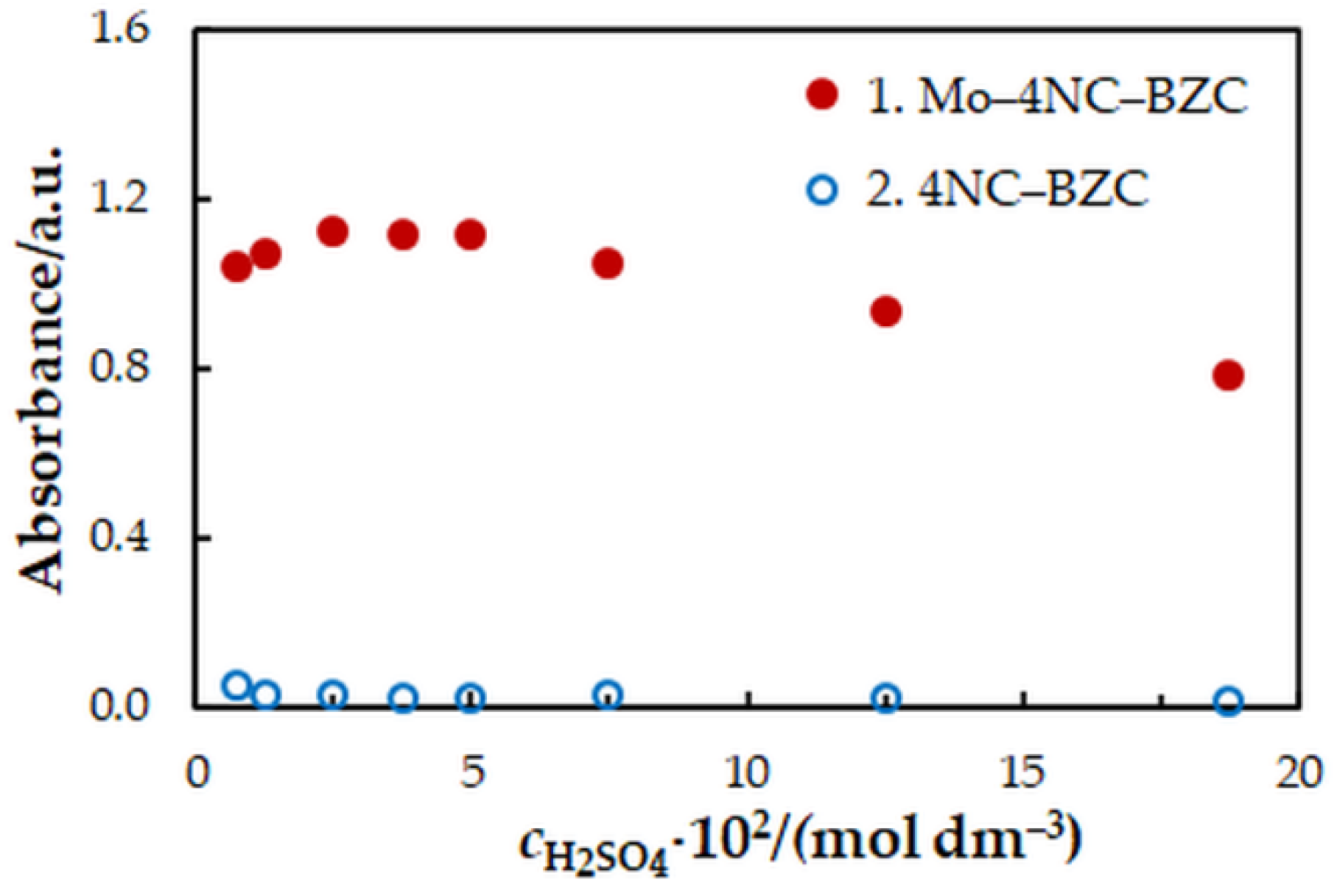
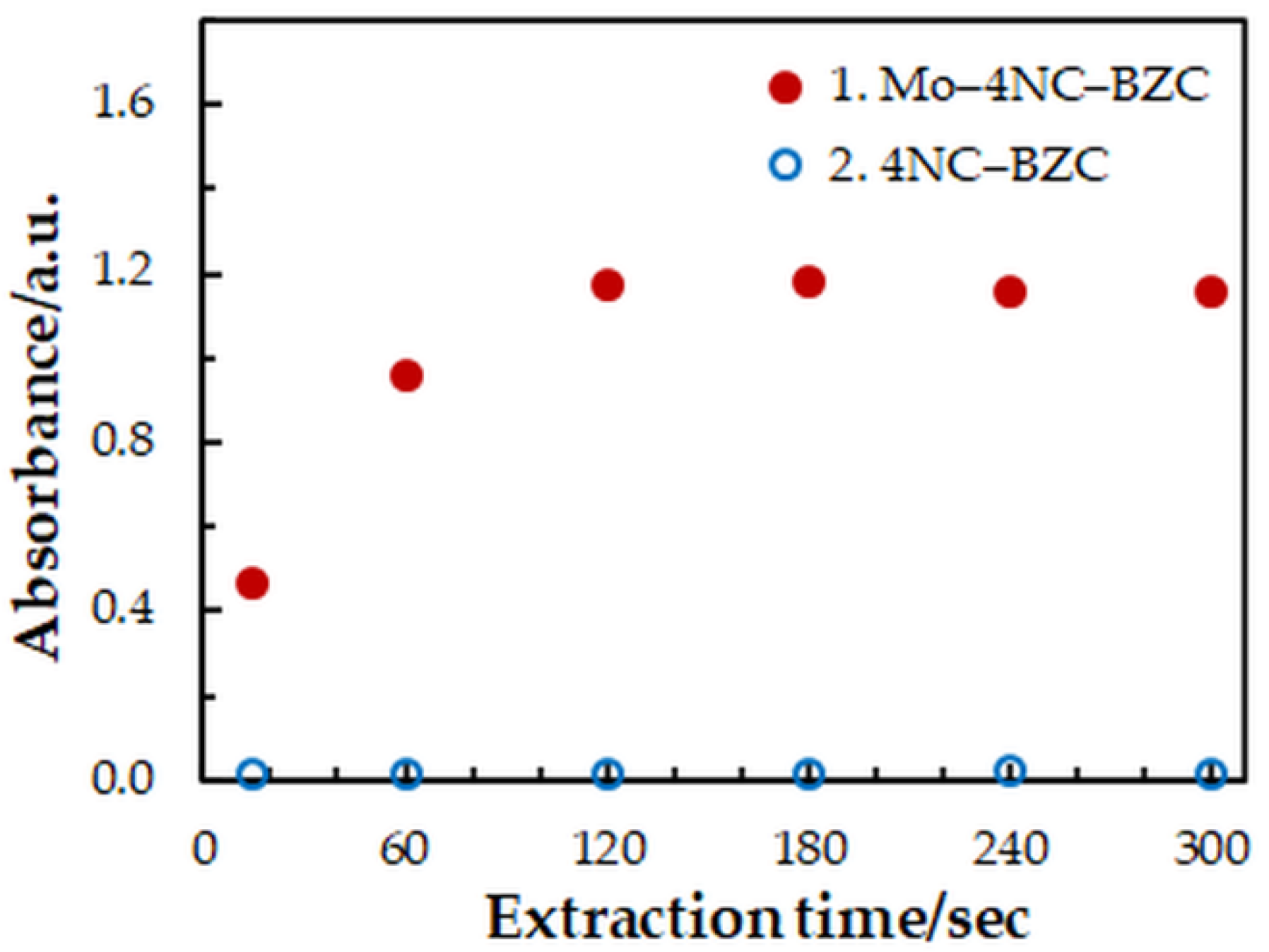
One-factor-at-a-time optimization was carried out at room temperature to find the optimum values of the following extraction—spectrophotometric parameters: wavelength of spectrophotometric measurement (Figure 1), concentration of 4NC (Figure 2), concentration of BZC (Figure 2), concentration of H2SO4 (Figure 3), and extraction time (Figure 4). Under the optimum conditions (Table 1), the complex has an absorption maximum at 439 nm, where the blank absorbs insignificantly.
