Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Honeybee Products
2.2. Amino Acid Composition and Score (AAS%)
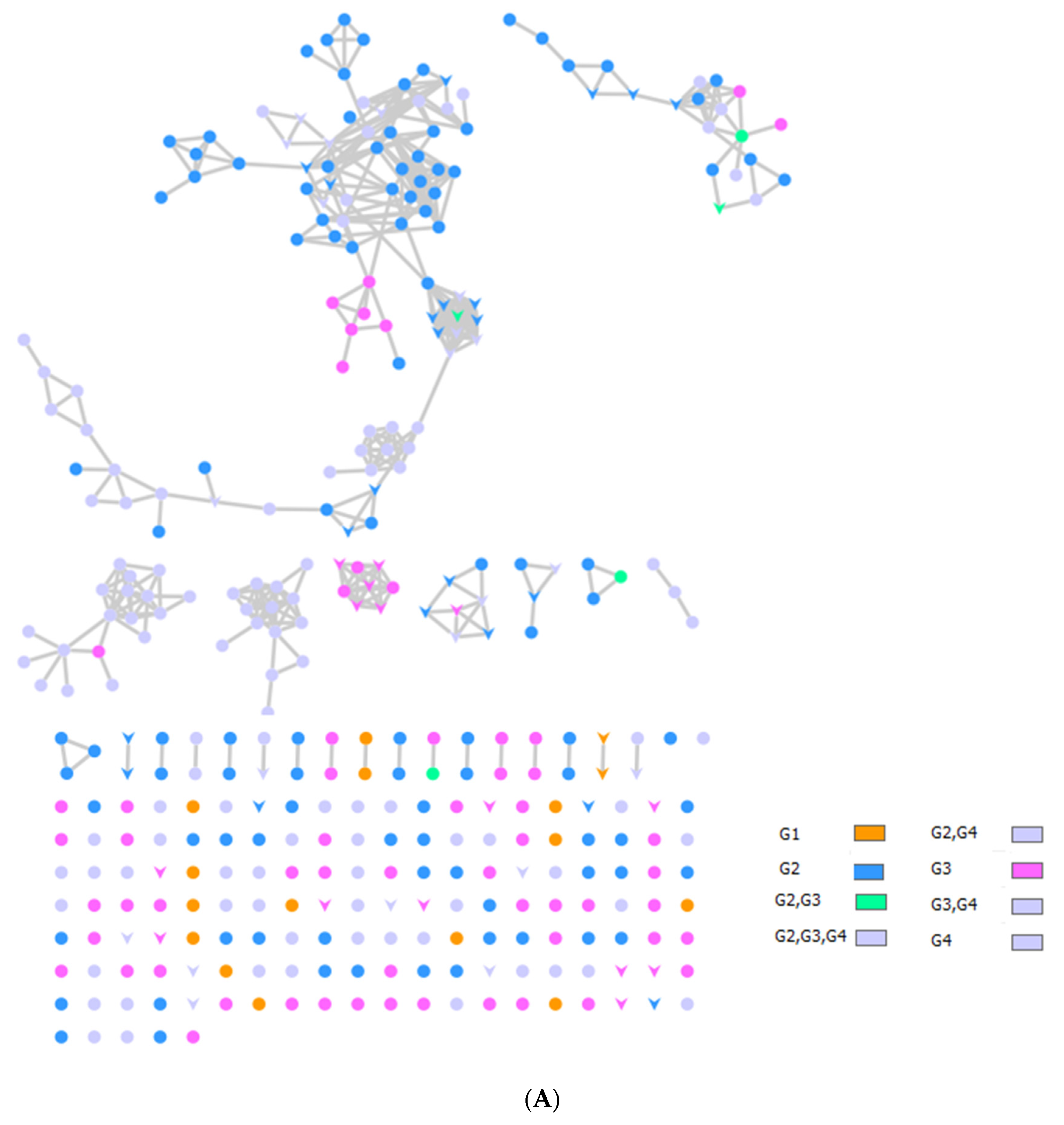
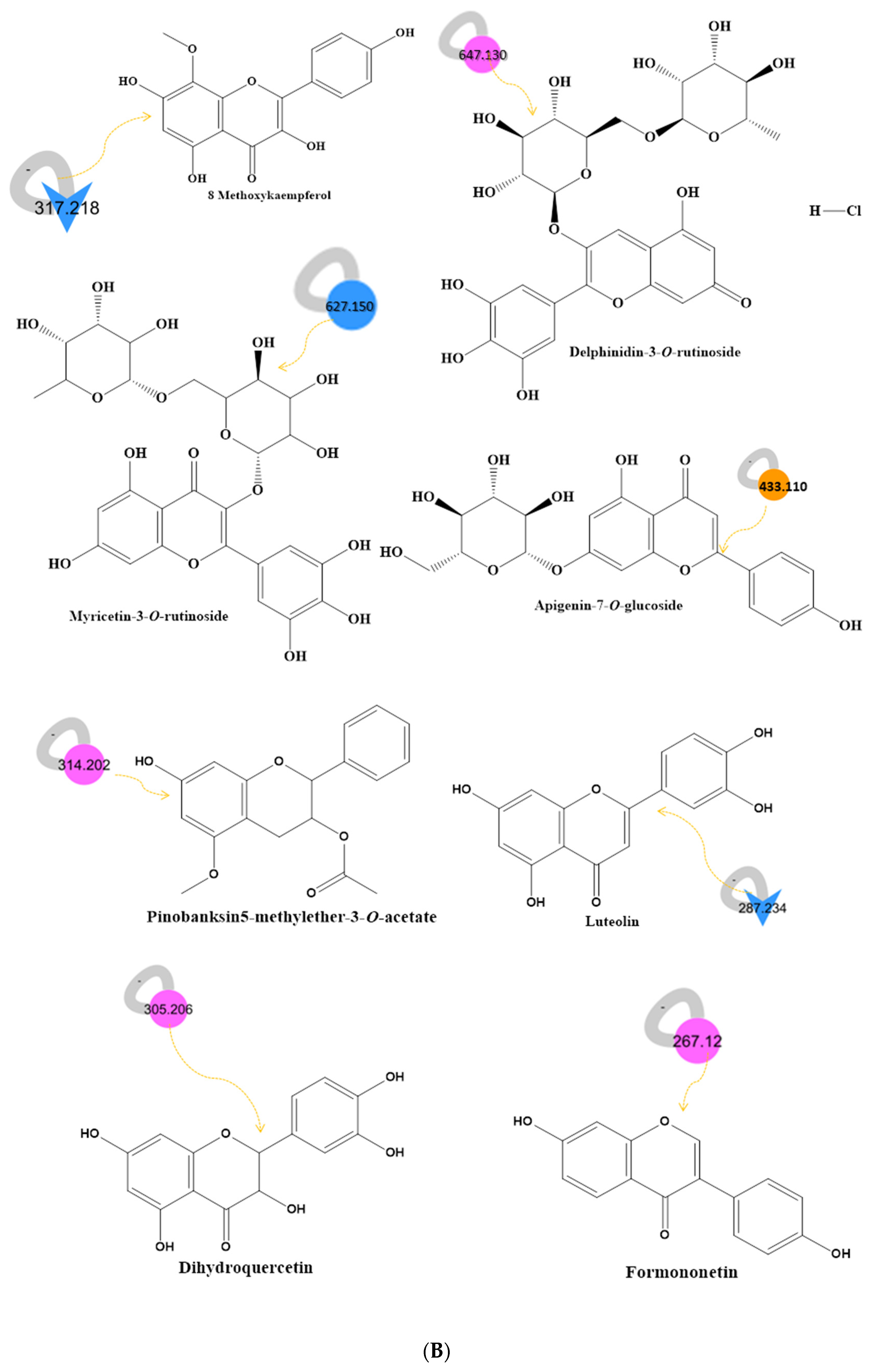
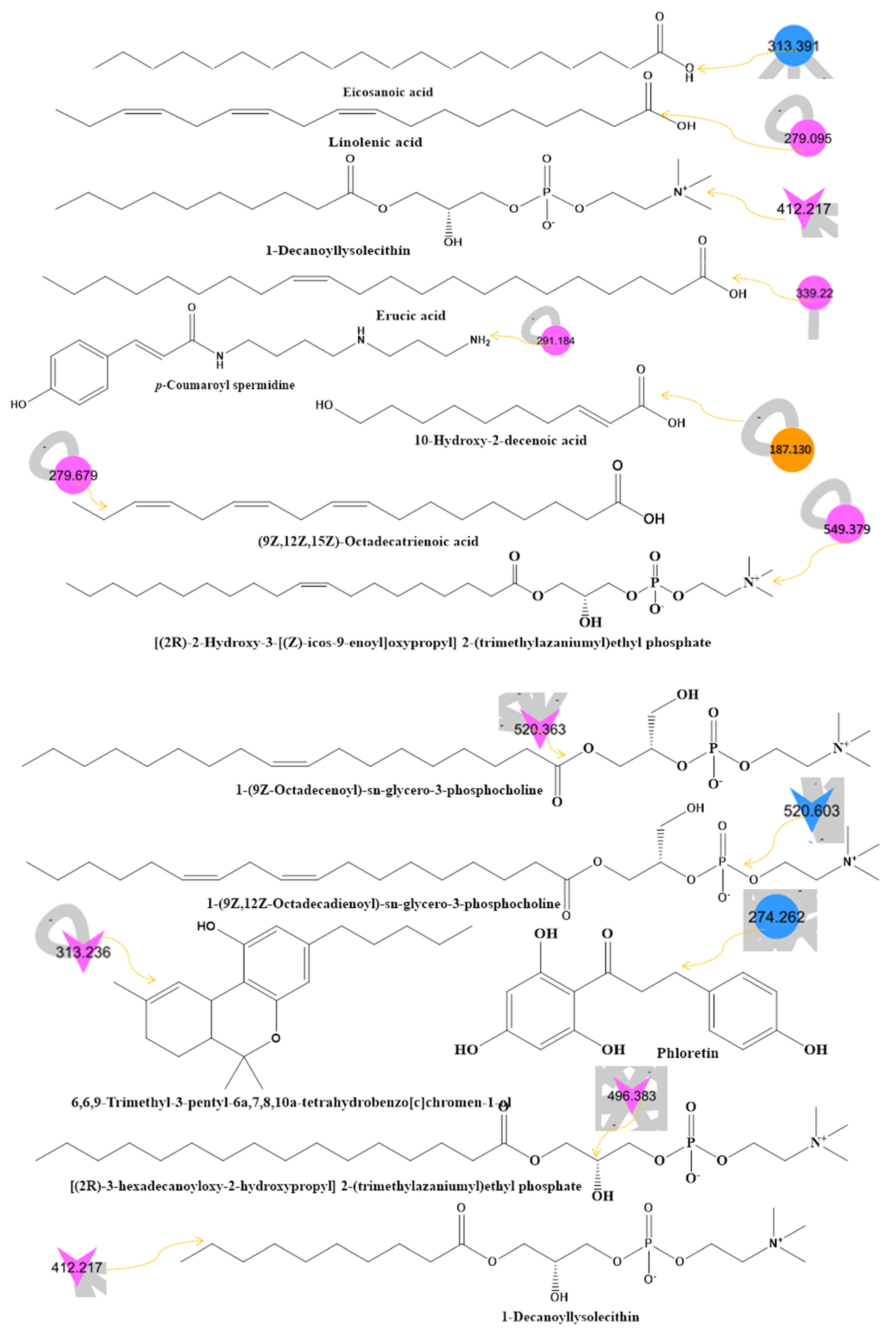
2.3. LC–MS–MS Analysis and International Molecular Networking GNPS Database of Bee Product Metabolites
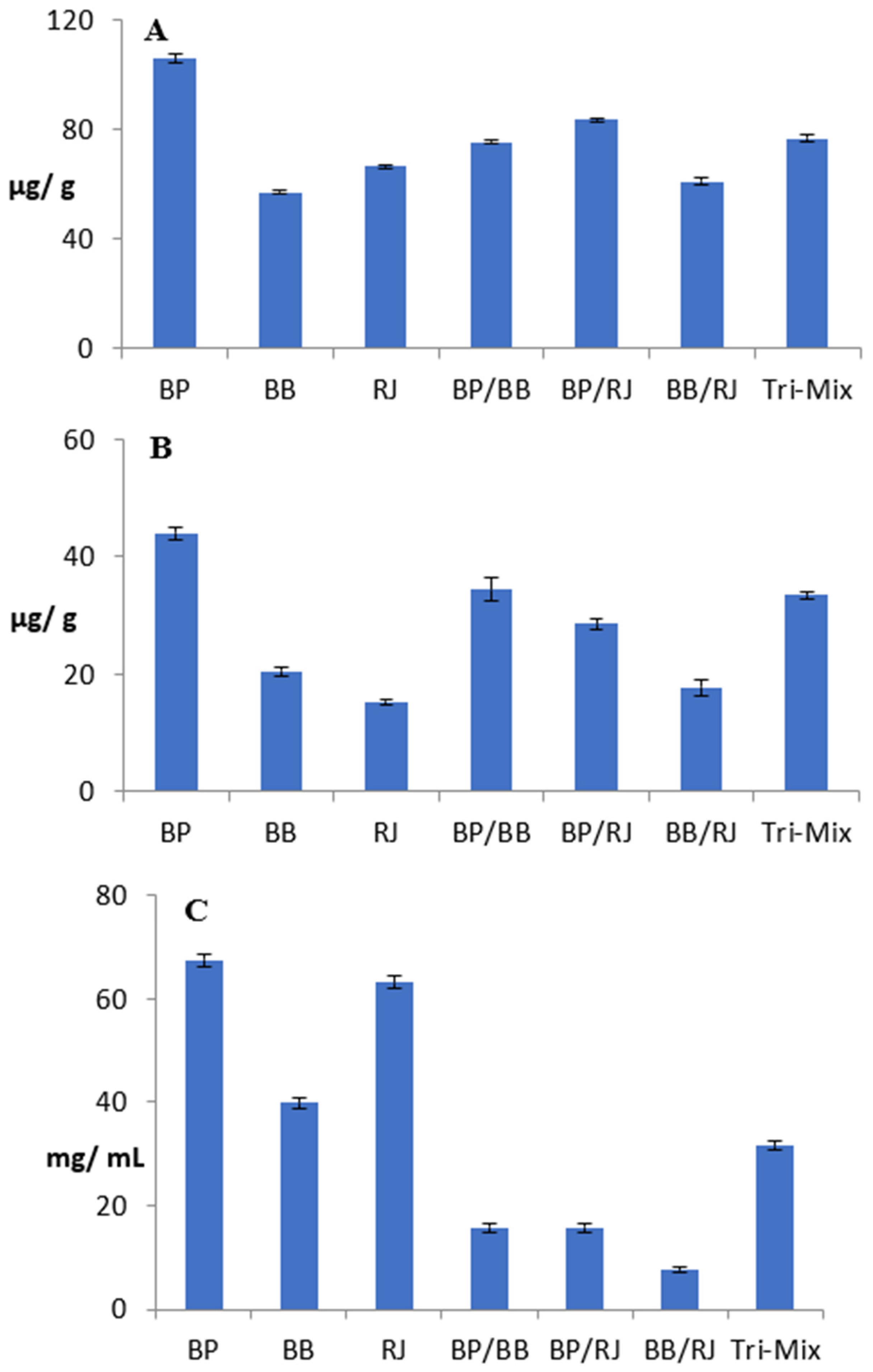
2.4. Antioxidant Potentials
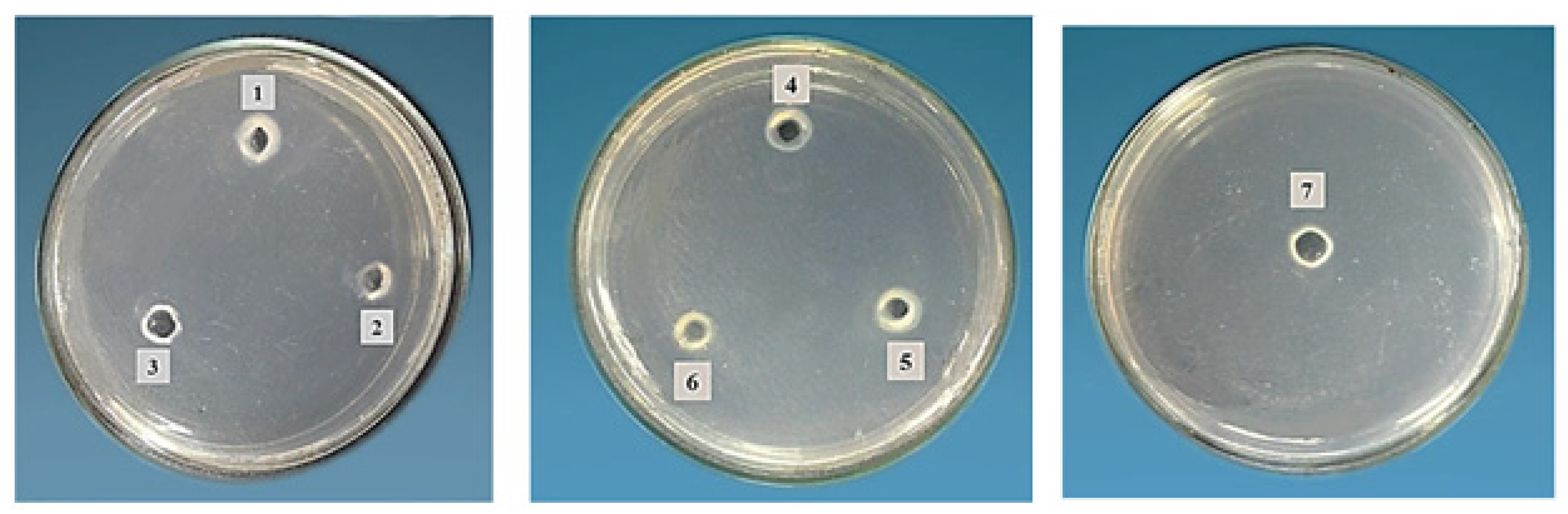
2.5. Protease Activity of Honeybee Products
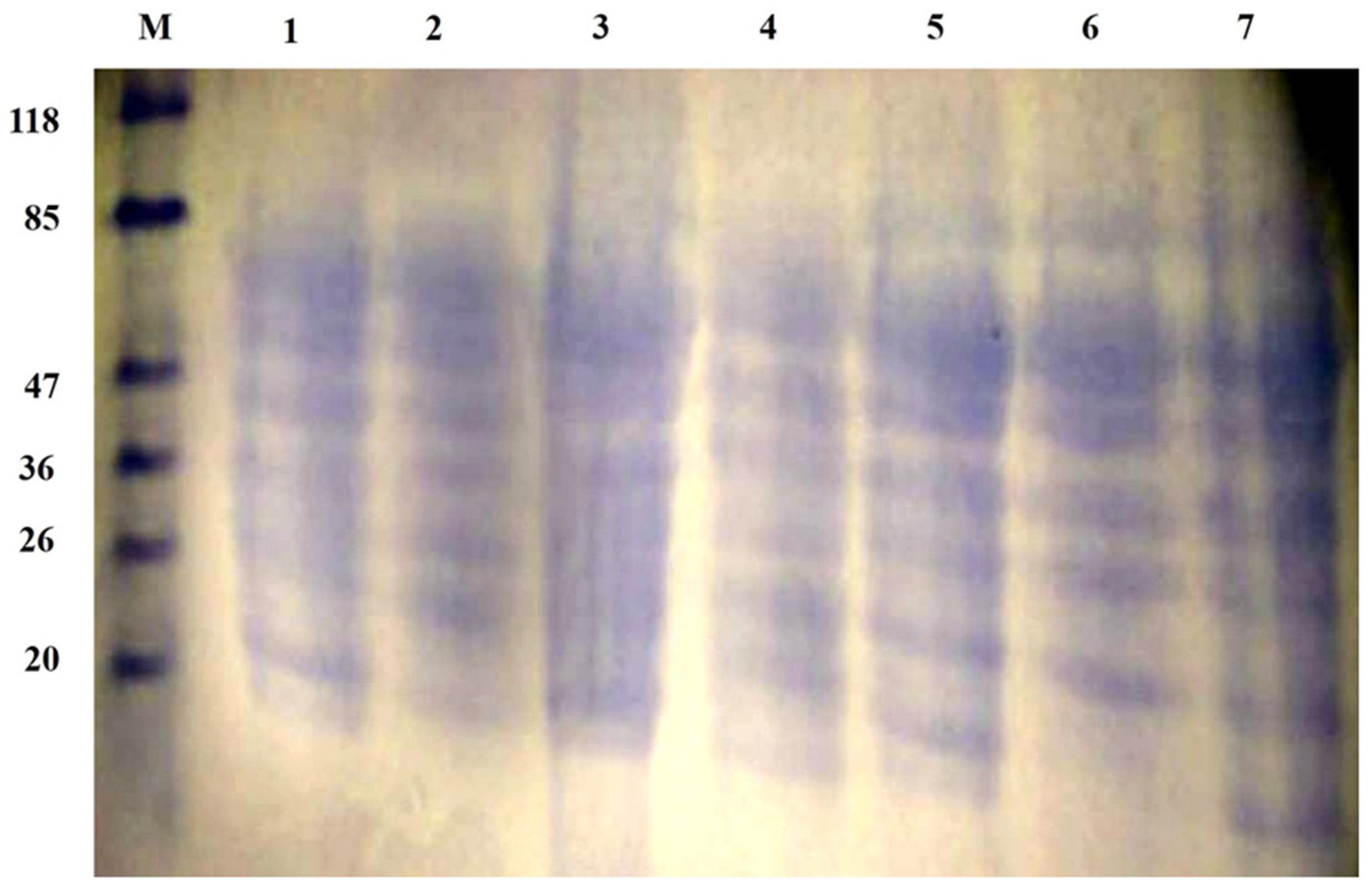
2.6. SDS–PAGE of Honeybee Products
2.7. Physicochemical Characteristics of Developed Functional Fermented Dairy Products
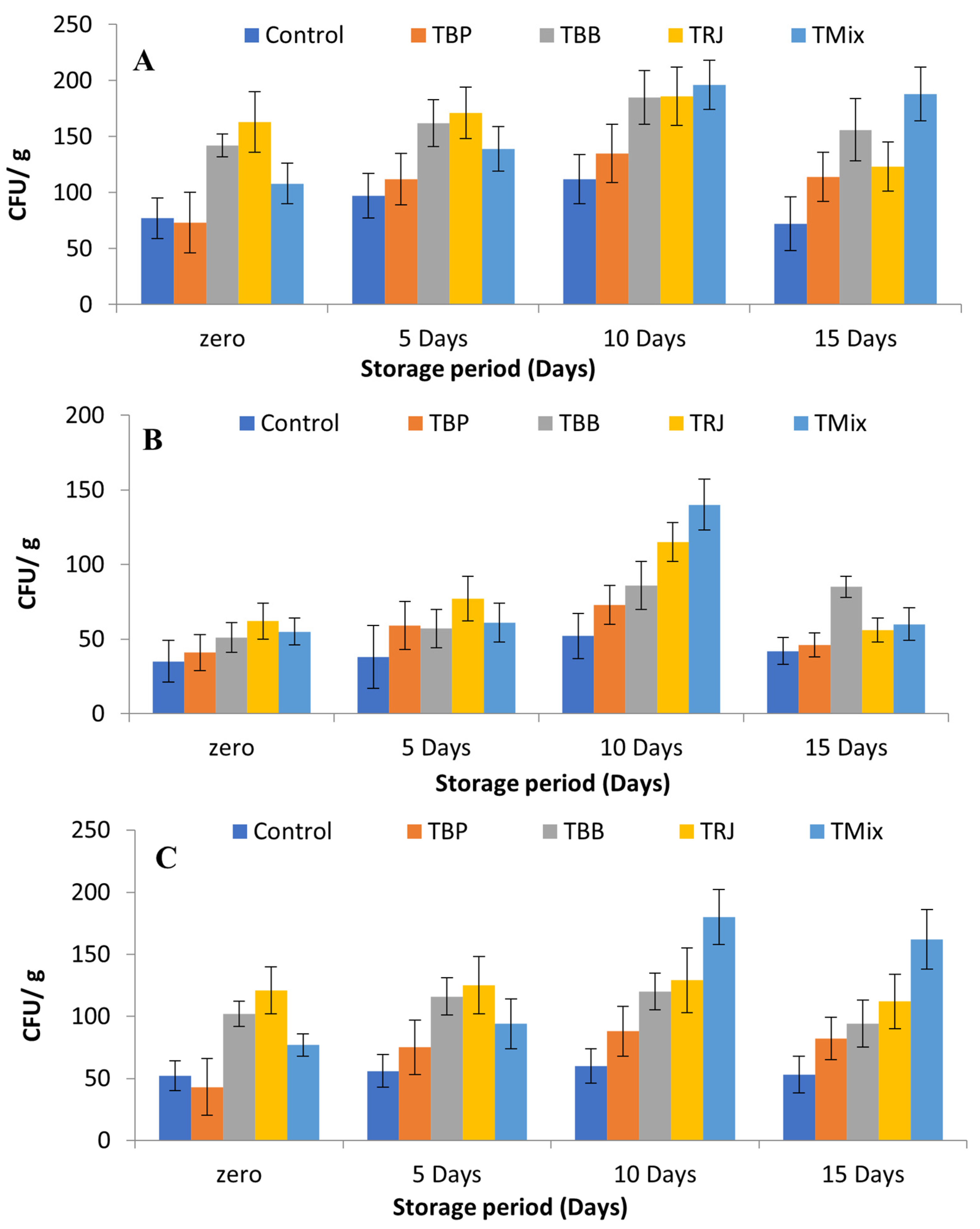
2.8. Effects of Fortification on LAB Starter Culture
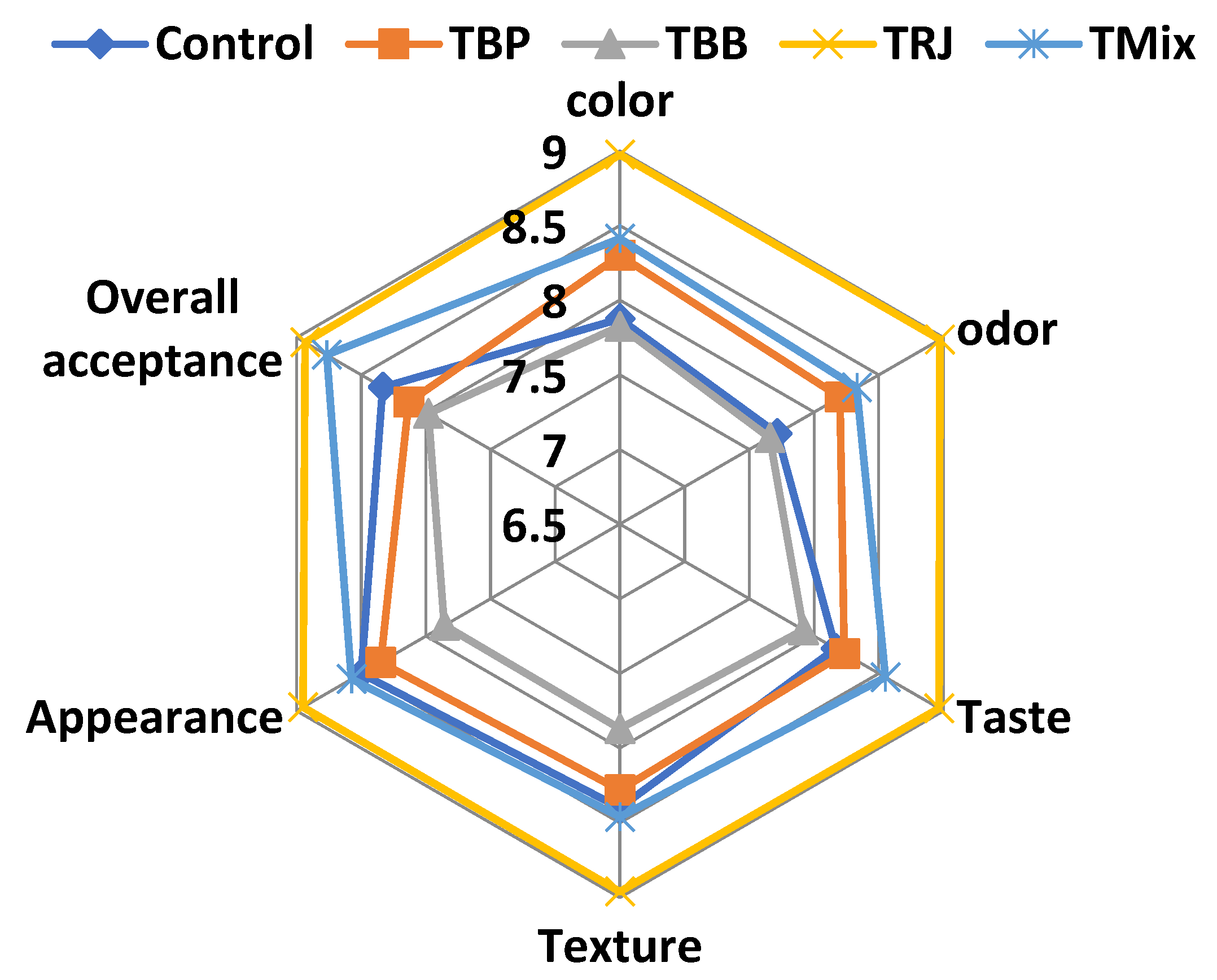
2.9. Sensory Evaluation
3. Materials and Methods
3.1. Sampling and Extracting
3.2. Chemical Compositions of Honeybee Products
3.3. Mineral Contents of Honeybee Products
3.4. Daily Values (DVs%)
3.5. Amino Acid Compositions of Honeybee Products
3.6. Liquid Chromatography–Mass Spectrometry (LC-MS-MS) Analysis
3.7. Antioxidant Potentials of Honeybee Products
3.7.1. Total Phenolic Content
3.7.2. Total Flavonoid Content
3.7.3. DPPH Scavenging Activity
3.8. Screening and Quantitative Determination of Proteolytic Activity
3.9. SDS-PAGE
3.10. Application in Fermented Dairy Products
3.10.1. Preparation of Honeybee-Product-Fortified Fermented Dairy Products
3.10.2. Physicochemical Characterization of Fortified Fermented Dairy Products
3.10.3. Microbiological Analysis
3.10.4. Sensory Evaluation
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical opportunities and challenges. Anal. Chem. 2022, 94, 366–381. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Schmid, R.; Gauglitz, J.M.; Wang, M.; Dorrestein, P.C. FoodMASST a mass spectrometry search tool for foods and beverages. NPJ Sci. Food 2022, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- El-Garawani, I.; Hassab El-Nabi, S.; El Kattan, A.; Sallam, A.; Elballat, S.; Abou-Ghanima, S.; El Azab, I.H.; El-Seedi, H.R.; Khalifa, S.A.M.; El-Shamy, S. The ameliorative role of Acacia senegal gum against the oxidative stress and genotoxicity induced by the radiographic contrast medium (Ioxitalamate) in albino rats. Antioxidants 2021, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- El-Din, M.I.G.; Fahmy, N.M.; Wu, F.; Salem, M.M.; Khattab, O.M.; El-Seedi, H.R.; Korinek, M.; Hwang, T.L.; Osman, A.K.; El-Shazly, M.; et al. Comparative LC–LTQ–MS–MS analysis of the leaf extracts of Lantana camara and Lantana montevidensis growing in Egypt with insights into their anti-Inflammatory, and cytotoxic activities. Plants 2022, 11, 1699. [Google Scholar] [CrossRef]
- Özkök, D.; Silici, S. Antioxidant activities of honeybee products and their mixtures. Food Sci. Biotechnol. 2017, 26, 201–206. [Google Scholar] [CrossRef]
- Bakour, M.; Campos, M.d.G.; Imtara, H.; Lyoussi, B. Antioxidant content and identification of phenolic/flavonoid compounds in the pollen of fourteen plants using HPLC-DAD. J. Apic. Res. 2020, 59, 35–41. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Ka, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Sabir, J.S.M.; Battino, M.; Xiao, J.; Zou, X.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal jelly: Beneficial properties and synergistic effects with chemotherapeutic drugs with particular emphasis in anticancer strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; de Almeida-Muradian, L.B. Quality and standardisation of royal jelly. J. ApiProduct ApiMedical Sci. 2009, 1, e78298. [Google Scholar] [CrossRef]
