4. Experimental
NMR spectra were recorded using the Varian spectrometers (Darmstadt, Germany) DD2 and VNMRS (400 and 500 MHz, respectively). MS spectra were taken on a Advion expressionL CMS mass spectrometer (Ithaca, NY, USA); positive ion polarity mode, solvent: methanol, solvent flow: 0.2 mL/min, spray voltage: 5.17 kV, source voltage: 77 V, APCI corona discharge: 4.2 μA, capillary temperature: 250 °C, capillary voltage: 180 V, sheath gas: N2). Thin-layer chromatography was performed on pre-coated silica gel plates supplied by Macherey-Nagel (Düren, Germany). IR spectra were recorded on a Spectrum 1000 FT-IR-spectrometer from Perkin Elmer (Rodgau, Germany). The UV/Vis-spectra were recorded on a Lambda 14 spectrometer from Perkin Elmer (Rodgau, Germany); optical rotations were measured at 20 °C using a JASCO-P2000 instrument (JASCO Germany GmbH, Pfungstadt, Germany) The melting points were determined using the Leica hot stage microscope Galen III (Leica Biosystems, Nussloch, Germany) and are uncorrected. The solvents were dried according to usual procedures. Microanalyses were performed with an Elementar Vario EL (CHNS) instrument (Elementar Analysensysteme GmbH, Elementar-Straße 1, D-63505, Langenselbold, Germany).
4.1. General Procedure for the Synthesis of Acetates 1–4 (GPA)
To a solution of the triterpenoic acid (OA, UA, BA, PA, 1 equiv.) in dry DCM, acetic anhydride (3 equiv.), triethylamine (3 equiv.), and DMAP (cat.) were added, and the mixture was stirred at 20 °C for 1 day. Usual aqueous work-up followed by re-crystallization from ethanol furnished products 1–4.
4.2. 3β-Acetyloxy-lup-20(29)-en-28-oic Acid (1)
Following GPA, compound
1 (4.90 g, 90%) was obtained from
BA as a colorless solid; R
f = 0.59 (n-hexane/ethyl acetate, 4:1); m.p. 277–279 °C (lit.: [
28] 277–278 °C); [α]
D = +20.7° (
c 0.42, CHCl
3) [(lit.: [
29] [α]
D = +20.7° (
c 0.42, CHCl
3)]; MS (ESI, MeOH):
m/
z 487.1 (32%, [M − H]
−, 995.0 (100%, [2M − H]
−, 1018.6 (29% [2M − 2H + Na]
−).
4.3. 3β-Acetyloxy-olean-12-en-oic Acid (2)
Following GPA, compound
2 (2.45 g, 89%) was obtained from
OA as a colorless solid; R
f = 0.72 (toluene/ethyl acetate/heptane/formic acid, 80:26:10:5); m.p. 286–289 °C (lit.: [
30] 264–265 °C); [α]
D = +69.4° (
c 0.30, CHCl
3) [(lit.: [
31] [α]
D = +74.0° (
c 1.0, CHCl
3)]; MS (ESI, MeOH):
m/
z 499.4 ([100%, M + H]
+), 516.3 (36%, [M + NH
4]
+, 521.5 [35%, [M + Na]
+.
4.4. 3β-Acetyloxy-urs-12-en-oic Acid (3)
Following GPA, compound
3 (2.41 g, 89%) was obtained from
UA as a colorless solid; R
f = 0.57 (n-hexane/ethyl acetate, 3:1); m. p. 281–283 °C (lit.: [
32] 280 °C); [α]
D = +66.5° (
c 0.42, CHCl
3) [lit.: [
33] [α]
D = +63.5° (
c 0.5, CHCl
3)]; MS (ESI, MeOH):
m/
z 499.3 (17%, [M + H]
+), 521.2 (31%, [M + H]
+), 1019.4 (100%, [2M + Na]
+).
4.5. 3β-Acetyloxy-20-oxo-30-norlupan-28-oic Acid (4)
Following GPA, compound
4 (6.9 g, 84%) was obtained from
PA as a colorless solid; R
f = 0.50 (toluene/ethyl acetate/heptane/formic acid, 80:26:10.5); m.p. 265–268 °C (lit.: [
34] 252–255 °C); [α]
D = −9.4° (
c 0.32, CHCl
3) [(lit.: [
34] [α]
D = −9.5° (
c 0.5, CHCl
3)]; MS (ESI, MeOH):
m/
z 999.5 (100%, [2M − H]
−).
4.6. General Procedure for the Synthesis of Acetylated (homo)piperazinyl Amides 5–12 (GPB)
To a solution of the acetylated triterpenoic acid 1–4 (1 equiv.) in dry DCM, DMF (cat.) and oxalyl chloride (4 equiv.) were added followed by the addition of (homo)piperazine (4 equiv.). After stirring for 1h at 20 °C followed by usual aqueous work-up and column chromatography, products 5–12 were obtained.
4.7. 3β-Acetyloxy-28-(1-piperazinyl)-lup-20(29)en-28-one (5)
Following GPB from
1 (2.5 g, 5 mmol) and piperazine (1.6 g, 20.0 mmol), compound
5 (2.07 g, 73%) was obtained as a colorless solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. = 166–173 °C (lit.: [
35] 162–167 °C); [α]
D = −1.4° (
c 0.21, MeOH), [lit.: [
35] [α]
D = −1.8° (
c 0.32, MeOH); MS (ESI, MeOH):
m/
z (%) = 567.3 ([100%, M + H]
+).
4.8. 3β-Acetyloxy-28-(1-piperazinyl)-olean-12-en-28-one (6)
Following GPB from
2 (2.5 g, 5.0 mmol) and piperazine (1.6 g, 29 mmol),
6 (2.16 g, 76%) was obtained as a colorless solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. = 172–175 °C (lit.: [
35] 173–175 °C); [α]
D = +23.4° (
c 0.18, CHCl
3), [lit.: [
35] [α]
D = +26.6° (
c 0.35, MeOH); MS (ESI, MeOH):
m/
z (%) = 567.4 (100%, [M + H]
+).
4.9. 3β-Acetyloxy-28-(1-piperazinyl)-urs-12-en-28-one (7)
Following GPB from
3 (2.5 g, 5.0 mmol) and piperazine (1.6 g, 20.0 mmol),
7 (1.84 g, 65%) was obtained as a colorless solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. = 187–190 °C (lit.: [
35] 187–188 °C); [α]
D = +25.1° (
c 0.24, MeOH), [lit.: [
35] [α]
D = +24.5° (
c 0.29, MeOH); MS (ESI, MeOH):
m/
z (%) = 567.4 (100%, [M + H]
+).
4.10. 3β-Acetyloxy-28-(1-piperazinyl)-30-norlupane-20,28-dione (8)
Following GPB from
4 (2.5 g, 5.0 mmol) and piperazine (1.6 g, 20.0 mmol),
17 (1.93 g, 68%) was obtained as a colorless solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. = 127–130 °C (lit.: [
20] 115–125 °C); [α]
D = −20.3° (
c 0.13, CHCl
3); MS (ESI, MeOH):
m/
z (%) = 569.3 (100%, [M + H]
+).
