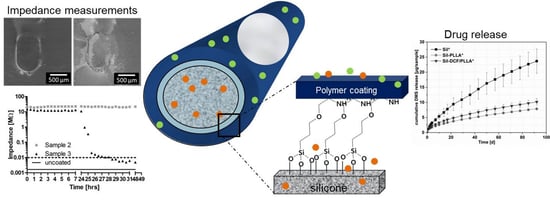
PLLA Coating of Active Implants for Dual Drug Release
Abstract
:1. Introduction
2. Results
2.1. Cell Culture
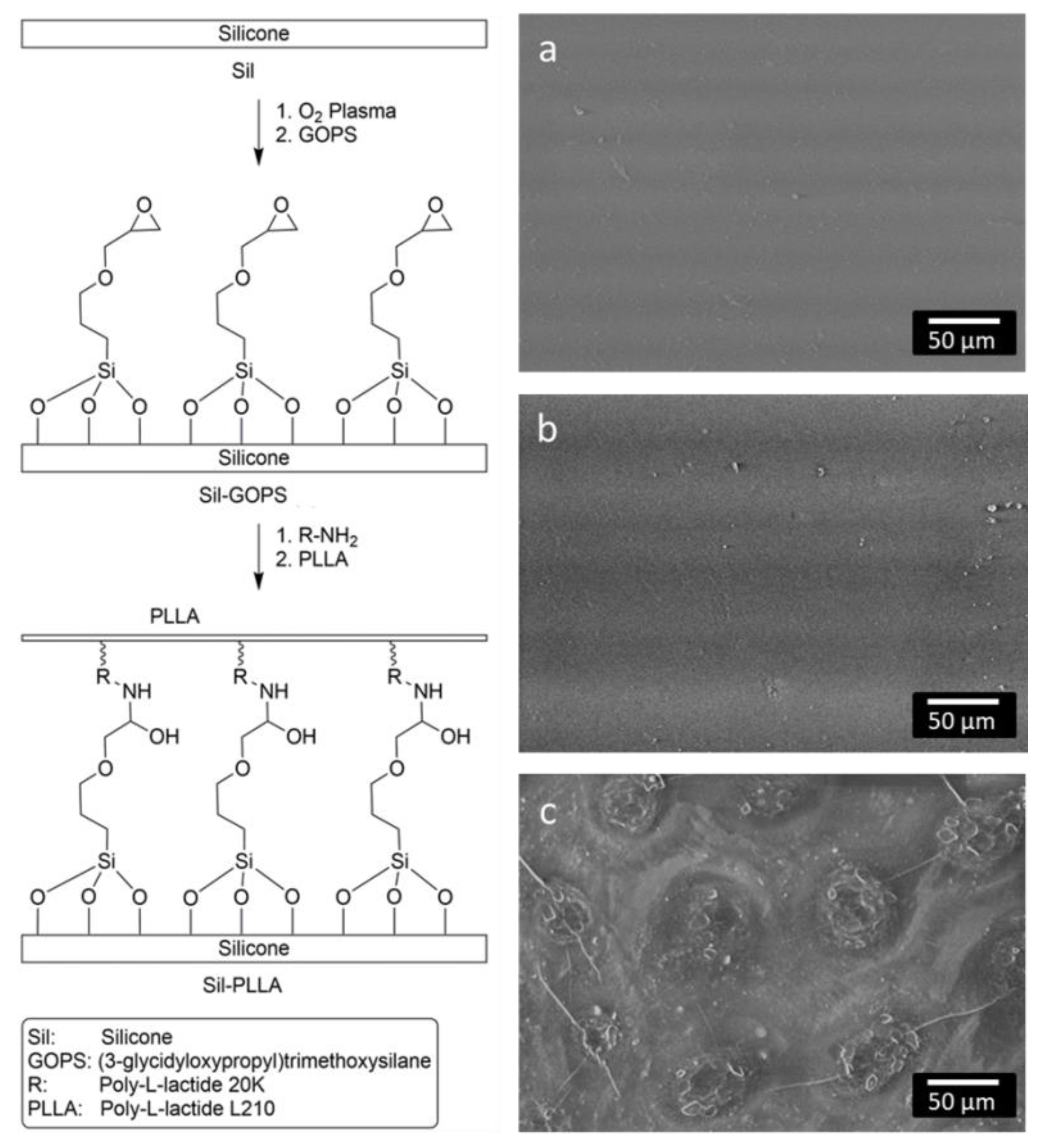
2.2. PLLA Coating
2.3. Contact Angle Measurements
2.4. ATR-FTIR
2.5. Drug Release
2.6. Impedance Results
2.7. Effect of Electrical Stimulation on Coating
2.8. Prevention of Coating of Contacts
3. Discussion
3.1. Cell Culture
3.2. PLLA Coating
3.3. Drug Release
3.4. Impedance Measurements
4. Materials and Methods
4.1. Ethical Statement
4.2. Materials
4.3. Preparation of Substances
4.4. Spiral Ganglion Cell Culture
4.5. Immunhistochemistry
4.6. Coating of the Silicone Surface
4.7. In Vitro Drug Release
4.8. Impedance Measurements of Coated Samples
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anderson, I.; Baumgartner, W.-D.; Böheim, K.; Nahler, A.; Arnoldner, C.; Arnolder, C.; D’Haese, P. Telephone use: What benefit do cochlear implant users receive? Int. J. Audiol. 2006, 45, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.; Webster, D.B. Spiral ganglion neuron loss following organ of corti loss: A quantitative study. Brain Res. 1981, 212, 17–30. [Google Scholar] [CrossRef]
- Scheper, V.; Paasche, G.; Miller, J.M.; Warnecke, A.; Berkingali, N.; Lenarz, T.; Stöver, T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J. Neurosci. Res. 2009, 87, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Shepherd, R.K.; Millard, R.E.; Clark, G.M. Chronic electrical stimulation of the auditory nerve at high stimulus rates: A physiological and histopathological study. Hear. Res. 1997, 105, 1–29. [Google Scholar] [CrossRef]
- Somdas, M.A.; Li, P.M.M.C.; Whiten, D.M.; Eddington, D.K.; Nadol, J.B. Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiol. Neurootol. 2007, 12, 277–284. [Google Scholar] [CrossRef]
- Paasche, G.; Bockel, F.; Tasche, C.; Lesinski-Schiedat, A.; Lenarz, T. Changes of postoperative impedances in cochlear implant patients: The short-term effects of modified electrode surfaces and intracochlear corticosteroids. Otol. Neurotol. 2006, 27, 639–647. [Google Scholar] [CrossRef]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef]
- Linke, I.; Fadeeva, E.; Scheper, V.; Esser, K.-H.; Koch, J.; Chichkov, B.N.; Lenarz, T.; Paasche, G. Nanostructuring of cochlear implant electrode contacts induces delayed impedance increase in vivo. Phys. Status Solidi A 2015, 212, 1210–1215. [Google Scholar] [CrossRef]
