Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours
Abstract
:1. Introduction
2. Materials and Experimental Methodology
2.1. Raw Material
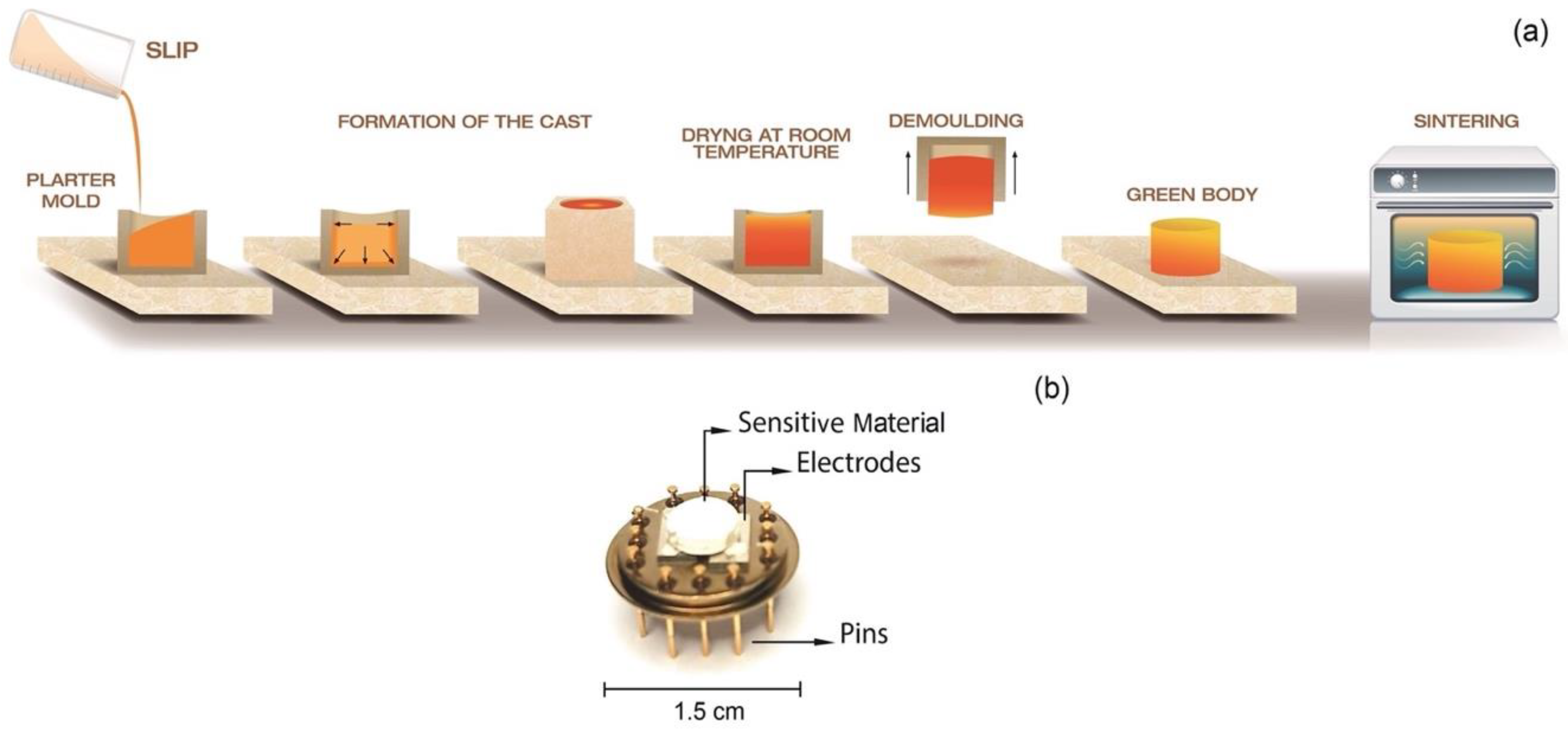
2.2. Sensor Fabrication
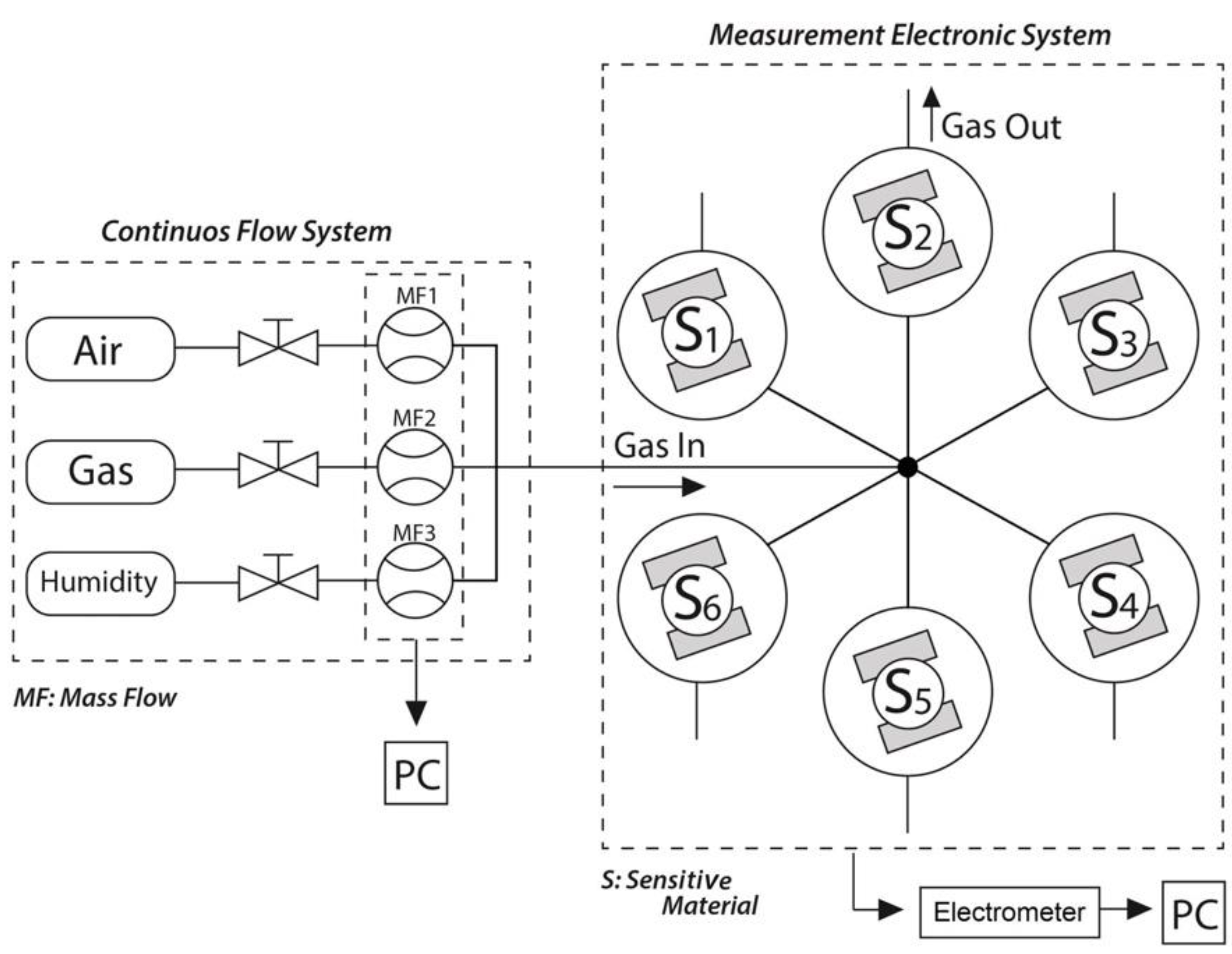
2.3. Sensor Testing
3. Results and Discussion
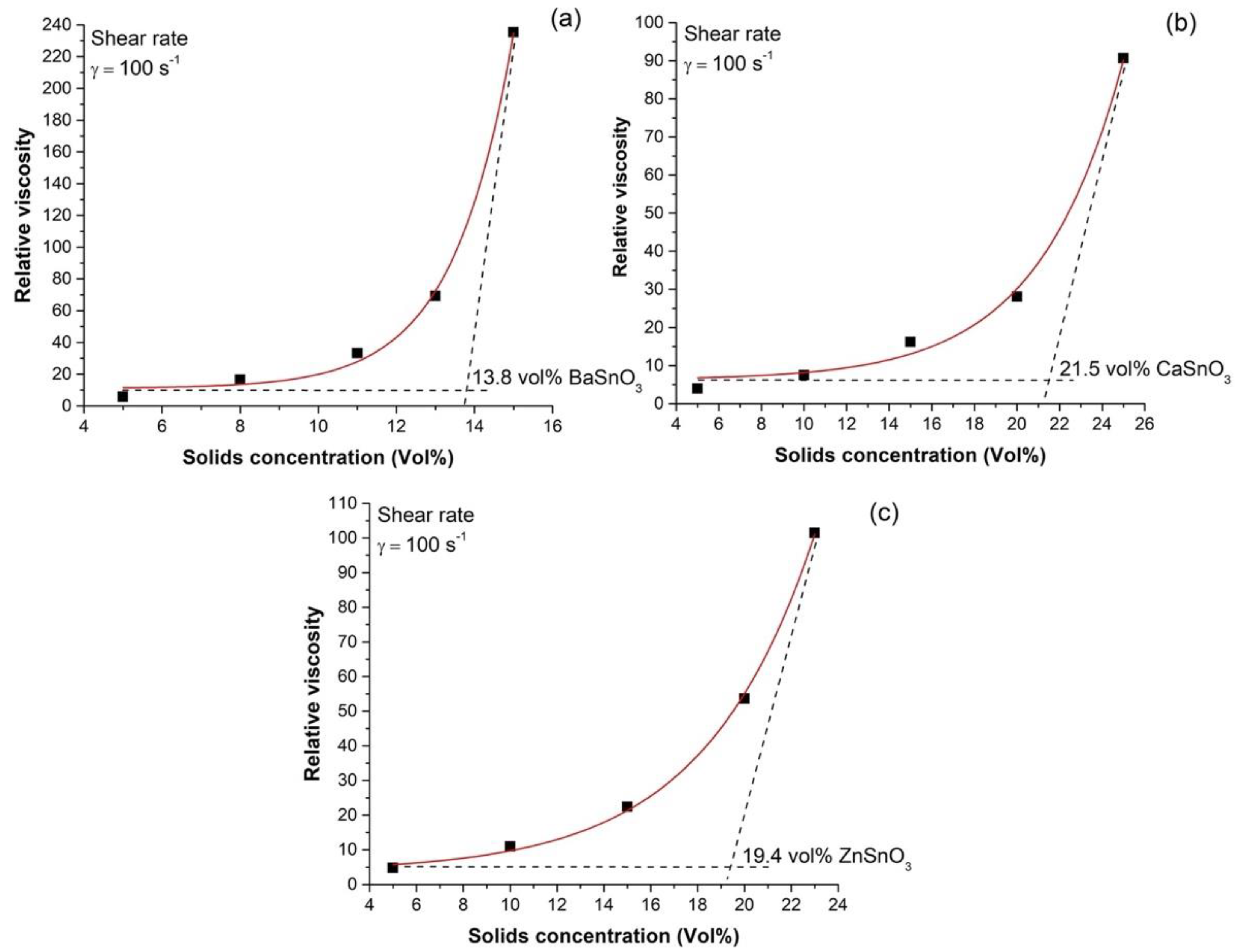
3.1. Optimum Loading of Solids in the Suspensions
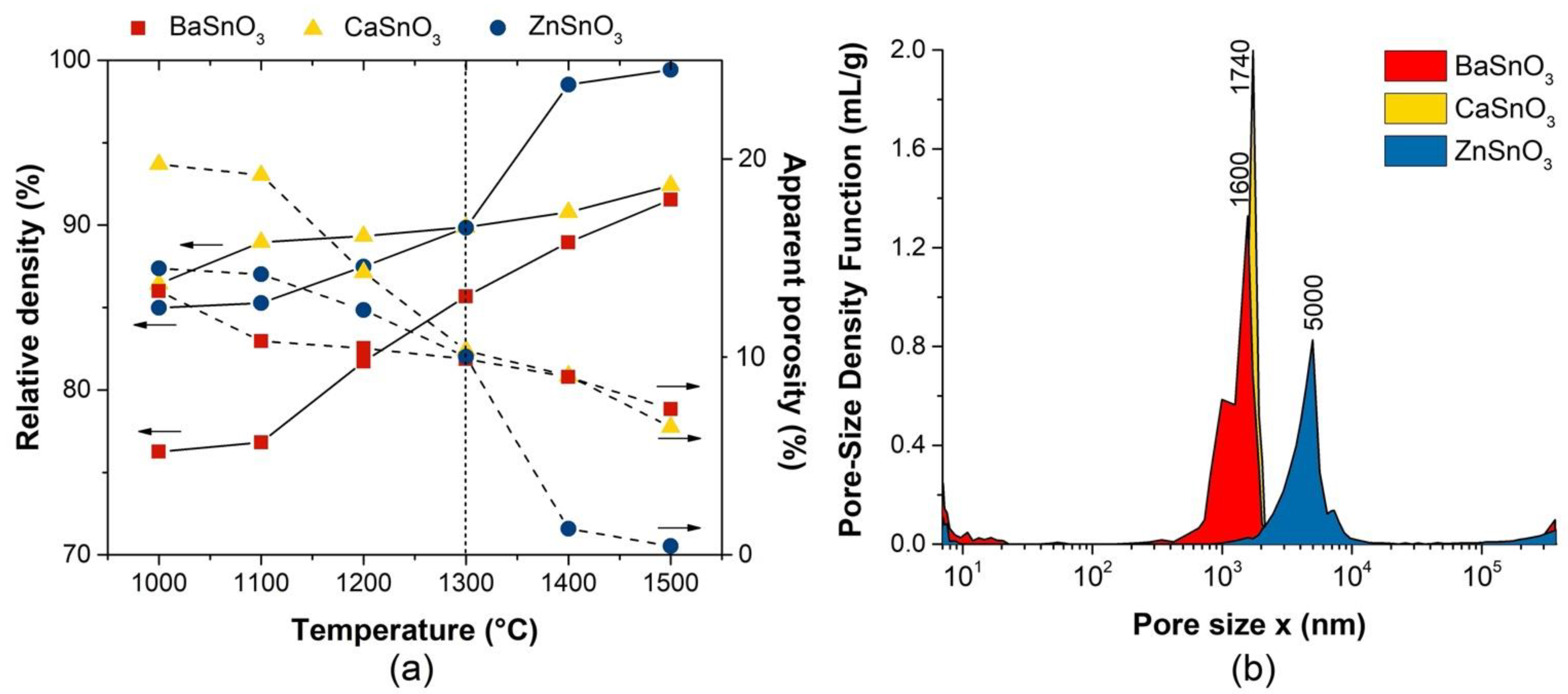
3.2. Densification and Porosity of Gas-Sensitive Materials
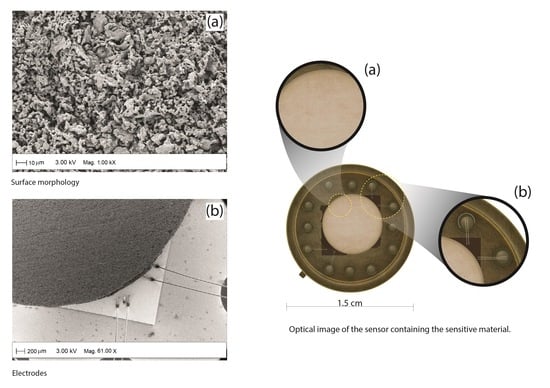
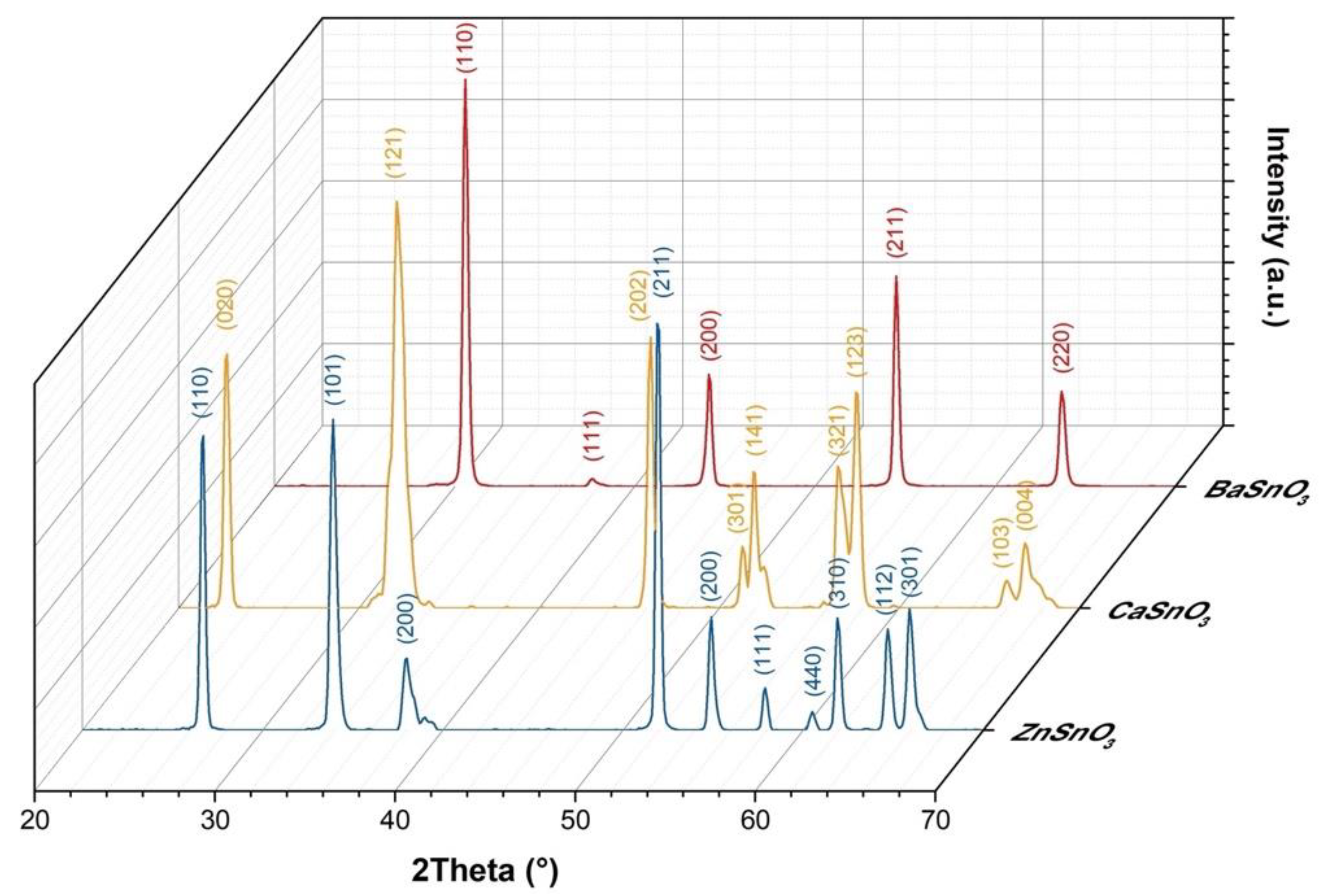
3.3. Structural, Compositional, and Morphological Studies
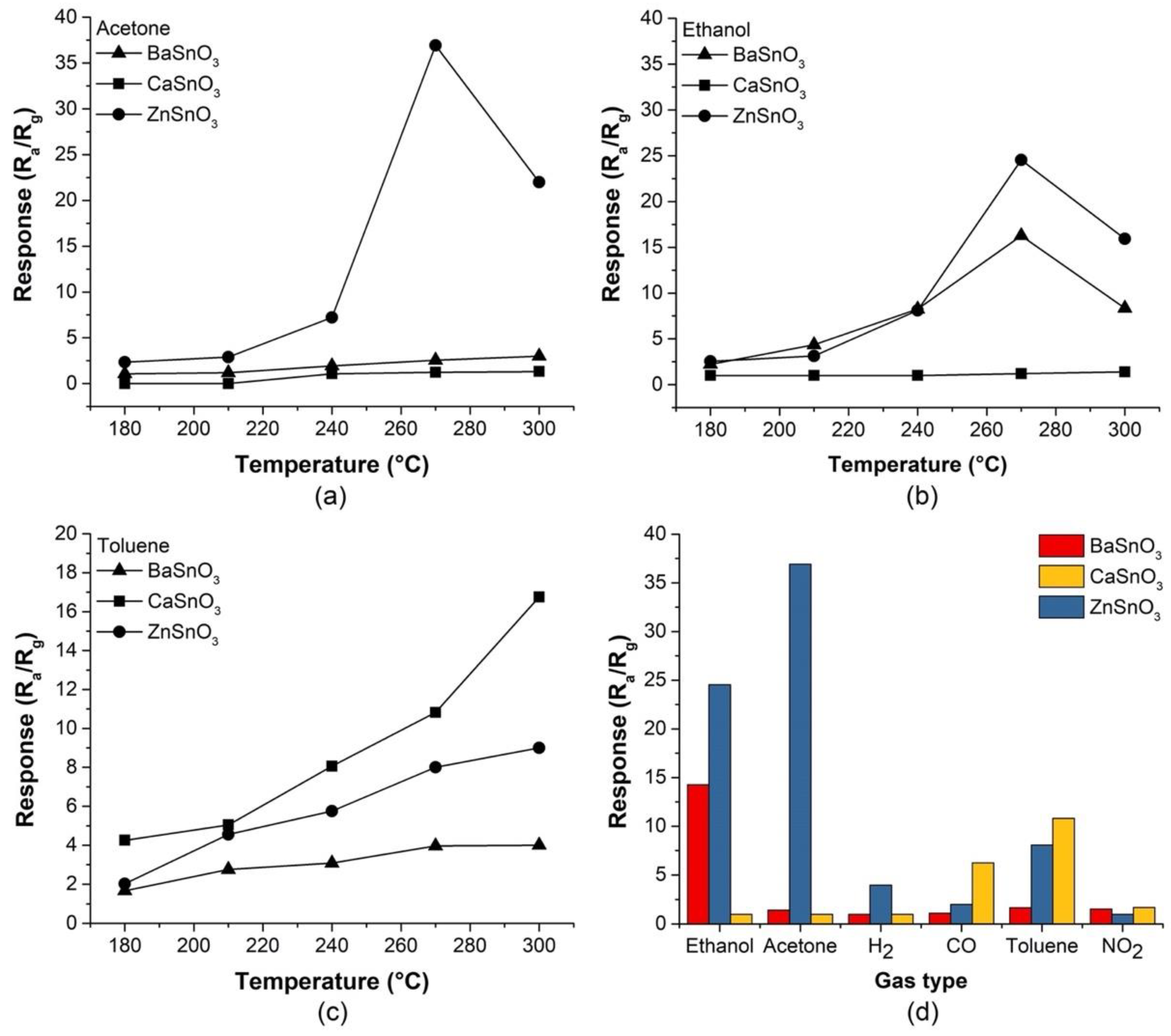
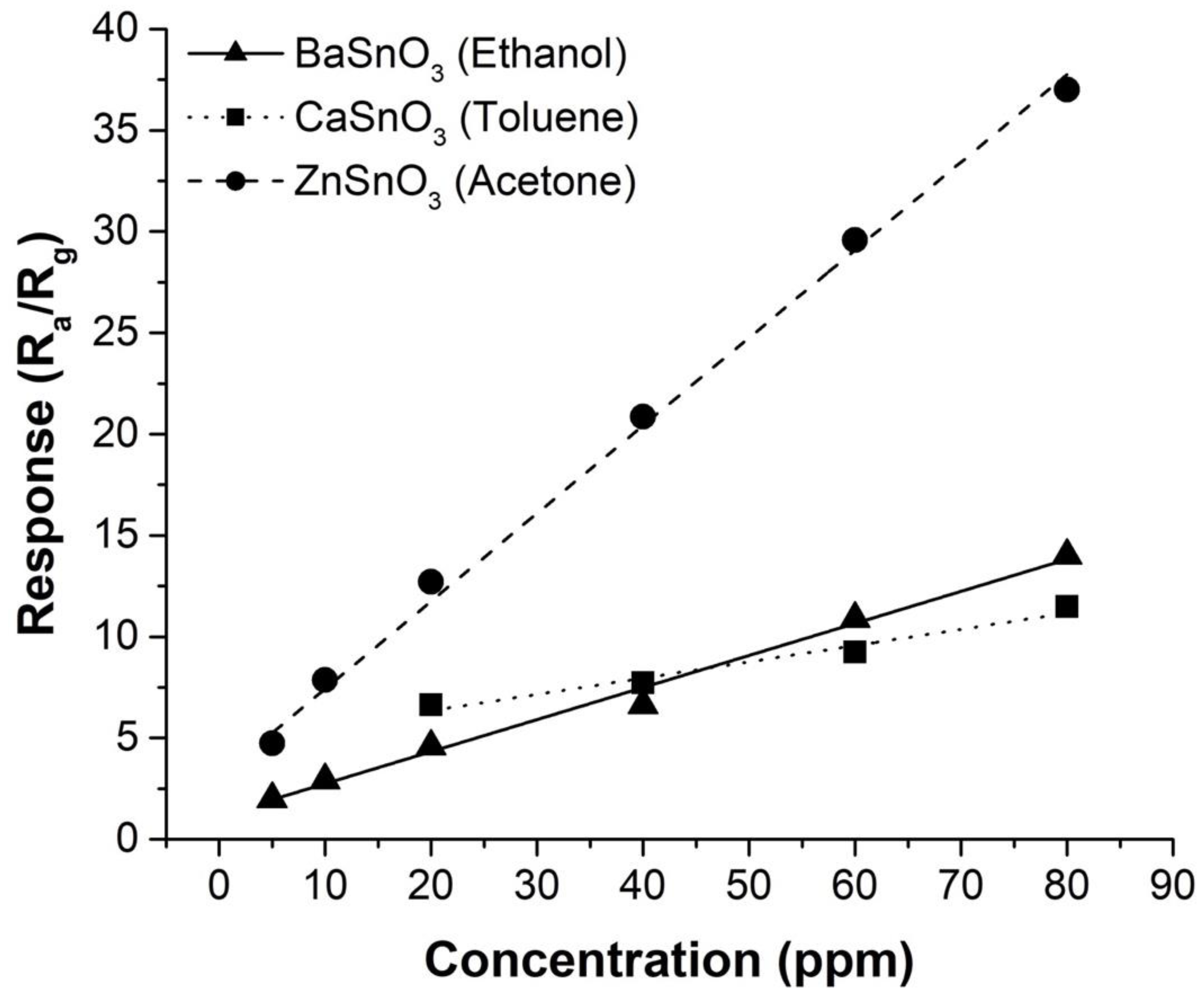
3.4. Gas Sensing Properties
3.5. Gas Sensing Mechanism
3.6. The Influence of Humidity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Burgués, J.; Marco, S. Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors. Sensors 2018, 18, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdcharoen, T.; Wongchoosuk, C. Carbon nanotube and metal oxide hybrid materials for gas sensing. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 386–407. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spannhake, J.; Helwig, A.; Schulz, O.; Müller, G. Micro-Fabrication of Gas Sensors. In Solid State Gas Sensing; Springer: Boston, MA, USA, 2009; pp. 1–46. [Google Scholar] [CrossRef]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. A Metal Oxide Gas Sensors Array for Lung Cancer Diagnosis Through Exhaled Breath Analysis. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1584–1587. [Google Scholar] [CrossRef]
- Ram, S. Metal Oxide Nanostructures as Gas Sensing Devices, G. Eranna. Mater. Manuf. Process. 2013, 28, 1277–1278. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Yamani, Z.H. Silver Nanoparticle-Decorated Tin Oxide Thin Films: Synthesis, Characterization, and Hydrogen Gas Sensing. Front. Mater. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: A case study on the {221} high-index faceted SnO2 octahedra. CrystEngComm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Kakati, N.; Jee, S.H.; Kim, S.H.; Lee, H.-K.; Yoon, Y.S. Sensitivity Enhancement of ZnO Nanorod Gas Sensors with Surface Modification by an InSb Thin Film. Jpn. J. Appl. Phys. 2009, 48, 105002. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.; Lee, J.