4.11. 3β-Acetyloxy-28-(1-homopiperazinyl)-lup-20(29)en-28-one (9)
Following GPB from
1 (1.0 g, 2.02 mmol) and homopiperazine (9.8 g, 8.0 mmol),
9 (1.02 g, 88%) was obtained as a colorless solid; R
f = 0.4 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 190–193°C (lit.: [
21] 196–199 °C); [α]
D = +107.6° (
c 0.20, CHCl
3), [lit.: [
21] [α]
D = +109.8° (
c 0.38, CHCl
3); MS (ESI, MeOH):
m/
z = 581.4 (100%, [M + H]
+).
4.12. 3β-Acetyloxy-28-(1-homopiperazinyl)-olean-12-en-28-one (10)
Following GPB from
2 (1.0 g, 2.02 mmol) and homopiperazine (0.8 g, 8.0 mmol),
10 (1.4 g, 90%) was obtained as a colorless solid; R
f = 0.4 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 182–185 °C (lit.: [
21] 187–190 °C); [α]
D = 12.4° (
c 0.14, CHCl
3), [lit.: [
21] [α]
D = +9.9° (
c 0.35, CHCl
3); MS (ESI, MeOH):
m/
z = 581.4 (100%, [M + H]
+).
4.13. 3β-Acetyloxy28-(1-homopierazinyl)-urs-12-en-28-one (11)
Following GPB from
3 (1.0 g, 2.02 mmol) and homopiperazine (0.8 g, 8.0 mmol),
11 (1.1 g, 86%) was obtained as a colorless solid; R
f = 0.4 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 171–175 °C (lit.: [
21] 178–180 °C); [α]
D = +27.0° (
c 0.21, CHCl
3), [lit.: [
21] [α]
D = +29.7° (
c 0.34, CHCl
3); MS (ESI, MeOH):
m/
z = 581.4 (100%, [M + H]
+, 100%).
4.14. 3β-Acetyloxy-28-(1-homopiperazinyl)-30-norlupane-20,28-dione (12)
Following GPB from 4 (1.0 g, 2.02 mmol) and homopiperazine (0.8 g, 8.0 mmol), 12 (1.1 g, 94%) was obtained as a colorless solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. 162–165 °C; [α]D = −29.2° (c 0.16, CHCl3); IR (ATR): ν = 2941m, 2866w, 1732m, 1708m, 1622m, 1464m, 1453m, 1408m, 1367m, 1244vs, 1191m, 1149m, 1133m, 1109w, 1026m, 979m, 947w, 934w, 900w, 750s, 665m, 610w, 557m, 506m, 455w, 410w cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.44 (dd, J = 10.5, 5.5 Hz, 1H, 3-H), 3.89–3.60 (m, 4H, 33-H), 3.40–3.09 (m, 5H, 19-H, 33-H), 2.62 (td, J = 12.1, 4.1 Hz, 1H, 13-H), 2.25 (s, 2H, 35-H), 2.17 (s, 3H, 29-H), 2.14–2.06 (m, 2H, 16-Ha + 18-H), 2.02 (s, 3H, 32-H), 1.97–1.84 (m, 2H, 21-Ha + 22-Ha), 1.68–1.14 (m, 15H + 1-Ha + 2-H + 6-Ha + 6-Hb + 7-H + 9-H + 11-Ha + 11-Hb + 15-H + 16-Hb + 21-Hb + 22-Hb), 1.06–0.98 (m, 2H, 12-H), 0.96 (s, 4H, 1-Hb + 27-H), 0.89 (s, 3H, 26-H), 0.82 (s, 3H, 24-H), 0.82 (s, 3H, 25-H), 0.81 (s, 3H, 23-H), 0.79–0.74 (m, 1H, 5-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 213.0 (C-20), 175.0 (C-28), 171.2 (C-31), 81.0 (C-3), 55.4 (C-5), 54.9 (C-17), 52.6 (C-18), 50.6 (C-9), 49.9 (C-19), 46.6 (C-33 + C-34), 41.8 (C-14), 40.6 (C-8), 38.3 (C-1), 37.8 (C-4), 37.1 (C-10), 36.0 (C-13), 35.7 (C-22), 34.1 (C-7), 31.7 (C-16), 30.3 (C-29), 29.9 (C-15), 28.6 (C-21), 27.9 (C-24), 27.3 (C-12), 25.7 (C-35), 23.6 (C-2), 21.3 (C-32), 21.1 (C-11), 18.1 (C-6), 16.4 (C-23), 16.2 (C-25), 15.9 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH): m/z = 583.3 (100%, [M + H]+; analysis calcd for C36H58N2O4 (582.87): C 74.18, H 10.03, N 4.81; found: C 73.95, H 10.34, N 4.63.
4.15. General Procedure for the Synthesis of the Rhodamine Conjugates 13–28 (GPC)
To a solution of the respective rhodamine (1.15 eq.) in dry DCM at 0 °C, DMF (cat.) and oxalyl chloride (4 eq.) were added (vide supra). This acid chloride was slowly added to the solution of the respective triterpene (1 eq. in DCM) in the presence of triethylamine (1 eq.). After stirring for 1 day at 20 °C, aqueous work-up was carried out as usual and the residue was purified by column chromatography.
4.16. 9-[2-[[4-(3β-Acetyloxy-28-oxo-lup-20(29)en-28-yl)-1-piperazinyl]carbonyl]phenyl]-3,6-bis(diethylamino)-xanthylium chloride (13)
Following GPC from
5 (180 mg, 0.32 mmol) and rhodamine B,
13 (220 mg, 67%) was obtained as a pink solid; R
f = 0.39 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 247–252 °C (lit.: [
20] m.p. 246–250 °C); MS (ESI, MeOH):
m/
z = 991.6 (100%, [M − Cl]
+).
4.17. 9-[2-[[4-(3β-Acetyloxy-28-oxo-olean-12-en-28-yl)-1-piperazinyl]carbonyl]phenyl]-3,6-bis(diethylamino)-xanthylium chloride (14)
Following GPC from
6 (180 mg, 0.32 mmol) and rhodamine B,
14 (250 mg, 76%) was obtained as a pink solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 246–252 °C (lit.: [
20] 245–248 °C); MS (ESI, MeOH):
m/
z = 991.9 (100%, [M − Cl]
+).
4.18. 9-[2-[[4-(3β-Acetyloxy-28-oxo-ursan-12-en-28-yl)-1-piperazinyl]carbonyl]phenyl]-3,6-bis(diethylamino)-xanthylium chloride (15)
Following GPC from
7 (180 mg, 0.32 mmol) and rhodamine B,
15 (190 mg, 58%) was obtained as a pink solid; R
f = 0.40 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 245–251 °C (lit.: [
20] 243–245 °C); MS (ESI, MeOH):
m/
z = 991.7 (100%, [M − Cl]
+).