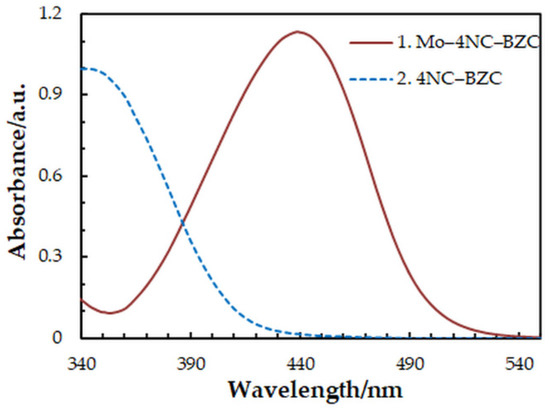
Figure 1.
Absorption spectra of the complex (1) and blank (2): cMo = 2 × 10−5 mol dm−3, c4NC = 7.5 × 10−4 mol dm−3, cBZC = 1.9 × 10−4 mol dm−3, cH2SO4 = 3.75 × 10−2 mol dm−3, tex = 3 min.
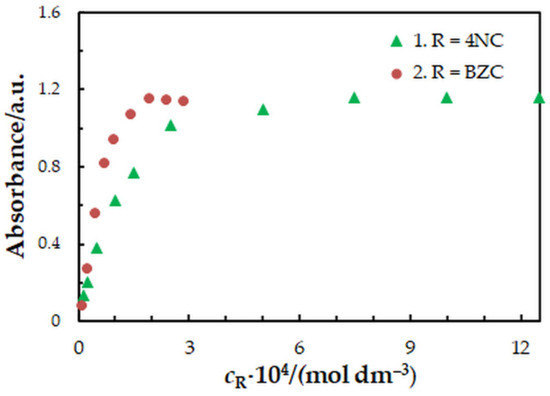
Figure 2.
Effect of the 4NC (1) and BZC (2) concentration: cMo = 2 × 10−5 mol dm−3, tex = 3 min, λ = 439 nm. 1—cBZC = 1.9 × 10−4 mol dm−3; 2—c4NC = 7.5 × 10−4 mol dm−3.
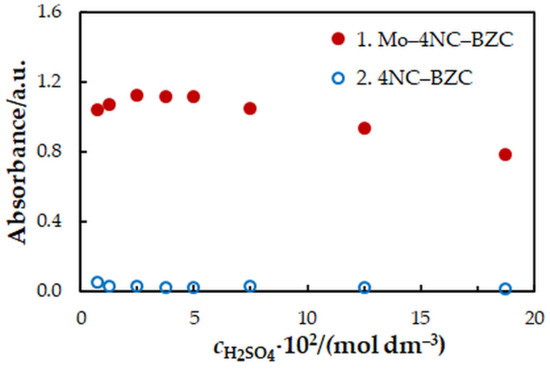
Figure 3.
Effect of the H2SO4 concentration: cMo = 2 × 10−5 mol dm−3, c4NC = 7.5 × 10−4 mol dm−3, cBZC = 1.9 × 10−4 mol dm−3, tex = 3 min, λ = 439 nm.
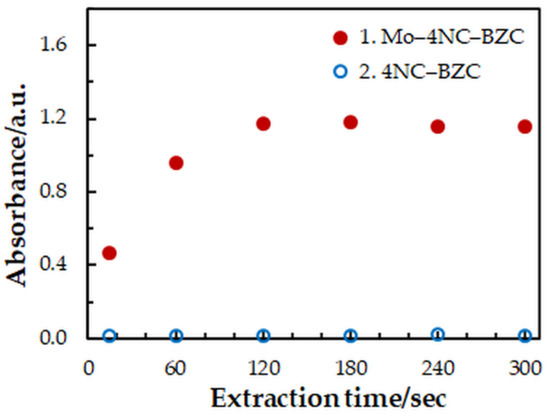
Figure 4.
Effect of the extraction time: cMo = 2 × 10−5 mol dm−3, c4NC = 7.5 × 10−4 mol dm−3, cBZC = 1.9 × 10−4 mol dm−3, cH2SO4 = 3.75 × 10−2 mol dm−3, λ = 439 nm.

Table 1.
Extraction—spectrophotometric optimization of the Mo(VI)–4NC–BZC–water–chloroform system.
2.2. Molar Ratios and Formula of the Ternary Complex
The complexation in acidic medium between the tetrahedral molybdate MoO42− and reagents bearing a catechol moiety (L) leads to the production of octahedral anionic products in which the molar L-to-Mo ratio is 1-to-1: [MoVIO3(OH)L]3−, [MoVIO2(OH)2L]2−, and [MoVIO(OH)3L]−. As the acidity decreases, complexes with a molar ratio of 2:1 (L:Mo) are also formed [31,32,33].
