Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle
Abstract
:1. Introduction
2. Antimicrobial Activity
3. Antifungal Activity
4. Antiviral Activity
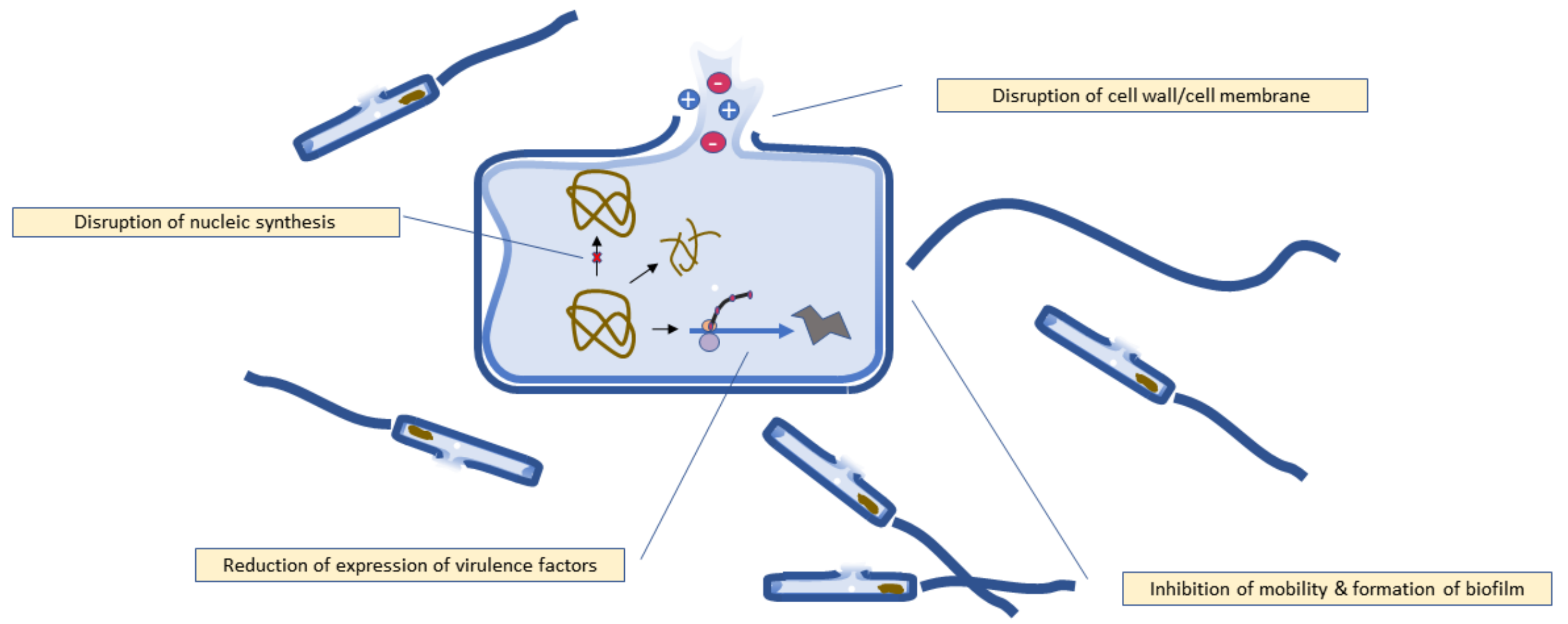
5. Mechanism of Antibacterial Activity
5.1. Disruption of Bacterial Cell Walls and Cell Membrane
5.2. Disruption of Nucleic Acid Synthesis
5.3. Inhibition of Biofilm Formation
5.4. Reduction of Expression of Virulence Factors
6. Mechanism of Antifungal Activity
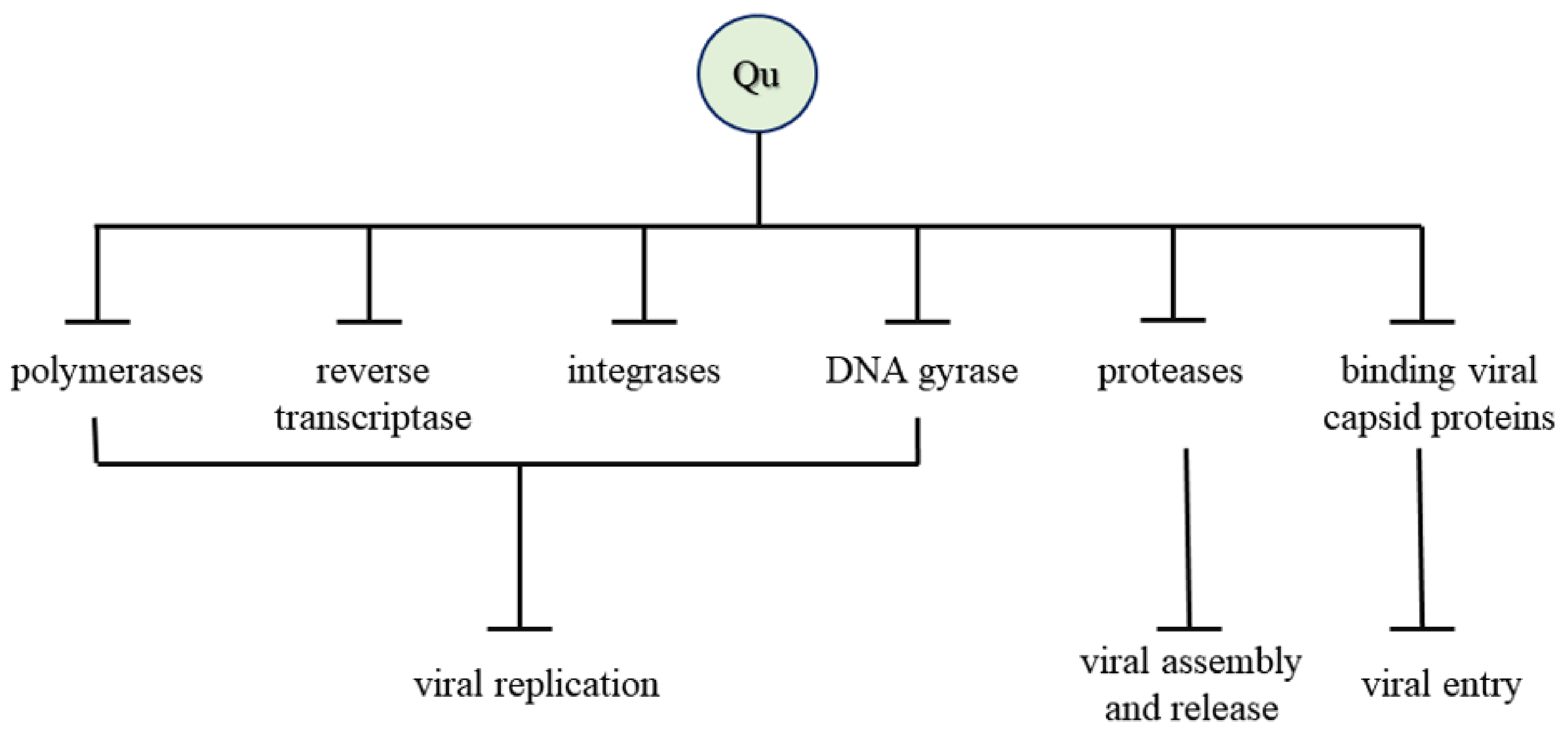
7. Mechanism of Antiviral Activity
8. Bioavailability of Quercetin
9. Merits and Demerits
10. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell Longev. 2020, 30, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, G.H. Quantification of Quercetin in different parts of onion and its DPPH radical scavenging and antibacterial activity. Food Sci. Biotechnol. 2006, 15, 39–43. [Google Scholar]
- Ozgen, S.; Kilinc, O.K.; Selamoglu, Z. Antioxidant Activity of Quercetin: A Mechanistic Review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, S.; Asakura, M.; Neogi, S.B.; Hinenoya, A.; Iwaoka, E.; Aoki, S. Inhibition of virulence potential of Vibrio cholerae by natural compounds. Indian J. Med. Res. 2011, 133, 232–239. [Google Scholar]
- Shu, Y.; Liu, Y.; Li, L.; Feng, J.; Lou, B.; Zhou, X.; Wu, H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Braz. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooda, H.; Singh, P.; Bajpai, S. Effect of quercetin impregnated silver nanoparticle on growth of some clinical pathogens. Mater. Today Proc. 2020, 31, 625–630. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive–dentin interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef] [Green Version]
- Martini, N.D.; Katerere, D.R.P.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef]
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplement and antimicrobial activities of flavonoids from Entada phaseoloides. Nat. Prod. Commun. 2012, 7, 867–871. [Google Scholar] [CrossRef] [Green Version]
- Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, quercetin, and hydroxyapatite act synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Peng, X.; Lin, J.; Zhang, Y.; Zhang, J.; Gao, H.; Tian, X.; Zhang, R.; Zhao, G. Quercetin ameliorates Aspergillus fumigatus keratitis by inhibiting fungal growth, toll-like receptors and inflammatory cytokines. Int. Immunopharmacol. 2021, 93, 107435. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, W.E.; Awad, H.M.; AbdelMohsen, M.M. HPLC Analysis of Quercetin and Antimicrobial Activity of Comparative Methanol Extracts of Shinus molle L. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 550–558. [Google Scholar]
- Gao, M.; Wang, H.; Zhu, L. Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]