4.19. 9-[2-[[4-(3β-Acetyloxy-20,28-dioxo-30-norlupan-28-yl)-1-piperazinyl]carbonyl]phenyl]-3,6-bis(diethylamino)-xanthylium chloride (16)
Following GPC from
8 (180 mg, 0.32 mmol) and rhodamine B,
16 (230 mg, 70%) was obtained as a pink solid; R
f = 0.37 (SiO
2, CHCl
3/MeOH, 9:1); m.p. 247–254 °C (lit.: [
20] 235–243 °C); MS (ESI, MeOH):
m/
z = 993.7 (100%, [M − Cl]
+).
4.20. 9-[2-[[4-(3β-Acetyloxy-28-oxo-30-norlupan-28-yl)-1-homopiperazinyl]carbonyl]phenyl]- 3,6-bis(diethylamino)-xanthylium chloride (17)
Following GPC from
9 (260 mg, 0.32 mmol) and rhodamine B,
17 (229 mg, 69%) was obtained as a pink solid; R
f = 0.50 (SiO
2, MeCN/CH
2Cl
2/H
2O, 10:1:1); m.p. 261–266 °C (lit.: [
21] 256–260 °C); MS (ESI, MeOH):
m/
z = 1005.7 (100%, [M − Cl]
+).
4.21. 9-[2-[[4-(3β-Acetyloxy-28-oxo-olean-12-en-28-yl]-1-homopiperazinyl]carbonyl]phenyl]- 3,6-bis(diethylamino)-xanthylium chloride (18)
Following GPC from
10 (270 mg, 0.32 mmol) and rhodamine B,
18 (267 mg, 80%) was obtained as a pink solid; R
f = 0.52 (SiO
2, MeCN/CH
2Cl
2/H
2O, 10:1:1); m.p. 235–241 °C (lit.: [
21] 238–245 °C); MS (ESI, MeOH):
m/
z = 1005.8 (100%, [M − Cl]
+).
4.22. 9-[2-[[4-(3β-Acetyloxy-28-oxo-urs-12-en-28-yl]-1-homopiperazinyl]carbonyl]phenyl]- 3,6-bis(diethylamino)-xanthylium chloride (19)
Following GPC from
11 (270 mg, 0.32 mmol) and rhodamine B,
19 (253 mg, 76%) was obtained as a pink solid; R
f = 0.51 (SiO
2, MeCN/CH
2Cl
2/H
2O, 10:1:1); m.p. 238–246 °C (lit.: [
21] 238–245 °C); MS (ESI, MeOH):
m/
z = 1005.7 (100%, [M − Cl]
+).
4.23. 9-[2-[[4-(3β-Acetyloxy-20,28-dioxo-30-norlupan-28-yl)-1-homopiperazinyl]carbonyl]phenyl]-3,6-bis(diethylamino)-xanthylium chloride (20)
Following GPC from 10 (125 mg, 0.21 mmol) and rhodamine B, 18 (190 mg, 87%); was obtained as a pink solid; m.p. 248–250 °C (decomp.); Rf = 0.30 (SiO2, CHCl3/MeOH, 9:1); UV-Vis (CHCl3): λmax (log ε) = 562 nm (5.05); IR (ATR): ν = 2937w, 1730w, 1585s, 1466m, 1411m, 1334s, 1273m, 1244m, 1178s, 1131m, 1072m, 977w, 920w, 822w, 746m, 683m cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.66–7.57 (m, 2H, 42-H, 43-H), 7.42–7.36 (m, 1H, 40-H), 7.31–7.27 (m, 1H, 41-H), 7.25–7.15 (m, 2H, 50-H), 6.99–6.83 (m, 2H, 49-H), 6.79–6.68 (m, 2H, 47-H), 4.40 (dd, J = 10.4, 5.6 Hz, 1H, 3-H), 3.85–3.31 (m, 16H, 32-H, 33-H, 34-H, 36-H, 51-H), 3.27–3.12 (m, 1H, 13-H), 2.72–2.57 (m, 1H, 19-H), 2.11 (s, 3H, 29-H), 2.18–2.06 (m, 1H, 16-Ha), 1.98 (s, 3H, 31-H), 1.96 (s, 1H, 18-H), 1.95–0.97 (m, 21H, 22-Ha, 21-Ha, 35-H, 1-Ha, 2-H, 16-Hb, 21-Hb, 6-H, 22-Hb, 11-Ha, 7-H, 11-Hb, 9-H, 15-H, 12-H), 1.32–1.26 (m, 12H, 52-H), 0.92 (s, 3H, 27-H), 0.89 (s, 3H, 24-H), 0.94–0.86 (m, 1H, 1-Hb), 0.79 (s, 9H, 23-H, 25-H, 26-H), 0.73 (s, 1H, 5-Hb) ppm; 13C NMR (126 MHz, CDCl3): δ = 212.8 (C-20), 174.7 (C-28), 170.7 (30 C-), 168.3 (C-37), 157.5 (C-48), 157.5 (C-44), 155.4 C- (46), 136.5 (C-38) 135.9 (C-39), 132.1 (C-50), 130.1 (C-41), 129.6 (C-42), 129.4 (C-43), 126.3 (C-40), 113.9 (C-49), 113.4 (C-45), 96.2 (C-47), 80.7 (C-3), 55.3 (C-5), 54.7 (C-17), 52.7 (C-18), 50.4 (C-9), 50.0 (C-19), 46.0 (C-51), 41.6 (C-14), 40.4 (C-8), 38.2 (C-1), 37.6 (C-4), 36.9 (C-10), 35.8 (C-13), 35.3 (C-22), 34.1 (C-7), 31.7 (C-35), 31.2 (C-16), 30.0 (C-29), 29.7 (C-15), 28.7 (C-21), 27.7 (C-23), 27.2 (C-12), 23.5 (C-2), 21.1 (C-31), 21.0 (C-11), 18.0 (C-6), 16.3 (C-25), 16.1 (C-26), 16.0 (C-24), 14.4 (C-27), 12.5 (C-52). ppm; MS (ESI, MeOH): m/z 1007 (100%, [M]+); analysis calculated for C64H85ClN4O6 (1041.84): C 73.78, H 8.22, N 5.38; found: C 73.55, H 8.41, N 5.19.
