Rapid Sample Screening Method for Authenticity Controlling of Vanilla Flavours Using Liquid Chromatography with Electrochemical Detection Using Aluminium-Doped Zirconia Nanoparticles-Modified Electrode
Abstract
:1. Introduction
2. Results
2.1. Characterisation of Aluminium-Doped ZrO2 Nanoparticles (Al-ZrO2-NPs)
2.2. Optimisation the Experimental Parameters
2.3. Validation of the Methodology
2.4. Analytical Application
3. Materials and Methods
3.1. Materials and Standards
3.2. Instruments and Apparatus
3.3. Preparation of Aluminum-Doped ZrO2 Nanoparticles (Al-ZrO2-NPs)
3.4. Preparation of Modified Electrode
3.5. Sample Preparation
3.6. Chromatographic Conditions and Amperometric Detection at Al-ZrO2-NPs/SPCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. Simultaneous determination of tocopherols, retinol, ester derivatives and β-carotene in milk- and soy-juice based beverages by HPLC with diode-array detection. LWT-Food Sci. Technol. 2014, 58, 557–562. [Google Scholar] [CrossRef]
- Murtada, K.; Jodeh, S.; Zougagh, M.; Ríos, Á. Development of an Aluminium Doped TiO2 Nanoparticles-modified Screen Printed Carbon Electrode for Electrochemical Sensing of Vanillin in Food Samples. Electroanalysis 2018, 30, 969–974. [Google Scholar] [CrossRef]
- Sinha, A.K.; Verma, S.C.; Sharma, U.K. Development and validation of an RP-HPLC method for quantitative determination of vanillin and related phenolic compounds in Vanilla planifolia. J. Sep. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Paillat, L.; Périchet, C.; Lavoine, S.; Meierhenrich, U.; Fernandez, X. Validated high-performance thin-layer chromatography (HPTLC) method for quantification of vanillin β-d-glucoside, and Four major phenolic compounds in vanilla (Vanilla planifolia) fruits, beans, and extracts. JPC-J. Planar Chromatogr.-Mod. TLC 2012, 25, 295–300. [Google Scholar] [CrossRef]