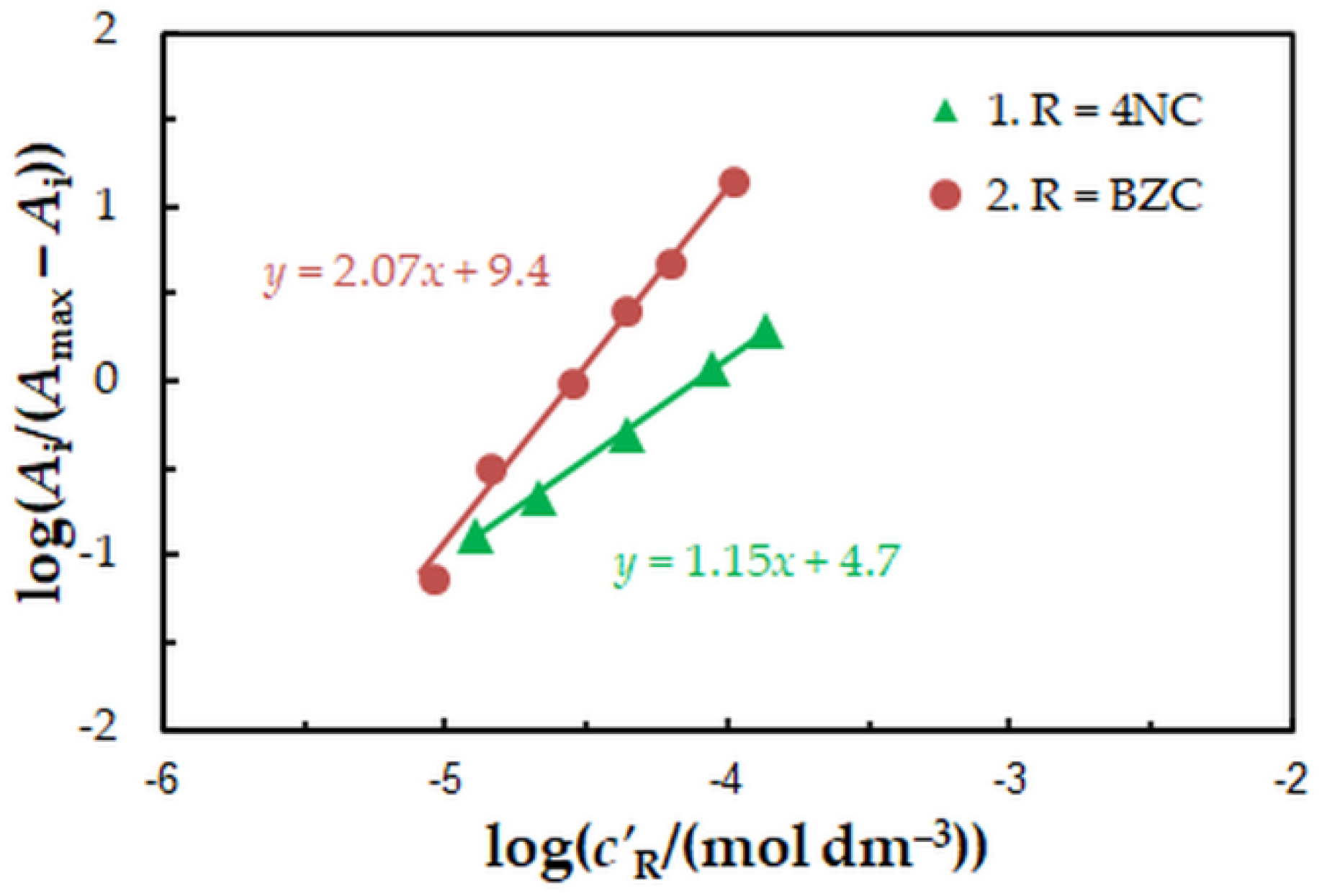
To find the 4NC-to-Mo(VI) and BZC-to-Mo(VI) molar ratios in the examined ternary complex, we used the mobile equilibrium method [34], and the straight-line method of Asmus [35]. The results (Figure 5 and Figure 6) show a composition of 1:1:2 (Mo:4NC:BZC). Since BZC forms monovalent cations (BZ+), the ternary complex can be represented by the following formula: (BZ+)2[MoVIO2(OH)2(4NC)].
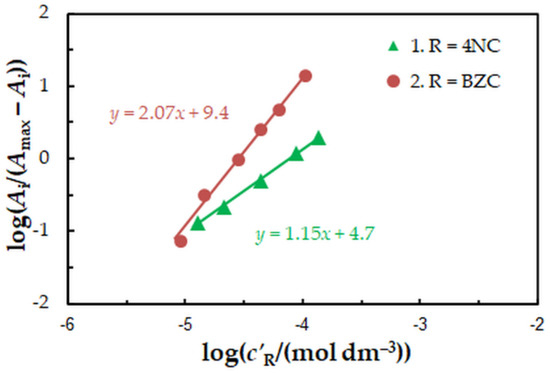
Figure 5.
Determination of the 4NC:Mo (1) and BZC:Mo (2) molar ratios by the mobile equilibrium method. The data are derived from the experimental points in Figure 2.
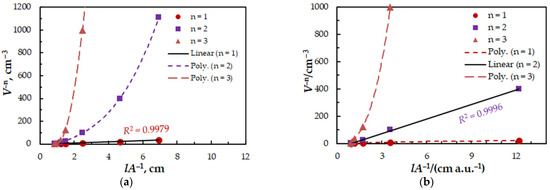
Figure 6.
Determination of the 4NC:Mo (a) and BZC:Mo (b) molar ratios by the straight-line method of Asmus. The data are derived from the experimental points in Figure 2.
2.3. Extraction Constant, Distribution Ratio and Fraction Extracted
The equation of ion-association and subsequent extraction of the ternary complex is as follows:
[MoVI(4NC)O2(OH)2]2−(aq) + 2BZ+(aq) ↔ (BZ+)2[MoVI(4NC)O2(OH)2](org)
The equilibrium constant characterizing this equation was determined by three methods based on the saturation curve with BZC (Figure 2, series 2): the mobile equilibrium method [34] (Figure 5, straight line 2), the Holme—Langmyhr method [36], and the Harvey—Manning method [37]. The obtained values are given in Table 2, along with values of other extraction characteristics: distribution ratio (D) and fraction extracted (E). The fact that the extraction constants (K) obtained by the above-mentioned methods (which operate with points located in different sections of the experimental saturation curve) are statistically identical, shows that the proposed equation is correct and there are no significant side processes.

Table 2.
Extraction characteristics.