- Brix, K.; Stocker, W. Proteases: Structure and Function; Springer: Wien, Austria, 2013; Volume 9783709108, ISBN 9783709108857. [Google Scholar]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef]
- Matsuoka, T.; Kawashima, T.; Nakamura, T.; Kanamaru, Y.; Yabe, T. Isolation and characterization of proteases that hydrolyze royal jelly proteins from queen bee larvae of the honeybee, Apis mellifera. Apidologie 2012, 43, 685–697. [Google Scholar] [CrossRef]
- Ismail, B.; Mohammed, H.; Nair, A.J. Influence of Proteases on Functional Properties of Food. In Green Bio-Processes, Energy, Environment, and Sustainability; Parameswaran, B., Varjani, S., Raveendran, S., Eds.; Springer: Singapore, 2019; pp. 31–53. ISBN 9789811332630. [Google Scholar]
- Sagona, S.; Coppola, F.; Giannaccini, G.; Betti, L.; Palego, L.; Tafi, E.; Casini, L.; Piana, L.; Dall’Olio, R.; Felicioli, A. Impact of Different Storage Temperature on the Enzymatic Activity of Apis mellifera Royal Jelly. Foods 2022, 11, 3165. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Al Naggar, Y.; Algethami, A.F.; Shou, Q.; Alsharif, S.M. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- Spence, S.; Delve, J.; Stamp, E.; Matthews, J.N.S.; White, M.; Adamson, A.J. The impact of food and nutrient-based standards on primary school children’s lunch and total dietary intake: A natural experimental evaluation of government policy in England. PLoS ONE 2013, 8, e78298. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.K.; Zawawi, N. Botanical origin and nutritional values of bee bread of stingless bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020, 2020, 15–17. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Kaplan, M.; Karaoglu, Ö.; Eroglu, N.; Silici, S. Fatty acid and proximate composition of bee bread. Food Technol. Biotechnol. 2016, 54, 497–504. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine: A Review. Lipids 2011, 38, 19. [Google Scholar]
- Nehete, J.; Bhambar, R.; Narkhede, M.; Gawali, S. Natural proteins: Sources, isolation, characterization and applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Energy and Protein Requirements—FAO/WHO/UNU Expert Consultation; Food and Agriculture Organization of the United States/World Health Organization/United Nations University, Energy and Protein requirements. Report of a joint FAO/WHO/UNU meeting; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- FAO/WHO. Energy and Protein Requirements; World Health Organization: Geneva, Switzerland, 1973; p. 118. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances—Food and Nutrition/ Board Commission on Life/Sciences National Research Council, 10th ed.; National Academy Press: Washington, DC, USA, 1989; ISBN 0309536065. [Google Scholar]
- Watford, M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J. Nutr. 2008, 138, 2003S–2007S. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bărnuţiu, L.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Mihai, C.M.; Bobiş, O. Chemical composition and antimicrobial activity of royal jelly. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets—A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Bagameri, L.; Baci, G.-M.; Dezmirean, D.S. Royal jelly as a nutraceutical natural product with a focus on its antibacterial activity. Pharmaceutics 2022, 14, 1142. [Google Scholar] [CrossRef]
