Selective Consumption of Fish Oil at End of the Day Increases the Physiological Fatty Acid Compositions of Eicosapentaenoic Acid and Docosahexaenoic Acid in Mice
Abstract
:1. Introduction
2. Results
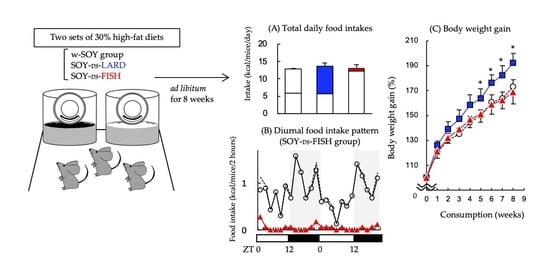
2.1. Preferential Consumption of High-Fat Diets Composed of Different Fat Sources
2.2. Diurnal Feeding Patterns
2.3. Effects on Biological Parameters
2.4. Effects on Plasma Biochemical and Hepatic Lipid Parameters
2.5. Effects on Plasma and Hepatic Fatty Acid Composition
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Diets
4.3. Animal Experiments
4.3.1. Institutional Approval of the Study Protocol
4.3.2. Animals and Housing Environments
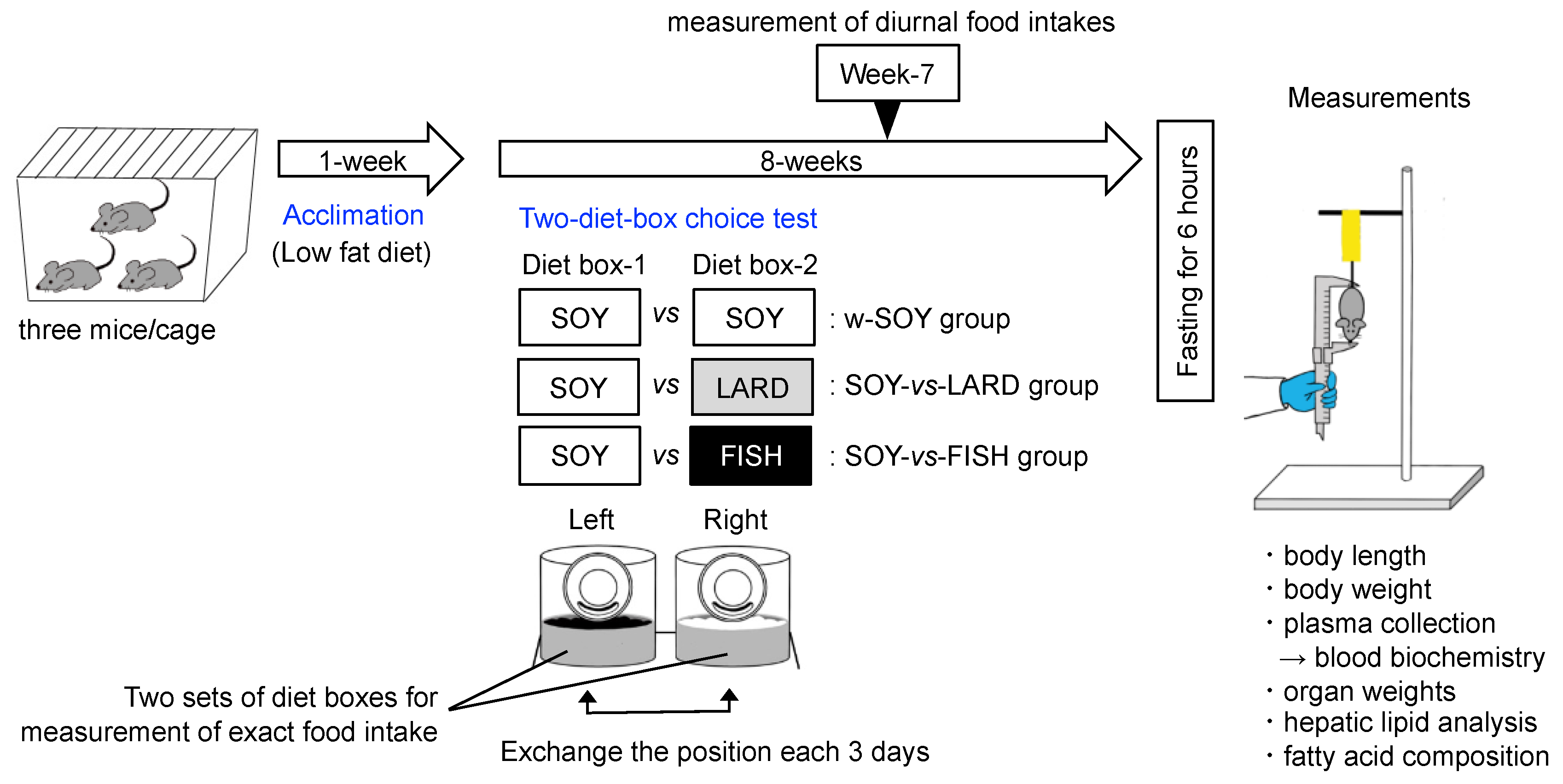
4.3.3. Experimental Design
4.3.4. Sample Collection
4.4. Biochemical Parameters
4.4.1. Blood Biochemistry
4.4.2. Hepatic Lipid Analysis
4.4.3. Plasma and Hepatic Fatty Acid Composition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchie, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Fruhbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and anti-inflammatory potential of polyphenols contained in mediterranean diet in obesity: Molecular mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Zhao, F.; Song, S.; Li, Y.; Xu, X.; Zhou, G.; Li, C. Fish oil, lard and soybean oil differentially shape gut microbiota of middle-aged rats. Sci. Rep. 2017, 7, 826. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 2018, 28, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.A.; Lovejoy, J.C.; Smith, S.R.; DeLany, J.P.; Lefevre, M.; Hwang, D.; Ryan, D.H.; York, D.A. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J. Nutr. 2002, 132, 2488–2491. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina lipids on high-fat and high-sucrose diet induced obesity and hepatic lipid accumulation in C57BL/6J mice. J. Funct. Food 2020, 65, 103741. [Google Scholar] [CrossRef]
- Na, B.R.; Lee, J.H. In vitro and in vivo digestibility of soybean, fish, and microalgal oils, and their influences on fatty acid distribution in tissue lipid of mice. Molecules 2020, 25, 5357. [Google Scholar] [CrossRef]
- Du, S.; Jin, J.; Fang, W.; Su, Q. Does fish oil have an anti-obesity effect in overweight/obese adults? A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0142652. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, M.; Takeda, M.; Suzuki, A.; Sawano, S.; Fushiki, T. Preference for high-fat food in mice: Fried potatoes compared with boiled potatoes. Appetite 2001, 36, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Imaizumi, M.; Fushiki, T. Preference for vegetable oils in the two-bottle choice test in mice. Life Sci. 2000, 67, 197–204. [Google Scholar] [CrossRef]
