Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects
Abstract
:1. Introduction
2. Royal Jelly: Chemical Characterization, Quantitative Determination, and Storage
2.1. General Remarks
2.2. Sugar Composition
2.3. Proteins, Peptides, and Amino Acids
2.4. Lipids and Fatty Acids
2.5. Minerals, Flavonoids, Vitamins, and Other Components
2.6. Conditions for Maintaining RJ Quality
3. Main Bioactive Compounds in Royal Jelly
3.1. Major Royal Jelly Proteins (MRJPs) Family
3.1.1. MRJP1
3.1.2. Therapeutic Impact of MRJPs
3.1.3. Purification of MRJPs
3.2. 10-Hydroxy-2-Decenoic Acid (10-HDA)
3.3. Hormones
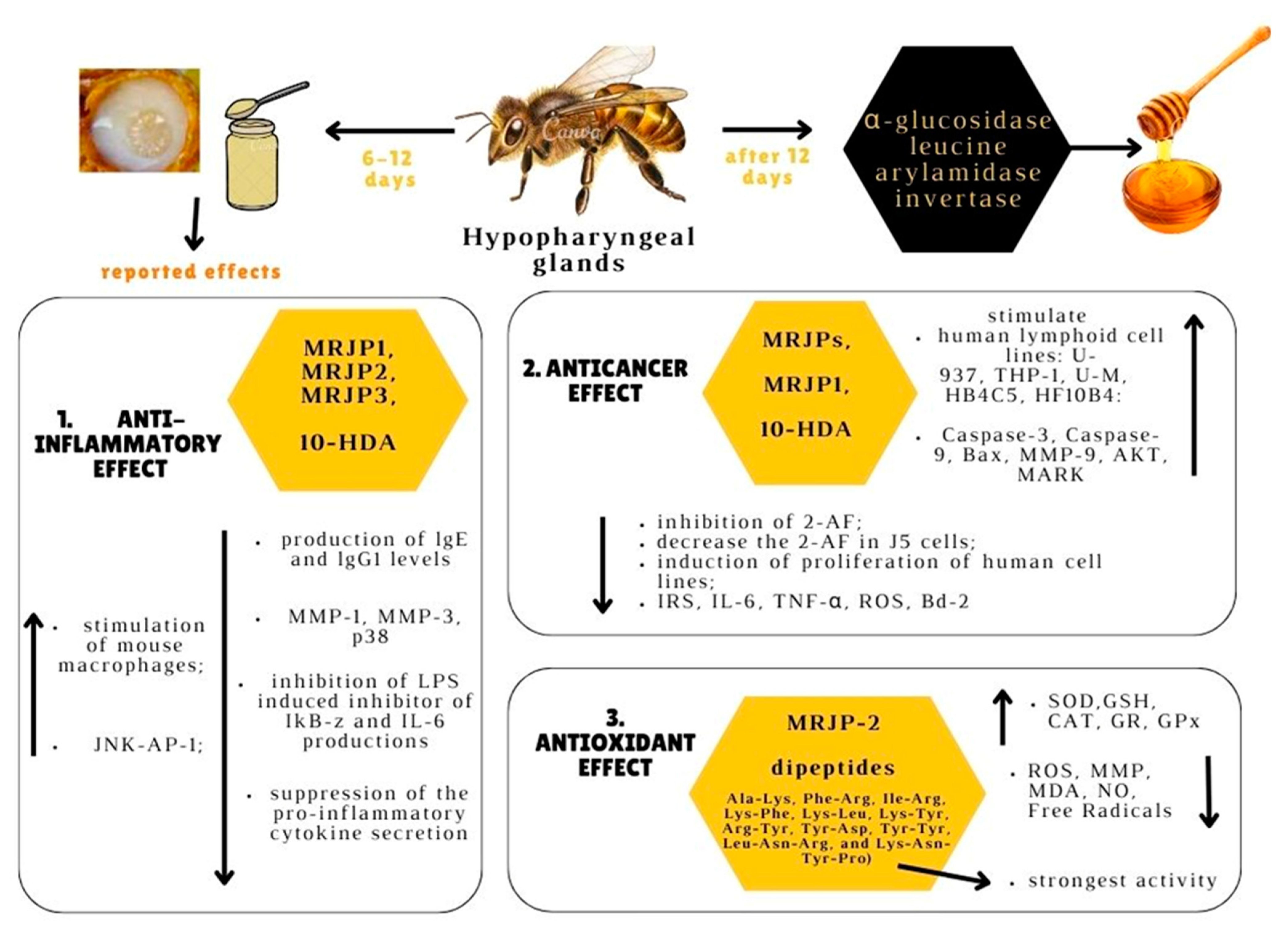
4. Functional Properties of Royal Jelly
4.1. Anticancer Role
4.2. Anti-Inflammatory Activity
4.3. Antioxidant Effect
5. The Apitherapeutic Potential of Royal Jelly
5.1. RJ as a Nutraceutical
5.2. Wound Healing
5.3. Aging and Longevity
5.4. Side Effects of Royal Jelly Administration
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weis, W.A.; Ripari, N.; Conte, F.L.; Honorio, M.S.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine 2022, 2, 100239. [Google Scholar] [CrossRef]
- Bobis, O.; Bonta, V.; Varadi, A.; Strant, M.; Dezmirean, D. Bee Products and Oxidative Stress: Bioavailability of Their Functional Constituents. Mod. Appl. Bioequiv. Availab. 2017, 1, 555565. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Gu, H.; Song, I.B.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Anti-inflammatory and immune-enhancing effects of enzyme-treated royal jelly. J. Appl. Biol. Chem. 2018, 61, 227–233. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef]
- Zhu, F.; Wongsiri, S. A brief introduction to apitherapy health care. J. Thai Trad. Alt. Med. 2008, 6, 303–312. [Google Scholar]
- Strant, M.; Yücel, B.; Topal, E.; Puscasu, A.M.; Margaoan, R.; Varadi, A. Use of Royal Jelly as Functional Food on Human and Animal Health. Hayvansal Üretim 2019, 60, 131–144. [Google Scholar] [CrossRef]
- Sharma, A.; Pant, K.; Brar, D.S.; Thakur, A.; Nanda, V. A review on Api-products: Current scenario of potential contaminants and their food safety concerns. Food Control 2023, 145, 109499. [Google Scholar] [CrossRef]
- Garcia-Amoedo, L.H.; Almeida-Muradian, L.B. Physicochemical composition of pure and adulterated royal jelly. Quim. Nova 2007, 30, 257–259. [Google Scholar] [CrossRef]
- Hu, F.L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Espindola, F.S.; Feng, M.; Guan, C.; Han, B.; Kraková, T.K.; Li, J.K.; et al. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudlou, A.; Mahoonak, A.S.; Mohebodini, H.; Toldra, F. Royal Jelly: Chemistry, Storage and Bioactivities. J. Apic. Sci.. 2019, 63, 17–40. [Google Scholar] [CrossRef]
- Hu, H.; Qiaohong, W.; Zhihua, S.; Xufeng, Z.; Chuan, M.; Mao, F.; Lifeng, M.; Jianke, L.; Bin, H.J. Development of a Freshness Assay for Royal Jelly Based on the Temperature- and Time-Dependent Changes of Antimicrobial Effectiveness and Proteome Dynamics of Royal Jelly Proteins. Agric. Food Chem. 2021, 69, 10731–10740. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight into the chemical composition and biological properties of Mediterranean royal jelly. J. Apic. Res. 2020, 59, 890–909. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 2017, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.I.; Dezmirean, D.S.; Marc, B.D.; Suharoschi, R.; Pop, O.L.; Buttstedt, A. Biological properties and activities of major royal jelly proteins and their derived peptides. J. Funct. Foods 2022, 98, 105286. [Google Scholar] [CrossRef]