-H. Design of Highly Selective Gas Sensors via Physicochemical Modification of Oxide Nanowires: Overview. Sensors 2016, 16, 1531. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sensors Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Tanaka, H.; Misono, M. Advances in designing perovskite catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Zaza, F.; Pallozzi, V.; Serra, E. Optimization of Working Conditions for Perovskite-Based Gas Sensor Devices by Multiregression Analysis. J. Nanotechnol. 2019, 2019, 4628765. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiliute, R.M.; Seibutas, P.; Ivanov, M.; Grigalaitis, R.; Stanulis, A.; Banys, J.; Kareiva, A. Dielectric and impedance spectroscopy of BaSnO3 and Ba2SnO4. Ferroelectrics 2014, 464, 49–58. [Google Scholar] [CrossRef]
- Deepa, K.; Angel, S.L.; Rajamanickam, N.; Jayakumar, K.; Ramachandran, K. Structural and dielectric studies on Ag doped nano ZnSnO3. AIP Conf. Proc. 2018, 1942, 3–7. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, B.; Zeng, H. Microstructure and Properties of Functional Ceramics. In Microstructure, Property and Processing of Functional Ceramics; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–111. [Google Scholar] [CrossRef]
- Choi, Y.-Y.; Choi, K.-H.; Lee, H.; Lee, H.; Kang, J.-W.; Kim, H.-K. Nano-sized Ag-inserted amorphous ZnSnO3 multilayer electrodes for cost-efficient inverted organic solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1615–1623. [Google Scholar] [CrossRef]
- Xie, F.; Li, Y.; Xiao, T.; Shen, D.; Wei, M. Efficiency improvement of dye-sensitized BaSnO3 solar cell based surface treatments. Electrochim. Acta 2018, 261, 23–28. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, Y.; Zhang, X.; Guo, H.; Lu, A.; Wang, L.; Yue, G.; Peng, D.-L. Synthesis of amorphous ZnSnO3-C hollow microcubes as advanced anode materials for lithium ion batteries. Electrochim. Acta 2014, 141, 374–383. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Reddy, M.V.; Ozoemena, K.I.; Ezema, F.I.; Balakrishna, R.G.; Chowdari, B.V.R. Electrochemical Performance of BaSnO3 Anode Material for Lithium-Ion Battery Prepared by Molten Salt Method. J. Electrochem. Soc. 2016, 163, A540–A545. [Google Scholar] [CrossRef]