4.24. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij] pyrido[1”,2”,3”:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]piperazine-1-yl]-28-oxo-lup-20(29)-en chloride (21)
Following GPC from 5 (0.25 g, 0.44 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 21 (0.24 g, 52%) was obtained as a pink colored solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 3400w, 2939w, 2862w, 1728w, 1630m, 1595m, 1542w, 1493m, 1434m, 1360m, 1294vs, 1267s, 1248s, 1195s, 1182s, 1099s, 1035m, 1003m, 978m, 896w, 827w, 772m, 747m, 637m, 560m, 506m, 421s cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.67 (dd, J = 5.7, 3.3 Hz, 2H, 37-H, 39-H), 7.53–7.48 (m, 1H, 38-H), 7.31 (dd, J = 5.7, 3.2 Hz, 1H, 40-H), 6.68 (d, J = 6.0 Hz, 2H, 48-H), 4.68 (s, 1H, 29-Ha), 4.56 (s, 1H, 29-Hb), 4.44 (dd, J = 10.2, 5.9 Hz, 1H, 3-H), 3.60–3.48 (m, 4H, 49-H), 3.48–3.40 (m, 4H, 52-H), 3.40–3.31 (m, 8H, 33-H + 34-H), 3.04–2.97 (m, 4H, 54-H), 2.91 (dt, J = 11.9, 6.1 Hz, 1H, 19-H), 2.81–2.74 (m, 1H, 13-H), 2.74–2.60 (m, 4H, 51-H), 2.14–2.04 (m, 4H, 53-H), 2.02 (s, 3H, 32-H), 1.98–1.91 (m, 4H, 50-H), 1.77 (q, J = 33.5, 10.4 Hz, 4H, 12-H + 16-Ha + 22-Ha), 1.65 (s, 3H, 30-H), 1.63–1.44 (m, 8H, 1-H + 2-H + 6-Ha + 11-H + 16-Hb + 18-H), 1.44–1.08 (m, 10H, 6-Hb + 7-H + 9-H + 15-H + 21-H + 22-Hb), 0.92 (s, 3H, 27-H), 0.88 (s, 3H, 26-H), 0.82 (s, 6H, 23-H + 24-H), 0.81 (s, 3H, 25-H), 0.77 (s, 1H, 5-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 174.1 (C-28), 171.0 (C-31), 167.9 (C-35), 162.7 (C-20), 152.0 (C-43), 151.2 (C-44), 150.9 (C-46), 134.8 (C-41), 131.8 (C-36), 130.7 (C-40), 130.2 (C-39), 129.7 (C-37), 127.5 (C-38), 126.5 (C-48), 123.6 (C-45), 123.6 (C-45), 113.2 (C-42), 109.4 (C-29), 105.4 (C-47), 80.9 (C-3), 55.5 (C-5), 54.6 (C-17), 52.5 (C-18), 51.0 (C-49), 50.7 (C-9), 50.5 (C-52,), 45.7 (C-19), 41.9 (C-14), 40.6 (C-8), 38.4 (C-1), 37.8 (C-4), 37.1 (C-10), 36.9 (C-13), 35.8 (C-22), 34.3 (C-7), 32.5 (C-16), 31.2 (C-21), 29.8 (C-15), 27.9 (C-24), 27.7 (C-51), 25.5 (C-12), 23.7 (C-2), 21.3 (C-32), 20.6 (C-11, C-50), 19.9 (C-54), 19.6 (C-53), 19.5 (C-30), 18.1 (C-6), 16.5 (C-23), 16.2 (C-25), 16.1 (C-26), 14.6 (C-27) ppm; MS (ESI, MeOH): m/z = 1039.3 (100%, [M − Cl]+); analysis calculated for C68H87N4O5Cl (1075.92): C 75.91, H 8.15, N 5.21; found: C 75.77, H 8.36, N 5.05.
4.25. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij]pyrido[1”,2”,3”:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]piperazine-1-yl]-28-oxoolean-12-en chloride (22)
Following GPC from 6 (0.25 g, 0.44 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 22 (0.33 g, 71%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 3398w, 2941w, 2859w, 1728w, 1631m, 1594s, 1543w, 1494s, 1458m, 1435m, 1361m, 1295Vs, 1267s, 1248s, 1196s, 1182s, 1100s, 1077m, 1035m, 1002m, 897w, 862w, 773w, 733m, 640m, 560m, 498m, 421s cm−1; 1H NMR (500 MHz, CDCl3) δ = 7.70–7.66 (m, 2H, 37-H, 39-H), 7.53–7.49 (m, 1H, 38-H), 7.34–7.30 (m, 1H, 40-H), 6.67 (d, J = 7.5 Hz, 2H, 48-H), 5.23 (t, J = 3.5 Hz, 1H, 12-H), 4.48 (dd, J = 9.9, 6.0 Hz, 1H, 3-H), 3.63–3.51 (m, 4H, 49-H), 3.52–3.40 (m, 4H, 52-H), 3.39–3.29 (m, 8H, 33-H + 34-H), 3.09–3 (m, 4H, 54-H), 3.00–2.96 (m, 1H, 18-H), 2.76–2.62 (m, 4H, 51-H), 2.15–2.07 (m, 5H, 16-Ha + 53-H), 2.04 (s, 3H, 32-H), 2.01–1.91 (m, 4H, 50-H), 1.89–1.81 (m, 1H, 11-H), 1.71–1.48 (m, 10H, 1-Ha + 2-H + 6-Ha + 7-H + 9-H + 15-Ha + 16-Hb + 19-Ha), 1.48–1.13 (m, 7H, 6-Hb + 19-Hb + 21-H + 22-Ha + 22-Hb), 1.11 (s, 3H, 27-H), 1.08–0.94 (m, 2H, 1-Hb + 15-Hb), 0.91 (s, 3H, 25-H), 0.89 (s, 3H, 29-H), 0.88 (s, 3H, 30-H), 0.86 (s, 3H, 24-H), 0.85 (s, 3H, 23-H), 0.82 (s, 1H, 5-H), 0.66 (s, 3H, 26-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 176.4 (C-28), 171.0 (C-31), 167.8 (C-35), 152.8 (C-43), 152.0 (C-44), 152.0 (C-44), 151.3 (C-46), 151.2 (C-46), 144.4 (C-13), 134.8 (C-41), 131.8 (C-36), 130.8 (C-40), 130.2 (C-39), 129.7 (C-37), 127.4 (C-36), 126.5 (C-48), 126.5 (C-48), 123.6 (C-45), 123.5 (C-45), 121.7 (C-12), 113.3 (C-42), 105.5 (C-47), 105.5 (C-47), 80.8 (C-3), 55.3 (C-5), 51.0 (C-49, 49), 50.6 (C-52), 47.6 (C-9, 33, 34), 47.5 (C-17), 46.2 (C-19), 43.5 (C-18), 41.8 (C-14), 39.1 (C-8), 38.0 (C-1), 37.7 (C-4), 37.0 (C-10), 33.9 (C-21), 32.9 (C-30), 32.8 (C-22), 30.3 (C-20), 30.0 (C-7), 28.0 (C-24), 27.8 (C-15), 27.7 (C-51), 25.9 (C-27), 24.0 (C-29), 23.5 (C-2), 23.3 (C-11), 22.5 (C-16), 21.3 (C-32), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 18.2 (C-6), 16.9 (C-26), 16.7 (C-23), 15.4 (C-25) ppm; MS (ESI, MeOH): m/z = 1039.3 (100%, [M − Cl]+); analysis calculated for C68H87N4O5Cl (1075.64): C 75.91, H 8.15, N 5.21; found: C 75.72, H 8.29, N 5.01.