- Borenstein, J.T. Intracochlear drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Roemer, A.; Köhl, U.; Majdani, O.; Klöß, S.; Falk, C.; Haumann, S.; Lenarz, T.; Kral, A.; Warnecke, A. Biohybrid cochlear implants in human neurosensory restoration. Stem Cell Res. Ther. 2016, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Ma, D.; Chen, D.; Cai, J.; He, Q.; Shu, F.; Tang, J.; Zhang, H. Preparation, characterization and application research of a sustained dexamethasone releasing electrode coating for cochlear implantation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; O ’Leary, S.; Birman, C.; Plant, K.; English, R.; Dawson, P.; Risi, F.; Gavrilis, J.; Needham, K.; Cowan, R. Comparison of electrode impedance measures between a dexamethasone-eluting and standard Cochlear™ Contour Advance® electrode in adult cochlear implant recipients. Hearing Research 2020, 390, 107924. [Google Scholar] [CrossRef] [PubMed]
- Bas, E.; Bohorquez, J.; Goncalves, S.; Perez, E.; Dinh, C.T.; Garnham, C.; Hessler, R.; Eshraghi, A.A.; van de Water, T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear. Res. 2016, 337, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Prenzler, N.K.; Salcher, R.; Lenarz, T.; Gaertner, L.; Warnecke, A. Dose-Dependent Transient Decrease of Impedances by Deep Intracochlear Injection of Triamcinolone With a Cochlear Catheter Prior to Cochlear Implantation-1 Year Data. Front. Neurol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Bohl, A.; Rohm, H.W.; Ceschi, P.; Paasche, G.; Hahn, A.; Barcikowski, S.; Lenarz, T.; Stöver, T.; Pau, H.-W.; Schmitz, K.-P.; et al. Development of a specially tailored local drug delivery system for the prevention of fibrosis after insertion of cochlear implants into the inner ear. J. Mater. Sci. Mater. Med. 2012, 23, 2151–2162. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, H.; Zhuang, J.; Thayumanavan, S. Tunable enzyme responses in amphiphilic nanoassemblies through alterations in the unimer-aggregate equilibrium. Chem. Sci. 2019, 10, 3018–3024. [Google Scholar] [CrossRef] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Wulf, K.; Teske, M.; Matschegewski, C.; Arbeiter, D.; Bajer, D.; Eickner, T.; Schmitz, K.-P.; Grabow, N. Novel approach for a PTX/VEGF dual drug delivery system in cardiovascular applications-an innovative bulk and surface drug immobilization. Drug Deliv. Transl. Res. 2018, 8, 719–728. [Google Scholar] [CrossRef]
- Wulf, K.; Arbeiter, D.; Matschegewski, C.; Teske, M.; Huling, J.; Schmitz, K.-P.; Grabow, N.; Kohse, S. Smart releasing electrospun nanofibers-poly: L.lactide fibers as dual drug delivery system for biomedical application. Biomed. Mater. 2020, 16, 15022. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tan, H.; Huang, Y.; Pan, J.; Yao, J.; Liang, M.; Yang, J.; Jia, H. Development of a rapidly made, easily personalized drug-eluting polymer film on the electrode array of a cochlear implant during surgery. Biochem. Biophys. Res. Commun. 