Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
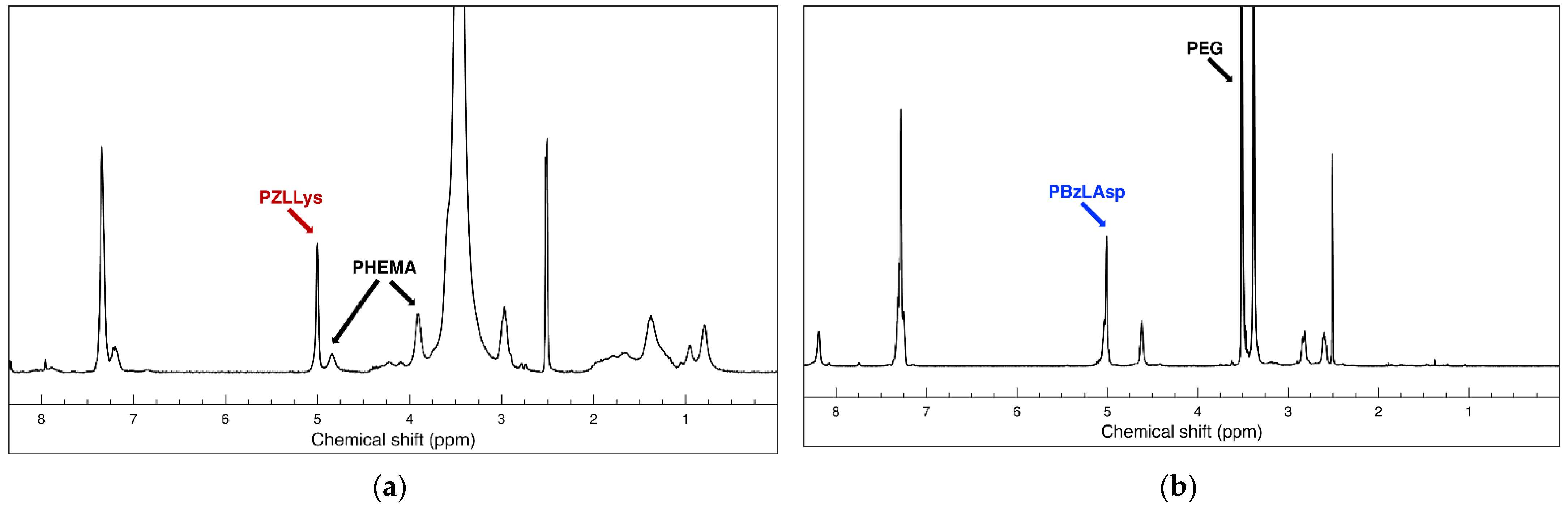
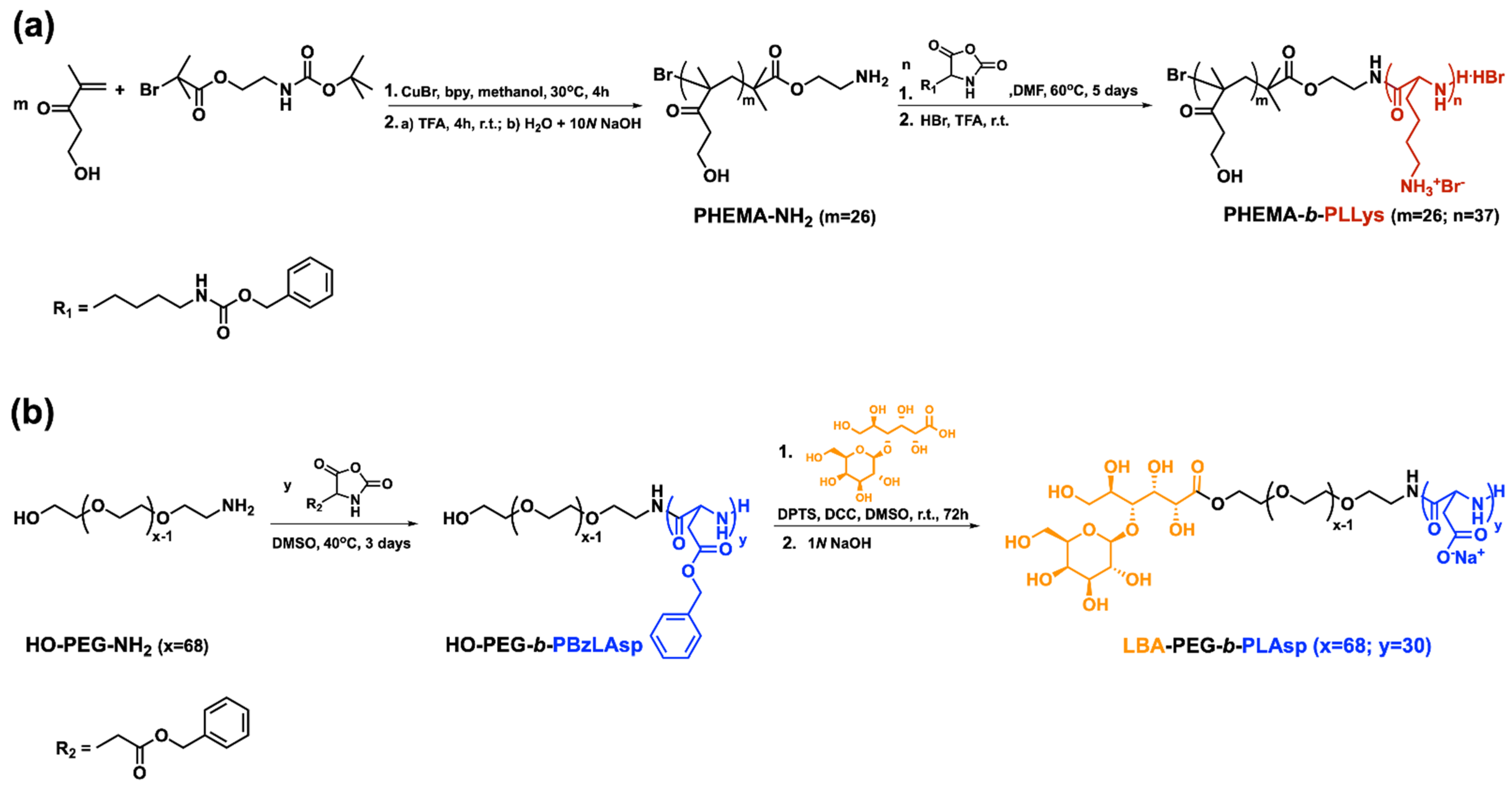
2.2. Synthesis of PHEMA-b-PLLys Block Copolymer
2.2.1. Synthesis of Poly(2-Hydroxyethyl Methacrylate) with Protected Amine End-Group (PHEMA-N-Boc)
2.2.2. Synthesis of Poly(2-Hydroxyethyl Methacrylate) Macroinitiator (PHEMA-NH2)
2.2.3. Synthesis of PHEMA-b-PZLLys Block Copolymer
2.2.4. Primary Amine Groups Deprotection
2.3. Synthesis of LBA-PEG-b-PLAsp Functional Block Copolymer
2.3.1. Synthesis of HO-PEG-b-PBzLAsp Block Copolymer
2.3.2. End-Functionalization with LBA and Carboxyl Groups Deprotection
2.4. Characterization Methods
2.5. Polyion Complex (PIC) Micelles Preparation
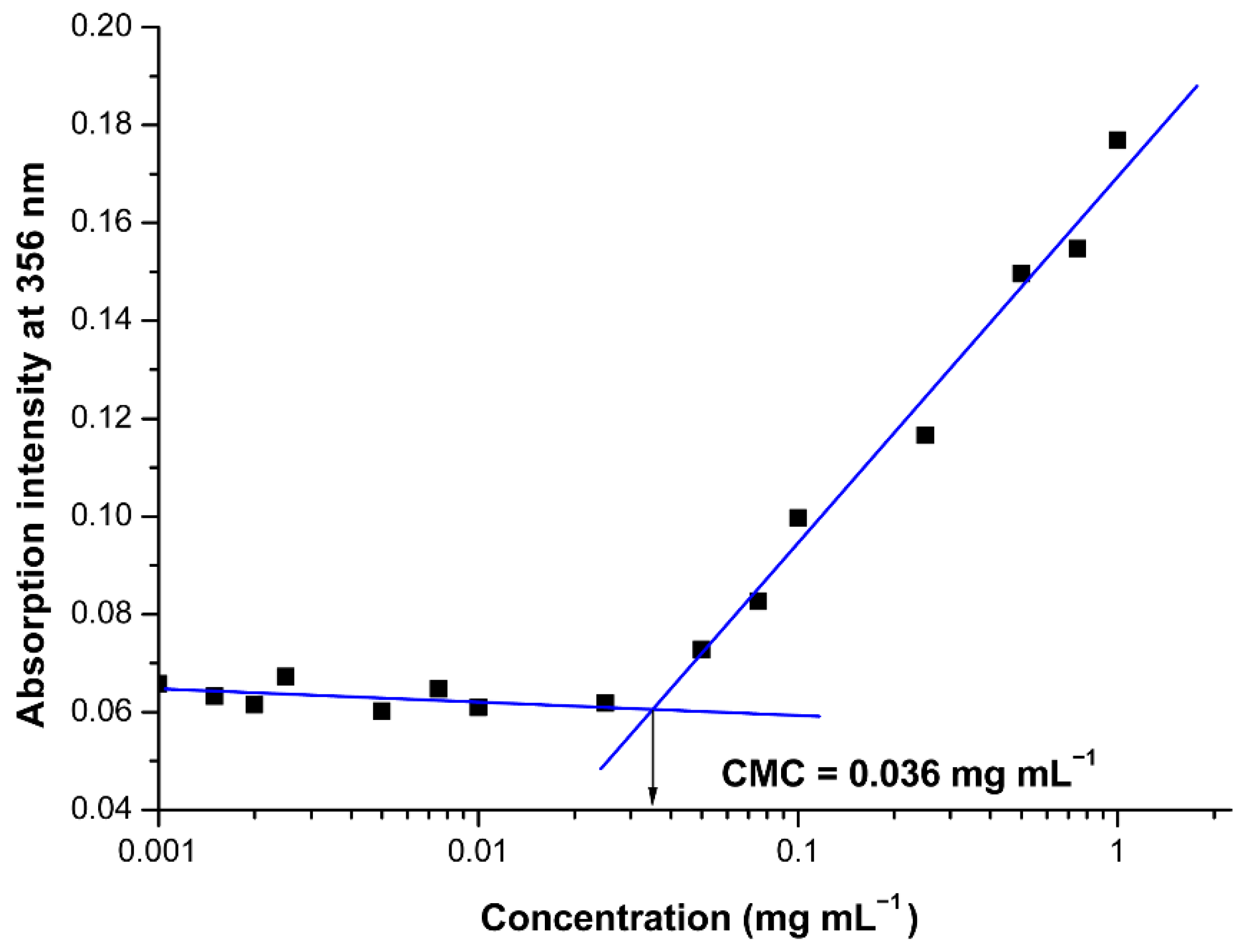
2.6. Critical Micelle Concentration (CMC) Estimation
2.7. Drug Loading and In Vitro Drug Release
2.8. Antioxidant Activity Estimation via DPPH• Radical Scavenging Assay
3. Results and Discussion
3.1. Synthesis and Characterization of Functional Oppositely Charged Hybrid Block Copolymers
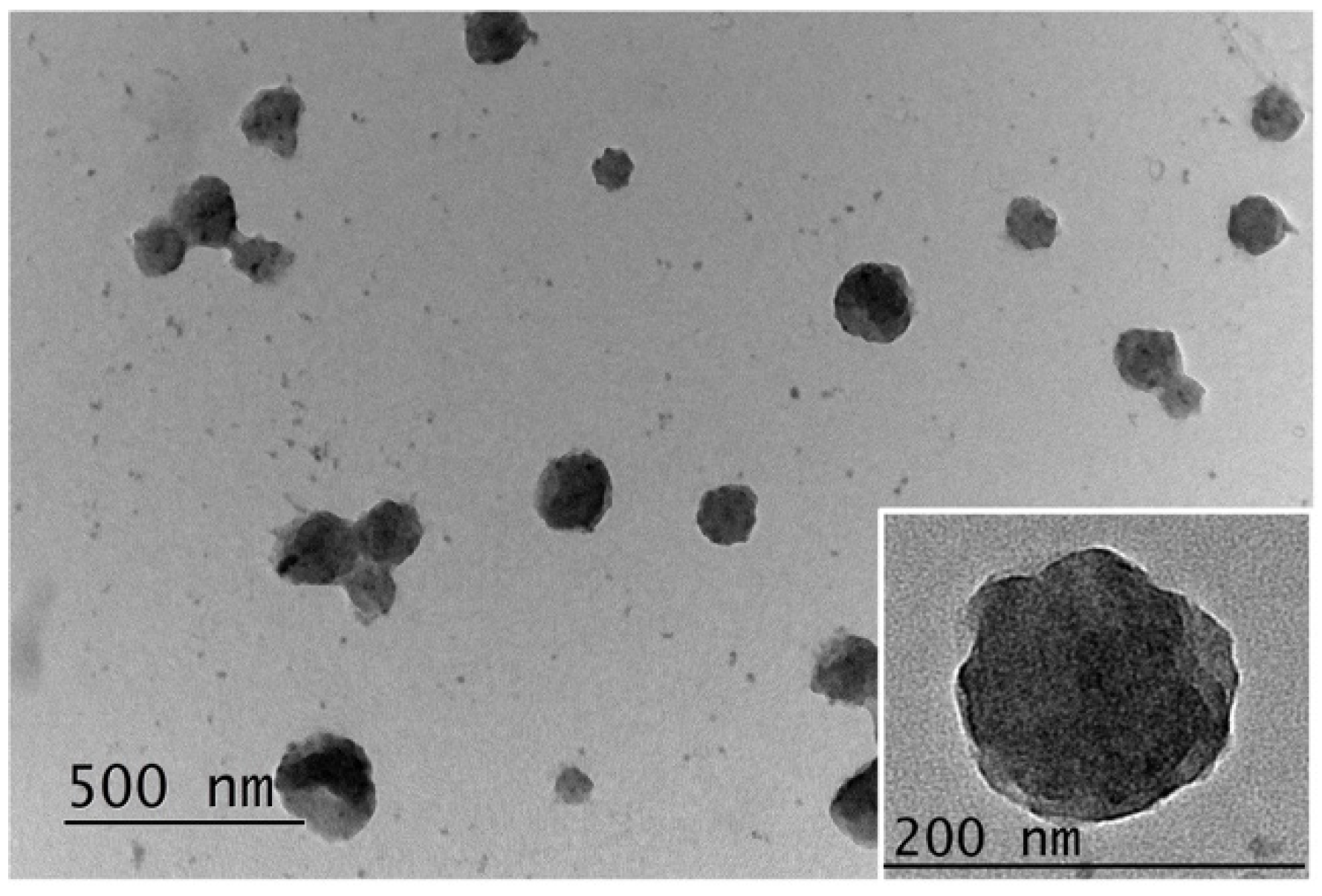
3.2. Formation of Polyion Complex Micelles from the Synthesized Oppositely Charged Hybrid Block Copolymers
3.3. Hydrophobic Drug Loading into the Polyion Complex Micelles