- De Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Comparison of headspace-SPME-GC-MS and LC-MS for the detection and quantification of coumarin, vanillin, and ethyl vanillin in vanilla extract products. Food Chem. 2008, 107, 1701–1709. [Google Scholar] [CrossRef]
- Maruenda, H.; Vico, M.D.L.; Householder, J.E.; Janovec, J.P.; Cañari, C.; Naka, A.; Gonzalez, A.E. Exploration of Vanilla pompona from the Peruvian Amazon as a potential source of vanilla essence: Quantification of phenolics by HPLC-DAD. Food Chem. 2013, 138, 161–167. [Google Scholar] [CrossRef]
- Lahouidak, S.; Salghi, R.; Zougagh, M.; Ríos, A. Capillary electrophoresis method for the discrimination between natural and artificial vanilla flavor for controlling food frauds. Electrophoresis 2018, 39, 1628–1633. [Google Scholar] [CrossRef]
- Cantalapiedra, A.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Sensitive and selective determination of phenolic compounds from aromatic plants using an electrochemical detection coupled with HPLC method. Phytochem. Anal. 2014, 25, 247–254. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Mümin, Y. Simultaneous Electrochemical Determination of Caffeine and Vanillin by Using Poly(Alizarin Red S) Modified Glassy Carbon Electrode. Food Anal. Methods 2017, 10, 31–40. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H.C. An Amperometric Sensor Based on Tin Dioxide and Cetylpyridinium Bromide Nanoparticles for the Determination of Vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Ávila, M.; González, M.C.; Zougagh, M.; Escarpa, A.; Ríos, Á. Rapid sample screening method for authenticity controlling vanilla flavors using a CE microchip approach with electrochemical detection. Electrophoresis 2007, 28, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.; Zougagh, M.; Escarpa, A.; Ríos, Á. Fast single run of vanilla fingerprint markers on