2020, 526, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.S.; Nakagawa, T.; Ying, L.; Tabata, Y.; Tsubouchi, H.; Ido, A.; Ito, J. Growth factor-eluting cochlear implant electrode: Impact on residual auditory function, insertional trauma, and fibrosis. J. Transl. Med. 2014, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Chikar, J.A.; Hendricks, J.L.; Richardson-Burns, S.M.; Raphael, Y.; Pfingst, B.E.; Martin, D.C. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials 2012, 33, 1982–1990. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.T.; Wise, A.K.; Thompson, B.C.; Flynn, B.O.; Atkinson, P.J.; Fretwell, N.J.; Fallon, J.B.; Wallace, G.G.; Shepherd, R.K.; Clark, G.M.; et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials 2009, 30, 2614–2624. [Google Scholar] [CrossRef] [Green Version]
- Al-Nimer, M.S.; Hameed, H.G.; Mahmood, M.M. Antiproliferative effects of aspirin and diclofenac against the growth of cancer and fibroblast cells: In vitro comparative study. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2015, 23, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Jayant, R.D.; McShane, M.J.; Srivastava, R. In vitro and in vivo evaluation of anti-inflammatory agents using nanoengineered alginate carriers: Towards localized implant inflammation suppression. Int. J. Pharm. 2011, 403, 268–275. [Google Scholar] [CrossRef]
- Botta, R.; Lisi, S.; Marcocci, C.; Sellari-Franceschini, S.; Rocchi, R.; Latrofa, F.; Menconi, F.; Altea, M.A.; Leo, M.; Sisti, E.; et al. Enalapril reduces proliferation and hyaluronic acid release in orbital fibroblasts. Thyroid 2013, 23, 92–96. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, Y.; Sun, H.-X.; Yu, D.-J. Inhibitory effects of enalaprilat on rat cardiac fibroblast proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules 2012, 17, 2738–2751. [Google Scholar] [CrossRef] [Green Version]
- Todd, P.A.; Sorkin, E.M. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Diclofenac sodium: A review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 1980, 20, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Borchert, T.; Hess, A.; Lukačević, M.; Ross, T.L.; Bengel, F.M.; Thackeray, J.T. Angiotensin-converting enzyme inhibitor treatment early after myocardial infarction attenuates acute cardiac and neuroinflammation without effect on chronic neuroinflammation. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1757–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, S.T.; Nguyen, H.T.-L.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Miller, F.; Hinze, U.; Chichkov, B.; Leibold, W.; Lenarz, T.; Paasche, G. Validation of eGFP fluorescence intensity for testing in vitro cytotoxicity according to ISO 10993-5. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 715–722. [Google Scholar] [CrossRef]