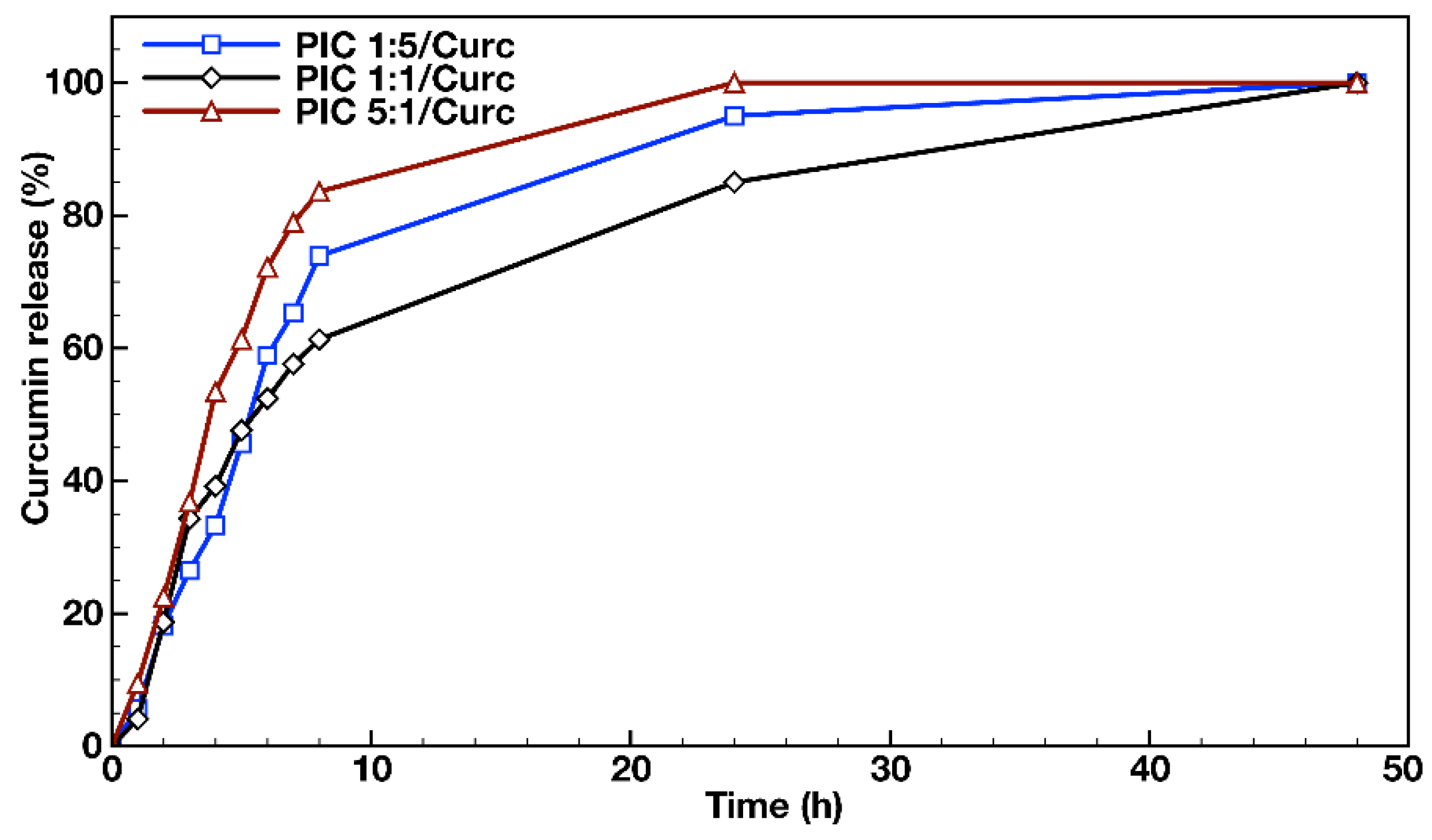
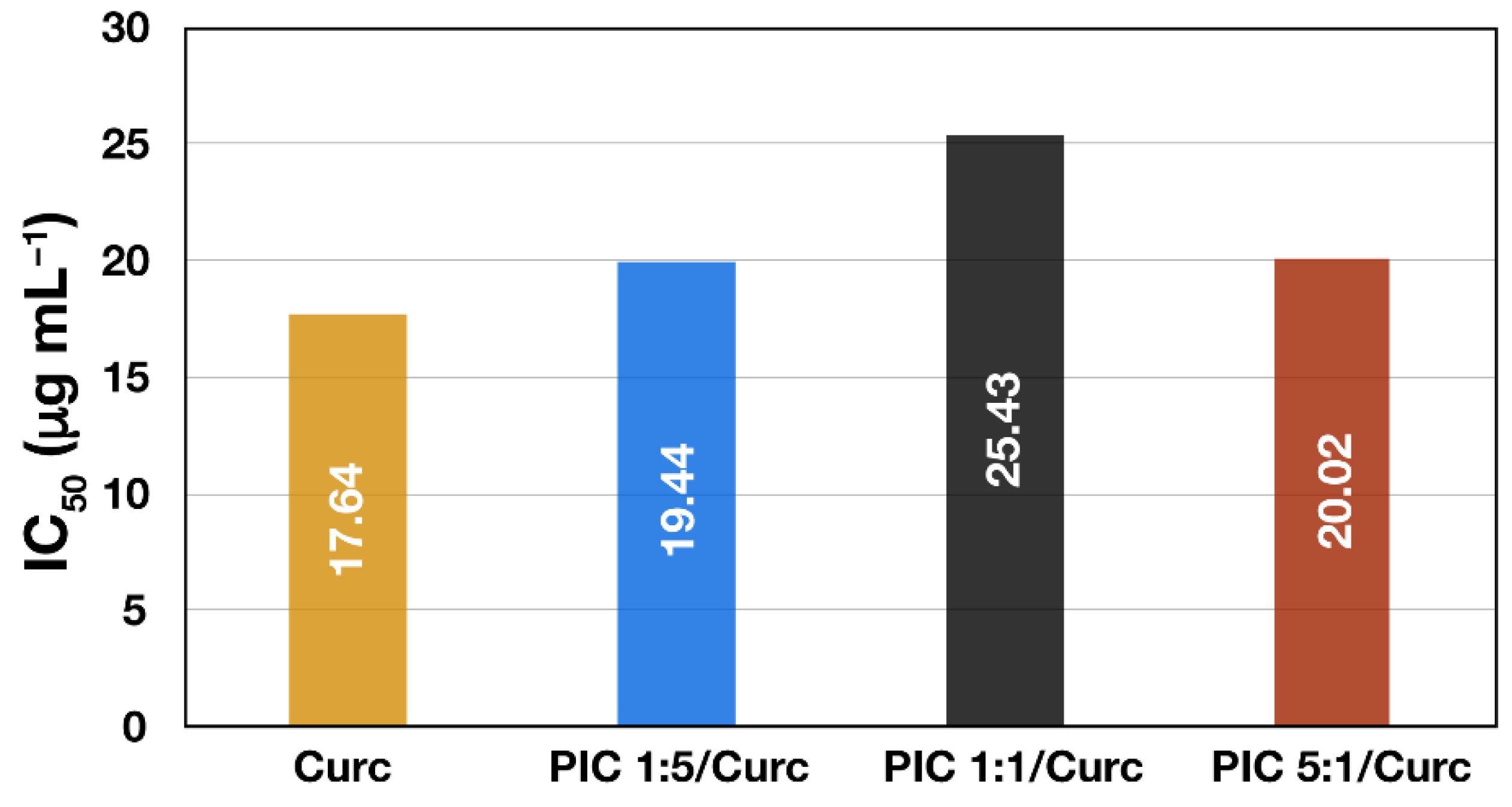
3.4. In Vitro Drug Release and Antioxidant Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schacher, F.; Rupar, P.; Manners, J. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 2012, 51, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Orilall, M.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Astruc, D. Supramolecular nanoreactors for catalysis. Coord. Chem. Rev. 2016, 324, 106–122. [Google Scholar] [CrossRef]
- Mi, P.; Miyata, K.; Kataoka, K.; Cabral, H. Clinical translation of self-assembled cancer nanomedicines. Adv. Therap. 2021, 4, 2000159. [Google Scholar] [CrossRef]
- Torchilin, V. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-B.; Binkhathlan, Z.; Molavi, O.; Lavasanifar, A. Amphiphilic block co-polymers: Preparation and application in nanodrug and gene delivery. Acta Biomater. 2012, 8, 2017–2033. [Google Scholar] [CrossRef] [PubMed]
- Toscanini, M.; Limeres, M.; Garrido, A.; Cagel, M.; Bernabeu, E.; Moretton, M.; Chiappetta, D.; Cuestas, M. Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Mitchell, M.; Billingsley, M.; Haley, R.; Wechsler, M.; Peppas, N.