4.26. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij] pyrido[1”,2”,3”:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]piperazine-1-yl]-28-oxo-urs-12-en chloride (23)
Following GPC from 7 (0.25 g, 0.44 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 23 (0.27 g, 58%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 3392w, 2926w, 2867w, 1728w, 1626m, 1594s, 1542w, 1493s, 1457m, 1435m, 1361m, 1294vs, 1266s, 1246s, 1196s, 1181s, 1099s, 1035m, 1004m, 897w, 863w, 773m, 732m, 653m, 561m, 420s cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.71–7.65 (m, 2H, 37-H, 39-H), 7.54–7.48 (m, 1H, 38-H), 7.34–7.30 (m, 1H, 40-H), 6.67 (d, J = 4.7 Hz, 2H, 48-H), 5.17 (s, 1H, 12-H), 4.48 (dd, J = 10.4, 5.5 Hz, 1H, 3-H), 3.56 (dt, J = 17.2, 6.1 Hz, 4H, 49-H), 3.51–3.41 (m, 4H, 52-H), 3.33 (s, 8H, 33-H + 34-H), 3.02 (q, J = 6.0 Hz, 4H, 54-H), 2.68 (ddt, J = 22.7, 15.6, 7.3 Hz, 4H, 51-H), 2.39–2.32 (m, 1H, 18-H), 2.15–2.06 (m, 4H, 53-H), 2.04 (s, 3H, 32-H), 1.99–1.94 (m, 4H, 50-H), 1.91–1.87 (m, 1H + 11-Ha), 1.79–1.57 (m, 7H + 1-Ha + 2-H + 11-Hb + 16-H + 22-Ha), 1.56–1.40 (m, 5H, 6-Ha + 7-Ha + 9-H + 21-Ha + 22-Hb), 1.40–1.20 (m, 4H, 6-Hb + 7-Hb + 19-H + 21-Hb), 1.05 (s, 6H, 1-Hb + 15-H + 27-H), 0.99–0.95 (m, 1H, 20-H), 0.93 (s, 3H, 30-H), 0.91 (s, 3H, 25-H), 0.86 (s, 3H, 24-H), 0.86 (s, 3H, 26-H), 0.84 (s, 3H, 29-H), 0.81–0.80 (m, 1H, 5-H), 0.67 (s, 3H, 23-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 174.8 (C-28), 171.0 (C-31), 167.8 (C-35), 152.8 (C-46), 152.0 (C-43), 151.3 (C-44), 151.2 (C-44), 134.8 (C-41), 130.8 (C-40), 130.2 (C-37), 129.8 (C-39), 127.5 (C-38), 126.5 (C-48), 125.2 (C-12), 123.6, 123.5 (C-45), 113.2 (C-42), 105.5 (C-47), 105.5 (C-47), 80.8 (C-3), 55.3 (C-5, 18), 51.0 (C-49), 50.6 (C-52), 48.6 (C-17), 47.5 (C-9, C-33, C-34), 42.1 (C-14), 39.8 (C-19), 39.4 (C-8), 38.7 (C-20), 34.4 (C-22), 32.9 (C-7), 30.4 (C-21), 28.1 (C-15), 28.1 (C-24), 27.7 (C-51), 27.7 (C-51), 23.5 (C-2, 16, 27), 23.3 (C-11), 21.3 (C-30), 21.2 (C-32), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 18.2 (C-6), 17.4 (C-29), 16.9 (C-26), 16.7 (C-23), 15.5 (C-25) ppm; MS (ESI, MeOH): m/z = 1039.4 ([M − Cl]+, 100%); analysis calculated for C68H87N4O5Cl (1075.64): C 75.91, H 8.15, N 5.21; found: C 75.64, H 8.29, N 4.96.
4.27. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij]pyrido[1”,2”,3”:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]piperazine-1-yl]-20,28-dioxo-30-norlupan-12-en chloride (24)
Following GPC from 8 (0.25 g, 0.44 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 24 (0.30 g, 65%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 3396w, 2940w, 2863w, 1727w, 1627m, 1594s, 1543w, 1493s, 1459m, 1446m, 1439m, 1419m, 1361m, 1295Vs, 1267s, 1195s, 1182s, 1099s, 1035m, 1004m, 979m, 897w, 863w, 773m, 732m, 561m, 505m, 420s cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.70–7.67 (m, 2H, 37-H, 39-H), 7.53–7.49 (m, 1H, 38-H), 7.33–7.30 (m, 1H, 40-H), 6.66 (d, J = 6.8 Hz, 2H, 48-H), 4.44 (dd, J = 10.9, 5.2 Hz, 1H, 3-H), 3.62–3.51 (m, 4H, 49-H), 3.51–3.43 (m, 4H, 52-H), 3.43–3.29 (m, 8H, 33-H + 34-H), 3.15 (td, J = 11.1, 3.1 Hz, 1H, 19-H), 3.06–2.98 (m, 4H, 54-H), 2.78–2.63 (m, 4H, 51-H), 2.58 (td, J = 12.1, 3.8 Hz, 1H, 13-H), 2.14 (s, 3H, 29-H), 2.13–2.08 (m, 4H, 53-H), 2.08–2.04 (m, 1H, 18-H), 2.02 (s, 3H, 32-H), 1.97 (q, J = 6.9 Hz, 5H, 16-Ha + 50-H), 1.88–1.80 (m, 2H, 21-Ha + 22-Ha), 1.67–1.54 (m, 4H, 1-Ha + 2-H + 16-Hb), 1.54–0.98 (m, 14H, 6-Ha + 6-Hb + 7-H + 9-H + 11-Ha + 11-Hb + 12-H + 15-H + 21-Hb + 22-Hb), 0.95 (s, 4H, 1-Hb + 27-H), 0.87 (s, 3H, 26-H), 0.82 (s, 3H, 24-H), 0.82 (s, 3H, 25-H), 0.81 (s, 3H, 23-H), 0.79–0.75 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3) δ = 212.6 (C-20), 173.9 (C-28), 170.9 (C-31), 167.8 (C-35), 152.8 (C-43), 152.0 (C-44), 152.0 (C-44), 151.3 (C-46), 151.2 (C-46), 134.7 (C-41), 131.9 (C-36), 130.8 (C-40), 130.3 (C-39), 129.7 (C-37), 127.4 (C-38), 126.5 (C-48), 123.6 (C-45), 123.5 (C-45), 113.2 (C-42), 105.5 (C-47), 55.4 (C-5), 54.5 (C-17), 52.5 (C-18), 51.0 (C-49), 51.0 (C-49), 50.6 (C-52), 50.6 (C-52), 50.5 (C-9), 50.0 (C-19), 41.7 (C-14), 40.5 (C-8), 38.3 (C-1), 37.8 (C-4), 37.1 (C-10), 35.9 (C-13), 35.6 (C-22), 34.2 (C-7), 32.0 (C-16), 30.2 (C-29), 29.8 (C-15), 28.7 (C-21), 27.9 (C-24), 27.7 (C-51), 27.4 (C-12), 23.6 (C-2), 21.3 (C-32), 21.1 (C-11), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 18.1 (C-6), 16.5 (C-23), 16.2 (C-25), 16.0 (C-26), 14.6 (C-27) ppm; MS (ESI, MeOH): m/z = 1041.3 ([M − Cl]+, 100%); analysis calculated for C67H85N4O6Cl (1077.89): C 74.66, H 7.95, N 5.20; found: C 74.50, H 8.14, N 5.03.