- Becerril-s, A.L.; Quintero-Salazar, B.; Dubl, O.; Escalona-Buend, B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-Hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, D.S.; Zhang, W.; Wang, J.M.; Li, C.Y.; Ye, H.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Mendes, M.A.; de Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Aguilera-Luiz, M.d.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Fuenmayor, B.C.; Zuluaga, D.C.; Díaz, M.C.; de Quicazán, C.M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Córdoba 2014, 19, 4003–4014. [Google Scholar] [CrossRef]
- Li, F.; Guo, S.; Zhang, S.; Peng, S.; Cao, W.; Ho, C.T.; Bai, N. Bioactive constituents of f. esculentum bee pollen and quantitative analysis of samples collected from seven areas by HPLC. Molecules 2019, 24, 2705. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Development and validation of a liquid chromatography-tandem mass spectrometry method to determine intact glucosinolates in bee pollen. J. Chromatogr. B 2015, 1000, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Zurek, G.; Bäßmann, C.; Almaraz-Abarca, N.; Quirantes, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds from pollen extracts using capillary electrophoresis-electrospray time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Serra Bonvehi, J.; Soliva Torrentó, M.; Centelles Lorente, E. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Aylanc, V.; Falcão, S.I.; Ertosun, S.; Vilas-Boas, M. From the hive to the table: Nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread. Trends Food Sci. Technol. 2021, 109, 464–481. [Google Scholar] [CrossRef]
- Ismail, N.I.; Kadir, M.R.A.; Zulkifli, R.M. Isolation and identification of potential antineoplastic bioactive phenolic compounds in malaysian honeys. J. Appl. Pharm. Sci. 2015, 5, 59–66. [Google Scholar] [CrossRef]
- Šarić, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D.; et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- El Ghouizi, A.; El Menyiy, N.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical composition, antioxidant activity, and diuretic effect of Moroccan fresh bee pollen in rats. Vet. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- Wei, X.E.; Korth, J.; Brown, S.H.J.; Mitchell, T.W.; Truscott, R.J.W.; Blanksby, S.J.; Willcox, M.D.P.; Zhao, Z. Rapid quantification of free cholesterol in tears using direct insertion/electron ionization-Mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8027–8035. [Google Scholar] [CrossRef]
- Bárbara, M.S.; Machado, C.S.; Sodré, G.D.S.; Dias, L.G.; Estevinho, L.M.; De Carvalho, C.A.L. Microbiological assessment, nutritional characterization and phenolic compounds of bee pollen from Mellipona mandacaia Smith, 1983. Molecules 2015, 20, 12525–12544. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.K.; Zawawi, N. Stingless bee-collected pollen (Bee Bread): Chemical and microbiology properties and health Benefits. Molecules 2021, 26, 957. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Agra, M.d.F.; Silva, E.M.S.; Reis, I.T.; Freitas, B.M. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). An. Acad. Bras. Cienc. 2009, 81, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Caçador, I.; Duarte, B. Heavy metal pre-conditioning history modulates Spartina patens physiological tolerance along a salinity gradient. Plants 2021, 10, 2072. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319596891. [Google Scholar]