- Singh, B.N.; Upreti, D.K.; Singh, B.R.; Pandey, G.; Verma, S.; Roy, S.; Naqvi, A.H.; Rawat, A.K.S. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother. 2015, 59, 2153–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, C.L.; Alvear, M.; Barrientos, L.; Montenegro, G.; Salazar, L.A. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Cien. Inv. Agric. 2010, 37, 75–84. [Google Scholar] [CrossRef]
- Biancatelli, R.M.L.C.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Uchide, N.; Toyoda, H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit Vectors 2014, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Pierro, F.D.; Iqtadar, S.; Khan, A.; Mumtaz, S.U.; Chaudhry, M.M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Ishitsuka, H.; Ohsawa, C.; Ohiwa, T.; Umeda, I.; Suhara, Y. Antipicornavirus flavone Ro 09-0179. Antimicrob. Agents Chemother. 1982, 22, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, W.; Zhao, Z.; Li, N.; Mou, Z.; Sun, D.; Cai, Y.; Wang, W.; Lin, Y. Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. J. Inorg. Biochem. 2017, 167, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and mode of action of ceftazidime plus quercetin or luteolin on Streptococcus pyogenes. Evid. Based Complement. Alternat. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, A.; Tripathi, A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Tripathi, A. Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem resistant Escherichia coli and Klebsiella pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorg. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Hossion, A.M.; Zamami, Y.; Kandahary, R.K.; Tsuchiya, T.; Ogawa, W.; Iwado, A.; Sasaki, K. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. J. Med. Chem. 2011, 54, 3686–3703. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Feng, X.A.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron Complex: Synthesis, Characterization, Antioxidant, DNA Binding, DNA Cleavage, and Antibacterial Activity Studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.S.; Luo, R.H.; Huang, C.Y. A Complexed Crystal Structure of a Single-Stranded DNA-Binding Protein with Quercetin and the Structural Basis of Flavonol Inhibition Specificity. Int. J. Mol. Sci. 2022, 23, 588. [Google Scholar] [CrossRef]
- Qayyum, S.; Sharma, D.; Bisht, D.; Khan, A.U. Identification of factors involved in Enterococcus faecalis biofilm under quercetin stress. Microb. Pathog. 2019, 126, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Júnior, S.D.D.C.; Santos, J.V.D.O.; Campos, L.A.D.A.; Pereira, M.A.; Magalhães, N.S.S.; Cavalcanti, I.M.F. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int. J. Environ. Agric. Biotech. 2018, 3, 1948–1958. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manner, S.; Fallarero, A. Screening of natural product derivatives identifies two structurally related flavonoids as potent quorum sensing inhibitors against gram-negative bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Hadinoto, K. A Potential Quorum-Sensing Inhibitor for Bronchiectasis Therapy: Quercetin-Chitosan Nanoparticle Complex Exhibiting Superior Inhibition of Biofilm Formation and Swimming Motility of Pseudomonas aeruginosa to the Native Quercetin. Int. J. Mol. Sci. 2021, 22, 1541. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaempferol against Streptococcus mutans biofilm. Arch. Oral Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Tapia-Rodriguez, M.R.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Martinez-Tellez, M.A.; Hernandez-Oñate, M.A.; Ayala-Zavala, J.F. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 2018, 90, 266–273. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Hernandez-Oñate, M.A.; Martinez-Tellez, M.A.; Lopez-Zavala, A.A.; Gonzalez-Aguilar, G.A.; Gutierrez-Pacheco, M.M.; Ayala-Zavala, J.F. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes. Food Microbiol. 2020, 87, 103377. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, T.G.; Jung, M.; Park, K.H.; Chong, Y. In Vitro Synergism and Anti-biofilm Activity of Quercetin-Pivaloxymethyl Conjugate against Staphylococcus aureus and Enterococcus Species. Chem. Pharm. Bull. 2018, 66, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, E.; Mazinani, S.; Nguyen, D.; Betancourt, F.; Yan, H. Abrasive treatment of microtiter plates improves the reproducibility of bacterial biofilm assays. RSC Adv. 2018, 8, 32434–32439. [Google Scholar] [CrossRef] [Green Version]
- Vanaraj, S.; Keerthana, B.B.; Preethi, K. Biosynthesis, Characterization of Silver Nanoparticles Using Quercetin from Clitoria ternatea L to Enhance Toxicity against Bacterial Biofilm. J. Inorg. Organomet. Polym. 2017, 27, 1412–1422. [Google Scholar] [CrossRef]
- Vipin, C.; Mujeeburahiman, M.; Ashwini, P.; Arun, A.B.; Rekha, P.D. Anti-biofilm and cytoprotective activities of quercetin against Pseudomonas aeruginosa isolates. Lett. Appl. Microbiol. 2019, 68, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Feng, W.; Lai, X.; Chen, Y.; Zhang, X.; Rong, L.; Sun, F.; Chen, Y. Quercetin inhibits Pseudomonas aeruginosa biofilm formation via the vfr-mediated lasIR system. Microb. Pathog. 2020, 149, 104291. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Mu, Y.; Zeng, H.; Chen, W. Quercetin Inhibits Biofilm Formation by Decreasing the Production of EPS and Altering the Composition of EPS in Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 631058. [Google Scholar] [CrossRef]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, B.; Si, X.; Liu, X.; Deng, X.; Niu, X.; Jin, Y.; Wang, D.; Wang, J. Quercetin protects rats from catheter-related Staphylococcus aureus infections by inhibiting coagulase activity. J. Cell. Mol. Med. 2019, 23, 4808–4818. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Zhang, P.; Quan, P.; Cui, M.; Liu, T.; Yin, Y.; Chi, G. Quercetin, a pneumolysin inhibitor, protects mice against Streptococcus pneumoniae infection. Microb. Pathog. 2020, 140, 103934. [Google Scholar] [CrossRef] [PubMed]