2.4. Ground-State Equilibrium Geometries of the Anionic Part, Spectral Comparison, Energies, and Kinetics
The spectral characteristics of the ion-association complex in the visible range are determined mainly by its anionic part [27]. Therefore, it is interesting to make a comparison between the experimental spectrum and simulated spectra of different isomers of the complex anion [MoVIO2(OH)2(4NC)]2−. For this purpose, the ground-state equilibrium geometries of three possible structures of the anionic part were optimized at the B3LYP/3-21G level of theory (Figure 7). Then, the vertical excitation energies were calculated with the TD Hamiltonian to simulate theoretical UV/Vis absorption spectra (Figure 8).
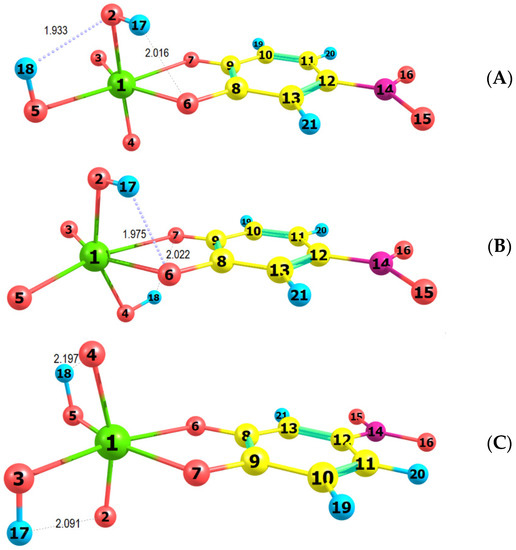
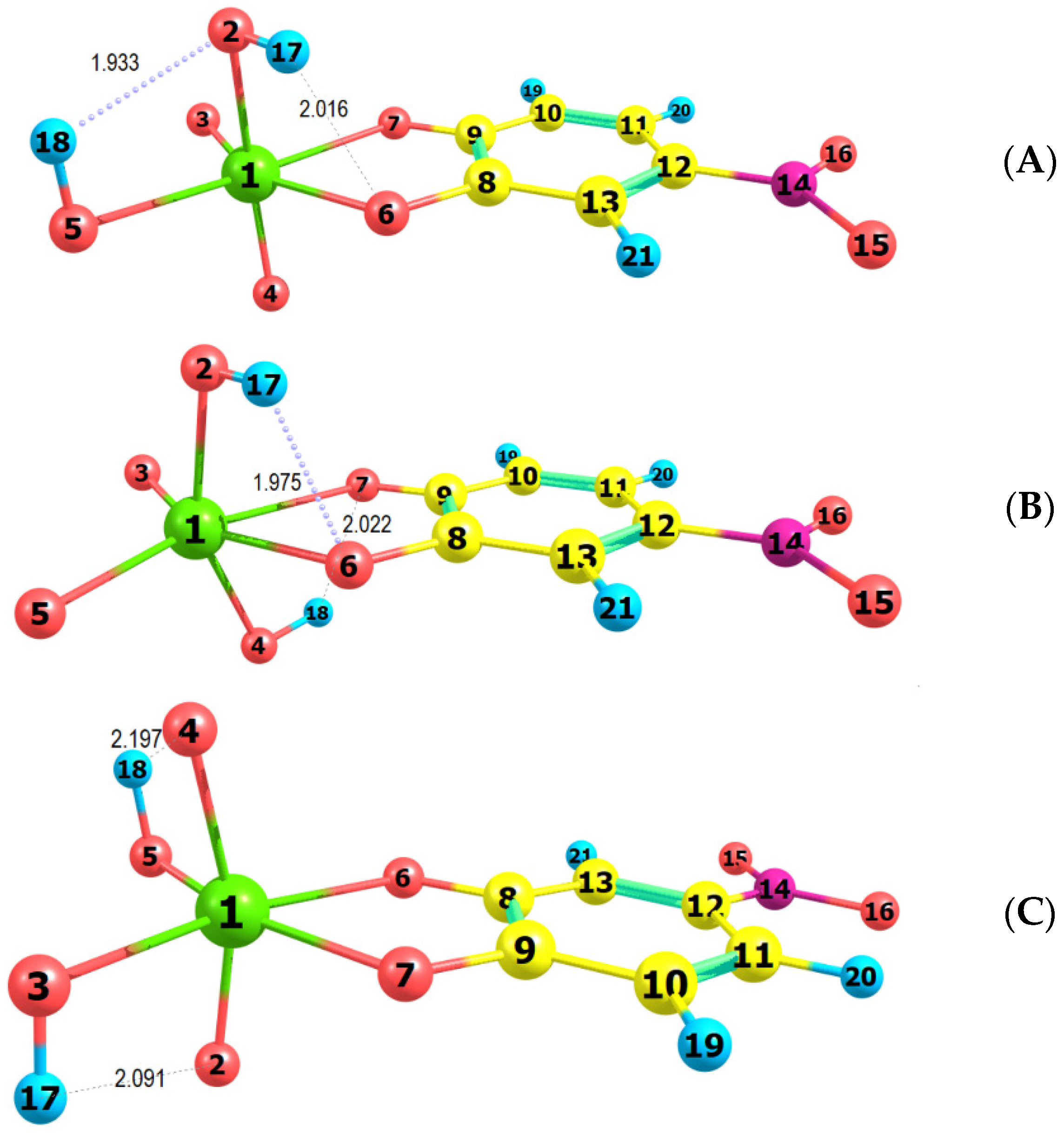
Figure 7.
Optimized ground-state equilibrium geometries of the three possible structures, (A–C).