- Chang, C.L.; Wu, R.T. Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC-MS. Food Chem. 2011, 126, 710–717. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic-mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Lu, Q. Separation and characterization of phenolamines and flavonoids from rape bee pollen, and comparison of their antioxidant activities and protective effects against oxidative stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P. Original article UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2019, 54, 335–346. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. A multivariate approach based on physicochemical parameters and biological potential for the botanical and geographical discrimination of Brazilian bee pollen. Food Biosci. 2018, 25, 91–110. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT-Food Sci. Technol. 2019, 112, 108244–108250. [Google Scholar] [CrossRef]
- Čeksterytė, V.; Jansen, E.H.J.M. Composition and content of fatty acids in beebread of various floral origin, collected in Lithuania and prepared for storage in different ways. Chemine Technol. 2012, 2, 57–61. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Production, characterization and antioxidant potential of protease from Streptomyces sp. MAB18 using poultry wastes. Biomed Res. Int. 2013, 2013, 496586. [Google Scholar] [CrossRef] [PubMed]
- Koka, R.; Weimer, B.C. Isolation and characterization of a protease from Pseudomonas fluorescens RO98. J. Appl. Microbiol. 2000, 89, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sumantha, A.; Larroche, C.; Pandey, A. Microbiology and industrial biotechnology of food-grade proteases: A perspective. Food Technol. Biotechnol. 2006, 44, 211–220. [Google Scholar]
- Azarkan, M.; El Moussaoui, A.; Van Wuytswinkel, D.; Dehon, G.; Looze, Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J. Chromatogr. B 2003, 790, 229–238. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.P.; Hemmings, A.M.; Buttle, D.J.; Pearl, L.H. Crystal Structure of Glycyl Endopeptidase from Carica papaya: A Cysteine Endopeptidase of Unusual Substrate Specificity. Biochemistry 1995, 34, 13190–13195. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Metry, W.A.; Owayss, A.A. Influence of incorporating honey and royal jelly on the quality of yoghurt during storage. Egypt. J. Food Sci. 2009, 37, 115–131. [Google Scholar]
- Atallah, A.A. The production of bio-yoghurt with probiotic bacteria, royal jelly and bee pollen grains. J. Nutr. Food Sci. 2016, 6, 1000510–1000516. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Gatzias, I.; Riganakos, K.A. Bio-functional properties of bee pollen: The case of “bee pollen yoghurt”. Coatings 2018, 8, 423. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection—A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.M.; Elenany, Y.E.; Nassrallah, A.; Cheng, W.; Abd El-Maksoud, A.A. Royal jelly improves the physicochemical properties and biological activities of fermented milk with enhanced probiotic viability. LWT 2022, 155, 112912. [Google Scholar] [CrossRef]
- Laidlaw, H.H.; Page, R.E. Queen Rearing and Bee Breeding; Wicwas Press: New York, NY, USA, 1997. [Google Scholar]
- AOAC. AOAC: Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Food Analysis Laboratory Manual, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-1-4419-1462-0. [Google Scholar]
- Beaty, R.D.; Kerber, J.D. Concepts, Instrumentation and Techniques in Atomic Absorption Spectrophotometry, 2nd ed.; The Perkin-Elmer Corporation: Norwalk, CT, USA, 1993. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; Agronomy Monographs; ASA, CSSA, SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- FDA. 14. Appendix F: Calculate the Percent Daily Value for the Appropriate Nutrients. In Guidance for Industry: A Food Labeling Guide; FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- INGOS. Automatic Amino Acid Analyser AAA 400 User Manual; INGOS: Strasbourg, France, 2006. [Google Scholar]