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complement. Altern. Med. 2013, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef]
- Cassetta, A.; Stojan, J.; Krastanova, I.; Kristan, K.; Brunskole Švegelj, M.; Lamba, D.; Lanišnik Rižner, T. Structural basis for inhibition of 17 β -hydroxysteroid dehydrogenases by phytoestrogens: The case of fungal 17β-HSDcl. J. Steroid Biochem. Mol. Biol. 2017, 171, 80–93. [Google Scholar] [CrossRef]
- da Silva, C.R.; de Andrade Neto, J.B.; de Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.; Cavalcanti, B.C.; Gaspar, D.M.; de Andrade, G.M.; Lima, I.S.; et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Woo, E.R.; Shin, C.G.; Park, H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 1998, 61, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E., Jr.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Li, W.; Shen, L.; Huang, S.; Tang, J.; Duan, J.; Fang, F.; Huang, Y.; Chang, H.; et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016, 6, 19095. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, J.; Li, W.; Wang, X.; Xu, J.; Xie, J.; Tao, K.; Shen, L.; Zhang, R. Molecular docking of potential inhibitors for influenza H7N9. Comput. Math. Methods Med. 2015, 2015, 480764. [Google Scholar] [CrossRef]
- Fatima, K.; Mathew, S.; Suhail, M.; Ali, A.; Damanhouri, G.; Azhar, E.; Qadri, I. Docking studies of Pakistani HCV NS3 helicase: A possible antiviral drug target. PLoS ONE 2014, 9, e106339. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Fatima, K.; Fatmi, M.Q.; Archunan, G.; Ilyas, M.; Begum, N.; Azhar, E.; Damanhouri, G.; Qadri, I. Computational Docking Study of p7 Ion Channel from HCV Genotype 3 and Genotype 4 and Its Interaction with Natural Compounds. PLoS ONE 2015, 10, e0126510. [Google Scholar] [CrossRef] [Green Version]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Rojas, Á.; del Campo, J.A.; Clement, S.; Lemasson, M.; García-Valdecasas, M.; Gil-Gómez, A.; Ranchal, I.; Bartosch, B.; Bautista, J.D.; Rosenberg, A.R.; et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci. Rep. 2016, 6, 31777. [Google Scholar] [CrossRef]
- Hung, P.Y.; Ho, B.C.; Lee, S.Y.; Chang, S.Y.; Kao, C.L.; Lee, S.S.; Lee, C.N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, e0115475. [Google Scholar]