- Mouyane, M.; Womes, M.; Jumas, J.C.; Olivier-Fourcade, J.; Lippens, P.E. Original electrochemical mechanisms of CaSnO3 and CaSnSiO5 as anode materials for Li-ion batteries. J. Solid State Chem. 2011, 184, 2877–2886. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. Biomorphic synthesis of ZnSnO3 hollow fibers for gas sensing application. Sens. Actuators B Chem. 2011, 156, 983–989. [Google Scholar] [CrossRef]
- Kumar, S.; Pugh, D.; Dasgupta, D.; Sarin, N.; Parkin, I.; Luthra, V. Tuning operating temperature of BaSnO3 gas sensor for reducing and oxidizing gases. AIP Conf. Proc. 2018, 1953, 090059. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Lu, Z. Synthesis and gas-sensing properties of CaSnO3 microcubes. Solid State Sci. 2008, 10, 1042–1048. [Google Scholar] [CrossRef]
- Zheng, J.; Hou, H.; Fu, H.; Gao, L.; Liu, H. Size-controlled synthesis of porous ZnSnO3 nanocubes for improving formaldehyde gas sensitivity. RSC Adv. 2021, 11, 20268–20277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, X.; Lian, D.; Yang, J.; Wang, S.; Li, Y.; Song, H. Enhanced selective acetone gas sensing performance by fabricating ZnSnO3/SnO2 concave microcube. Appl. Surf. Sci. 2021, 542, 148555. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zhu, X.; Xia, M.; Tao, T.; Leng, B.; Xu, W. Improving ethanol sensitivity of ZnSnO3 sensor at low temperature with multi-measures: Mg doping, nano-TiO2 decoration and UV radiation. Sens. Actuators B Chem. 2019, 297, 126745. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Leng, B.; Ma, J.; Qi, Z.; Tao, T.; Wang, M. Enhanced gas sensing properties for ethanol of Ag@ZnSnO3 nano-composites. J. Mater. Sci. Mater. Electron. 2020, 31, 18649–18663. [Google Scholar] [CrossRef]
- Bing, Y.; Zeng, Y.; Liu, C.; Qiao, L.; Sui, Y.; Zou, B.; Zheng, W.; Zou, G. Assembly of hierarchical ZnSnO3 hollow microspheres from ultra-thin nanorods and the enhanced ethanol-sensing performances. Sens. Actuators B Chem. 2014, 190, 370–377. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, B.; Fu, M.; Wang, C.; Peng, R. One pot synthesis of hierarchical and porous ZnSnO3 nanocubes and gas sensing properties to formaldehyde. Results Phys. 2019, 15, 102606. [Google Scholar] [CrossRef]
- Kim, H.; Jin, C.; Park, S.; Lee, C. Synthesis, Structure and Gas-Sensing Properties of Pd-Functionalized ZnSnO3 Rods. J. Nanosci. Nanotechnol. 2013, 13, 533–536. [Google Scholar] [CrossRef]
- Dabbabi, S.; Nasr, T.B.; Madouri, A.; Cavanna, A.; Garcia-Loureiro, A.; Kamoun, N. Fabrication and Characterization of Sensitive Room Temperature NO2 Gas Sensor Based on ZnSnO3 Thin Film. Phys. Status Solidi 2019, 216, 1900205. [Google Scholar] [CrossRef]