4.28. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij] pyrido[1”,2”,3”:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]homopiperazine-1-yl]-28-oxo-lup-20(29)-en chloride (25)
Following GPC from 9 (0.35 g, 0.60 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 25 (0.37 g, 68%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 2940w, 2865w, 1730w, 1624m, 1595s, 1542w, 1493s, 1459m, 1376m, 1361m, 1294vs, 1268s, 1247m, 1196s, 1180s, 1098s, 1035m, 1018m, 978m, 895w, 772w, 747m, 622m, 421s cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.63–7.51 (m, 2H, 37-H, 39-H), 7.41 (t, J = 9.2 Hz, 1H, 38-H), 7.21 (d, J = 7.4 Hz, 1H, 40-H), 6.68 (dd, J = 26.0, 20.3 Hz, 2H, 48-H), 4.70 (d, J = 18.2 Hz, 1H, 29-Ha), 4.55 (d, J = 17.7 Hz, 1H, 29-Hb), 4.45–4.38 (m, 1H, 3-H), 3.85–3.62 (m, 4H, 33-H), 3.55–3.45 (m, 8H, 49-H + 52-H), 3.44–3.24 (m, 4H, 34-H), 3.08–2.91 (m, 5H, 19-H + 54-H), 2.89–2.80 (m, 1H, 13-H), 2.78–2.59 (m, 4H), 2.07 (s, 7H, 16-Ha + 53-H + 55-H), 1.99 (s, 3H, 32-H), 1.93 (s, 4H, 50-H), 1.83 (d, J = 4.6 Hz, 2H, 21-Ha + 22-Ha), 1.66 (s, 2H, 12-H), 1.62 (s, 3H, 30-H), 1.55 (dd, J = 22.6, 11.1 Hz, 3H, 1-H + 2-H), 1.50–1.40 (m, 3H, 6-Ha + 16-Hb + 18-H), 1.40–1.30 (m, 5H, 7-H + 11-Ha + 21-Hb + 22-Hb), 1.30–1.04 (m, 5H, 6-Hb + 9-H + 11-Hb + 15-Ha + 15-Hb), 0.93–0.89 (m, 5H, 1-H + 12-H + 27-H), 0.88 (s, 3H, 26-H), 0.82–0.78 (m, 9H, 23-H + 24-H + 25-H), 0.77–0.72 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 175.5 (C-28), 170.9 (C-31), 168.9 (C-35), 152.8, 152.0 (C-43), 151.9 (C-43), 151.3 (C-44), 151.3 (C-44), 151.2 (C-20), 151.1 (C-46), 136.0 (C-41), 131.1 (C-36), 130.5 (C-40), 129.8 (C-39), 129.5 (C-37), 127.6 (C-38), 126.6 (C-48), 126.4 (C-48), 123.4 (C-45), 123.4 (C-45), 112.9 (C-42), 109.4 (C-29), 105.4 (C-47), 105.3 (C-47), 80.9 (C-3), 55.5 (C-5), 54.8 (C-17), 52.7 (C-18), 51.0 (C-49), 50.5 (C-52), 46.1 (C-33 + 34), 45.9 (C-19), 41.9 (C-14), 40.7 (C-8), 38.4 (C-1), 37.8 (C-4), 37.1 (C-10), 36.8 (C-13), 36.0 (C-22), 34.3 (C-7), 32.3 (C-16), 31.5 (C-21), 29.9 (C-15), 28.9 (C-55), 27.9 (C-24), 27.5 (C-51), 25.5 (C-12), 23.6 (C-2), 21.3 (C-32), 21.1 (C-11), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 19.5 (C-30), 18.2 (C-6), 16.4 (C-23), 16.2 (C-25), 16.1 (C-26), 14.6 (C-27) ppm; MS (ESI, MeOH): m/z = 1052.9 ([M − Cl]+, 100%); analysis calculated for C69H89N4O5Cl (1089.94): C 76.04, H 8.23, N 5.14; found: C 75.76, H 8.31, N 5.02.
4.29. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij] pyrido[1′′,2′′,3′′:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]homopiperazine-1-yl]-28-oxo-olean-12-en chloride (26)
Following GPC from 10 (0.25 g, 0.60 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 26 (0.38 g, 70%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. > 300 °C; IR (ATR): ν = 3369vw, 2942w, 2863w, 1729w, 1623m, 1595s, 1543w, 1493s, 1459m, 1361m, 1294vs, 1268s, 1246s, 1195s, 1180s, 1150m, 1098s, 1035m, 985m, 896w, 862w, 771m, 746m, 622m, 575w, 560w, 498m, 421s cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.63–7.58 (m, 2H, 37-H + 39-H), 7.55–7.50 (m, 1H, 38-H), 7.23–7.17 (m, 1H, 40-H), 6.72–6.60 (m, 2H, 48-H), 5.25–5.21 (m, 1H, 12-H), 4.45 (t, J = 7.8 Hz, 1H, 3-H), 3.95–3.60 (m, 4H, 33-H + 34-H), 3.58–3.43 (m, 8H, 49-H + 52-H), 3.41–3.11 (m, 4H, 34-H), 3.06 (s, 1H, 18-H), 3.03–2.95 (m, 4H, 54-H), 2.74–2.61 (m, 4H, 51-H), 2.14–2.02 (m, 7H, 16-Ha + 53-H + 55-H), 2.00 (s, 3H, 32-H), 1.94 (dq, J = 11.1, 5.3 Hz, 4H, 50-H), 1.88–1.79 (m, 2H + 11-H), 1.71–1.41 (m, 10H, 1-Ha + 2-H + 6-Ha + 7-H + 9-H + 15-Ha + 16-Hb + 19-Ha), 1.41–1.26 (m, 3H, 6-Hb, 21 + 22-Ha), 1.26–1.12 (m, 3H, 19-Hb + 22-Hb), 1.09 (s, 3H, 27-H), 1.06–0.96 (m, 2H, 1-Hb + 15-Hb), 0.94 (s, 3H, 29-H), 0.88 (d, J = 2.0 Hz, 6H, 25-H + 30-H), 0.82 (s, 3H, 24-H), 0.81 (s, 3H, 23-H), 0.79–0.76 (m, 1H, 5-H), 0.66 (s, 3H, 26-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 176.1 (C-28), 171.0 (C-31), 169.0 (C-35), 152.8 (C-43), 152.0 (C-44), 151.9 (C-44), 151.3 (C-46), 151.2 (C-46), 144.8 (C-13), 136.0 (C-41), 130.4 (C-40), 129.9 (C-39), 129.5 (C-37), 127.5 (C-36), 126.5 (C-48), 126.2 (C-48), 123.6 (C-45), 123.5 (C-45), 121.4 (C-12), 113.3 (C-42), 105.5 (C-47), 105.4 (C-47), 80.9 (C-3), 55.3, 51.0 (C-49), 50.5 (C-52), 47.7 (C-17), 47.6 (C-9), 47.6 (C-33 + 34), 46.6 (C-19), 43.5 (C-18), 42.0 (C-14), 39.0 (C-8), 38.0 (C-1), 37.7 (C-4), 37.0 (C-10), 34.0 (C-21), 32.9 (C-30), 32.7 (C-22), 30.5 (C-7), 30.3 (C-20, C-55), 28.0 (C-24), 27.8 (C-15), 27.6 (C-51), 25.9 (C-27), 24.2 (C-29), 23.5 (C-2), 23.3 (C-11), 22.5 (C-16), 21.3 (C-32), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 19.6 (C-53), 18.2 (C-6), 17.0 (C-26), 16.6 (C-23), 15.4 (C-25) ppm; MS (ESI, MeOH): m/z = 1053.0 (100%, [M − Cl]+); analysis calculated for C69H89N4O5Cl (1089.94): C 76.04, H 8.23, N 5.14; found: C 75.81, H 8.40, N 4.86.