- Moskot, M.; Jakóbkiewicz-Banecka, J.; Kloska, A.; Piotrowska, E.; Narajczyk, M.; Gabig-Cimińska, M. The role of dimethyl sulfoxide (DMSO) in gene expression modulation and glycosaminoglycan metabolism in lysosomal storage disorders on an example of mucopolysaccharidosis. Int. J. Mol. Sci. 2019, 20, 304. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-X.; Yoshikawa, C.; Welch, N.G.; Pasic, P.; Thissen, H.; Voelcker, N.H. Spatially Controlled Surface Modification of Porous Silicon for Sustained Drug Delivery Applications. Sci. Rep. 2019, 9, 1367. [Google Scholar] [CrossRef] [Green Version]
- Festag, G.; Steinbrück, A.; Wolff, A.; Csaki, A.; Möller, R.; Fritzsche, W. Optimization of gold nanoparticle-based DNA detection for microarrays. J. Fluoresc. 2005, 15, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Udayakumar, M.; Kollár, M.; Kristály, F.; Leskó, M.; Szabó, T.; Marossy, K.; Tasnádi, I.; Németh, Z. Temperature and time dependence of the solvent-induced crystallization of poly(l-lactide). Polymers 2020, 12, 1065. [Google Scholar] [CrossRef]
- Peter, M.N.; Warnecke, A.; Reich, U.; Olze, H.; Szczepek, A.J.; Lenarz, T.; Paasche, G. Influence of in vitro electrical stimulation on survival of spiral ganglion neurons. Neurotox. Res. 2019, 36, 204–216. [Google Scholar] [CrossRef]
- Schulze, J.; Kaiser, O.; Paasche, G.; Lamm, H.; Pich, A.; Hoffmann, A.; Lenarz, T.; Warnecke, A. Effect of hyperbaric oxygen on BDNF-release and neuroprotection: Investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS ONE 2017, 12, e0178182. [Google Scholar] [CrossRef]







| 2.5 µm | 5 µm | 10 µm | |||
|---|---|---|---|---|---|
| <10 kΩ | >1 MΩ | <10 kΩ | >1 MΩ | <10 kΩ | >1 MΩ |
| 2/10 | 4/10 | 6/10 | 4/10 | 1/10 | 4/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulf, K.; Goblet, M.; Raggl, S.; Teske, M.; Eickner, T.; Lenarz, T.; Grabow, N.; Paasche, G. PLLA Coating of Active Implants for Dual Drug Release. Molecules 2022, 27, 1417. https://doi.org/10.3390/molecules27041417
Wulf K, Goblet M, Raggl S, Teske M, Eickner T, Lenarz T, Grabow N, Paasche G. PLLA Coating of Active Implants for Dual Drug Release. Molecules. 2022; 27(4):1417. https://doi.org/10.3390/molecules27041417
Chicago/Turabian StyleWulf, Katharina, Madeleine Goblet, Stefan Raggl, Michael Teske, Thomas Eickner, Thomas Lenarz, Niels Grabow, and Gerrit Paasche. 2022. "PLLA Coating of Active Implants for Dual Drug Release" Molecules 27, no. 4: 1417. https://doi.org/10.3390/molecules27041417
APA StyleWulf, K., Goblet, M., Raggl, S., Teske, M., Eickner, T., Lenarz, T., Grabow, N., & Paasche, G. (2022). PLLA Coating of Active Implants for Dual Drug Release. Molecules, 27(4), 1417. https://doi.org/10.3390/molecules27041417