- Smith, A.J. Post column amino acid analysis. In Methods in Molecular Biology (Clifton, N.J.); Smith, A.J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2003; Volume 211, pp. 133–141. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteau Reagent. Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 9, 569–575. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Larsen, M.D.; Kristiansen, K.R.; Hansen, T.K. Characterization of the proteolytic activity of starter cultures of Penicillium roqueforti for production of blue veined cheeses. Int. J. Food Microbiol. 1998, 43, 215–221. [Google Scholar] [CrossRef]
- Ramakrishna, T.; Pandit, M.W. Self-association of α-chymotrypsin: Effect of amino acids. J. Biosci. 1988, 13, 215–222. [Google Scholar] [CrossRef]
- Rodarte, M.P.; Dias, D.R.; Vilela, D.M.; Schwan, R.F. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Sci.-Agron. 2011, 33, 457–464. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Beguin, F.; Gujer-Kellenberger, G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 1970, 47, 69–85. [Google Scholar] [CrossRef]
- Sutikno, B.T.; Rizal, S.; Marniza. Effects of sugar type and concentration on the characteristics of fermented turi (Sesbania grandiflora (L.) poir) milk. Emirates J. Food Agric. 2013, 25, 576–584. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robenson, R.K. Tamime and Robenson’s Yoghurt Science and Technology, 3rd ed.; Woodhead Publishing Ltd. and CRC Press LLC: Cambridge, UK, 2007; ISBN 9781855735842. [Google Scholar]
- Ling, E.R. A Textbook of Dairy Chemistry, 2nd ed.; Chapman and Hall LTD: London, UK, 1945. [Google Scholar]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Marth, E.H. Standard Methods for the Examination of Dairy Products, 14th ed.; American Public Health Association: Washington, DC, USA, 1978; p. 20036. [Google Scholar]
- Darwish, A.M.G.; Khalifa, R.E.; El Sohaimy, S.A. Functional Properties of Chia Seed Mucilage Supplemented in Low Fat Yoghurt. Alexandria Sci. Exch. J. An Int. Q. J. Sci. Agric. Environ. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Senaka Ranadheera, C.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- 103. ISO 22935-3|IDF 099-3: 2009; Milk and Milk Products—Sensory Analysis—Part 3: Guidance on a Method for Evaluation of Compliance with Product Specifications for Sensory Properties by Scoring, First Edition. ISO and IDF: Geneva, Switzerland, 2009; ISBN 2831886376.
- IBM Corp. IBM SPSS Statistics for Windows, Version 23.0; IBM Corp: Armonk, NY, USA, 2015.




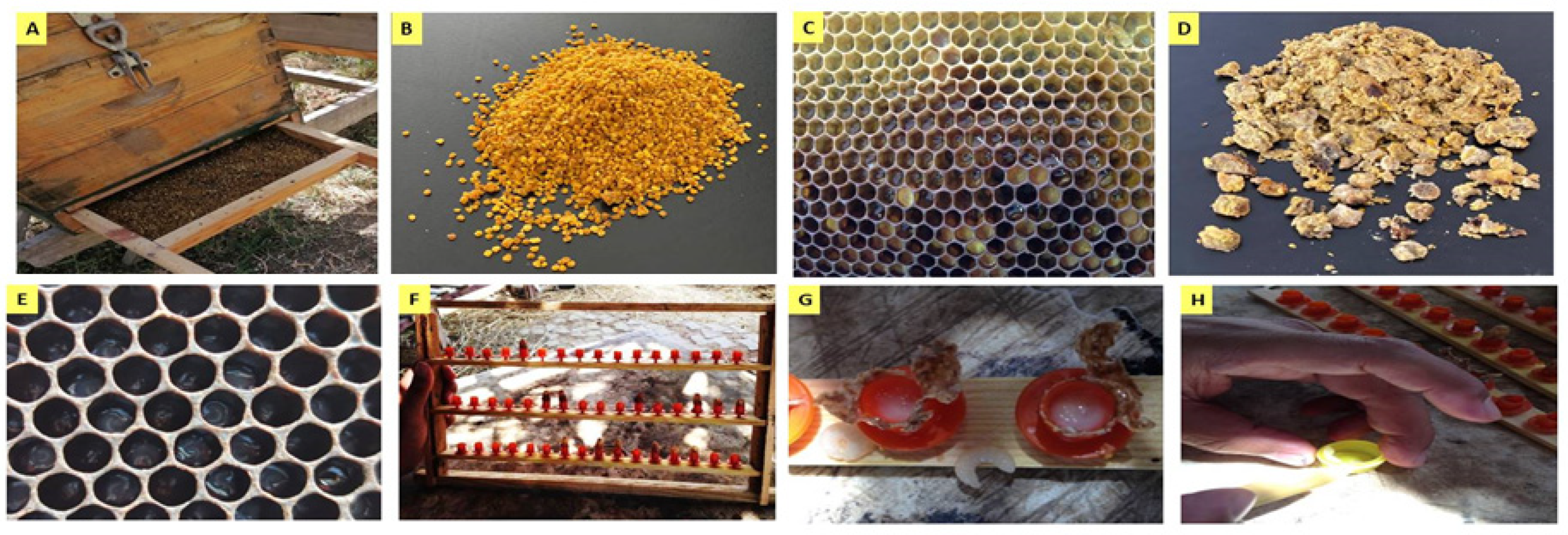
| Component | Bee Pollen | Bee Bread | Royal Jelly | Tri-Mix | ||||
|---|---|---|---|---|---|---|---|---|
| Content | 1 DV% | Content | 1 DV% | Content | 1 DV% | Content | 1 DV% | |
| Nutrients (g/100 g) | ||||||||
| Moisture | 17.41 ± 1.74 | - | 25.92 ± 0.14 | - | 63.81 ± 1.24 | - | 26.38 ± 0.58 | - |
| Ash | 2.35 ± 0.16 | - | 3.02 ± 0.07 | - | 3.25 ± 0.09 | - | 3.35 ± 0.19 | - |
| Protein | 21.09 ± 0.01 | 42.18 | 22.57 ± 0.07 | 45.14 | 12.89 ± 0.21 | 25.78 | 18.69 ± 0.05 | 37.38 |
| Total sugars | 19.69 ± 0.51 | 39.38 | 26.78 ± 1.80 | 53.56 | 14.78 ± 1.29 | 29.56 | 19.69 ± 2.06 | 39.38 |
| Minerals (mg/100 g) | ||||||||
| Calcium, Ca | 138.31 | 13.83 | 282.87 | 28.29 | 52.44 | 5.24 | 272.29 | 27.23 |
| Copper, Cu | 0.97 | 48.50 | 1.40 | 70.00 | 0.50 | 25.00 | 1.31 | 65.50 |
| Iron, Fe | 25.40 | 141.11 | 35.73 | 198.50 | 17.09 | 94.94 | 40.94 | 227.44 |
| Potassium, K | 480.41 | 13.73 | 518.77 | 14.82 | 124.01 | 3.54 | 504.71 | 14.42 |