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Khan, M.; Singh, D.; Ahmad, A.; Siddique, H. Revisiting inorganic nanoparticles as promising therapeutic agents: A paradigm shift in oncological theranostics. Eur. J. Pharm. Sci. 2021, 164, 105892. [Google Scholar] [CrossRef]
- Deng, C.; Liu, Y.; Zhou, F.; Wu, M.; Zhang, Q.; Yi, D.; Yuan, W.; Wang, Y. Engineering of dendritic mesoporous silica nanoparticles for efficient delivery of water-insoluble paclitaxel in cancer therapy. J. Colloid Interface Sci. 2021, 593, 424–433. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.; Perez, L.; Battaglia, G. Block copolymer nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.; Kabanov, A. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Ali, A.; Fatima, M.; Apurva, N.; Kumar, A.; Sumi, M.; Sattar, R.; Mahajan, B.; Saluja, S. Ligand decorated biodegradable nanomedicine in the treatment of cancer. Pharmacol. Res. 2021, 167, 105544. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Kabanov, A.; Bronich, T.; Kabanov, V.; Yu, K.; Eisenberg, A. Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules 1996, 29, 6797–6802. [Google Scholar] [CrossRef] [Green Version]
- Cohen Stuart, M.; Besseling, N.; Fokkink, R. Formation of micelles with complex coacervate cores. Langmuir 1998, 14, 6946–6949. [Google Scholar] [CrossRef]