- Cho, W.K.; Weeratunga, P.; Lee, B.H.; Park, J.S.; Kim, C.J.; Ma, J.Y.; Lee, J.S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses 2015, 7, 352–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaabi, M. Antiviral Effects of Quercetin and Related Compounds. Naturopathic Currents, Special Edition, April 2020, Antiviral Effects of Quercetin and Related Compounds. 2020. Available online: https://naturopathiccurrents.com/sites/default/files/AntiviralEffectsofQuercetinandRelatedCompounds_0.pdf (accessed on 11 April 2022).
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Alomair, L.; Almsned, F.; Ullah, A.; Jafri, M.S. In Silico Prediction of the Phosphorylation of NS3 as an Essential Mechanism for Dengue Virus Replication and the Antiviral Activity of Quercetin. Biology 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, Q.; Xiao, H.; Li, M.; Huang, Y.; Zhang, Q.; Li, P. The Inhibitory Activities and Antiviral Mechanism of Medicinal Plant Ingredient Quercetin Against Grouper Iridovirus Infection. Front. Microbiol. 2020, 11, 586331. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]


| Fungus Name | Mechanism of Action | References |
|---|---|---|
| Trichophyton rubrum | Downregulated the enzyme fatty acid synthase and reduced ergosterol levels, thereby causing plasma membrane disruption | [66] |
| C. albicans | Induced apoptosis with increase in intracellular magnesium along with mitochondrial dysfunction. Mitochondrial antioxidant system was disrupted due to increased levels of intracellular ROS and decreased intracellular redox levels. DNA damage was also observed. | [67] |
| Cochliobolus lunatus | Inhibition of nucleic acid synthesis | [68] |
| Candida tropicalis | Induced apoptosis, caused morphological changes, disruption of membrane integrity, increase in intracellular ROS, mitochondrial depolarization and DNA damage in combination with the antibiotic fluconazole. | [69] |
| C. albicans | When combined with fluconazole, quercetin inhibited biofilm formation by downregulating the expression of biofilm-forming genes. The combination also inhibited cell adhesion, cell surface hydrophobicity (CSH), flocculation, fungal metabolism, yeast-to-hypha transition. | [23] |
| C. albicans | Downregulated virulence factors such as biofilm formation, hemolytic activity, activities of the enzymes, proteinase, phospholipase, and esterase, as well as hyphal development. Quercetin in combination with fluconazole induced fungal cell death by apoptosis. | [24] |
| Candida parapsilosis complex | Inhibited biofilm formation | [70] |
| Virus Name | Mechanism of Action | References |
|---|---|---|
| Human Immunodeficiency Virus (HIV)-1 strain | Inhibited the enzyme integrase | [72] |
| Herpes Simplex Virus (HSV), Poliovirus, Respiratory Syncytial Virus (RSV), Sindbis virus | Inhibited viral polymerase and binding of viral capsid proteins or viral nucleic acid | [13] |
| HSV-1 | Reduced infectivity, intracellular replication | [73] |
| Polio-virus type 1 | Reduced infectivity, intracellular replication | [73] |