- Khan, P.; Siddiqui, J.A.; Lakshmanan, I.; Ganti, A.K.; Salgia, R.; Jain, M.; Batra, S.K.; Nasser, M.W. RNA-based therapies: A cog in the wheel of lung cancer defense. Mol. Cancer Res. 2021, 20, 54. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell Longev. 2018, 2018, 1–29. [Google Scholar] [CrossRef]
- Miyata, Y.; Sakai., H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.; Gerçek, Y.C.; Özkök, A.; Bayram, N.E. Organic acids and their derivatives: Minor components of bee pollen, bee bread, royal jelly and bee venom. Eur. Food Res. Technol. 2022, 248, 3037–3057. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Food 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A.; Brescia, M.; Caprio, E. Assessment of geographical origin and production period of royal jelly by NMR metabolomics. Chem. Biol. Technol. Agric. 2020, 7, 24. [Google Scholar] [CrossRef]
- Lazarevska, S.; Makreski, P. Insights into the infrared and Raman spectra of fresh and lyophilized Royal Jelly and protein degradation IR spectroscopy study during heating. Maced. J. Chem. Chem. Eng. 2015, 34, 87–88. [Google Scholar] [CrossRef]
- Cebi, N.; Bozkurt, F.; Yilmaz, M.T.; Sagdic, O. An evaluation of FTIR spectroscopy for prediction of royal jelly content in hive products. J. Apic. Res. 2020, 59, 146–155. [Google Scholar] [CrossRef]
- Balkanska, R. Correlations of Physicochemical Parameters, Antioxidant Activity and Total Polyphenol Content of Fresh Royal Jelly Samples. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3744–3750. [Google Scholar] [CrossRef]
- Wytrychowski, M.; Chenavas, S.; Daniele, G.; Casabianca, H.; Batteau, M.; Guibert, S.; Brion, B. Physicochemical characterisation of French royal jelly: Comparison with commercial royal jellies and royal jellies produced through artificial bee-feeding. J. Food Compost. Anal. 2013, 29, 126–133. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L.B. Quality and standardisation of Royal Jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Machado, A.M.; Aazza, S.; Lyoussi, B.; Miguel, M.G.; Mateus, M.C.; Figueiredo, A.C. Chemical Characterization and Biological Properties of Royal Jelly Samples From the Mediterranean Area. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Kunugi, H.; Ali, A.M. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Bílikova, K.; Huang, S.C.; Lin, I.P.; Šimuth, J.; Peng, C.C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine. J. Bee Prod. Sci. 2011, 3, 8–19. [Google Scholar]
- Stocker., A.; Rossmann., A.; Kettrup., A.; Bengsch, E. Detection of royal jelly adulteration using carbon and nitrogen stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2010, 20, 181–184. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, P.; Wu, Y.; He, Q. Recent advances in analytical techniques for the detection of adulteration and authenticity of bee products—A review. Food Addit. Contam. Part A 2021, 38, 533–549. [Google Scholar] [CrossRef]