4.30. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij]pyrido[1′′,2′′,3′′:1′,8′]quinolino[6′,5′:5,6]pyrano[2,3-f]quinolin-4-ium-9-yl)benzoyl]homopiperazine-1-yl]-28-oxo-urs-12-en chloride (27)
Following GPC from 11 (0.35 g, 0.60 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 27 (0.39 g, 72%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. >300 °C; IR (ATR): ν = 2934w, 2866w, 1730w, 1622w, 1594w, 1543w, 1493w, 1459w, 1435w, 1376w, 1361w, 1293w, 1268w, 1246w, 1195w, 1180w, 1143w, 1098w, 1035w, 985w, 966w, 941w, 896w, 862w, 772w, 744w, 715w, 661w, 622w, 574w, 560w, 496w cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.59 (dd, J = 5.7, 3.3 Hz, 2H, 37-H + 39-H), 7.43–7.34 (m, 1H, 38-H), 7.22 (dd, J = 5.7, 3.3 Hz, 1H, 40-H), 6.61 (d, J = 24.5 Hz, 2H, 48-H), 5.18–5.11 (m, 1H-H), 4.44 (dd, J = 11.0, 5.2 Hz, 1H, 3-H), 4.08–3.66 (m, 4H, 34-H), 3.59–3.41 (m, 8H, 49-H + 52-H), 3.38–3.07 (m, 4H, 33-H), 3.02–2.93 (m, 4H, 54-H), 2.67 (h, J = 9.5, 8.8 Hz, 4H, 51-H), 2.45–2.34 (m, 1H, 18-H), 2.11–2.03 (m, 4H,55-H + 53-H), 2.00 (s, 3H, 32-H), 1.96–1.81 (m, 6H, 11-H + 50-H), 1.79–1.52 (m, 7H, 1-Ha + 2-H + 16-H + 22-H), 1.44 (dt, J = 14.3, 7.8 Hz, 4H, 6-Ha + 7-Ha + 9-H + 21-Ha), 1.37–1.11 (m, 4H, 6-Hb + 7-Hb + 19-H + 21-Hb), 1.01 (d, J = 11.5 Hz, 6H, 1-Hb + 15-H + 27-H), 0.92 (s, 1H, 20-H), 0.88 (s, 6H, 25-H + 30-H), 0.81 (s, 6H, 24-H + 29-H), 0.80 (s, 3H, 23-H), 0.75 (s, 1H, 5-H), 0.66 (s, 3H, 26-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 176.4 (C-28), 170.9 (C-31), 168.1 (C-35), 152.9 (C-46), 151.9 (C-43), 151.3 (C-44), 138.7 (C-13), 135.7 (C-41), 131.4 (C-36), 130.4 (C-40), 129.8, 129.6 (C-37), 129.5 (C-39), 127.5 (C-38), 126.8 (C-48), 125.0 (C-12), 123.7 (C-45), 113.3 (C-42), 105.3 (C-47), 105.1 (C-47), 80.9 (C-3), 55.3 (C-5, 18), 51.0 (C-49), 50.5 (C-52), 48.8 (C-17), 47.5 (C-9, 33, 34), 42.8 (C-14), 39.3 (C-8, 19), 38.7 (C-20), 38.2 (C-1), 37.6 (C-4), 36.9 (C-10), 34.4 (C-22), 32.9 (C-7), 30.4 (C-21), 30.3 (C-55), 28.0 (C-15, 24), 27.6 (C-51), 27.5 (C-51), 23.5 (C-2, 16, 27), 23.2 (C-11), 21.3 (C-30), 21.2 (C-32), 20.6 (C-50), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 19.6 (C-53), 18.1, 17.4 (C-29), 17.0 (C-26), 16.7 (C-23), 15.5 (C-25) ppm; MS (ESI, MeOH): m/z = 1053.1 (100%, [M − Cl]+); analysis calculated for C69H89N4O5Cl (1089.94): C 76.04, H 8.23, N 5.14; found: C 75.76, H 8.51, N 4.97.
