Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species
Abstract
:1. Introduction
2. Results
2.1. Disk Diffusion Susceptibility Test
2.2. Minimum Inhibitory Concentration (MIC)
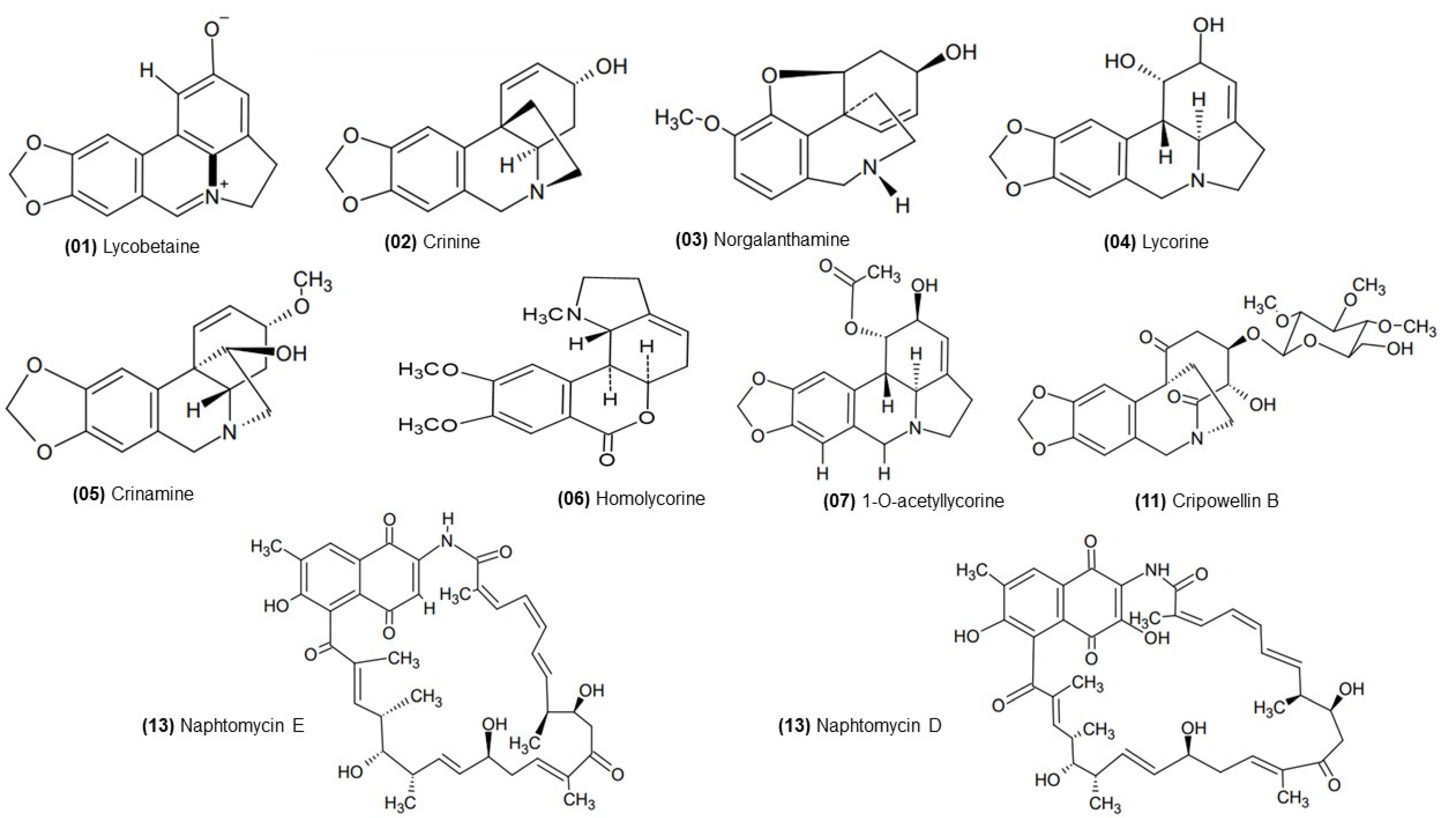
2.3. ESI (+) FT-ICR MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Collection
4.2. Preparation of Extracts and Fraction
4.3. Antifungal Disk Diffusion Susceptibility Testing
4.4. Determination of Minimum Inhibitory Concentration (MIC)
4.5. ESI (+) FT-ICR MS Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lacaz, C.d.S.; Porto, E.; Martins, J.E.C.; Heins-Vaccari, E.M.; Takahashi, N.M. Tratado de Micologia Médica; SciELO: Brasil, Brazil, 2002. [Google Scholar]
- Agência Nacional de Vigilância Sanitária. Detecção e Identificação dos Fungous de Importância Médica; Agência Nacional de Vigilância Sanitária: Brasil, Brazil, 2004; p. 24.