- Patil, L.A.; Pathan, I.G.; Suryawanshi, D.N.; Bari, A.R.; Rane, D.S. Spray Pyrolyzed ZnSnO3 Nanostructured Thin Films for Hydrogen Sensing. Procedia Mater. Sci. 2014, 6, 1557–1565. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, Y.H.; Schipani, F.; Aldao, C.M.; Rodríguez-Páez, J.E.; Ponce, M.A. Modification of sensitivity of BaSnO3 sensor due to parameters of synthesis and formation of the device. J. Mater. Res. 2015, 30, 3423–3430. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Baranchikov, A.; Gaskov, A. Nanocrystalline BaSnO3 as an Alternative Gas Sensor Material: Surface Reactivity and High Sensitivity to SO2. Materials 2015, 8, 6437–6454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaff, G. Wet chemical synthesis of BaSnO3 and Ba2SnO4 powders. J. Eur. Ceram. Soc. 1993, 12, 159–164. [Google Scholar] [CrossRef]
- Pfaff, G. Chemical synthesis of calcium stannates from peroxo precursors. Mater. Sci. Eng. B 1995, 33, 156–161. [Google Scholar] [CrossRef]
- Azad, A.M.; Hashim, M.; Baptist, S.; Badri, A.; Haq, A.U. Phase evolution and microstructural development in sol-gel derived MSnO3 (M = Ca, Sr and Ba). J. Mater. Sci. 2000, 35, 5475–5483. [Google Scholar] [CrossRef]
- Köferstein, R.; Jäger, L.; Zenkner, M.; Ebbinghaus, S.G. Nano-sized BaSnO3 powder via a precursor route: Comparative study of sintering behaviour and mechanism of fine and coarse-grained powders. J. Eur. Ceram. Soc. 2009, 29, 2317–2324. [Google Scholar] [CrossRef] [Green Version]
- Köferstein, R.; Jäger, L.; Zenkner, M.; Müller, T.; Ebbinghaus, S.G. The influence of the additive BaGeO3 on BaSnO3 ceramics. J. Eur. Ceram. Soc. 2010, 30, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Rogatz, A.L. Ceramic Condenser Dielectric. U.S. Patent 2815291, 3 December 1957. [Google Scholar]
- Abe, Y.; Nakayama, T.; Ohara, G.; Wake, R. Oxide Sintered Body Comprising Zinc Oxide Phase and Zinc Stannate Compound Phase. U.S. Patent 7976738B2, 12 July 2011. [Google Scholar]
- Pugh, R.J.; Bergstrom, L. Surface and Colloid Chemistry in Advanced Ceramics Processing; Pugh, R.J., Bergstrom, L., Eds.; Surfactant Science Series; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 1994; Volume 51. [Google Scholar]