| Parainfluenza virus type 3 (Pf-3) | Reduced infectivity, intracellular replication | [73] |
| RSV | Reduced infectivity, intracellular replication | [73] |
| Influenza A H1N1 | Inhibited neuraminidase | [74] |
| Influenza H7N9 | Inhibited neuraminidase | [75] |
| Hepatitis C virus (HCV) | Inhibited nonstructural protein 3 (NS3) of HCV helicase | [76] |
| HCV genotypes 3 and 4 | Inhibited the function of p7 proteins | [77] |
| HCV | Inhibited NS3 protease | [78] |
| HCV | Downregulated diacylglycerol acyltransferase (DGAT) | [79] |
| HSV-1 | Blocked viral binding and viral penetration to the host cell as well as inhibited the activation of NF-κB at the beginning of infection. | [80] |
| HSV-2 | Blocked viral binding and viral penetration to the host cell as well as inhibited the activation of NF-κB at the beginning of infection. | [80] |
| Acyclovir-resistant HSV-1 | Blocked viral binding and viral penetration to the host cell as well as inhibited the activation of NF-κB at the beginning of infection. | [80] |
| Influenza A Virus (PR8) | Reduced replication, induced the secretion of type I interferon (IFN) and other pro-inflammatory cytokines in vitro | [81] |
| Vesicular Stomatitis Virus (VSV) | Reduced replication, induced the secretion of type I interferon (IFN) and other pro-inflammatory cytokines in vitro | [81] |
| HSV | Reduced replication, induced the secretion of type I interferon (IFN) and other pro-inflammatory cytokines in vitro | [81] |
| Newcastle Disease Virus (NDV) | Reduced replication, induced the secretion of type I interferon (IFN) and other pro-inflammatory cytokines in vitro | [81] |
| Influenza A subtypes (H1N1, H5N2, H7N3, and H9N2) | Reduced replication, induced the secretion of type I interferon (IFN) and other pro-inflammatory cytokines in vivo | [81] |
| Dengue virus type-2 (DENV-2) | Inhibited replication, reduced the levels of ribonucleic acid (RNA) | [82] |
| Influenza virus | Inferred with viral replication by blocking endocytosis, inhibiting the activity of phosphatidylinositol 3-kinase, inhibiting RNA polymerase and other proteins, increasing antiviral response of mitochondria. | [83] |
| Influenza A viruses (IAVs) | Inhibited the activity of hemagglutinin | [84] |
| Dengue virus | Phosphorylation of NS3 | [85] |
| Singapore grouper iridovirus (SGIV) | Interfered with viral binding to target host cells | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. https://doi.org/10.3390/molecules27082494
Nguyen TLA, Bhattacharya D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules. 2022; 27(8):2494. https://doi.org/10.3390/molecules27082494
Chicago/Turabian StyleNguyen, Thi Lan Anh, and Debanjana Bhattacharya. 2022. "Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle" Molecules 27, no. 8: 2494. https://doi.org/10.3390/molecules27082494