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans Morphogenesis and Host Defence: Discriminating Invasion from Colonization. Nat. Rev. Microbiol. 2011, 10, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliev, I.D.; Underhill, D.M. Striking a Balance: Fungal Commensalism versus Pathogenesis. Curr. Opin. Microbiol. 2013, 16, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- States, U. Drug-Resistant Candida auris. Antibiotic Resistance Threats Report. 2021. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/candida-auris-508.pdf (accessed on 29 March 2021).
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Lora, I.; Bras, G.; Karkowska-Kuleta, J.; González-González, M.; Ceballos, K.; Sidlo, W.; Rapala-Kozik, M. Plant-Derived Substances in the Fight Against Infections Caused by Candida species. Int. J. Mol. Sci. 2020, 21, 6131. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of Phenolic Compounds from Plant Origin Against Candida species. Ind. Crops Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal Drug Resistance Among Candida species: Mechanisms and Clinical Impact. Mycoses 2015, 58 (Suppl. 2), 2–13. [Google Scholar] [CrossRef]
- Su, H.; Han, L.; Huang, X. Potential Targets for the Development of New Antifungal Drugs. J. Antibiot. 2018, 71, 978–991. [Google Scholar] [CrossRef]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.D.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A Decade of Antifungal Leads from Natural Products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felice, M.R.; Giuffrè, L.; El Aamri, L.; Hafidi, M.; Criseo, G.; Romeo, O.; Scordino, F. Looking for New Antifungal Drugs from Flavonoids: Impact of the Genetic Diversity of Candida albicans on the In-Vitro Response. Curr. Med. Chem. 2019, 26, 5108–5123. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Eom, Y.B. Antifungal and Anti-Biofilm Effects of 6-Shogaol Against Candida auris. J. Appl. Microbiol. 2021, 130, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Toenjes, K.A.; Stark, B.C.; Brooks, K.M.; Johnson, D.I. Inhibitors of Cellular Signalling Are Cytotoxic or Block the Budded-to-Hyphal Transition in the Pathogenic Yeast Candida albicans. J. Med. Microbiol. 2009, 58, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Cytotoxicity Studies of Lycorine Alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Sui, Y.; Ma, Z.; Feng, X.; Wang, F.; Ma, T. Lycorine Hydrochloride Inhibits the Virulence Traits of Candida albicans. BioMed Res. Int. 2019, 2019, 1851740. [Google Scholar] [CrossRef]
- Thi Ngoc Tram, N.T.N.; Titorenkova, T.V.; St Bankova, V.; Handjieva, N.V.; Popov, S.S. Crinum L. (Amaryllidaceae). Fitoterapia 2002, 73, 183–208. [Google Scholar] [CrossRef]
- Maroyi, A. A Review of Ethnoboatany, Therapeutic Value, Phytochemistry and Pharmacology of Crinum macowanii Baker: A Highly Traded Bulbous Plant in Southern Africa. J. Ethnopharmacol. 2016, 194, 595–608. [Google Scholar] [CrossRef]
- Ding, Y.; Qu, D.; Zhang, K.M.; Cang, X.X.; Kou, Z.N.; Xiao, W.; Zhu, J.B. Phytochemical and Biological Investigations of Amaryllidaceae Alkaloids: A Review. J. Asian Nat. Prod. Res. 2017, 19, 53–100. [Google Scholar] [CrossRef]
- Gomes, J.V.D.; Tosta, C.L.; Cunha Neto, Ád; Fagg, C.W.; Silva, C.A.G.; Gomes-Copeland, K.K.P.; Magalhães, P.O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Silveira, D. Chemical Profile and Biological Activity of Crinum americanum L. (Amaryllidaceae). S. Afr. J. Bot. 2022, 146, 25–35. [Google Scholar] [CrossRef]
- M44-A; Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. NCCLS: Malvern, PA, USA, 2004.