- Arfa, A.; Reyad, Y.M.; El Nikeety, M. Quality Parameters of Royal Jelly in national and international standards: Specifications, differences and suggestions. Ann. R.S.C.B. 2021, 25, 7977–7997. [Google Scholar]
- Sidor, E.; Miłek, M.; Zaguła, G.; Bocian, A.; Dzuga, M. Searching for Differences in Chemical Composition and Biological Activity of Crude Drone Brood and Royal Jelly Useful for Their Authentication. Foods 2021, 10, 2233. [Google Scholar] [CrossRef]
- Campos, M.G.; Markham, K.R. Structure information from HPLC and on-line measuredabsorption spectra: Flavones, Flavonols and Phenolic Acids, 1st ed.; Imprensa da Universidade: Coimbra, Portugal, 2017; p. 14. [Google Scholar]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 36—Vitamin Metabolism. In Essentials of Medical Biochemistry; Bhagavan, N.V., Chung-Eun, H., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 683–699. ISBN 9780124166875. [Google Scholar]
- Chiang, S.H.; Yang, K.M.; Sheu, S.C.; Chen, C.W. The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants 2021, 10, 580. [Google Scholar] [CrossRef]
- Kausar, S.; More, V. Royal Jelly: Organoleptic Characteristics and Physicochemical Properties. Pharm. Chem. J. 2019, 6, 20–24. [Google Scholar]
- Buttstedt, A.; Moritz, R.F.; Erler, S. More than royal food-Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.H.; Zhou, Q.X.; Yu, L.L.; Li, W.G.; Yi, Y.Z.; Zhang, Y.Z.; Zhang, Z.F. Identification and analysis of YELLOW protein family genes in the silkworm, Bombyx mori. BMC Genom. 2006, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the native major royal jelly protein 1 oligomer. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maori, E.; Navarro, I.C.; Boncristiani, H.; Seilly, D.J.; Rudolph, K.L.M.; Sapetschnig, A.; Lin, C.C.; Ladbury, J.E.; Evans, J.D.; Heeney, J.L.; et al. A Secreted RNA Binding Protein Forms RNA-Stabilizing Granules in the Honeybee Royal Jelly. Mol. Cell 2019, 74, 598–608.e6. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Kagami, T.; Sasak, T.; Nakamura, J.; Sasaki, M. Reduced expression of major royal jelly protein 1 gene in the mushroom bodies of worker honeybees with reduced learning ability. Apidologie 2010, 41, 194–202. [Google Scholar] [CrossRef]
- Mandacaru, S.C.; do Vale, L.H.F.; Vahidi, S.; Xiao, Y.; Skinner, O.S.; Ricart, C.A.O.; Kelleher, N.L.; de Sousa, M.V.; Konermann, L. Characterizing the Structure and Oligomerization of Major Royal Jelly Protein 1 (MRJP1) by Mass Spectrometry and Complementary Biophysical Tools HHS Public Access. Biochemistry 2017, 56, 1645–1655. [Google Scholar] [CrossRef]
- Moriyama, T.; Ito, A.; Omote, S.; Miura, Y.; Tsumoto, H. Heat resistant characteristics of major royal jelly protein 1 (MRJP1) oligomer. PLoS ONE 2015, 10, 0119169. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Mureşan, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.H.; Pietzsch, M.; Moritz, R.F. How Honeybees Defy Gravity with Royal Jelly to Raise Queens. Curr. Biol. 2018, 28, 1095–1100.e3. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Aumer, D.; Fuszard, M.; Erler, S.; Buttstedt, A. The rise and fall of major royal jelly proteins during a honeybee (Apis mellifera) workers’ life. Ecol. Evol. 2019, 9, 8771–8782. [Google Scholar] [CrossRef]