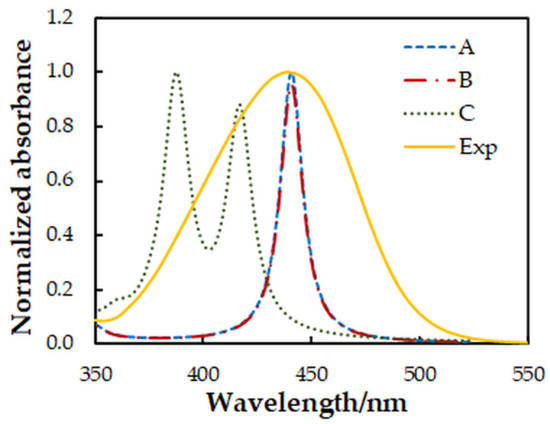
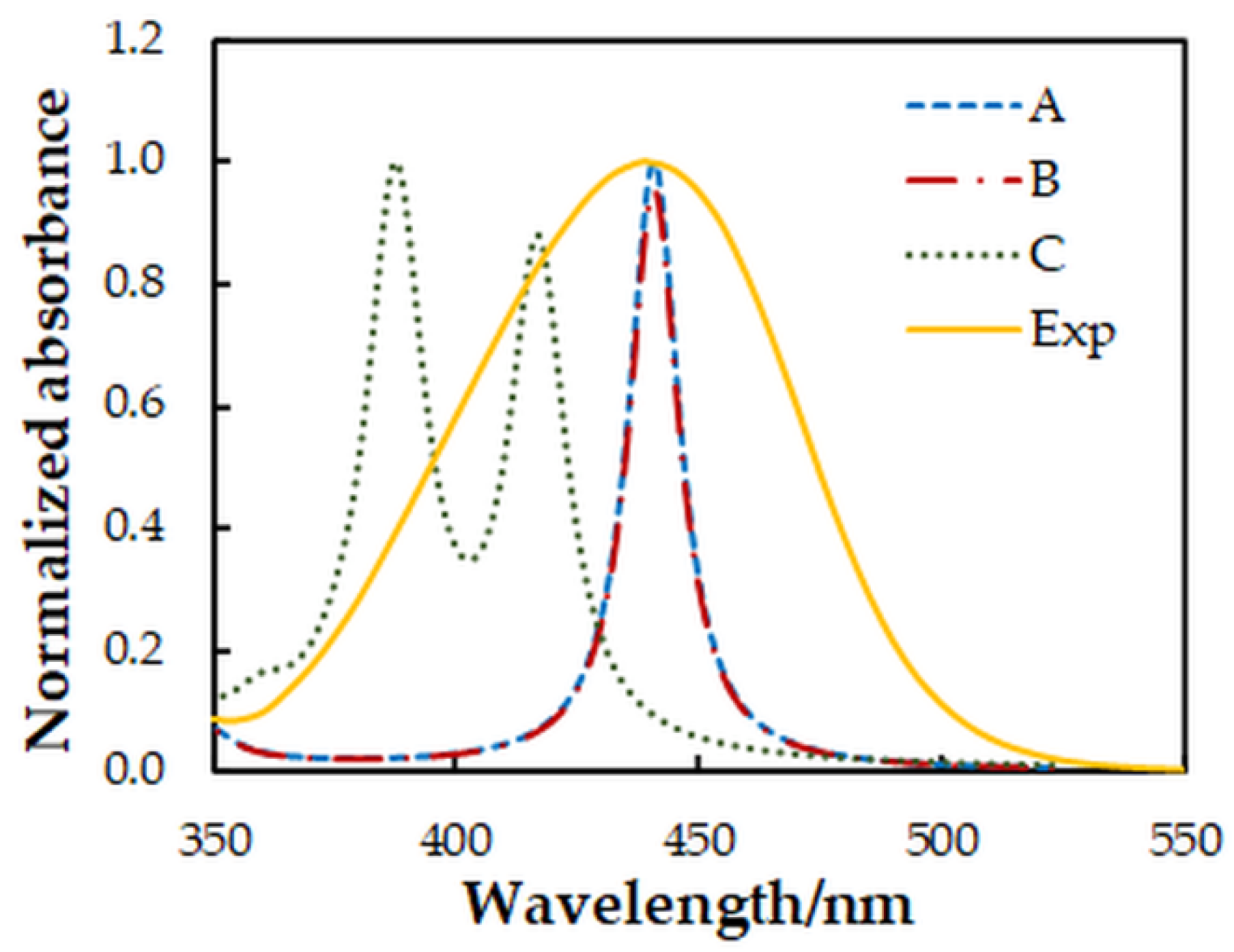
Figure 8.
Comparison between experimental (Exp) and simulated (A–C) absorption spectra. A Lorentzian broadening and a scaling factor of 0.76 were used for the theoretical spectra.
Figure 7 shows that two intramolecular H-bonds are observed in each of the examined structures. The shortest are the H18…O2 (1.933 Å) in structure A and H17…O6 (1.975 Å) in structure B. The two hydrogen atoms in structure B are located at oxygens outside the 4NC plane (axial position) and form H-bonds with the catechol oxygens. The octahedron in this structure is the most distorted: <C9O7Mo1O3 = 168.9°, <O2Mo1O4 = 153.9°. The least distorted is the octahedron in structure C (<C9O7Mo1O3 = 179.9°, <O2Mo1O4 = 163.5°).
The comparison of the spectra (Figure 8) suggests that structures A and B are more probable than structure C. Further energy analysis shows that B is more stable than A. The energy difference between the two structures is 21 kJ mol−1. The changes in the standard enthalpy ΔH° and Gibbs free energy ΔG° for the transition of H18 from O5 to O4 are −21.82 kJ mol−1 and −21.88 kJ mol−1, respectively. The rate constants, calculated using the Eyring equation (transmission coefficient equal to unity) after the optimization of the transition state of the reaction, are kforward = 8.45 × 10−8 s−1 and kreverse = 1.24 × 10−11 s−1. This also gives grounds to conclude that the anionic part of the ternary complex is dominated by structure B.
2.5. Effect of Foreign Ions and Masking Agents
The effect of foreign ions was studied under the optimum conditions. The results are shown in Table 3. The most serious interferences are caused by W(VI), which forms stable chloroform-extractable complexes with 4NC and cationic ion-association reagents [38]. Therefore, in the analysis of tungsten-containing samples (e.g., steels), the sample preparation methodology must include a step for its separation (as described below).

Table 3.
Effect of foreign ions on the determination of 7.1 μg Mo(VI).
It was found that Na2EDTA is an effective masking agent for several ions, including Fe(III). This makes it possible to apply the proposed procedure for the determination of molybdenum in steels and environmental samples.
2.6. Analytical Characteristics and Application
The relationship between the absorbance and the Mo(VI) concentration was studied under the optimal conditions. A good linearity was observed up to 3100 ng cm−3 Mo(VI) (R2 = 0.9995, n = 11). The linear regression equation was A = 0.577γ + 0.007, where γ is the mass concentration (μg cm−3). The standard deviations of the slope and intercept were 0.004 and 0.008, respectively. The molar absorption coefficient was 5.5 × 104 dm3 mol−1 cm−1. The limit of detection (LOD) and limit of quantitation (LOQ) calculated as 3- and 10-times standard deviation of the blank divided by the slope, were 5.6 ng cm−3 and 18.6 ng cm−3, respectively.
The developed procedure was applied to the analysis of referent standard steels (RSS) and synthetic mixtures (SM), imitating typical molybdenum-containing steels [2]. The results are given in Table 4. The relative standard deviation (RSD) for these determinations was in the range of 0.8–2.4%.

Table 4.
Determination a of molybdenum in synthetic mixtures (SM) and referent standard steels (RSS).