- Pergushov, D.; Remizova, E.; Gradzielski, M.; Lindner, P.; Feldthusen, J.; Zezin, A.; Müller, A.; Kabanov, V. Micelles of polyisobutylene-block-poly(methacrylic acid) diblock copolymers and their water-soluble interpolyelectrolyte complexes formed with quaternized poly(4-vinylpyridine). Polymer 2004, 45, 367–378. [Google Scholar] [CrossRef]
- Gohy, J.-F.; Creutz, S.; Garcia, M.; Mahltig, B.; Stamm, M.; Jérôme, R. Aggregates formed by amphoteric diblock copolymers in water. Macromolecules 2000, 33, 6378–6387. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Magana, J.; Sproncken, C.; Voets, I. On complex coacervate core micelles: Structure-function perspectives. Polymers 2020, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Kalinova, R.; Dimitrov, I.; Pispas, S.; Demetzos, C. Insulin/poly(ethylene glycol)-block-poly(L-Lysine) complexes: Physicochemical properties and protein encapsulation. J. Phys. Chem. B. 2015, 119, 6813–6819. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, C.; Hexum, J.; Tan, Z.; Dalal, R.; Reineke, T. Nonviral gene delivery with cationic glycopolymers. Acc. Chem. Res. 2019, 52, 1347–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-H.; Lin, M.-W.; Chien, M.-C.; Ke, G.-M.; Wu, I.-E.; Lin, R.-L.; Lin, C.-Y.; Hu, Y.-C. Polyplex nanomicelle delivery of self-amplifying RNA vaccine. J. Control. Release 2021, 338, 694–704. [Google Scholar] [CrossRef]