- Barbora, M. Honeybee (Apis mellifera L.) mrjp gene family: Computational analysis of putative promoters and genomic structure of mrjp1, the gene coding for the most abundant protein of larval food. Gene 2003, 303, 165–175. [Google Scholar]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 11, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. BioSci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties’. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.Q.; Shen, L.R. Supplementation with Major Royal-Jelly Proteins Increases Lifespan, Feeding, and Fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Qiao, J.; Zhang, G.; Zhang, H. Purification and characteristics of individual major royal jelly protein 1–3. J. Apic. Res. 2020, 59, 1049–1060. [Google Scholar] [CrossRef]
- Tefan Albert, S.; Klaudiny, J.; Imúth, J.S. Molecular characterization of MRJP3, highly polymorphic protein of honeybee (Apis mellifera) royal jelly. Insect Biochem. Mol. Biol. 1999, 29, 427–434. [Google Scholar] [CrossRef]
- Jiang-Hong, L.; Qing-Huan, L.; Da-Fu, C.; Qin, L. Differences in the expression of the major royal jelly protein 8 gene in different castes of Apis mellifera. Acta Entomol. Sin. 2014, 57, 1–7. [Google Scholar]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Lee, S.; Kwang, S.L.; Min, O.; Bo, K.Y.; Byung, R.J. Antimicrobial activity of major royal jelly protein 8 and 9 of honeybee (Apis mellifera) venom. J. Asia Pac. Entomol. 2022, 25, 101964. [Google Scholar] [CrossRef]
- Kim, B.Y.; Jin, B.R. Antimicrobial activity of the C-terminal of the major royal jelly protein 4 in a honeybee (Apis cerana). J. Asia Pac. Entomol. 2019, 22, 561–564. [Google Scholar] [CrossRef]
- Sato, A.; Unuma, H.; Ebina, K. Royal Jelly Proteins Inhibit Macrophage Proliferation: Interactions with Native- and Oxidized-Low Density Lipoprotein. Protein J. 2021, 40, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, X.; Li, C.; Qian, H.; Chen, D.; Lai, C.; Shen, L. Anti-senescence effect and molecular mechanism of the major royal jelly proteins on human embryonic lung fibroblast (HFL-I) cell line. J. Zhejiang Univ. Sci. B 2018, 19, 960–972. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, C.; Chen, Y.; Shi, F.; Lai, C.; Shen, L. Major royal jelly proteins accelerate onset of puberty and promote ovarian follicular development in immature female mice. Food Sci. Hum. Wellness 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Kim, B.Y. Antiapoptotic role of major royal jelly protein 8 of honeybee (Apis mellifera) venom. J. Asia Pac. Entomol. 2021, 24, 666–670. [Google Scholar] [CrossRef]
- Kamakura, M.; Suenobu, N.; Fukushima, M. Fifty-seven-kDa Protein in Royal Jelly Enhances Proliferation of Primary Cultured Rat Hepatocytes and Increases Albumin Production in the Absence of Serum. Biochem. Biophys. Res. Commun. 2001, 282, 865–874. [Google Scholar] [CrossRef]
- Mureșan, C. Biochemical Characterization of the Most Abundant Proteins Purified from Royal Jelly (Apis mellifera); University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Lin, X.M.; Liu, S.B.; Luo, Y.H.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Lu, B.X.; et al. 10-HDA Induces ROS-Mediated Apoptosis in A549 Human Lung Cancer Cells by Regulating the MAPK, STAT3, NF-κB, and TGF-β1 Signaling Pathways. Biomed. Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules 2021, 26, 7021. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.F.; You, M.M.; Liu, Y.C.; Shi, Y.Z.; Wang, K.; Lu, Y.Y.; Hu, F.L. Potential protective effect of Trans-10-hydroxy-2-decenoic acid on the inflammation induced by Lipoteichoic acid. J. Funct. Foods 2018, 45, 491–498. [Google Scholar] [CrossRef]