microfluidic-electrochemistry chip for confirmation of common frauds. Electrophoresis 2009, 30, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- Ekabutr, P.; Chailapakul, O.; Supaphol, P. Modification of Disposable Screen-Printed Carbon Electrode Surfaces with Conductive Electrospun Nanofibers for Biosensor Applications. J. Appl. Polym. Sci. 2013, 130, 3885–3893. [Google Scholar] [CrossRef]
- Bettazzi, F.; Palchetti, I.; Sisalli, S.; Mascini, M. A disposable electrochemical sensor for vanillin detection. Anal. Chim. Acta 2006, 555, 134–138. [Google Scholar] [CrossRef]
- Murtada, K.; Moreno, V.; Ríos, Á.; Zougagh, M. Decoration of graphene oxide with copper selenide in supercritical carbon dioxide medium as a novel approach for electrochemical sensing of eugenol in various samples. J. Supercrit. Fluids 2019, 153, 104597. [Google Scholar] [CrossRef]
- Peng, J.; Hou, C.; Hu, X. A Graphene-Based Electrochemical Sensor for Sensitive Detection of Vanillin. Int. J. Electrochem. 2012, 7, 1724–1733. [Google Scholar]
- Murtada, K.; Salghi, R.; Ríos, A.; Zougagh, M. A sensitive electrochemical sensor based on aluminium doped copper selenide nanoparticles-modified screen printed carbon electrode for determination of L-tyrosine in pharmaceutical samples. J. Electroanal. Chem. 2020, 874, 114466. [Google Scholar] [CrossRef]
- Huang, L.; Hou, K.; Jia, X.; Pan, H.; Du, M. Preparation of novel silver nanoplates/graphene composite and their application in vanillin electrochemical detection. Mater. Sci. Eng. C 2014, 38, 39–45. [Google Scholar] [CrossRef]
- Lopes, F.; Pacheco, J.G.; Rebelo, P.; Delerue-matos, C. Molecularly imprinted electrochemical sensor prepared on a screen printed carbon electrode for naloxone detection. Sens. Actuators B Chem. 2017, 243, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Alahi, M.E.E.; Mukhopadhyay, S.C.; Liu, Z. Multi-walled carbon nanotubes-based sensors for strain sensing applications. Sensors 2021, 21, 1261. [Google Scholar] [CrossRef]