- Mizunoya, W.; Ohnuki, K.; Baba, K.; Miyahara, H.; Shimizu, N.; Tabata, K.; Kino, T.; Sato, Y.; Tatsumi, R.; Ikeuchi, Y. Effect of dietary fat type on anxiety-like and depression-like behavior in mice. Springerplus 2013, 2, 165. [Google Scholar] [CrossRef] [Green Version]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Shu, G.; Zhou, L.; Zhu, X.; Liao, W.; Wang, S.; Yang, J.; Zhou, G.; Xi, Q.; Gao, P.; et al. Selective transport of long-chain fatty acids by FAT/CD36 in skeletal muscle of broilers. Animal 2013, 7, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Pavlisova, J.; Bardova, K.; Stankova, B.; Tvrzicka, E.; Kopecky, J.; Rossmeisl, M. Corn oil versus lard: Metabolic effects of omega-3 fatty acids in mice fed obesogenic diets with different fatty acid composition. Biochimie 2016, 124, 150–162. [Google Scholar] [CrossRef]
- Gondim, P.N.; Rosa, P.V.; Okamura, D.; Silva, V.O.; Andrade, E.F.; Biihrer, D.A.; Pereira, L.J. Benefits of fish oil consumption over other sources of lipids on metabolic parameters in obese rats. Nutrients 2018, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Kim, H.J.; Chiba, H.; Matsumoto, A. Anti-obesity effect of fish oil and fish oil-fenofibrate combination in female KK mice. J. Atheroscler Thromb 2009, 16, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, L.; Loh, D.H.; Colwell, C.S.; Tache, Y.; Reue, K.; Arnold, A.P. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm. Behav. 2015, 75, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, D.; Tanaka, W.; Hashizume, Y.; Tandia, M.; Sakono, M.; Shimoi, K.; Sakakibara, H. Daily consumption of monoglucosyl-rutin prevents high-fat diet-induced obesity by suppressing gastric inhibitory polypeptide secretion in mice. Funct. Food Health Dis. 2018, 8, 353–371. [Google Scholar] [CrossRef] [Green Version]
- Balogun, K.A.; Albert, C.J.; Ford, D.A.; Brown, R.J.; Cheema, S.K. Dietary omega-3 polyunsaturated fatty acids alter the fatty acid composition of hepatic and plasma bioactive lipids in C57BL/6 mice: A lipidomic approach. PLoS ONE 2013, 8, e82399. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Wang, Q.; You, Q.L.; Li, Z.L.; Hu, N.Y.; Wang, Y.; Jin, Z.L.; Li, S.J.; Li, X.W.; Yang, J.M.; et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020, 11, 5465. [Google Scholar] [CrossRef]