| Magnesium, Mg | 96.41 | 24.10 | 85.13 | 21.28 | 30.13 | 7.53 | 80.20 | 20.05 |
| Manganese, Mn | 2.12 | 106.00 | 1.83 | 91.50 | 0.16 | 8.00 | 1.66 | 83.00 |
| Sodium, Na | 98.65 | 4.11 | 120.16 | 5.01 | 74.42 | 3.10 | 99.11 | 4.13 |
| Phosphorus, P | 226.15 | 22.62 | 192.25 | 19.23 | 104.44 | 10.44 | 241.00 | 24.10 |
| Zinc, Zn | 5.91 | 39.40 | 4.72 | 31.47 | 1.42 | 9.47 | 4.57 | 30.47 |
| Amino Acid | Symbol | 1 Pattern | Bee Pollen | Bee Bread | Royal Jelly | |||
|---|---|---|---|---|---|---|---|---|
| mg/g | 2 AAS | mg/g | 2 AAS | mg/g | 2 AAS | |||
| Essential Amino Acids | ||||||||
| Histidine | His | 12.00 | 3.35 | 27.91 | 4.66 * | 16.71 | 2.85 | 17.07 |
| Leucine | Leu | 14.00 | 5.34 | 38.13 | 9.46 | 24.80 | 4.04 | 16.30 |
| Isoleucine | Ile | 10.00 | 2.93 | 29.32 | 4.97 | 16.95 | 2.21 | 13.04 |
| Lysine | Lys | 12.00 | 5.04 | 42.03 | 9.28 | 22.07 | 3.98 | 18.05 |
| Methionine + cysteine | Met + Cys | 13.00 | 2.63 * | 20.26 | 5.44 | 26.85 | 1.13 * | 4.22 |
| Phenylalanine + tyrosine | Phe + Tyr | 14.00 | 5.02 | 35.86 | 9.53 | 26.58 | 4.87 | 18.31 |
| Threonine | Thr | 7.00 | 4.17 | 59.50 | 6.33 | 10.64 | 2.50 | 23.54 |
| Valine | Val | 10.00 | 3.97 | 39.74 | 6.48 | 16.30 | 2.96 | 18.13 |
| Non-Essential Amino Acids | ||||||||
| Alanine | Ala | - | 5.94 | - | 7.79 | - | 1.99 | - |
| Arginine | Arg | - | 4.23 | - | 7.63 | - | 2.96 | - |
| Aspartic acid | Asp | - | 8.66 | - | 14.74 | - | 11.56 | - |
| Glutamic acid | Glu | - | 9.01 | - | 39.32 | - | 5.99 | - |
| Glycine | Gly | - | 4.39 | - | 6.71 | - | 1.80 | - |
| Proline | Pro | - | 6.63 | - | 41.32 | - | 4.22 | - |
| Serine | Ser | - | 4.76 | - | 9.37 | - | 3.00 | - |
| No. | Compound Name | RT (min) | Parent Mass (g/moleg) | Molecular Formula | Fragments from the Raw Mass Spectrum (MS–MS of [M+] | Class | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Apigenin-7-O-glucoside 1 | 0.41 | 433.110 | C21H20O10 | 385.9840, 265.0950, 221.0030 | Flavonoid | [43] |
| 2 | Glycerophosphorylcholine 2 | 1.50 | 258.240 | C8H20NO6P | 184.0270, 103.9920 | Glycerophospholipids | https://bit.ly/3cCJup1 (accessed on 8 November 2022) |
| 3 | Linolenic acid 3 | 2.29 | 279.095 | C18H30O2 | 205.0880, 149.0250, 135.1179 | Fatty acid | [44] |
| 4 | (9Z,12Z,15Z)-Octadecatrienoic acid 3 | 2.29 | 279.679 | C18H30O2 | 223.1450, 206.0780, 169.0890, 128.1160 | Fatty acid | [45] |
| 5 | Gluconapin 1 | 6.69 | 373.312 | C11H19NO9S2 | 133.1230, 113.0610, 85.0760 | Alkylglucosinolate | [46] |
| 6 | Erucifoline 2 | 7.95 | 350.205 | C18H23NO6 | 332.0370, 303.9810, 259.8500, 186.0020, 122.0490 | Pyrrolizidine alkaloid | [47] |
| 7 | Galloyl-glucose 2 | 8.85 | 332.250 | C13H15O10 | 314.0940, 286.0230, 242.0300, 167.9630 | Tannins | [48] |
| 8 | Glucotropaeolin 2 | 9.48 | 410.478 | C14H19NO9S2 | 284.0570, 206.0510, 163.9885, 149.0770, | Alkylglucosinolate | [46] |
| 9 | 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine 2 | 10.10 | 469.383 | C21H44NO8P | 313.2810, 258.1590, 184.0740, 125.090, 104.1170, 86.1320, 71.1090, | Fatty acid | https://bit.ly/3Kx8UkB (accessed on 8 November 2022) |
| 10 | Trans-cinnamic acid 3 | 10.38 | 148.050 | C9H8O2 | 149.0472, 131.9598 | Cinnamic acid | [49] |
| 11 | Quercetin 3,4′-diglucoside 2 | 10.97 | 627.2240 | C27H30O17 | 464.9100, 302.9680, 257.0180, 229.0340 | Flavonoid | https://bit.ly/3CLx0G8 (accessed on 8 November 2022) |
| 12 | Myricetin-3-O-rutinoside 2 | 11.96 | 627.150 | C27H30O17 | 478.9240, 463.0380, 316.9320, | Flavonoid | [50] |
| 13 | Kaempferol-7-O-neohesperidoside 2 | 11.97 | 595.204 | C27H30O15 | 448.9148, 329.0170, 286.9450 | Flavonoid | https://bit.ly/3KH5MTi (accessed on 8 November 2022) |