- Dimitrov, I.; Petrova, E.; Kozarova, R.; Apostolova, M.; Tsvetanov, C. A mild and versatile approach for DNA encapsulation. Soft Matter 2011, 7, 8002–8004. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011, 63, 152–160. [Google Scholar] [CrossRef]
- Bronich, T.; Keifer, P.; Shlyakhtenko, L.; Kabanov, A. Polymer micelle with cross-linked ionic core. J. Am. Chem. Soc. 2005, 127, 8236–8237. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, A.; Yuan, J.; Gao, Q. Preparation, characterization and drug release behavior of polyion complex micelles. Int. J. Pharm. 2009, 374, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-L.; Yuan, J.-F.; Shi, J.-H.; Gao, Q.-Y. Synthesis and characterization of polyion complex micelles and their controlled release of folic acid. J. Colloid Interface Sci. 2010, 350, 140–147. [Google Scholar] [CrossRef]
- Abolmaali, S.; Tamaddon, A.; Salmanpour, M.; Mohammadi, S.; Dinarvan, R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur. J. Pharm. Sci. 2017, 104, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Yu, J.; Zeng, J.; Chang, P.; Xu, H.; Huang, J. Fabrication and reduction-sensitive behavior of polyion complex nano-micelles based on PEG-conjugated polymer containing disulfide bonds as a potential carrier of anti-tumor paclitaxel. Colloids Surf. B 2013, 110, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Shen, Y.; Tang, L.; Sun, R.; Shi, D.; Webster, T.; Tu, J.; Sun, C. Electrostatic interactions between polyglutamic acid and polylysine yields stable polyion complex micelles for deoxypodophyllotoxin delivery. Int. J. Nanomed. 2017, 12, 7963–7977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Jiao, Y.; Ge, Y.; Liu, G.; Xu, W.; Li, L. The study of tacrolimus-loaded polyion complex micelles for oral delivery. J. Biomed. Nanotechnol. 2017, 13, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Wang, W.-T.; Hsiue, G.-H. Development of polyion complex micelles for encapsulating and delivering amphotericin B. Biomaterials 2009, 30, 3352–3358. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, S.; Harrison, W.; Harder, J.; Wen, X.; Gelovani, J.; Qiao, A.; Li, C. Long-circulating near-infrared fluorescence core-cross-linked polymeric micelles: Synthesis, characterization, and dual nuclear/optical imaging. Biomacromolecules 2007, 8, 3422–3428. [Google Scholar] [CrossRef] [Green Version]