- Soni, N.; Ross, A.B.; Scheers, N.; Nookaew, I.; Gabrielsson, B.G.; Sandberg, A.S. The omega-3 fatty acids EPA and DHA, as a part of a murine high-fat diet, reduced lipid accumulation in brown and white adipose tissues. Int. J. Mol. Sci. 2019, 20, 5895. [Google Scholar] [CrossRef] [Green Version]
- Pavlisova, J.; Horakova, O.; Kalendova, V.; Buresova, J.; Bardova, K.; Holendova, B.; Plecita-Hlavata, L.; Vackova, S.; Windrichova, J.; Topolcan, O.; et al. Chronic n-3 fatty acid intake enhances insulin response to oral glucose and elevates GLP-1 in high-fat diet-fed obese mice. Food Funct. 2020, 11, 9764–9775. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumi, S.H.; Mahmoud, A.A.A.; Fahmy, A.; Ibrahim, I. Effect of omega-3 fatty acids on glucose homeostasis: Role of free fatty acid receptor 1. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1797–1808. [Google Scholar] [CrossRef]
- Matsuyama, H.; Tanaka, W.; Yokoyama, D.; Matsumoto, S.; Sano, T.; Yamashita, T.; Nishimura, S.; Sakono, M.; Sakakibara, H. Suitability of a 10% fat diet for use in time-restricted feeding experiments with C57BL/6 mice. Bioact. Compd. Health Dis. 2020, 3, 55–65. [Google Scholar] [CrossRef]
- Kappel, S.; Hawkins, P.; Mendl, M.T. To group ornot to group? Good practice for housing male laboratory mice. Animals 2017, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- Mertens, S.; Vogt, M.A.; Gass, P.; Palme, R.; Hiebl, B.; Chourbaji, S. Effect of three different forms of handling on the variation of aggression-associated parameters in individually and group-housed male C57BL/6NCrl mice. PLoS ONE 2019, 14, e0215367. [Google Scholar] [CrossRef] [Green Version]
- Jirkof, P.; Bratcher, N.; Medina, L.; Strasburg, D.; Ebert, P.; Gaskill, B.N. The effect of group size, age and handling frequency on inter-male aggression in CD 1 mice. Sci. Rep. 2020, 10, 2253. [Google Scholar] [CrossRef] [Green Version]
- Swennes, A.G.; Alworth, L.C.; Harvey, S.B.; Jones, C.A.; King, C.S.; Crowell-Davis, S.L. Human handling promotes compliant behavior in adult laboratory rabbits. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 41–45. [Google Scholar] [PubMed]
- Eskola, S.; Lauhikari, M.; Voipio, H.M.; Nevalainen, T. The use of aspen blocks and tubes to enrich the cage environment of laboratory rats. Scand. J. Lab. Anim. Sci. 1999, 26, 1–10. [Google Scholar]