- Adair, J.; Casey, J.A. Science, Technology, and Application of Colloidal Suspensions (Ceramic Transactions); American Ceramic Society: Columbus, OH, USA, 1995. [Google Scholar]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.-B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef] [Green Version]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.-P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Tohver, V.; Morissette, S.L.; Lewis, J.A.; Tuttle, B.A.; Voigt, J.A.; Dimos, D.B. Direct-Write Fabrication of Zinc Oxide Varistors. J. Am. Ceram. Soc. 2004, 85, 123–128. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-printing inks for the fabrication of solid oxide fuel cell films: A review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Nishihora, R.K.; Rachadel, P.L.; Quadri, M.G.N.; Hotza, D. Manufacturing porous ceramic materials by tape casting—A review. J. Eur. Ceram. Soc. 2018, 38, 988–1001. [Google Scholar] [CrossRef]
- Aguilar-Paz, C.J.; Ochoa-Muñoz, Y.; Ponce, M.A.; Rodríguez-Páez, J.E. Electrical Behavior of SnO2 Polycrystalline Ceramic Pieces Formed by Slip Casting: Effect of Surrounding Atmosphere (Air and CO). J. Electron. Mater. 2016, 45, 576–593. [Google Scholar] [CrossRef]
- Ochoa, Y.H.; Schipani, F.; Aldao, C.M.; Ponce, M.A.; Savu, R.; Rodríguez-Páez, J.E. Electrical behavior of BaSnO3 bulk samples formed by slip casting: Effect of synthesis methods used for obtaining the ceramic powders. Mater. Res. Bull. 2016, 78, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.P.; Kim, J.H.; Park, J.K.; Huh, M.Y. Microstructure and gas-sensing properties of thick film sensor using nanophase SnO2 powder. Sens. Actuators B Chem. 2004, 99, 18–24. [Google Scholar] [CrossRef]
- Bârsan, N.; Grigorovici, R.; Ionescu, R.; Motronea, M.; Vancu, A. Mechanism of gas detection in polycrystalline thick film SnO2 sensors. Thin Solid Film. 1989, 171, 53–63. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Tin dioxide gas sensors. Part 1.—Aspects of the surface chemistry revealed by electrical conductance variations. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 1323. [Google Scholar] [CrossRef]
- Ochoa-Muñoz, Y.H.; Rodríguez-Páez, J.E.; Mejía de Gutiérrez, R. Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route. Mater. Chem. Phys. 2021, 266, 124557. [Google Scholar] [CrossRef]
- Ochoa-Muñoz, Y.H.; Álvarez-Láinez, M.L.; Rodríguez-Páez, J.E.; Mejía de Gutiérrez, R.; Universidad del Valle, Cali, Colombia. Unpublished work. 2021.