- Tallini, L.R.; Torras-Claveria, L.; Borges, W.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. N-Oxide Alkaloids from Crinum amabile (Amaryllidaceae). Molecules 2018, 23, 1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrath, E.L.; dos Santos Passos, C.; Klein, L.C.; Henriques, A.T. Alkaloids as a Source of Potential Anticholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef] [PubMed]
- Ločárek, M.; Nováková, J.; Klouček, P.; Hošt’álkoviá, A.; Kokoška, L.; Lucie, G.; Šafratová, M.; Opletal, L.; Cahliková, L. Antifungal and Antibacterial Activity of Extracts and Alkaloids of Selected Amaryllidaceae species. Nat. Prod. Commun. 2015, 10, 1537–1540. [Google Scholar] [CrossRef] [Green Version]
- Nair, J.J.; Van Staden, J. The Plant Family Amaryllidaceae as a Source of Cytotoxic Homolycorine Alkaloid Principles. S. Afr. J. Bot. 2021, 136, 157–174. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Abou-Donia, A.H.; Touema, S.M.; Hammoda, H.M.; Shawky, E.; Motta, A. (−)-Amarbellisine, a Lycorine-Type Alkaloid from Amaryllis belladonna L. Growing in Egypt. Phytochemistry 2004, 65, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Barthelmes, H.U.; Niederberger, E.; Roth, T.; Schulte, K.; Tang, W.C.; Boege, F.; Fiebig, H.H.; Eisenbrand, G.; Marko, D. Lycobetaine Acts as a Selective Topoisomerase IIβ Poison and Inhibits the Growth of Human Tumour Cells. Br. J. Cancer 2001, 85, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, S.A.; Olugbade, T.A.; Odebiyl, O.O.; Aladesanmi, J.A. Antibacterial Alkaloids in Crinum jagus. Int. J. Pharmacogn. 1992, 30, 303–307. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol Deniz, F.S.; Eren, G.; Sener, B. Molecular Approach to Promising Cholinesterase Inhibitory Effect of Several Amaryllidaceae Alkaloids: Further re-Investigation. S. Afr. J. Bot. 2021, 136, 175–181. [Google Scholar] [CrossRef]