- Bălan, A.; Moga, M.A.; Dima, L.; Toma, S.; Neculau, E.A.; Anastasiu, C.V. Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules 2020, 25, 3291. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit Sci. 2014, 22, 241. [Google Scholar] [CrossRef]
- Williams, M.J.; Sottoriva, A.; Graham, T.A. Measuring Clonal Evolution in Cancer with Genomics. Annu. Rev. Genom. Hum. Genet. 2019, 20, 309–329. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer Review evolve progressively from normalcy via a series of pre. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Garnis, C.; Lam, W.L. Genetic alteration and gene expression modulation during cancer progression. Mol. Cancer 2004, 3, 9. [Google Scholar] [CrossRef]
- Loeb, L.A.; Loeb, K.R.; Anderson, J.P. Multiple mutations and cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 776–781. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal Jelly: Beneficial Properties and Synergistic Effects with Chemotherapeutic Drugs with Particular Emphasis in Anticancer Strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef] [PubMed]
- Bincoletto, C.; Eberlin, S.; Figueiredo, C.A.; Luengo, M.B.; Queiroz, M.L. Effects produced by Royal Jelly on haematopoiesis: Relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharmacol. 2005, 5, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. BioSci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.W. Effects of royal jelly extracts on growth inhibition, differentiation human leukemic U937 cells and its immunomodulatory activity. Biocell 2019, 43, 29–41. [Google Scholar]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Q.; Geng, H.; Su, S. The effect of royal jelly on the growth of breast cancer in mice. Oncol. Lett. 2017, 14, 7615–7621. [Google Scholar] [CrossRef] [Green Version]
- Abandansari, R.M.; Parsian, H.; Kazerouni, F.; Porbagher, R.; Zabihi, E.; Rahimipour, A. Eect of Simultaneous Treatment with Royal Jelly and Doxorubicin on the Survival of the Prostate Cancer Cell Line (PC3): An In Vitro Study. Int. J. Cancer Manag. 2018, 11, e13780. [Google Scholar]
- Araki, K.; Miyata, Y.; Ohba, K.; Nakamura, Y.; Matsuo, T.; Mochizuki, Y.; Sakai, H. Oral intake of royal jelly has protective effects against tyrosine kinase inhibitor-induced toxicity in patients with renal cell carcinoma: A randomized, double-blinded, placebo-controlled trial. Medicines 2019, 6, 2. [Google Scholar] [CrossRef]
- Miyata, Y.; Ohba, K.; Matsuo, T.; Mitsunari, K.; Sakai, H. A randomized, double-blinded clinical trial of royal jelly intake for anticancer effects and suppressing adverse events in renal cell carcinoma patients treated with tyrosine kinase inhibitors. J. Clin. Oncol. 2020, 38, 697. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Antitumor Activity of Royal Jelly and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. BioMed. Res. Int. 2022, 2022, 7233997. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh. Hig. Rada. Toksikol. 2015, 66, 269–274. [Google Scholar] [CrossRef]