- Oizumi, H.; Kuriyama, N.; Imamura, S.; Tabuchi, M.; Omiya, Y.; Mizoguchi, K.; Kobayashi, H. Influence of aging on the behavioral phenotypes of C57BL/6J mice after social defeat. PLoS ONE 2019, 14, e0222076. [Google Scholar] [CrossRef] [Green Version]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Takashima, M.; Tanaka, W.; Matsuyama, H.; Tajiri, H.; Sakakibara, H. Maternal quercetin consumption during pregnancy may help regulate total cholesterol/HDL-cholesterol ratio without effect on cholesterol levels in male progeny consuming high-fat diet. Nutrients 2021, 13, 1242. [Google Scholar] [CrossRef]
- Monguchi, T.; Hara, T.; Hasokawa, M.; Nakajima, H.; Mori, K.; Toh, R.; Irino, Y.; Ishida, T.; Hirata, K.I.; Shinohara, M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J. Cardiol. 2017, 70, 121–127. [Google Scholar] [CrossRef] [Green Version]





| Fatty Acids | Common Name | w-SOY | SOY-vs-LARD | SOY-vs-FISH |
|---|---|---|---|---|
| C12:0 | lauric acid | <1.0 | <1.0 | <1.0 |
| C14:0 | myristic acid | <1.0 | 7.1 ± 0.4 | 2.5 ± 0.2 # |
| C16:0 | palmitic acid | 76.2 ± 1.2 | 148.8 ± 9.8 * | 78.6 ± 8.3 # |
| C18:0 | stearic acid | 22.4 ± 0.4 | 71.6 ± 4.5 * | 22.6 ± 2.4 # |
| C16:1 (ω 9) | palmitoleic acid | <1.0 | 18.4 ± 1.2 | 6.0 ± 0.4 # |
| C18:1 (ω 9) | oleic acid | 182.6 ± 2.9 | 272.3 ± 18.6 * | 171.0 ± 18.8 # |
| C18:2 (ω 6) | linoleic acid | 432.0 ± 6.9 | 263.2 ± 26.0 * | 388.0 ± 43.3 # |
| C18:3 (ω 6) | γ-linolenic acid | <1.0 | 1.2 ± 0.1 | <1.0 |
| C20:4 (ω 6) | arachidonic acid | <1.0 | <1.0 | <1.0 |
| C18:3 (ω 3) | α-linolenic acid | 42.1 ± 0.7 | 20.3 ± 2.4 * | 38.0 ± 4.2 # |
| C20:5 (ω 3) | eicosapentaenoic acid | <1.0 | <1.0 | 3.0 ± 0.2 |
| C22:6 (ω 3) | docosahexaenoic acid | <1.0 | <1.0 | 12.9 ± 0.9 |
| Sum of saturated fatty acids | 99.0 ± 1.6 | 227.9 ± 14.7 * | 103.7 ± 10.9 # | |
| Sum of monounsaturated fatty acids | 182.7 ± 2.9 | 290.7 ± 19.6 * | 177.0 ± 19.2 # | |
| Sum of omega-6 fatty acids | 432.0 ± 6.9 | 265.0 ± 26.1 * | 389.2 ± 43.4 # | |
| Sum of omega-3 fatty acids | 42.2 ± 0.7 | 20.4 ± 2.4 * | 53.9 ± 5.3 # | |
| omega-6/omega-3 ratio | 10.2 | 13.0 | 7.3 | |
| w-SOY | SOY-vs-LARD | SOY-vs-FISH | |
|---|---|---|---|
| Body weight (g) | |||
| week 0 | 17.5 ± 0.8 | 17.5 ± 0.6 | 17.5 ± 1.4 |
| week 8 | 30.3 ± 1.6 | 33.7 ± 1.6 | 29.8 ± 3.2 # |
| Body weight gain (g) | 12.8 ± 1.0 | 16.1 ± 1.3 * | 12.0 ± 2.0 # |
| Body length (cm) | 7.9 ± 0.3 | 8.1 ± 0.2 | 8.0 ± 0.1 |
| Body mass index (g/cm2) | 0.49 ± 0.03 | 0.52 ± 0.02 | 0.46 ± 0.03 # |