- Velten, R.; Erdelen, C.; Gehling, M.; Göhrt, A.; Gondol, D.; Lenz, J.; Lockhoff, O.; Wachendorff, U.; Wendisch, D. Cripowellin A and B, a Novel Type of Amaryllidaceae Alkaloid from Crinum powellii. Tetrahedron Lett. 1998, 39, 1737–1740. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Cytotoxic Agents in the Minor Alkaloid Groups of the Amaryllidaceae. Planta Med. 2021, 87, 916–936. [Google Scholar] [CrossRef]
- Presley, C.C.; Krai, P.; Dalal, S.; Su, Q.; Cassera, M.; Goetz, M.; Kingston, D.G.I. New Potently Bioactive Alkaloids from Crinum erubescens. Bioorg. Med. Chem. 2016, 24, 5418–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrieri, C.G.; Pigni, N.B.; de Andrade, J.P.; dos Santos, V.D.; Binns, F.; de Souza Borges, W.; Viladomat, F.; Bastida, J. Alkaloids from Crinum erubescens Aiton. Arab. J. Chem. 2016, 9, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Golubkina, N.; Tallarita, A.; Abdelhamid, M.T.; Sekara, A. Biodiversity, Ecology, and Secondary Metabolites Production of Endophytic Fungi Associated with Amaryllidaceae Crops. Agriculture 2020, 10, 533. [Google Scholar] [CrossRef]
- Lu, C.; Shen, Y. A Novel Ansamycin, Naphthomycin K from Streptomyces sp. J. Antibiot. 2007, 60, 649–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, J.J.; van Staden, J. Antifungal Constituents of the Plant Family Amaryllidaceae. Phytother. Res. 2018, 32, 976–984. [Google Scholar] [CrossRef]
- Alawode, T.T.; Lajide, L.; Owolabi, B.J.; Olaleye, M.T. Investigation of Bulb Extracts of Crinum jagus for Antibacterial and Antifungal Activities. J. Appl. Sci. Environ. Manag. 2021, 25, 113–117. [Google Scholar] [CrossRef]
- Udegbunam, S.O.; Udegbunam, R.I.; Nnaji, T.O.; Anyanwu, M.U.; Kene, R.O.; Anika, S.M. Antimicrobial and Antioxidant Effect of Methanolic Crinum jagus Bulb Extract in Wound Healing. J. Intercult. Ethnopharmacol. 2015, 4, 239–248. [Google Scholar] [CrossRef]
- Horn, C.; Vediyappan, G. Anticapsular and Antifungal Activity of α-Cyperone. Antibiotics 2021, 10, 51. [Google Scholar] [CrossRef]
- Bonvicini, F.; Antognoni, F.; Iannello, C.; Maxia, A.; Poli, F.; Gentilomi, G.A. Relevant and Selective Activity of Pancratium illyricum L. Against Candida albicans Clinical Isolates: A Combined Effect on Yeast Growth and Virulence. BMC Complement. Altern. Med. 2014, 14, 409. [Google Scholar] [CrossRef] [Green Version]