- Kamiya, T.; Watanabe, M.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Itoh, A.; Adachi, T. Induction of Human-Lung-Cancer-A549-Cell Apoptosis by 4-Hydroperoxy-2- decenoic Acid Ethyl Ester through Intracellular ROS Accumulation and the Induction of Proapoptotic CHOP Expression. J. Agric. Food Chem. 2018, 66, 10741–10747. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal Jelly Attenuates LPS-Induced Inflammation in BV-2 Microglial Cells through Modulating NF-κB and p38/JNK Signaling Pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [Green Version]
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z. Effects of Royal Jelly Administration on Lipid Profile, Satiety, Inflammation, and Antioxidant Capacity in Asymptomatic Overweight Adults. Evid. Based Complement. Altern. Med. 2019, 2019, 4969720. [Google Scholar] [CrossRef]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Res. 2017, 79, 299–307. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.N.; Rifaai, R.A.; Abdelzaher, W.Y. Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study. Biomed. Pharmacother. 2017, 90, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef]
- Karaca, T.; Şimşek, N.; Uslu, S.; Kalkan, Y.; Can, I.; Kara, A.; Yörük, M. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol. Immunopathol. 2012, 40, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Metwally, S.E.L.; Kosba, A.A. Royal jelly supplementation reduces skeletal muscle lipotoxicity and insulin resistance in aged obese rats. Pathophysiology 2018, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Carotenoids and chronic diseases. Drug. Metabol. Drug. Interact. 2000, 17, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, D.; Fujiwara, N.; Suzuki, K. Antioxidants: Benefits and risks for long-term health. Maturitas 2010, 67, 103–107. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative Effect of Royal Jelly in Cisplatin-induced Testes Damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Cemek, M.; Aymelek, F.; Büyükokuroğlu, M.E.; Karaca, T.; Büyükben, A.; Yilmaz, F. Protective potential of Royal Jelly against carbon tetrachloride induced-toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol. 2010, 48, 2827–2832. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef] [PubMed]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef] [PubMed]
- Eshtiyaghi, M.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 2016, 86, 2210–2221. [Google Scholar] [CrossRef]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Obaidat, M.M.; Ereifej, K.; Alhamad, M.N.; Mhaidat, N.; Andrade, J.E.; Johargy, A.; Ayadi, W. Probiotics in Milk as Functional Food: Characterization and Nutraceutical Properties of Extracted Phenolics and Peptides from Fermented Skimmed Milk Inoculated with Royal Jelly. J. Food Saf. 2015, 35, 509–522. [Google Scholar] [CrossRef]
- Mendoza, M.R.; Olano, N.O.; Villamiel, M. Chemical Indicators of Heat Treatment in Fortified and Special Milks. J. Agric. Food Chem. 2005, 53, 2995–2999. [Google Scholar] [CrossRef]
- Fern, B.; Mart, J.M.; Chas-Barbeito, C.; Bautista-Casta, I. Food & Function Determinants of specific food consumption in the Canary Islands (Spain). Food Funct. 2011, 2, 627–632. [Google Scholar]