| 14 | 1-Palmitoylglycerophosphocholine 2 | 15.14 | 497.621 | C24H51NO7 P+ | 479.2050, 183.9420 | Glycerophospholipid | https://bit.ly/3Tycvmz (accessed on 8 November 2022) |
| 15 | Kaempferol 2 | 15.63 | 287.234 | C15H10O6 | 270.930, 240.9860, 231.0140, 212.9970, 164.9480, 152.930, 120.9760 | Flavonoid | [51] https://bit.ly/3YMu2tH (accessed on 8 November 2022) |
| 16 | Luteolin 2 | 15.63 | 287.234 | C15H10O6 | 240.9860, 176.9550, 152.9390 | Flavonoid | [52,53] |
| 17 | Cholesterol 3 | 16.14 | 387.204 | C27H46O | 331.1450, 275.0680, 175,0180, 77.0670 | Steroid | [54] https://bit.ly/3ABPp5R (accessed on 8 November 2022) |
| 18 | Erucic acid 3 | 16.48 | 339.220 | C22H42O2 | 321.2420, 167.1070, 113.0830 | Fatty acid | [55] |
| 19 | 8 Methoxykaempferol 2 | 17.31 | 317.218 | C16H12O7 | 299.0680, 182.9630, | Flavonoid | [56]https://bit.ly/3FP7CAz |
| 20 | Isorhamnetin-3,7-di-O-glucoside 3 | 18.55 | 641.199 | C28H32O17 | 479.1630, 317.076, 145.0590, 127.0400 | Flavonoid | https://bit.ly/3cxxxRp (accessed on 8 November 2022) |
| 21 | 3-[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5-hydroxy-7-methoxychromen-4-one 3 | 18.66 | 641.199 | C28H32O17 | 317.0720, 228.0740, 127.0500, 109.0410, 85.0560 | Flavonoid | https://bit.ly/3cxQvaL (accessed on 8 November 2022) |
| 22 | Dihydroquercetin 3 | 19.40 | 305.208 | C15H12O7 | 153.0180, 135.0550, 95.1010 | Flavonoid | [57] |
| 23 | Antheraxanthin 3 | 19.99 | 584.340 | C40H56O3 | 584.3281, 566.2841, 548.3191 | Epoxycarotenol | [58] |
| 24 | Catechin 3 | 20.09 | 291.194 | C15H14O6 | 273.1880, 109.0810 | Flavonoid | [59,60] |
| 25 | Eicosanoic acid 2 | 20.28 | 313.391 | C20H40O2 | 295.097, 226.9930, 113.0180 | Fatty acid | [45] |
| 26 | Narcissin 2 | 20.40 | 625.140 | C28H32O16 | 478.9240, 463.0380, 316.9320 | Flavonoid | https://bit.ly/3R39BV2 (accessed on 8 November 2022) |
| 27 | Petunidin-3-O-rutinoside 3 | 20.40 | 627.170 | C28H35O16+ | 627.3573, 480.1067, 317.0757 | Flavonoid | [61] |
| 28 | 1-(9Z,12Z-Octadecadienoyl)-sn-glycero-3-phosphocholine 2 | 24.56 | 520.603 | C26H50NO7P | 520.2020, 335.1590, 183.9490165.9880 | Glycerophospholipid | https://bit.ly/3B1qeLd (accessed on 8 November 2022) |
| 29 | Pinobanksin5-methylether-3-O-acetate 3 | 20.80 | 314.202 | C18H17O5 | 254.1670, 236.1810, 180.1590, 162.9690 | Flavonoid | [59] |
| 30 | 10-Hydroxy-2-decenoic acid 1 | 21.53 | 187.130 | C10H18O3 | 187.1488, 169.1582, 153.1280, 142.1416, 129.1183, 87.9283 | Fatty acid | [62] |
| 31 | Jelleine II 1 | 21.79 | 1054.300 | C51H82N12O12 | 1053.8247, 527.9614 | Peptide | [42] |
| 32 | 1-Decanoyllysolecithin 3 | 23.20 | 412.217 | C18H38NO7P | 229.1340, 184.0640, 166.0410, 125.000, 104.0930, 86.1210, 59.0810 | Glycerophospholipid | https://bit.ly/3ednJwB (accessed on 8 November 2022) |
| 33 | p-Coumaroyl spermidine 3 | 23.26 | 291.184 | C16H25N3O2 | 233.1550, 219.1350, 177.1480 | Styrene | [63] |
| 34 | p-Coumaroyl glycerl 3 | 23.63 | 239.159 | C12H14O5 | 221.1500, 206.5031, 198.1296 | Phenol | [64] |
| 35 | Unknown 1 | 23.84 | 2586.186 | - | 1293.093, 863.3149, 647.7252, 518.5782, 432.4673 | Polypeptide | - |
| 36 | Formononetin3 | 24.77 | 267.120 | C16H12O4 | 131.0950, 119.0610 | Flavonoid | [65] |
| 37 | Delphinidin-3-O-rutinoside 3 | 24.91 | 647.130 | C27H31ClO16 | 484.2287, 322.1964, 308.2132, 291.1788, 177.0493 | Flavonoid | [61] |
| 38 | [(2R)-2-Hydroxy-3-[(Z)-icos-9-enoyl]oxypropyl] 2-(trimethylazaniumyl)ethyl phosphate 3 | 25.97 | 549.379 | C28H56NO7P | 349.3070, 184.0731, 142.0910, 125.0070, 86.1240, 60.1180 | Glycerophospholipid | https://bit.ly/3R8MTuW (accessed on 8 November 2022) |