- Poché, D.; Moore, M.; Bowles, J. An unconventional method for purifying the N-carboxyanhydride derivatives of γ-alkyl-L-glutamates. Synth. Commun. 2007, 29, 843–854. [Google Scholar] [CrossRef]
- Moore, J.; Stupp, S. Room temperature polyesterification. Macromolecules 1990, 23, 65–70. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.; Hatton, T. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Babikova, D.; Kalinova, R.; Zhelezova, I.; Momekova, D.; Konstantinov, S.; Momekov, G.; Dimitrov, I. Functional block copolymer nanocarriers for anticancer drug delivery. RSC Adv. 2016, 6, 84634–84644. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B 2014, 114, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Hathout, R.; Geneidi, A.; Mansour, S. Bisdemethoxycurcumin loaded polymeric mixed micelles as potential anti-cancer remedy: Preparation, optimization and cytotoxic evaluation in a HepG-2 cell model. J. Mol. Liq. 2016, 214, 162–170. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]

| Macroinitiators | Hybrid Diblock Copolymers | |||||||
|---|---|---|---|---|---|---|---|---|
| Code | DPna | Mna (g mol−1) | ÐMb | Code | t-DPn c | DPna | Mna (g mol−1) | ÐMb |
| PHEMA-NH2 | 26 | 3400 | 1.23 | PHEMA-b-PZLLys | 35 | 37 | 13,100 | 1.38 |
| HO-PEG-NH2 | 68 | 3000 | 1.12 | HO-PEG-b-PLBzAsp | 35 | 30 | 9150 | 1.30 |
| Empty PIC Micelles | Drug Loaded PIC Micelles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | d a (nm) | PdI a | ζ a (mV) | d a (nm) | PdI a | ζ a (mV) | DLE b (%) | DLC b (%) | IC50 c (μg mL−1) |
| PIC 1:5 | 95.85 ± 1.21 | 0.295 | −21.64 ± 2.10 | 109.99 ± 0.92 | 0.077 | −19.24 ± 2.00 | 71 | 6.4 | 19.94 |
| PIC 1:1 | 90.02 ± 3.45 | 0.318 | −4.41 ± 1.58 | 101.81 ± 1.83 | 0.077 | −2.06 ± 2.35 | 66 | 6.5 | 25.43 |
| PIC 5:1 | 79.14 ± 1.68 | 0.109 | 10.93 ± 1.31 | 89.35 ± 0.18 | 0.079 | 9.79 ± 1.73 | 60 | 5.5 | 20.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinova, R.; Dimitrov, I. Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery. Molecules 2022, 27, 2178. https://doi.org/10.3390/molecules27072178
Kalinova R, Dimitrov I. Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery. Molecules. 2022; 27(7):2178. https://doi.org/10.3390/molecules27072178
Chicago/Turabian StyleKalinova, Radostina, and Ivaylo Dimitrov. 2022. "Functional Polyion Complex Micelles for Potential Targeted Hydrophobic Drug Delivery" Molecules 27, no. 7: 2178. https://doi.org/10.3390/molecules27072178