Commercial potable water from three Bulgarian brands was also a subject of analysis. The result obtained for the “Devin” mineral water (20 ng cm−3, n = 4, RSD = 10%) was confirmed by the standard addition method (22.5 ng cm−3, RSD = 5.3%) at three spiked concentration levels. The Mo(VI) content in the “Sevtopolis” table water and “Gorna Banya” mineral water was below the limit of determination.
2.7. Comparison with Other Liquid—Liquid Extraction–Spectrophotometric Procedures
A comparison of the present method with other liquid—liquid extraction–spectrophotometric methods for molybdenum determination is made in Table 5. The proposed method is characterized by a low LOD, high molar absorption coefficient, and good linearity. It is reliable and robust because of the wide optimum intervals of the variables studied. In addition, the volume of organic solvent used (5 cm3 per sample) is smaller than that of most of the procedures described in Table 5.

Table 5.
Comparison with other liquid—liquid extraction—spectrophotometric methods for molybdenum determination.
3. Experimental Section
3.1. Reagents and Chemicals
The solution of Mo(VI) (2 × 10−4 mol dm−3) was prepared from (NH4)6Mo7O24⋅4H2O (99.98% trace metals basis, Merck, Schnelldorf, Germany). The other chemicals were 4NC (> 98%, Fluka AG, Buchs, Switzerland), BZC (>95.0%, Merck, Schnelldorf, Germany), sulfuric acid (puriss. p. a., Fluka AG, Germany), and disodium ethylenediaminetetraacetate dihydrate (Na2EDTA; > 99.5%, Fillab EOOD, Plovdiv, Bulgaria) The prepared aqueous solutions were at concentrations of 7.5 × 10−3 mol dm−3 (4NC), 2.4 × 10−3 mol dm−3 (BZC), 2.5 × 10−1 mol dm−3 (H2SO4), and 1.0 × 10−1 mol dm−3 (Na2EDTA). Distilled or deionized (18.2 MΩ cm, ELGA-Veolia LabWater, High Wycombe, UK) water and redistilled chloroform were used throughout the work.
3.2. Instrumentation
Absorbance was measured on an Ultrospec 3300 pro UV/Vis spectrophotometer (Little Chalfont, UK), equipped with 10 mm path-length quartz cells. The pH was checked using a WTW InoLab 720 pH-meter (Weilheim, Germany).
3.3. General Optimization Procedure
Solutions of Mo(VI), H2SO4, 4NC, and BZC were successively transferred into a separatory funnel. The total volume was adjusted to 10 cm3 with water. Then, 5 cm3 of chloroform was added and the mixture was shaken for a fixed period (15–300 s). After a short wait for phase separation, a portion of the organic layer was poured into the cell. The absorbance was measured against chloroform or a blank prepared at the same manner.
3.4. Determination of the Distribution Ratio and Fraction Extracted
The distribution ratio (D) and fraction extracted (%E) were calculated by comparing the absorbances obtained after a single extraction (A1) and a triple extraction (A3) under the optimal conditions given in Table 1. The total volume in both cases (single extraction and triple extraction) was 25 cm3. The following formulae were used:
D = A1/(A3 − A1)
%E = 100 × D/(D + 1).
3.5. Sample Preparation
The steels were prepared for analysis according to a procedure described previously [47,48]. A known amount of the sample (250–1000 μg) was dissolved in 100 cm3 of HCl (1:1). Then, 20 cm3 of HNO3 (1:1) was added and the mixture was heated for 5 min or until tungstic acid became light yellow (incase the steel contains tungsten). The mixture was diluted to 150 cm3, heated to boiling, and if necessary, filtered through a medium-fast filtering paper. The precipitate (tungstic acid) was carefully washed with HC1 (1:4). A 15 cm3 portion of H2SO4 (1:1) was added to the filtrate and the solution was heated until fumes of SO3 evolved. After cooling, 100 cm3 of water was added, and the mixture was heated to dissolve the salts. The filtrate together with the washing was transferred into a 1000 cm3 volumetric flask and diluted with water to the mark.
The bottled water from three Bulgarian brands (“Devin” mineral water, “Gorna Banya” mineral water, and “Sevtopolis” table water) was bought from a local supermarket (Plovdiv, Bulgaria) and analyzed on the same day.
3.6. Procedure for the Determination of Molybdenum(VI)
An aliquot (1 cm3 for the steel analysis or 5 cm3 for the water analysis) of the analyzed solution was placed in a separatory funnel and the pH was adjusted to 1.4–1.5 with 0.25 mol dm−3 H2SO4 (the needed H2SO4 volumes were 1 cm3 for the steel analysis and 1.5 cm3 for the water analysis). Next, 1 cm3 of 0.1 mol dm−3 Na2EDTA solution, 1 cm3 of 7.5 × 10−3 mol dm−3 4NC solution, and 0.8 cm3 of 2.4 × 10−3 mol dm−3 BZC solution were added, and the volume was brought to 10 cm3 with water. Then, 5 cm3 of chloroform was buretted and the mixture was shaken for 3 min. After phase separation, a portion of the organic extract was poured into the cell and the absorbance was measured at 439 nm against a blank. The Mo(VI) concentration was calculated from a calibration plot prepared by the same procedure using standard solutions.
4. Theoretical Section
The ground-state equilibrium geometries of the three possible structures of the anionic part of the ion-association complex were optimized at the B3LYP theoretical level using 3–21G basis functions. The charge and the spin multiplicity were set to −2 and singlet. Subsequent frequency calculations were carried out to check for the imaginary frequencies of the structures. The vertical excitation energies were calculated to make a comparison between experimental and theoretical spectra. The calculations were performed with the GAUSSIAN 03 commercial software [49]. The ChemCraft 1.8 program [50] was used for the visualization of the structures.
5. Conclusions
A new liquid—liquid extraction—chromogenic system for Mo(VI) involving 4-nitrocatechol and benzalkonium chloride was studied. Optimal conditions were found for the formation and extraction of a ternary ion-association complex, (BZ+)2[MoVI(4NC)O2(OH)2]. The structure of its anionic part was clarified with the help of theoretical TD DFT calculations. The complex is intensely colored and allows the determination of trace Mo(VI) in a simple and economical way, without the use of sophisticated instruments. The developed analytical procedure is characterized by a low LOD, good linearity, and high molar absorption coefficient. It is fast, selective, and robust. Its reliability is governed by the high masking efficiency of Na2EDTA and the wide optimal intervals of the investigated parameters.