- Chiu, H.F.; Chen, B.K.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef]
- Tahereh, F.N.; Nayereh, K.; Narjes, B.; Mojtaba, M.; Shirin, S.; Sareh,, D. The Fertility Outcome of Royal Jelly versus Intra Uterine Insemination: A Pilot Randomized Controlled Trial Study, Jundishapur. J. Nat. Prod. 2021, 16, e107420. [Google Scholar]
- Basmeh, K.; Farzad, S.; Shima, J.; Mojtaba, M.A.; Fatemeh, H. Effect of royal jelly intake on serum glucose, HbA1c, and total antioxidant capacity (TAC) in type 2 diabetic patients: A randomized, double blind clinical trial study. RJMS 2013, 19, 33–40. [Google Scholar]
- Ohba, K.; Miyata, Y.; Shinzato, T.; Funakoshi, S.; Maeda, K.; Matsuo, T.; Mitsunari, K.; Mochizuki, Y.; Nishino, T.; Sakai, H. Effect of oral intake of royal jelly on endothelium function in hemodialysis patients: Study protocol for multicenter, double-blind, randomized control trial. Trials 2022, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Oshvandi, K.; Aghamohammadi, M.; Kazemi, F.; Masoumi, S.Z.; Mazdeh, M.; Molavi, V.M. Effect of royal jelly capsule on quality of life of patients with multiple sclerosis: A double-blind randomized controlled clinical trial. Iran. Red Crescent Med. J. 2020, 22, e74. [Google Scholar] [CrossRef]
- Yumi, M.; Fumihiko, T.; Yuji, K.; Hiroyuki, H. The Effects of The Royal Jelly On Dry Mouth Sensation with Normal Saliva Function: A Double-Blind, Placebo-Controlled, Cross-Over Trial Clinical Study. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Shafiee, Z.; Hanifi, N.; Rashtchi, V. The effect of royal jelly on the level of consciousness in patients with traumatic brain injury: A double-blind randomized clinical trial. Nurs. Midwifery Stud. 2022, 11, 96–102. [Google Scholar]
- Peivandi., S.; Khalili, S.S.; Abbasi, Z.; Zamaniyan, M.; Gordani, N.; Moradi, S. Effect of Royal Jelly on Sperm Parameters and Testosterone Levels in Infertile Men. J. Maz. Univ. Med. Sci. 2022, 31, 43–52. [Google Scholar]
- Vahid, M.; Fatemeh, D. Royal jelly for genitourinary syndrome of menopause: A randomized controlled trial. Gyn. Obst. Clin. Med. 2021, 1, 211–215. [Google Scholar]
- Minegaki, N.; Koshizuka, T.; Nishina, S.; Kondo, H.; Takahashi, K.; Sugiyama, T.; Inoue, N. The carboxyl-terminal penta-peptide repeats of major royal jelly protein 3 enhance cell proliferation. Biol. Pharm. Bull. 2020, 43, 1911–1916. [Google Scholar] [CrossRef]
- Natarajan, O.; Angeloni, J.T.; Bilodeau, M.F.; Russi, K.E.; Dong, Y.; Cao, M. The immunomodulatory effects of royal jelly on defending against bacterial infections in the Caenorhabditis elegans model. J. Med. Food 2021, 24, 358–369. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Efects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Yiu, T.Y.; Argüelles, S. Bee Products: Royal Jelly and Propolis; Nabav, S.M., Silva, A.S., Eds.; Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; Chapter 4.1; pp. 475–484. ISBN 9780128124918. [Google Scholar] [CrossRef]
- Hata, T.; Furusawa-Horie, T.; Arai, Y.; Takahashi, T.; Seishima, M.; Ichihara, K. Studies of royal jelly and associated cross-reactive allergens in atopic dermatitis patients. PLoS ONE 2020, 15, e0233707. [Google Scholar] [CrossRef]
| Compound | Method of Determination | Levels [%] | References |
|---|---|---|---|