- Razmavar, S.; Abdulla, M.A.; Ismail, S.B.; Hassandarvish, P. Antibacterial Activity of Leaf Extracts of Baeckea frutescens against Methicillin-Resistant Staphylococcus aureus. BioMed Res. Int. 2014, 2014, 521287. [Google Scholar] [CrossRef] [Green Version]
- Mlozi, S.H.; Mmongoyo, J.A.; Chacha, M. Antimicrobial Activities of Tephrosia Vogelii against Selected Pathogenic Fungi and Bacteria Strains. Mycology 2020, 11, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Seidel, V.; Katerere, D.R.; Gray, A.I. Colorimetric Broth Microdilution Method for the Antifungal Screening of Plant Extracts Against Yeasts. Methods 2007, 42, 325–329. [Google Scholar] [CrossRef] [PubMed]
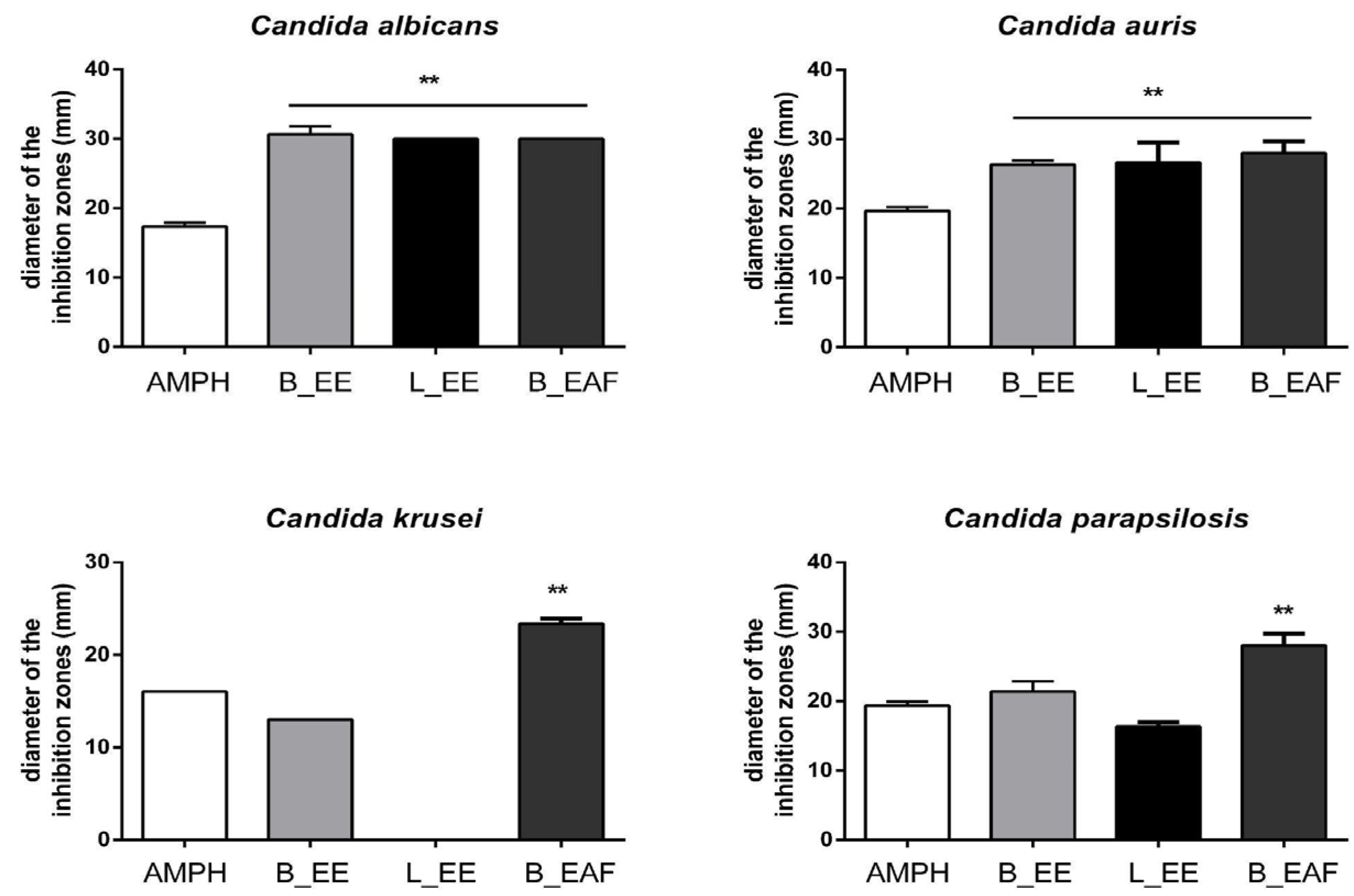
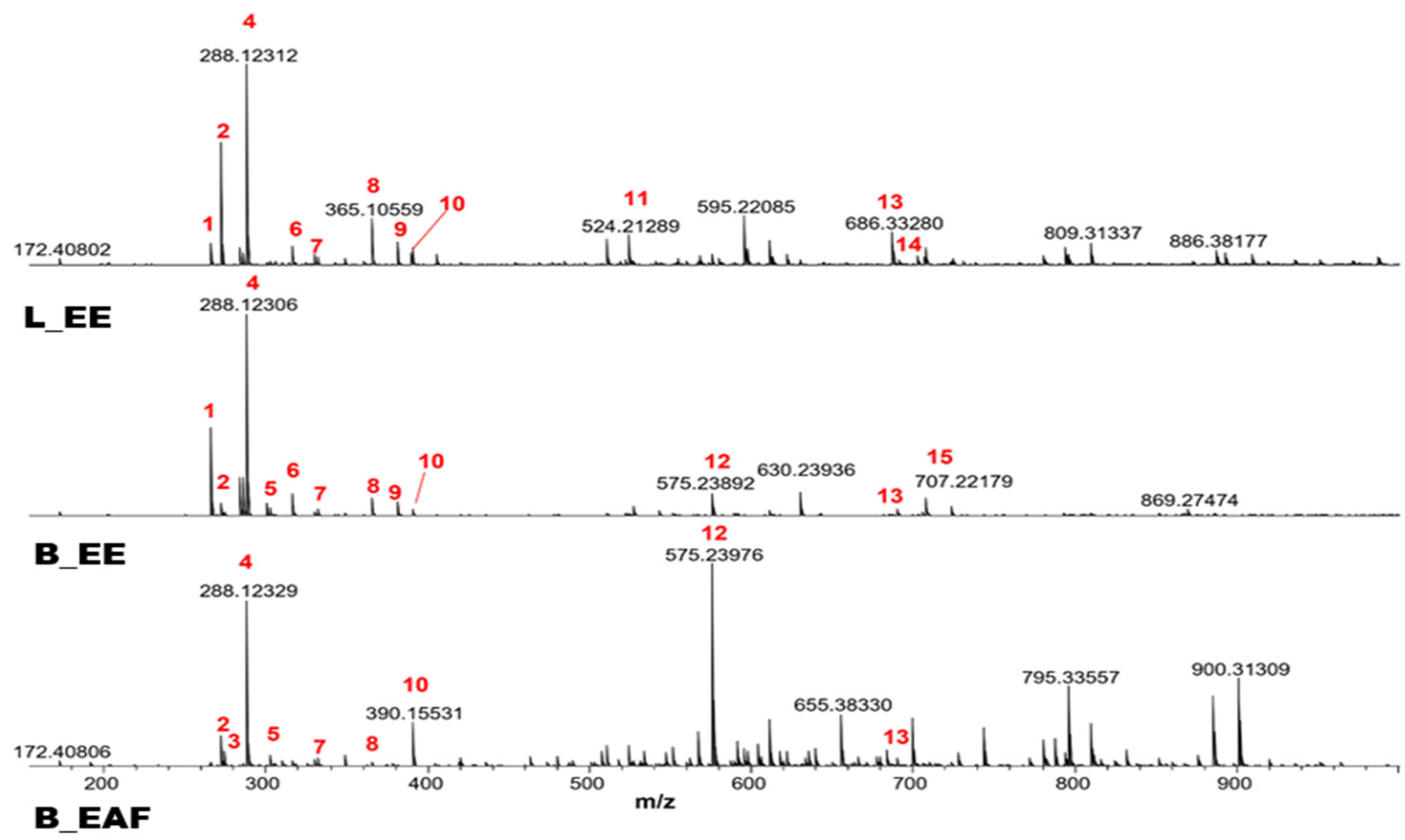
| Candida Strain | CIM L_EE | CIM B_EE | CIM B_EAF | CIM Lycorine | Positive Control | Negative Control | AMPH Control |
|---|---|---|---|---|---|---|---|
| C. auris | 805 | 422 | 344 | 162.5 | + | - | - |
| C. albicans | 805 | 422 | 86 | 40.6 | + | - | - |
| C. krusei | 221 | NA | 86 | 81.3 | + | - | - |
| C. parapsilosis | 441 | 422 | 172 | 20.3 | + | - | - |
| Num. | Chemical Compound | L_EE | B_EE | B_EAF | Molecular Formula | Theory m/z | Measured m/z | Error ppm | DBE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lycobetaine/Ungeremine | X | X | - | [C16H11NO3 + H]+ | 266.08117 | 266.08110 | 0.26 | 12 |
| 2 | Crinine | X | X | X | [C16H17NO3 + H]+ | 272.12812 | 272.12808 | −0.33 | 9 |
| 3 | Norgalanthamine | - | - | X | [C16H19NO3 + H]+ | 274.14377 | 274.14378 | −0.02 | 8 |
| 4 | Lycorine or Flexinine | X | X | X | [C16H17NO4 + H]+ | 288.12303 | 288.12312 | −0.31 | 9 |
| 5 | Crinamine or 8-O-demethyl-homolycorine | - | X | X | [C17H19NO4 + H]+ | 302.13868 | 302.13872 | −0.11 | 9 |
| 6 | Homolycorine | X | X | - | [C18H21NO4 + H]+ | 316.15433 | 316.15414 | −0.02 | 9 |