- Changmai, M.; Purkait, M.K. Chapter 10-Membrane adsorption. In Interface Science and Technology; Elsevier: London, UK, 2021; Volume 33, pp. 629–653. [Google Scholar] [CrossRef]
- Vallejos, S.; Grácia, I.; Chmela, O.; Figueras, E.; Hubálek, J.; Cané, C. Chemoresistive micromachined gas sensors based on functionalized metal oxide nanowires: Performance and reliability. Sens. Actuators B Chem. 2016, 235, 525–534. [Google Scholar] [CrossRef] [Green Version]
- James, K.K.; Krishnaprasad, P.S.; Hasna, K.; Jayaraj, M.K. Structural and optical properties of La-doped BaSnO3 thin films grown by PLD. J. Phys. Chem. Solids 2015, 76, 64–69. [Google Scholar] [CrossRef]
- Cerdà, J.; Arbiol, J.; Diaz, R.; Dezanneau, G.; Morante, J.R. Synthesis of perovskite-type BaSnO3 particles obtained by a new simple wet chemical route based on a sol-gel process. Mater. Lett. 2002, 56, 131–136. [Google Scholar] [CrossRef]
- Deepa, A.S.; Vidya, S.; Manu, P.C.; Solomon, S.; John, A.; Thomas, J.K. Structural and optical characterization of BaSnO3 nanopowder synthesized through a novel combustion technique. J. Alloys Compd. 2011, 509, 1830–1835. [Google Scholar] [CrossRef]
- Maul, J.; Erba, A.; Santos, I.M.G.; Sambrano, J.R.; Dovesi, R. In silico infrared and Raman spectroscopy under pressure: The case of CaSnO3 perovskite. J. Chem. Phys. 2015, 142, 014505. [Google Scholar] [CrossRef] [Green Version]
- Redfern, S.A.T.; Chen, C.-J.; Kung, J.; Chaix-Pluchery, O.; Kreisel, J.; Salje, E.K.H. Raman spectroscopy of CaSnO3 at high temperature: A highly quasi-harmonic perovskite. J. Phys. Condens. Matter 2011, 23, 425401. [Google Scholar] [CrossRef]
- Tarrida, M.; Larguem, H.; Madon, M. Structural investigations of (Ca,Sr)ZrO3 and Ca(Sn,Zr)O3 perovskite compounds. Phys. Chem. Miner. 2009, 36, 403–413. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, N.; Ren, Z.; Yang, B. Synthesis of High-Purity SnO2 Nanobelts by Using Exothermic Reaction. J. Nanomater. 2011, 2011, 526094. [Google Scholar] [CrossRef] [Green Version]
- Mayedwa, N.; Mongwaketsi, N.; Khamlich, S.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Green synthesis of zin tin oxide (ZnSnO3) nanoparticles using Aspalathus Linearis natural extracts: Structural, morphological, optical and electrochemistry study. Appl. Surf. Sci. 2018, 446, 250–257. [Google Scholar] [CrossRef]
- Leonardy, A.; Hung, W.-Z.; Tsai, D.-S.; Chou, C.-C.; Huang, Y.-S. Structural Features of SnO2 Nanowires and Raman Spectroscopy Analysis. Cryst. Growth Des. 2009, 9, 3958–3963. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhang, M.S.; Hong, J.M.; Yin, Z. Raman spectroscopic and photoluminescence study of single-crystalline SnO2 nanowires. Solid State Commun. 2006, 138, 242–246. [Google Scholar] [CrossRef]
- Lu, Y.M.; Jiang, J.; Becker, M.; Kramm, B.; Chen, L.; Polity, A.; He, Y.B.; Klar, P.J.; Meyer, B.K. Polycrystalline SnO2 films grown by chemical vapor deposition on quartz glass. Vacuum 2015, 122, 347–352. [Google Scholar] [CrossRef]
- Zhong, F.; Zhuang, H.; Gu, Q.; Long, J. Structural evolution of alkaline earth metal stannates MSnO3 (M = Ca, Sr, and Ba) photocatalysts for hydrogen production. RSC Adv. 2016, 6, 42474–42481. [Google Scholar] [CrossRef]