- Benmassaoud, Y.; Murtada, K.; Salghi, R.; Zougagh, M.; Ríos, Á. Surface polymers on multiwalled carbon nanotubes for selective extraction and electrochemical determination of rhodamine B in food samples. Molecules 2021, 26, 2670. [Google Scholar] [CrossRef] [PubMed]
- Murtada, K.; Moreno, V. Nanomaterials-based electrochemical sensors for the detection of aroma compounds—Towards analytical approach. J. Electroanal. Chem. 2020, 861, 113988. [Google Scholar] [CrossRef]
- Ranadive, A.S. Vanillin and related flavor compounds in vanilla extracts made from beans of various global origins. J. Agric. Food Chem. 1992, 40, 1922–1924. [Google Scholar] [CrossRef]
- Longares-Patrón, A.; Cañizares-Macías, M.P. Focused microwaves-assisted extraction and simultaneous spectrophotometric determination of vanillin and p-hydroxybenzaldehyde from vanilla fragans. Talanta 2006, 69, 882–887. [Google Scholar] [CrossRef]
- John, T.V.; Jamin, E. Chemical Investigation and Authenticity of Indian Vanilla Beans. J. Agric. Food Chem. 2004, 52, 7644–7650. [Google Scholar] [CrossRef]
- Huesgen, A.G. Analysis of natural and artificial vanilla preparations. Agil. Appl. Note 2011, 1–11. [Google Scholar]
- Hardcastle, J.L.; Paterson, C.J.; Compton, R.G. Biphasic Sonoelectroanalysis: Simultaneous Extraction from, and Determination of Vanillin in Food Flavoring. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 899–905. [Google Scholar] [CrossRef]
- Luque, M.; Luque-Pérez, E.; Ríos, Á.; Valcárcel, M. Supported liquid membranes for the determination of vanillin in food samples with amperometric detection. Anal. Chim. Acta 2000, 410, 127–134. [Google Scholar] [CrossRef]
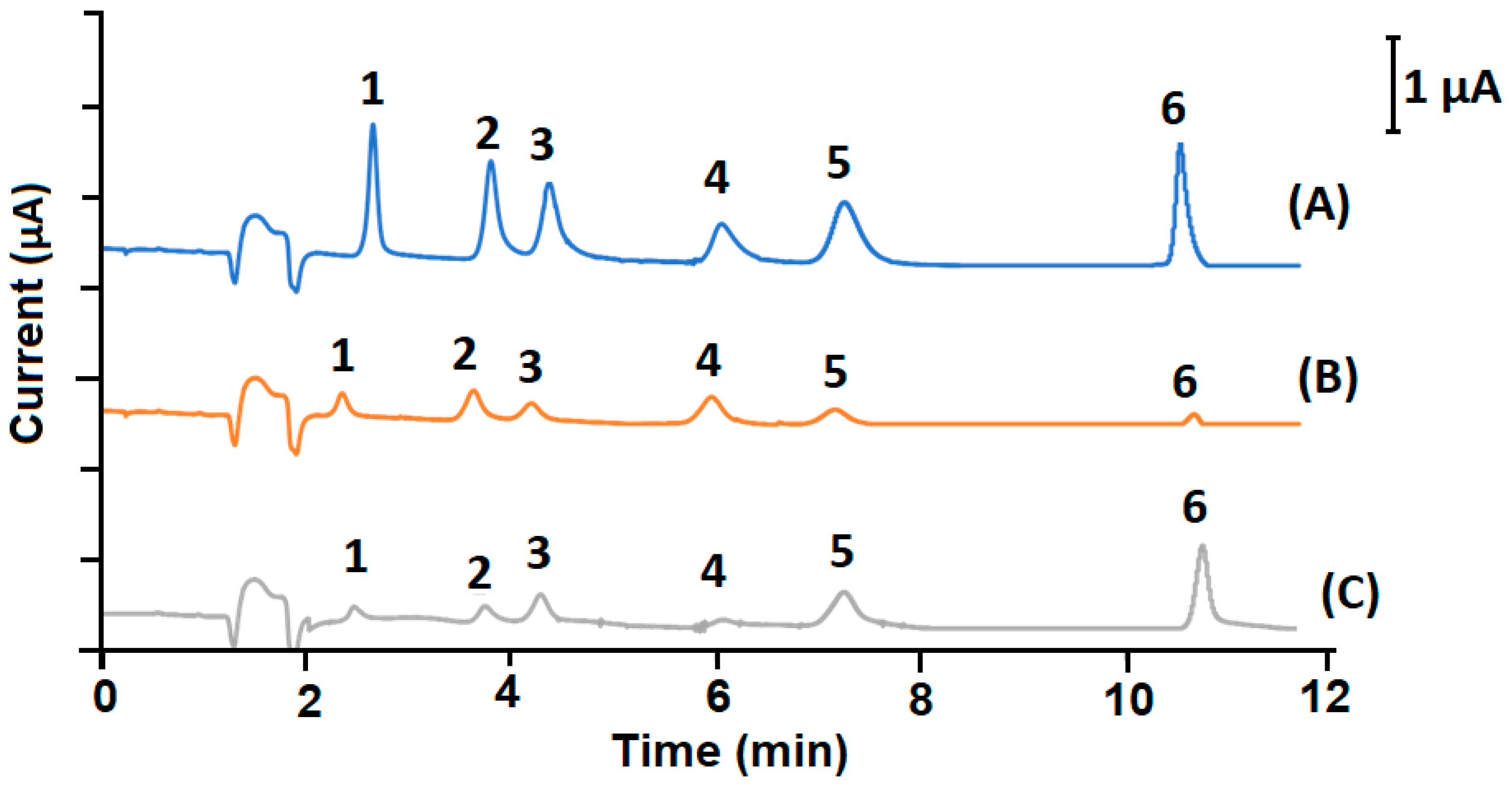

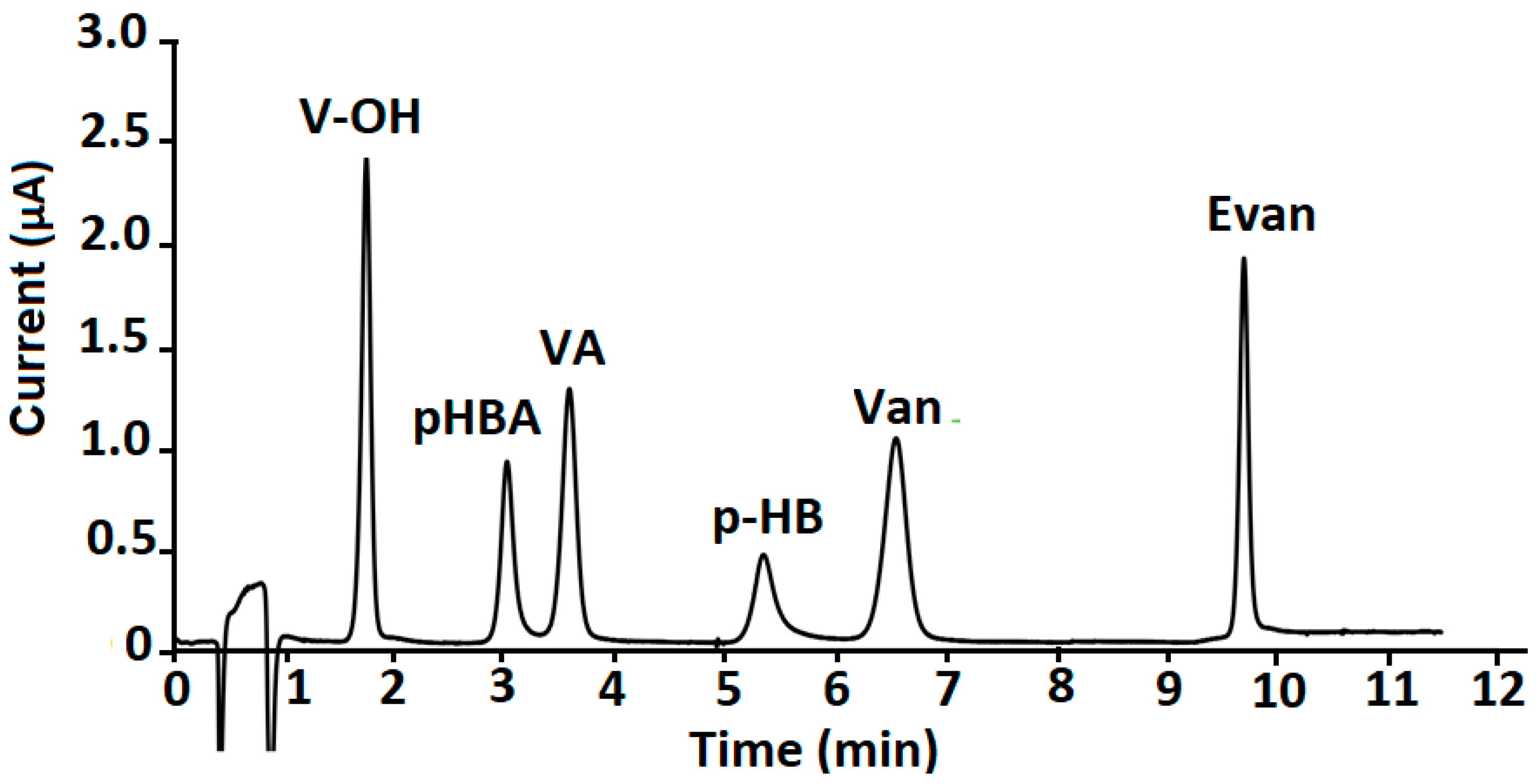