Author Contributions
Conceptualization, K.G., V.B.D. and V.V.D.; methodology, K.G., V.V.D. and V.B.D.; software, V.B.D.; validation, V.V.D., K.G. and N.M.; formal analysis, V.V.D., K.G. and V.B.D.; investigation, V.V.D., G.T., A.S., N.M., V.B.D. and K.G.; resources, V.V.D., A.S., N.M. and K.G.; writing—original draft preparation, K.G.; writing—review and editing, K.G. and N.M.; visualization, K.G. and V.B.D.; supervision, K.G.; project administration, K.G.; funding acquisition, K.G. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Medical University of Plovdiv and the Scientific Research Fund at the Plovdiv University ‘Paisii Hilendarski’ (grant SP-21-HF004) for the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. All data are in the form of tables and figures.
Conflicts of Interest
The authors declare that there are no conflict of interest regarding the publication of this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Not available.
References
- Outteridge, T.; Kinsman, N.; Ronchi, G.; Mohrbacher, H. Editorial: Industrial relevance of molybdenum in China. Adv. Manuf. 2020, 8, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.K. Uses of Metals and Metallic Minerals; New Age International (P) Ltd.: New Delhi, India, 2007; pp. 205–209. [Google Scholar]
- Lunk, H.-J.; Hartl, H. Discovery, properties and applications of molybdenum and its compounds. ChemTexts 2017, 3, 13. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Schwarz, G.; Belaidi, A.A. Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 415–450. [Google Scholar] [CrossRef]
- PSmedley, P.; Cooper, D.; Ander, E.L.; Milne, C.; Lapworth, D. Occurrence of molybdenum in British surface water and groundwater: Distributions, controls and implications for water supply. Appl. Geochem. 2014, 40, 144–154. [Google Scholar] [CrossRef]
- Novotny, J.A.; A Peterson, C. Molybdenum. Adv. Nutr. 2018, 9, 272–273. [Google Scholar] [CrossRef]
- Ghasemzadeh, N.; Karimi-Nazari, E.; Yaghoubi, F.; Zarei, S.; Azadmanesh, F.; Reza, J.Z.; Sargazi, S. Molybdenum Cofactor Biology and Disorders Related to Its Deficiency; A Review Study. J. Nutr. Food Secur. 2019, 4, 206–217. [Google Scholar] [CrossRef]
- EFSA, Overview on Dietary Reference Values for the EU Population as Derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Available online: https://www.efsa.europa.eu/sites/default/files/assets/DRV_Summary_tables_jan_17.pdf (accessed on 4 September 2021).
- Ivanov, V.M.; Kochelaeva, G.A.; Prokhorova, G.V. Methods for Determining Molybdenum. J. Anal. Chem. 2002, 57, 758–772. [Google Scholar] [CrossRef]
- Filik, H.; Tütem, E.; Apak, R. Use of the molybdenum–thiocyanate–rhodamine 6G ternary complex for spectrophotometric molybdenum determination without extraction. Anal. Chim. Acta 2004, 505, 77–82. [Google Scholar] [CrossRef]
- Pyrzynska, K. Determination of molybdenum in environmental samples. Anal. Chim. Acta 2007, 590, 40–48. [Google Scholar] [CrossRef]
- Kara, D.; Karadaş, C. A simple spectrophotometric method for the determination of trace levels of molybdenum using N,N′-bis(2-hydroxy-5-bromo-benzyl)1,2 diaminopropane. Spectrochim. Acta Part A 2015, 147, 158–162. [Google Scholar] [CrossRef]
- Temel, N.K.; Gürkan, R. Catalytic spectrophotometric determination of trace Mo(vi) in milk-based beverages in the presence of bromophenol blue and H2O2 using SDS as a sensitizer. Anal. Methods 2016, 8, 6284–6292. [Google Scholar] [CrossRef]
- Penner, M.H. Food Analysis; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 89–106. [Google Scholar] [CrossRef]
- Passos, M.L.; Saraiva, M.L.M. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Amin, A.S.; Moustafa, I.M.; El-Sharjawy, A.A. Utilization of Cloud-Point Preconcentration for Spectrophotometric Determination of Trace Amounts of Molybdenum(VI) in Plants and Water Samples. Can. Chem. Trans. 2015, 3, 486–496. [Google Scholar] [CrossRef]
- Snigur, D.; Chebotarev, A.; Dubovyiy, V.; Barbalat, D.; Bevziuk, K. Salicylic acid assisted cloud point extraction at room temperature: Application for preconcentration and spectrophotometric determination of molybdenum(VI). Microchem. J. 2018, 142, 273–278. [Google Scholar] [CrossRef]
- Agnihotri, R.; Singh, A.; Agnihotri, N. Extraction and Spectrophotometric Determination of Molybdenum(VI) using 3-hydroxy-2-[3-(4-methoxyphenyl)-1-phenyl-4-pyrazolyl]-4-oxo-4H-1-benzopyran as a Chelating Agent. J. Anal. Chem. 2019, 74, 81–86. [Google Scholar] [CrossRef]
- Kuliev, K.A.; Verdizade, N.A.; Aliev, S.G.; Abasquliyeva, U.B.; Efendieva, N.N. Analytical Application of Ion Associates of Molybdenum with Dithiolphenols and Aminophenols. J. Mater. Sci. Chem. Eng. 2019, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Klochkova, A.; Barbalat, D.; Chebotarev, A.; Snigur, D. Dispersive liquid–liquid semi-microextraction of molybdenum(VI) with 6,7-dihydroxy-2,4-diphenylbenzopyrylium chloride for its spectrophotometric determination. J. Iran. Chem. Soc. 2021, 18, 109–115. [Google Scholar] [CrossRef]
- Snigur, D.; Barbalat, D.; Chebotarev, A.; Synievyd, A.; Bevziuk, K. A rapid cloud point extraction of Molybdenum(VI) with 6,7-dihydroxy-2,4-diphenylbenzopyrylium perchlorate prior to its spectrophotometric determination. Chem. Pap. 2021, 75, 1823–1830. [Google Scholar] [CrossRef]
- Cornard, J.-P.; Rasmiwetti; Merlin, J.-C. Molecular structure and spectroscopic properties of 4-nitrocatechol at different pH: UV–visible, Raman, DFT and TD-DFT calculations. Chem. Phys. 2005, 309, 239–249. [Google Scholar] [CrossRef]
- Gavazov, K.B. Nitroderivatives of catechol: From synthesis to application. Acta Chim. Slov. 2012, 59, 1–17. [Google Scholar]