- Sharma, N.; Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Anodic behaviour and X-ray photoelectron spectroscopy of ternary tin oxides. J. Power Sources 2005, 139, 250–260. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Meas. Serv. Div. Natl. Inst. Stand. Technol. 2012, 19, 241–246. [Google Scholar] [CrossRef]
- Du, L.; Zhang, H.; Zhu, M.; Zhang, M. Construction of flower-like ZnSnO3/Zn2SnO4 hybrids for enhanced phenylamine sensing performance. Inorg. Chem. Front. 2019, 6, 2311–2317. [Google Scholar] [CrossRef]
- Barreca, D.; Garon, S.; Tondello, E.; Zanella, P. SnO2 Nanocrystalline Thin Films by XPS. Surf. Sci. Spectra 2000, 7, 81–85. [Google Scholar] [CrossRef]
- Stranick, M.A.; Moskwa, A. SnO2 by XPS. Surf. Sci. Spectra 1993, 2, 50–54. [Google Scholar] [CrossRef]
- Alammar, T.; Slowing, I.I.; Anderegg, J.; Mudring, A.-V. Ionic-Liquid-Assisted Microwave Synthesis of Solid Solutions of Sr1−xBaxSnO3 Perovskite for Photocatalytic Applications. ChemSusChem 2017, 10, 3387–3401. [Google Scholar] [CrossRef] [Green Version]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Ahlers, S.; Müller, G.; Doll, T. A rate equation approach to the gas sensitivity of thin film metal oxide materials. Sens. Actuators B Chem. 2005, 107, 587–599. [Google Scholar] [CrossRef]
- Upadhyay, S. High temperature impedance spectroscopy of barium stannate, BaSnO3. Bull. Mater. Sci. 2013, 36, 1019–1036. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.M.; Shyan, L.L.W.; Alim, M.A. Immittance response of CaSnO3 prepared by self-heat-sustained reaction. J. Mater. Sci. 1999, 34, 1175–1187. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Zhang, Y.; Wu, H.; Chu, X.; Dong, Y.; Liang, S.; Xu, J.; Chakraborty, A.K. Ethanol sensor based on microrod-like La-doped barium stannate. J. Mater. Sci. Mater. Electron. 2020, 31, 17461–17473. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chu, X.; Gao, Q.; Li, X.; Dong, Y.; Liang, S.; Chakraborty, A.K. Influence of Gd+3 incorporation on ethanol sensing properties of Barium Stannate microrod films prepared by coprecipitation method. Appl. Surf. Sci. 2020, 504, 144289. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, S.Y.; Jiao, H.Y.; Zhang, G.H.; Chen, H.; Xu, X.L.; Yang, H.M.; Qiang, Z. Synthesis of novel ZnSnO3 hollow polyhedrons with open nanoholes: Enhanced acetone-sensing performance. Ceram. Int. 2017, 43, 1617–1621. [Google Scholar] [CrossRef]
- Yin, Y.; Li, F.; Zhang, N.; Ruan, S.; Zhang, H.; Chen, Y. Improved gas sensing properties of silver-functionalized ZnSnO3 hollow nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, M.; Ma, S.; Wang, P.; Zhang, G.; Chen, W.; Jiao, H.; Liu, L.; Xu, X. Enhanced acetone sensor based on Au functionalized In-doped ZnSnO3 nanofibers synthesized by electrospinning method. J. Colloid Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Lv, L.; Wang, Y.; Zhang, B.; Zhang, Y.; Zhang, Y.; Lei, Z.; Xu, L. SnO2/ZnSnO3 double-shelled hollow microspheres based high-performance acetone gas sensor. Sens. Actuators B Chem. 2021, 332, 129212. [Google Scholar] [CrossRef]