| 39 | Royalisin 1 | 26.07 | 5532.370 | C240H378N66O72S6 | 1382.8334, 1106.0650, 921.9670, 790.4330, 691.6823 | Polypeptide | [41] |
| 40 | N′,N″,N′″-Tris-p-feruloylspermidine 3 | 27.420 | 674.130 | C37H43N3O9 | 674.3249, 498.2758, 0.2445, 305.2053, 289.1925, 177.0558 | Phenylamide | [66] |
| 41 | 1-(9Z-Octadecenoyl)-sn-glycero-3-phosphocholine 3 | 28.85 | 520.363 | C26H52NO7P | 184.0800, 125.0050, 104.1150, 86.1280, 60.1070 | Glycerophospholipid | https://bit.ly/3pWfJTh (accessed on 8 November 2022) |
| 42 | Phloretin 2 | 30.86 | 274.262 | C15H14O5 | 256.000, 238.0010, 154.0300 | Dihydrochalcone | [67] |
| 43 | Capric acid 3 | 39.20 | 171.098 | C10H20O | 153.0930, 125.1070, 97.1230, 83.1100, 67.0730 | Fatty acid | [68] |
| 44 | [(2R)-3-Hexadecanoyloxy-2-hydroxypropyl] 2-(trimethylazaniumyl)ethyl phosphate 3 | 45.32 | 496.383 | C24H50NO7P | 478.2009, 183.9610 | Glycerophosphocholine | https://bit.ly/3dZzCWC (accessed on 8 November 2022) |
| 45 | [(2R)-3-Octadecanoyloxy-2-[(Z)-octadec-11-enoyl]oxypropyl] 2-(trimethylazaniumyl)ethyl phosphate 1 | 47.22 | 787.830 | C44H86NO8P | 524.4550, 184.0970, 99.5210 | Fatty acid | https://bit.ly/3RjZLxM (accessed on 8 November 2022) |
| 46 | 6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol 3 | 52.55 | 313.236 | C21H30O2 | 105.0820, 105.0820, 91.0670 | Benzopyran | https://bit.ly/3RnuWIK (accessed on 8 November 2022) |
| Sample | Casein Hydrolysis(Diameter mm) | Proteolytic Activity (U) | ||
|---|---|---|---|---|
| pH 5.0 | pH 7.0 | pH 9.0 | ||
| Bee pollen | - | 1.03 ± 0.14 fC | 2.20 ± 0.1 fB | 3.65 ± 0.098 eA |
| Bee bread | - | 1.22 ± 0.092 fC | 2.73 ± 0.12 eB | 4.73 ± 0.7 cA |
| Royal jelly | 1.0 ± 0.1 a | 2.27 ± 0.13 dC | 5.22 ± 0.11 bB | 7.28 ± 0.17 bA |
| Bee pollen/bee bread | - | 2.00 ± 0.1 eC | 3.22 ± 0.01 dB | 4.96 ± 0.15 cA |
| Bee pollen/royal jelly | 0.5 ± 0.1 b | 3.23 ± 0.15 cC | 4.23 ± 0.12 cB | 4.29 ± 0.13 dA |
| Bee bread/royal jelly | 0.5 ± 0.1 b | 5.72 ± 0.07 aC | 6.52 ± 0.11 aB | 8.4 ± 0.17 aA |
| Tri-mix | 0.5 ± 0.2 b | 4.35 ± 0.13 bC | 5.21 ± 0.1 bB | 7.11 ± 0.16 bA |
| Sample | pH | TA% | Viscosity (cP) |
|---|---|---|---|
| Control | 4.93 ± 0.02 a | 0.350 ± 0.01 b | 10.55 ± 0.07 e |
| TBP | 4.90 ± 0.01 a | 0.399 ± 0.01 a | 11.55 ± 0.21 d |
| TBB | 4.83 ± 0.01 b | 0.399 ± 0.01 a | 12.15 ± 0/07 bc |
| TRJ | 4.62 ± 0.01 c | 0.399 ± 0.01 a | 12.50 ± 0.14 b |
| TMix | 4.62 ± 0.03 c | 0.399 ± 0.01 a | 12.65 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, A.M.G.; Abd El-Wahed, A.A.; Shehata, M.G.; El-Seedi, H.R.; Masry, S.H.D.; Khalifa, S.A.M.; Mahfouz, H.M.; El-Sohaimy, S.A. Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules 2023, 28, 227. https://doi.org/10.3390/molecules28010227
Darwish AMG, Abd El-Wahed AA, Shehata MG, El-Seedi HR, Masry SHD, Khalifa SAM, Mahfouz HM, El-Sohaimy SA. Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules. 2023; 28(1):227. https://doi.org/10.3390/molecules28010227
Chicago/Turabian StyleDarwish, Amira M. G., Aida A. Abd El-Wahed, Mohamed G. Shehata, Hesham R. El-Seedi, Saad H. D. Masry, Shaden A. M. Khalifa, Hatem M. Mahfouz, and Sobhy A. El-Sohaimy. 2023. "Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products" Molecules 28, no. 1: 227. https://doi.org/10.3390/molecules28010227
APA StyleDarwish, A. M. G., Abd El-Wahed, A. A., Shehata, M. G., El-Seedi, H. R., Masry, S. H. D., Khalifa, S. A. M., Mahfouz, H. M., & El-Sohaimy, S. A. (2023). Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules, 28(1), 227. https://doi.org/10.3390/molecules28010227