- Stojnova, K.T.; Lekova, V.D. Study on the Equilibria of Chelate Formation and the Ion-association of Anionic Chelate of Germanium(IV) with 4-Nitrocatechol and 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium Cation. Russ. J. Inorg. Chem. 2019, 64, 1235–1241. [Google Scholar] [CrossRef]
- Saravanska, A.D.; Racheva, P.V.; Divarova, V.V.; Toncheva, G.K.; Milcheva, N.P.; Delchev, V.B.; Gavazov, K.B. Extraction-Spectrophotometric and Theoretical Studies on a Ternary Complex Obtained from Vanadium(V) and 4-Nitrocatechol. Russ. J. Inorg. Chem. 2021, 66, 1880–1886. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. (Eds.) Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2009; pp. 56–58. [Google Scholar]
- Li, Y.; Xu, Z.; Yu, M.; Wang, Z.; Ma, W.; Dong, Z. Two Novel Methods for the Determination of Benzalkonium Chloride in Bandages by Resonance Light Scattering Technology. Tenside Surfact. Det. 2021, 58, 44–50. [Google Scholar] [CrossRef]
- Hristov, D.G.G.; Racheva, P.V.; Toncheva, G.K.; Gavazov, K.B. Extraction-Chromogenic System for Cobalt Based on 5-Methyl-4-(2-thiazolylazo) Resorcinol and Benzalkonium Chloride. Acta Chim. Slov. 2021, 68, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kustin, K.; Liu, S.-T. Kinetics and complex formation of molybdate with catechol. J. Am. Chem. Soc. 1973, 95, 2487–2491. [Google Scholar] [CrossRef]
- Natansohn, S.; Krugler, J.I.; Lester, J.E.; Chagnon, M.S.; Finocchiaro, R.S. Stability constants of complexes of molybdate and tungstate ions with o-hydroxy aromatic ligands. J. Phys. Chem. 1980, 84, 2972–2980. [Google Scholar] [CrossRef]
- Dimitrov, A.N.; Lekova, V.; Gavazov, K.B.; Boyanov, B.S. Investigation of the extraction equilibrium of ion-association complexes of molybdenum (VI) with some polyphenols and thiazolyl blue. Extraction-spectrophotometric determination of molybdenum. Cent. Eur. J. Chem. 2005, 3, 747–755. [Google Scholar] [CrossRef]
- Zhiming, Z.; Dongsten, M.; Cunxiao, Y. Mobile equilibrium method for determining composition and stability constant of coordination compounds of the form MmRn. J. Rare Earths 1997, 15, 216–219. [Google Scholar]
- Asmus, E. Eine neue Methode zur Ermittlung der Zusammensetzung schwacher Komplexe. Fresenius’ J. Anal. Chem. 1960, 178, 104–116. [Google Scholar] [CrossRef]
- Holme, A.; Langmyhr, F. A modified and a new straight-line method for determining the composition of weak complexes of the form AmBn. Anal. Chim. Acta 1966, 36, 383–391. [Google Scholar] [CrossRef]
- Harvey, A.E.; Manning, D.L. Spectrophotometric Methods of Establishing Empirical Formulas of Colored Complexes in Solution. J. Am. Chem. Soc. 1950, 72, 4488–4493. [Google Scholar] [CrossRef]
- Lekova, V.; Gavazov, K.B.; Dimitrov, A.N. Application of a ternary complex of tungsten(VI) with 4-nitrocatechol and thiazolyl blue for extraction-spectrophotometric determination of tungsten. Chem. Pap. 2006, 60, 283–287. [Google Scholar] [CrossRef]
- Kumar, A.; Dass, R.; Sharma, R.G. 3-Hydroxy-2-(2′-thienyl)-4H-chromen-4-one reagent for extractive spectrophotometric determination of molybdenum (V). Chem. Anal. 2005, 50, 625–630. [Google Scholar]
- Dimitrov, A.; Lekova, V.; Gavazov, K.; Boyanov, B.S. Ternary complex of molybdenum(VI) with 4-nitrocatechol and tetrazolium blue chloride and its application to extraction-spectrophotometric analysis of ferrous metallurgy products. J. Anal. Chem. 2007, 62, 122–125. [Google Scholar] [CrossRef]
- Shrivas, K.; Agrawal, K.; Harmukh, N. Trace level determination of molybdenum in environmental and biological samples using surfactant-mediated liquid–liquid extraction. J. Hazard. Mat. 2009, 161, 325–329. [Google Scholar] [CrossRef]
- Aswar, A.; Joshi, M. Extractive Spectrophotometric Determination of Molybdenum(VI) with 2-hydroxy-5-methylacetophenone-isonicotinoylhydrazone (HMAINH). Rev. Anal. Chem. 2010, 29, 69–80. [Google Scholar] [CrossRef]
- Dass, R.; Kapoor, J.K.; Gambhir, S. Extractive Spectrophotometric Method for Determination of Molybdenum in Steels and Environmental Samples. J. Chem. 2013, 2013, 420768. [Google Scholar] [CrossRef] [Green Version]
- Dass, R.; Kapoor, J.K.; Gambhir, S. Spectrophotometric determination of molybdenum using surfactant-mediated liquid--liquid extraction. Turk. J. Chem. 2014, 38, 328–337. [Google Scholar] [CrossRef]
- Zalov, A.Z.; Verdizade, N.V.; Hadjieva, A.B. Extraction and spectrophotometric determination of molybdenum with o-hydroxythiophenols and aromatic amines. Pak. J. Anal. Environ. Chem. 2015, 16, 16–23. [Google Scholar]
- Stojnova, K.; Racheva, P.; Divarova, V.; Yanev, P.; Lekova, V. Study on the Complex Formation and the Ion-Association of Anionic Chelate of Molybdenum(VI) with Bidentate Ligand and the Cation of 2,3,5-Triphenyl-2H-tetrazolium Chloride. Acta Chim. Slov. 2020, 67, 594–601. [Google Scholar] [CrossRef]
- Yerramilli, A.; Kavipurapu, C.S.; Manda, R.R.; Pillutha, C.M. Extractive spectrophotometric method for the determination of vanadium(V) in steels and titanium base alloy. Anal. Chem. 1986, 58, 1451–1453. [Google Scholar] [CrossRef]
- Stefanova, T.S.; Simitchiev, K.K.; Gavazov, K.B. Liquid–liquid extraction and cloud point extraction for spectrophotometric determination of vanadium using 4-(2-pyridylazo)resorcinol. Chem. Pap. 2015, 69, 495–503. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C. Gaussian 03, Revision C. 02, Gaussian, Inc., Wallingford CT; d) Becke, AD (1993). J. Chem. Phys. 2004, 98, 5648–5652. [Google Scholar]
- ChemCraft. Available online: http://www.chemcraftprog.com (accessed on 8 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).