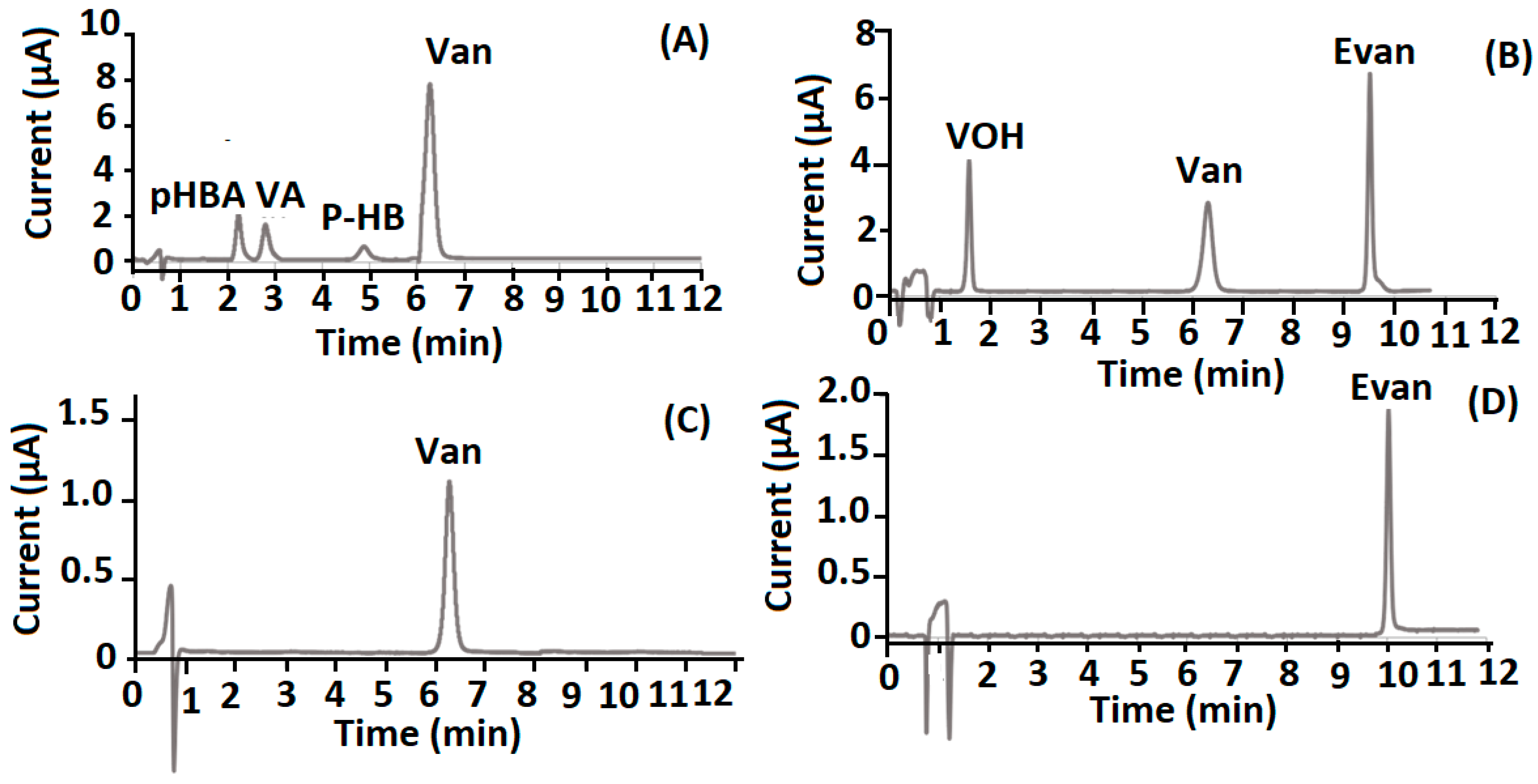
| Markers | Linear Range (µg g−1) | A = (a ± Sa)C + (b ± Sb) | R2 | LOD (µg g−1) | LOQ (µg g−1) | RSD (%) |
|---|---|---|---|---|---|---|
| V-OH | 0.5–25 | A = (9.76 × 10−7 ± 7.68 × 10−9 )C + (1.27 × 10−7 ± 3.59 × 10−8) | 0.9997 | 0.11 | 0.36 | 3.94 |
| p-HBA | 0.5–25 | A = (3.62 × 10−7 ± 3.4 × 10−9)C − (1.16 × 10−7 ± 1.73 × 10−8) | 0.9997 | 0.14 | 0.47 | 4.32 |
| VA | 0.5–25 | A = (1.06 × 10−6 ± 1.07 × 10−8)C − (1.01 × 10−8 ± 5.01 × 10−8) | 0.9995 | 0.14 | 0.46 | 2.89 |
| p-HB | 0.5–25 | A = (1.63 × 10−7 ± 1.07 × 10−9)C − (1.88 × 10−8 ± 5.46 × 10−9) | 0.9998 | 0.10 | 0.33 | 4.33 |
| Van | 0.5–25 | A = (1.31 × 10−6 ± 1.37 × 10−8)C + (1.15 × 10−7 ± 6.39 × 10−8) | 0.9995 | 0.14 | 0.48 | 4.76 |
| EVan | 0.5–25 | A = (1.39 × 10−6 ± 1.44 × 10−8)C + (3.68 × 10−7 ± 6.75 × 10−8) | 0.9995 | 0.13 | 0.48 | 3.54 |
| Markers | Vanilla Natural Extract (A); µg g−1 | Vanilla Extract Product (B); µg g−1 | Vanilla Extract Product (C); µg g−1 | Vanilla Extract Product (D); µg g−1 | ||||
|---|---|---|---|---|---|---|---|---|
| ED Detector | DAD Detector | ED Detector | DAD Detector | ED Detector | DAD Detector | ED Detector | DAD Detector | |
| V-OH | ND | ND | 31.07 ± 0.32 | 33.07 ± 1.21 | ND | ND | ND | ND |
| p-HBA | 6.67 ± 0.39 | 7.12 ± 0.5 | ND | ND | ND | ND | ND | ND |
| VA | 9.15 ± 0.19 | 8.84 ± 0.07 | ND | ND | ND | ND | ND | ND |
| p-HB | 5.10 ± 0.01 | 6.05 ± 0.14 | ND | ND | ND | ND | ND | ND |
| Van | 98.57 ± 3.61 | 101.5 ± 1.38 | 61.62 ± 0.36 | 59.2 ± 0.81 | 10.25 ± 0.31 | 11.06 ± 0.85 | ND | ND |
| EVan | ND | ND | 66.02 ± 0.9 | 68.41 ± 0.22 | ND | ND | 11.7 ± 0.08 | 10.2 ± 0.26 |
| Ratio Level Limits (1): Van/p-HBA = 110 − 53 | Ratio Level Limits (1): Van/p-HB = 20 − 10 | Ratio Level Limits (1): Van/VA = 29 − 15 | |||
|---|---|---|---|---|---|
| p-HBA (µg g−1) | p-HB (µg g−1) | VA (µg g−1) | |||