| Relative organ weights (g/100 g body weight) | |||
| liver | 3.54 ± 0.34 | 3.31 ± 0.48 | 3.65 ± 0.64 |
| kidney | 1.20 ± 0.16 | 1.14 ± 0.12 | 1.19 ± 0.15 |
| spleen | 0.31 ± 0.05 | 0.30 ± 0.07 | 0.34 ± 0.12 |
| visceral fat | 4.59 ± 1.31 | 5.59 ± 0.49 | 3.93 ± 1.43 |
| w-SOY | SOY-vs-LARD | SOY-vs-FISH | |
|---|---|---|---|
| Plasma biochemistry parameters | |||
| triglycerides (mg/dL) | 57.4 ± 14.4 | 74.3 ± 21.4 | 48.7 ± 24.0 |
| total cholesterol (mg/dL) | 96.5 ± 21.9 | 109.9 ± 32.4 | 85.7 ± 33.8 |
| total protein (g/dL) | 4.7 ± 0.3 | 4.7 ± 0.4 | 4.6 ± 0.5 |
| Hepatic lipids (mg/g liver) | |||
| triglycerides | 9.8 ± 1.6 | 12.5 ± 4.8 | 8.8 ± 2.1 |
| total cholesterol | 3.7 ± 0.7 | 5.4 ± 2.6 | 3.9 ± 0.7 |
| phospholipids | 5.4 ± 0.9 | 5.5 ± 0.9 | 5.0 ± 0.9 |
| 10% Soybean Oil | 30% Fat Diet | |||
|---|---|---|---|---|
| Soybean Oil (SOY) | Lard (LARD) | Fish Oil (FISH) | ||
| β-Cornstarch | 36.75 | 16.75 | 16.75 | 16.75 |
| α-Cornstarch | 13.2 | 13.2 | 13.2 | 13.2 |
| Casein | 20.0 | 20.0 | 20.0 | 20.0 |
| Soybean oil | 10.0 | 30.0 | - | - |
| Lard | - | - | 30.0 | - |
| Fish oil | - | - | - | 30.0 |
| Sucrose | 10.0 | 10.0 | 10.0 | 10.0 |
| Cellulose | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin mixture | 1.0 | 1.0 | 1.0 | 1.0 |
| Mineral mixture | 3.5 | 3.5 | 3.5 | 3.5 |
| L-Cysteine | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
| t-Butylhydroquinone | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Energy (kcal/g) | 4.098 | 5.098 | 5.098 | 5.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, H.; Matsuyama, H.; Tanaka, W.; Tajiri, H.; Sakakibara, H. Selective Consumption of Fish Oil at End of the Day Increases the Physiological Fatty Acid Compositions of Eicosapentaenoic Acid and Docosahexaenoic Acid in Mice. Molecules 2022, 27, 1271. https://doi.org/10.3390/molecules27041271
Matsuzaka H, Matsuyama H, Tanaka W, Tajiri H, Sakakibara H. Selective Consumption of Fish Oil at End of the Day Increases the Physiological Fatty Acid Compositions of Eicosapentaenoic Acid and Docosahexaenoic Acid in Mice. Molecules. 2022; 27(4):1271. https://doi.org/10.3390/molecules27041271
Chicago/Turabian StyleMatsuzaka, Hiroki, Hiroki Matsuyama, Wataru Tanaka, Hayato Tajiri, and Hiroyuki Sakakibara. 2022. "Selective Consumption of Fish Oil at End of the Day Increases the Physiological Fatty Acid Compositions of Eicosapentaenoic Acid and Docosahexaenoic Acid in Mice" Molecules 27, no. 4: 1271. https://doi.org/10.3390/molecules27041271