| 7 | 1-O-acetyllycorine | X | X | X | [C18H19NO5 + H]+ | 330.13360 | 330.13379 | −0.47 | 10 |
| 8 | Sucrose or isomers (sodium adduct) | X | X | X | [C12H22O11 + Na]+ | 365.10543 | 365.10545 | −0.04 | 2 |
| 9 | Sucrose or isomers (potassium adduct) | X | X | - | [C12H22O11 + K]+ | 381.07937 | 381.07951 | −0,47 | 2 |
| 10 | - | X | X | X | [C20H23NO7 + H]+ | 390.15473 | 390.15501 | −0.56 | 10 |
| 11 | Cripowellin B | X | - | - | [C25H33NO11 + H]+ | 524.21264 | 524.21289 | −0.48 | 10 |
| 12 | Lycorine Dimer | - | X | X | 2 [C16H17NO4 + H]+ | 575.23879 | 575.23933 | −0.93 | 17 |
| 13 | Naphtomycin E | X | X | X | [C40H47NO9 + H]+ | 686.33236 | 686.33242 | −0.64 | 18 |
| 14 | Naphtomycin D | X | - | - | [C40H47NO10 + H]+ | 702.32727 | 702.32807 | −1.13 | 18 |
| 15 | Sucrose Dimer or isomers (potassium adduct) | - | X | - | 2 [C12H22O11 + Na]+ | 707.22164 | 707.22179 | −0.21 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.C.; Correia, A.F.; Gomes, J.V.D.; Romão, W.; Motta, L.C.; Fagg, C.W.; Magalhães, P.O.; Silveira, D.; Fonseca-Bazzo, Y.M. Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species. Molecules 2022, 27, 2976. https://doi.org/10.3390/molecules27092976
Silva LC, Correia AF, Gomes JVD, Romão W, Motta LC, Fagg CW, Magalhães PO, Silveira D, Fonseca-Bazzo YM. Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species. Molecules. 2022; 27(9):2976. https://doi.org/10.3390/molecules27092976
Chicago/Turabian StyleSilva, Lorene Coelho, Amabel Fernandes Correia, João Victor Dutra Gomes, Wanderson Romão, Larissa Campos Motta, Christopher William Fagg, Pérola Oliveira Magalhães, Dâmaris Silveira, and Yris Maria Fonseca-Bazzo. 2022. "Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species" Molecules 27, no. 9: 2976. https://doi.org/10.3390/molecules27092976
APA StyleSilva, L. C., Correia, A. F., Gomes, J. V. D., Romão, W., Motta, L. C., Fagg, C. W., Magalhães, P. O., Silveira, D., & Fonseca-Bazzo, Y. M. (2022). Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species. Molecules, 27(9), 2976. https://doi.org/10.3390/molecules27092976