- Wang, G.; Bai, J.; Shan, C.; Zhang, D.; Lu, N.; Liu, Q.; Zhou, Z.; Wang, S.; Liu, C. Synthesis and ethanol gas sensing properties of mesoporous perovskite-type BaSnO3 nanoparticles interconnected network. Mater. Lett. 2017, 205, 169–172. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, S.; Huang, C.; Guo, X.; Zhu, Y.; Thomas, T.; Guo, H.; Attfield, J.P.; Yang, M. Surface Functionalized Sensors for Humidity-Independent Gas Detection. Angew. Chem. Int. Ed. 2021, 60, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J.R. Effect of air humidity on gas response of SnO2 thin film ozone sensors. Sens. Actuators B Chem. 2007, 122, 519–526. [Google Scholar] [CrossRef]



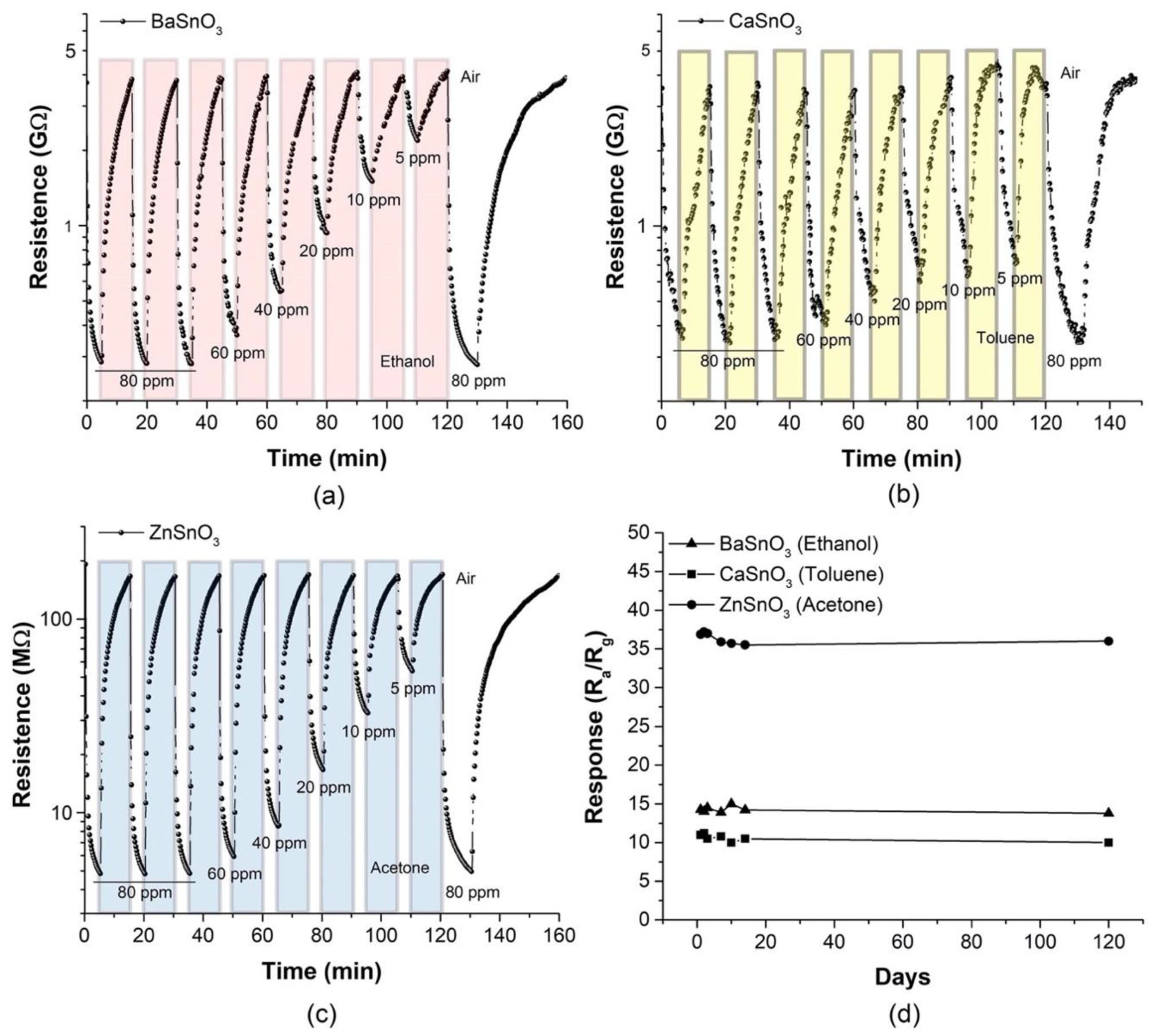
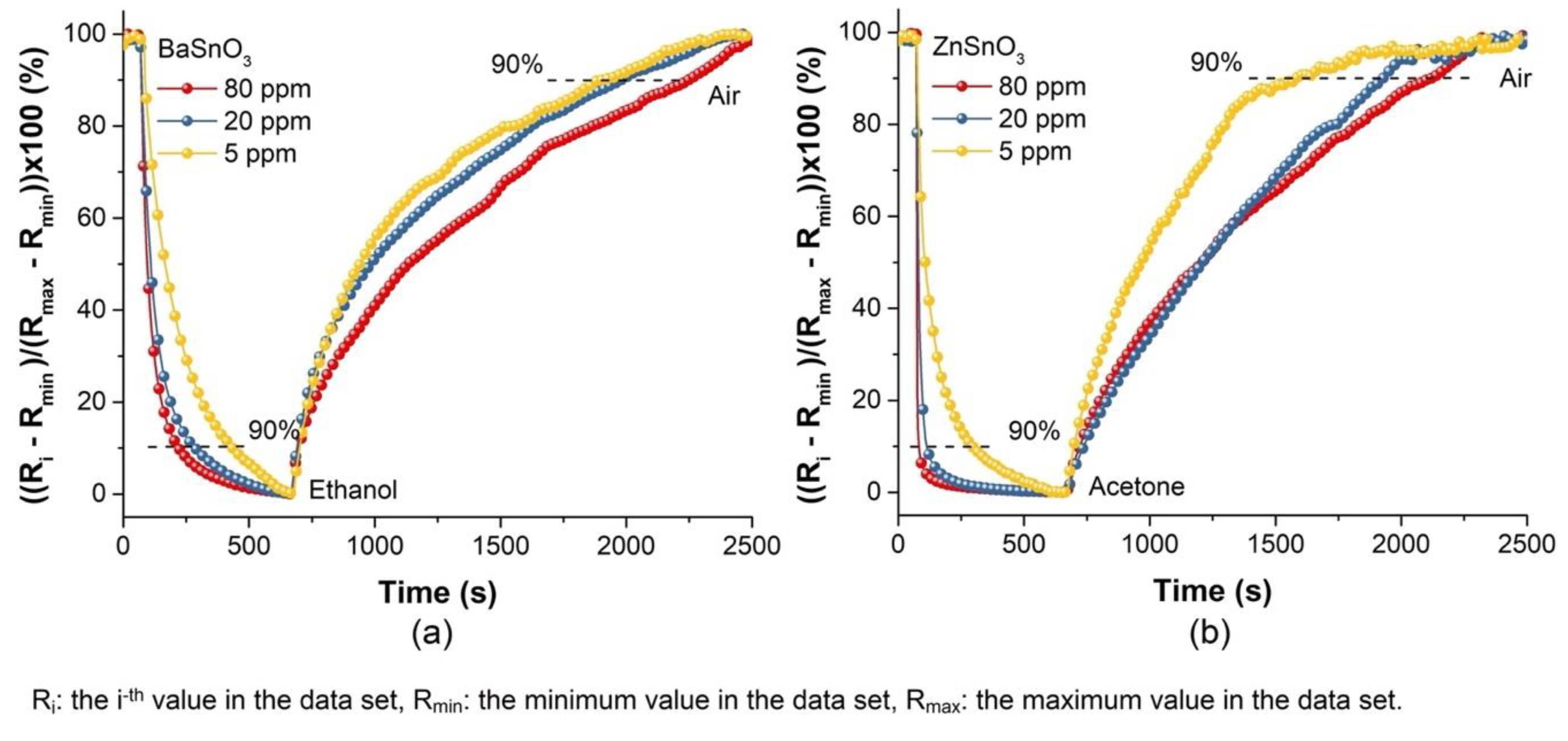
| Gas Concentration (ppm) | BaSnO3 (Ethanol) | ZnSO3 (Acetone) | ||
|---|---|---|---|---|
| tres (s) | trec (s) | tres (s) | trec (s) | |
| 80 | 72 | 1385 | 4 | 1285 |
| 20 | 98 | 1125 | 23 | 1159 |
| 5 | 219 | 1068 | 117 | 696 |
| Sensing Material | Operating Temperature (°C) | Concentration (ppm) | Response (Ra/Rg) | Response/Recovery Time (s) | Year/Ref. |
|---|---|---|---|---|---|
| ZnSnO3 porous bodies | 270 | 80 | 37 | 4/581 | In this work |
| ZnSnO3 hollow polyhedrons with open nanoholes | 240 | 50 | 12.48 | 17/10 | 2017/[84] |
| Silver-functionalized ZnSnO3 hollow nanocubes | 280 | 100 | 30 | 2/3 | 2018/[85] |
| Au functionalized In-doped ZnSnO3 nanofibers | 200 | 50 | 19.3 | 10/13 | 2019/[86] |
| Double-shell hollow SnO2/ZnSnO3 spheres | 290 | 100 | 30 | 5/115 | 2021/[87] |
| BaSnO3 porous bodies | 270 | 80 | 14.3 | 72/596 | In this work |
| Nanocrystalline BaSnO3 | 300 | 20 | ~ 12.5 | – | 2015/[36] |
| Mesoporous BaSnO3 nanoparticles interconnected network | 350 | 100 | 34.3 | 10/50 | 2017/[88] |
| La-doped BaSnO3 | 220 | 100 | 48 | 5/12 | 2020/[82] |
| Gd-doped BaSnO3 | 220 | 500 | 76 | – | 2020/[83] |
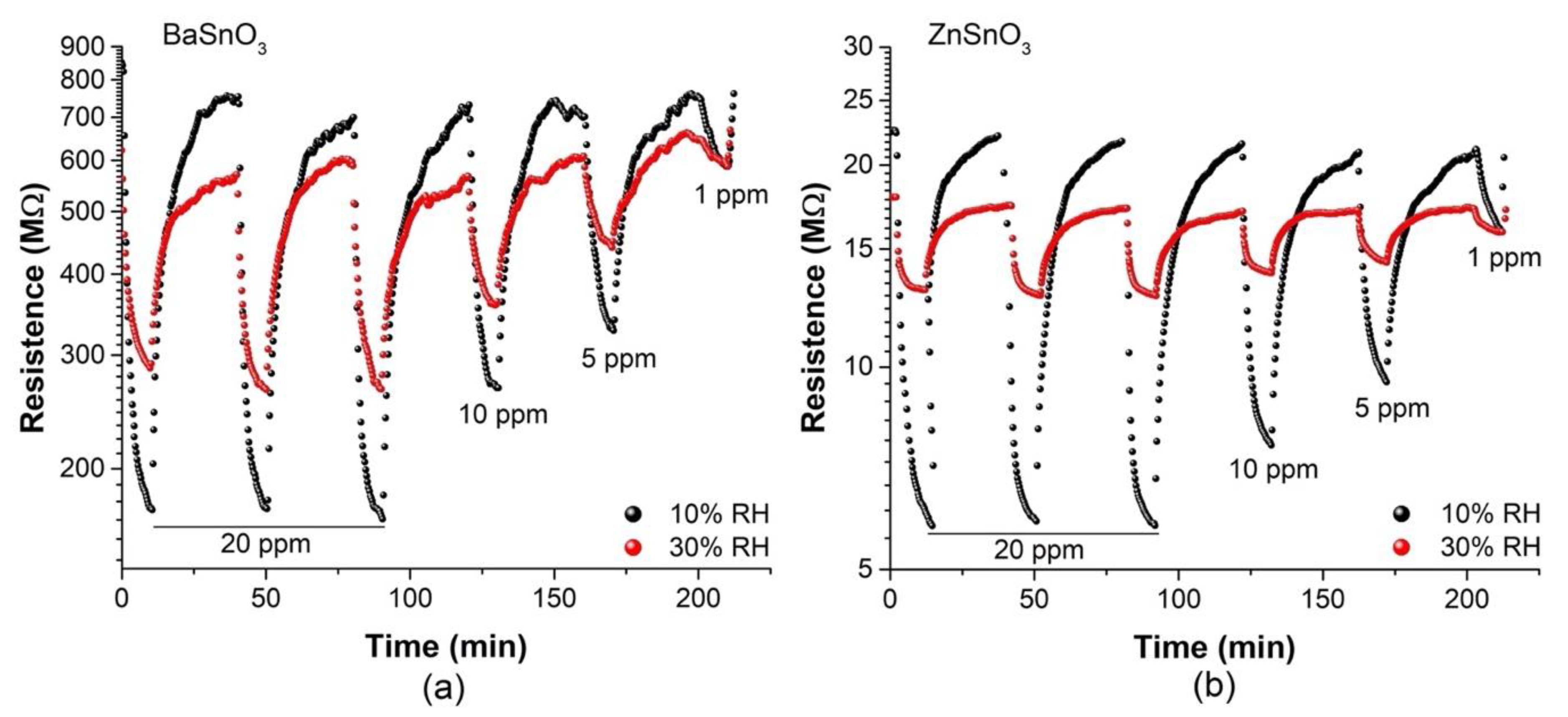
| Gas Concentration (ppm) | BaSnO3 (Ethanol) | ZnSO3 (Acetone) | ||
|---|---|---|---|---|
| tres (s) | trec (s) | tres (s) | trec (s) | |
| 10/30% RH | 10/30% RH | 10/30% RH | 10/30% RH | |
| 20 | 105/146 | 1065/971 | 45/52 | 1218/856 |
| 5 | 256/274 | 1003/873 | 146/158 | 1087/813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Muñoz, Y.H.; Mejía de Gutiérrez, R.; Rodríguez-Páez, J.E.; Gràcia, I.; Vallejos, S. Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours. Molecules 2022, 27, 2889. https://doi.org/10.3390/molecules27092889
Ochoa-Muñoz YH, Mejía de Gutiérrez R, Rodríguez-Páez JE, Gràcia I, Vallejos S. Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours. Molecules. 2022; 27(9):2889. https://doi.org/10.3390/molecules27092889
Chicago/Turabian StyleOchoa-Muñoz, Yasser H., Ruby Mejía de Gutiérrez, Jorge E. Rodríguez-Páez, Isabel Gràcia, and Stella Vallejos. 2022. "Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours" Molecules 27, no. 9: 2889. https://doi.org/10.3390/molecules27092889
APA StyleOchoa-Muñoz, Y. H., Mejía de Gutiérrez, R., Rodríguez-Páez, J. E., Gràcia, I., & Vallejos, S. (2022). Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours. Molecules, 27(9), 2889. https://doi.org/10.3390/molecules27092889