| Expected Concentration (2) | LOQ | Expected Concentration (2) | LOQ | Expected Concentration (2) | LOQ |
| 0.093–0.19 | 0.47 | 0.51–1.02 | 0.33 | 0.35–0.68 | 0.46 |
| Electrode | Electrochemical Detection | Detection Limit | Ref. |
|---|---|---|---|
| Glassy carbon electrode | Linear sweep voltammetry and square-wave voltammetry | 16 µM | [27] |
| PVC/graphite electrode | Amperometric | 290 µM | [28] |
| Al-ZrO2-NPs/SPCE | Amperometric | 0.6 µM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmassaoud, Y.; Murtada, K.; Salghi, R.; Zougagh, M.; Ríos, Á. Rapid Sample Screening Method for Authenticity Controlling of Vanilla Flavours Using Liquid Chromatography with Electrochemical Detection Using Aluminium-Doped Zirconia Nanoparticles-Modified Electrode. Molecules 2022, 27, 2915. https://doi.org/10.3390/molecules27092915
Benmassaoud Y, Murtada K, Salghi R, Zougagh M, Ríos Á. Rapid Sample Screening Method for Authenticity Controlling of Vanilla Flavours Using Liquid Chromatography with Electrochemical Detection Using Aluminium-Doped Zirconia Nanoparticles-Modified Electrode. Molecules. 2022; 27(9):2915. https://doi.org/10.3390/molecules27092915
Chicago/Turabian StyleBenmassaoud, Yassine, Khaled Murtada, Rachid Salghi, Mohammed Zougagh, and Ángel Ríos. 2022. "Rapid Sample Screening Method for Authenticity Controlling of Vanilla Flavours Using Liquid Chromatography with Electrochemical Detection Using Aluminium-Doped Zirconia Nanoparticles-Modified Electrode" Molecules 27, no. 9: 2915. https://doi.org/10.3390/molecules27092915
APA StyleBenmassaoud, Y., Murtada, K., Salghi, R., Zougagh, M., & Ríos, Á. (2022). Rapid Sample Screening Method for Authenticity Controlling of Vanilla Flavours Using Liquid Chromatography with Electrochemical Detection Using Aluminium-Doped Zirconia Nanoparticles-Modified Electrode. Molecules, 27(9), 2915. https://doi.org/10.3390/molecules27092915