4.31. 3β-Acetyloxy-28-[4-[3-(2,3,6,7,12,13,16,17-octahydro-1H,5H,11H,15H-pyrido[3,2,1-ij]pyrido[1′′,2′′,3′′:1′,8′]quinolino[6′,5′:5,6]pyrano [2,3-f]quinolin-4-ium-9-yl)benzoyl]homopiperazine-1-yl]-20,28-dioxo-30-norlupan-12-en chloride (28)
Following GPC from 12 (0.35 g, 0.60 mmol) and rhodamine 101 (0.25 g, 0.51 mmol), 28 (0.35 g, 66%) was obtained as a pink solid; Rf = 0.4 (SiO2, CHCl3/MeOH, 9:1); m.p. > 300 °C; IR (ATR): ν = 3383vw, 2940w, 2863w, 1731w, 1623m, 1594s, 1542w, 1493s, 1459m, 1436m, 1376m, 1361m, 1294vs, 1268s, 1247m, 1195s, 1180s, 1143m, 1099s, 1075m, 1035m, 1018m, 978w, 897w, 862w, 746m, 623w, 561m, 506m, 421s cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.63–7.57 (m, 2H, 37-H + 39-H), 7.41 (d, J = 23.5 Hz, 1H, 38-H), 7.29–7.21 (m, 1H, 40-H), 6.67 (dd, J = 27.4, 18.9 Hz, 2H, 48-H), 4.42 (dt, J = 10.6, 5.4 Hz, 1H, 3-H), 3.93–3.58 (m, 4H, 33-H), 3.58–3.40 (m, 8H, 49-H, 52-H), 3.40–3.08 (m, 5H, 19-H, 34-H), 3.06–2.91 (m, 4H, 54-H), 2.70 (d, J = 27.3 Hz, 5H, 13-H + 51-H), 2.12 (s, 3H, 29-H), 2.10–2.02 (m, 7H, 16-Ha + 53-H + 55-H), 2.00 (s, 4H, 18-H + 32-H), 1.98–1.88 (m, 5H, 22-Ha + 50-H), 1.87–1.80 (m, 1H, 21-Ha), 1.66–1.52 (m, 4H, 1-H + 16-Hb), 1.45 (q, J = 16.9, 12.1 Hz, 3H, 6-Ha + 21-Hb + 22-Hb), 1.39–1.30 (m, 5H, 6-Hb + 7-H + 11-Ha + 12-Ha), 1.29–1.19 (m, 4H, 9-H + 11-Hb + 15-H), 0.95–0.93 (m, 2H, 1-H + 12-Hb), 0.91 (s, 6H, 26-H + 27-H), 0.83–0.78 (m, 9H, 23-H + 24-H + 25-H), 0.76–0.72 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 213.0 (C-20), 174.5 (C-28), 170.9 (C-31), 168.9 (C-35), 152.6 (C-43), 151.9 (C-44), 151.3 (C-46), 136.1 (C-41), 131.1 (C-36), 130.5 (C-40), 129.8 (C-39), 129.5 (C-37), 127.5 (C-38), 126.7 (C-48), 126.2 (C-48), 123.7 (C-45), 123.5 (C-45), 112.9 (C-42), 105.3 (C-47), 80.9 (C-3), 55.5 (C-5), 54.9 (C-17), 52.8 (C-18), 51.0 (C-49), 50.6 (C-9), 50.5 (C-52), 50.2 (C-19), 48.0 (C-33 + 34), 41.8 (C-14), 40.6 (C-8), 38.4 (C-1), 37.8 (C-4), 37.1 (C-10), 36.0 (C-13), 35.6 (C-22), 34.2 (C-7), 31.9 (C-16), 30.2 (C-29), 29.9 (C-15), 29.6 (C-55), 28.9 (C-21), 27.9 (C-24), 27.6 (C-51), 27.6 (C-51), 27.3 (C-12), 23.6 (C-2), 21.3 (C-32), 21.2 (C-11), 20.6 (C-50), 19.9 (C-54), 19.7 (C-53), 19.6 (C-53), 18.2 (C-6), 16.5 (C-23), 16.2 (C-25), 16.1 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH): m/z = 1055.0 (100%, [M − Cl]+); analysis calcd for C68H87N4O6Cl (1091.92): C 74.80, H 8.03, N 5.13; found: C 74.61, H 8.27, N 4.95.
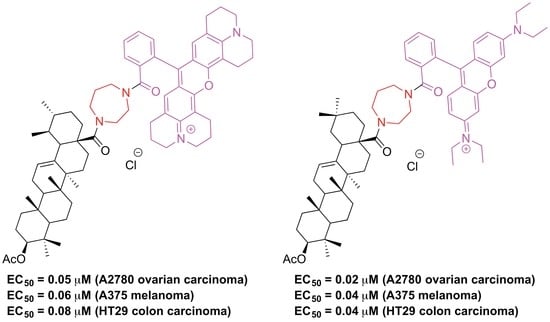
4.32. Cytotoxicity Assay (SRB)
The cell lines were obtained from Department of Oncology (Martin-Luther-University Halle Wittenberg; they were bought from ATCC; A 375 (CRL-1619), HT29 (HTB-38), MCF7 (HTM-22), A2780 (HTP-77), FaDu (HTP-43), NIH 3T3 (CRL-1658), HEK-293 (CRL-1573)). Cultures were maintained as monolayers in RPMI 1640 medium with l-glutamine (Capricorn Scientific GmbH, Ebsdorfergrund, Germany) supplemented with 10% heat inactivated fetal bovine serum (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and penicillin/streptomycin (1%, Capricorn Scientific GmbH, Ebsdorfergrund, Germany) at 37 °C in a humidified atmosphere with 5% CO2. The cytotoxicity of the compounds was evaluated using the sulforhodamine-B (Kiton-Red S, ABCR) micro culture colorimetric assay using confluent cells in 96-well plates with the seeding of the cells on day 0 applying appropriate cell densities to prevent confluence of the cells during the period of the experiment. On day 1, the cells were treated with six different concentrations (1, 3, 7, 12, 20, and 30 μM); thereby, the final concentration of DMSO was always <0.5%, generally regarded as non-toxic to the cells. On day 4, the supernatant medium was discarded; the cells were fixed with 10% trichloroacetic acid. After another day at 4 °C, the cells were washed in a strip washer and dyed with the SRB solution (100 μL, 0.4% in 1% acetic acid) for about 20 min to be followed by washing of the plates (four times, 1% acetic acid) and air-drying overnight. Furthermore, tris base solution (200 μL, 10 mM) was added to each well and absorbance was measured at λ = 570 nm employing a reader (96 wells, Tecan Spectra, Crailsheim, Germany). The EC50 values were averaged from three independent experiments performed each in triplicate calculated from semi logarithmic dose response curves applying a non-linear four-parameter Hills-slope equation (GraphPad Prism5; variables top and bottom were set to 100 and 0, respectively).
4.33. Acridine Orange (AO) Staining
On the first day, A375 cells were counted and seeded 1 × 105 in a Petri dish (diameter 4 cm) with coverslips (22 mm × 22 mm) in 2 mL medium. After 24 h, the medium was removed, and treatment was performed with 2 mL of new medium (control) and 2 mL each of 2 times the EC50 concentration of compounds 27. After 24 h, the medium was removed from the samples, the coverslip was rinsed with 1 mL of PBS (with Ca2+ and Mg2+), placed on a slide containing 20 µL of AO solution (2.5 µg/mL in PBS), and measured directly on the fluorescence microscope.
4.34. Hoechst 33,3342 and Rhodamine 123 Staining
On day 1, A375 cells were counted and seeded 1 × 105 in a Petri dish (diameter 4 cm) with coverslips (22 mm × 22 mm) in 2 mL medium. After 24 h, the medium was removed, and treatment was performed with 1 mL of new medium containing 1 µL of compound 27 (0.08 mM solution). After another 24 h, additional treatment was performed with 1 µL rhodamine (1 mg/mL in EtOH) and 2 µL Hoechst 33342 (100 µg/mL in DMSO) for (at least) 30 min. The medium was then removed, rinsed once with PBS (with Ca2+ and Mg2+), placed on a slide containing 20 µL PBS (with Ca2+ and Mg2+), and measured directly on the fluorescence microscope.