| Water | Refractometric analysis | min 62.0–max 68.5 | [32,33,34] |
| Lipids | Soxhlet method | min 2–max 8 | [32,35,36] |
| 10-HDA | High-performance liquid chromatography (HPLC); ultra-performance liquid chromatography, gas chromatography–mass spectrometry (GC/MS); NIR spectroscopy combined with chemometric methods | min 1.4 | [4,23,32,34,37] |
| Proteins (total protein/soluble protein fraction) | Bradford assay; bicinchoninic acid (BCA) method | min 11–max 18 | [32,34] |
| Nitrogen content using carbon/hydrogen/nitrogen analyzer TruSpec (LECO, Saint Joseph, MI, USA) | [38] | ||
| Total carbohydrates | colorimetric method | min 7–max 18 | [9,32,34] |
| Individual carbohydrates | Enzymatic method; HPLC method; GC method; Ionic chromatography; Infrared spectroscopy; Nuclear magnetic resonance | Fructose (2.3–7.6), glucose (2.9–8.1), sucrose (<0.1–2.1), maltose and maltotriose (0.0–1.0) | [9,34,39] |
| Compound | Levels [mg/100 g] | References |
|---|---|---|
| Minerals | ||
| Macroelements | [40,41] | |
| Na | 0.3–13.8 | |
| K | 321.1–357.4 | |
| Ca | 22.8–24.0 | |
| Mg | 44.0–50.4 | |
| P | 338.4–412.1 | |
| S | 153.2–169.3 | |
| Microelements | ||
| Fe | n.d. | |
| Mn | 0.01–0.08 | |
| Zn | 2.07–2.58 | |
| Cr | 0.03–0.15 | |
| Cu | 0.31–0.39 | |
| Vitamins | ||
| Vitamin A | 1.10 | [4,42] |
| Vitamin B1 | 2.06 | |
| Vitamin B2 | 2.77 | |
| Niacin (B3) | 42.42 | |
| Vitamin B5 (Pantothenic acid) | 52.80 | |
| Vitamin B6 | 11.90 | |
| Vitamin B9 (Folic acid) | 0.40 | |
| Vitamin B12 | 0.15 | |
| Vitamin C (Ascorbic acid) | 2.00 | |
| Vitamin D | 0.2 | |
| Vitamin E | 5.00 | |
| Flavonoids | ||
| Quercetin | 16.13 | [43] |
| Naringin | 0.47 | |
| Hesperetin | 0.85 | |
| Galangin | 0.51 | |
| Phenolic Acids | ||
| Chlorogenic Acid | 37.61 | [43] |
| Caffeic Acid | 5.14 | |
| Ferulic Acid | 68.42 |
| MRJP Member | Alternative Name | Amino Acids Number | Expression | Function | Reference |
|---|---|---|---|---|---|
| MRJP1 | Royalactin, apalbumin 1, D III | 413 | Upregulated in nurse bee’s head compared to foragers; Upregulated in mated queen spermathecal fluid and spermatheca of the mated queen | Essential amino acids absorption; Source of energy for stored sperm; Worker honeybees’ temporal polytheism and phenotypic plasticity’s regulation; Social behavior; Learning; Memory | [53,55,56,57] |
| MRJP2 | Apalbumin 2 | 435 | Upregulated in nurse bee’s head compared to foragers | Nitrogen reserve; Worker honeybees temporal polytheism and phenotypic plasticity’s regulation | [56,58] |
| MRJP3 | RJ protein RJP57-1 | 524 | Upregulated in nurse bee’s head compared to foragers | Nitrogen supply; Worker honeybees temporal polytheism and phenotypic plasticity’s regulation | [55,56,57,58] |
| MRJP4 | RJ protein RJP57-2 | 444 | Upregulated in nurse bee’s head compared to foragers; Upregulated in mated queen spermathecal fluid and spermatheca of mated queen | Essential amino acids absorption; Source of energy for stored sperm; Worker honeybees temporal polytheism and phenotypic plasticity’s regulation | [53,55,56] |
| MRJP5 | 578 | Upregulated in foragers’ head compared to nurses | Essential amino acids absorption; nitrogen reserve; Worker honeybees temporal polytheism and phenotypic plasticity’s regulation | [55,56] | |
| MRJP6 | Not applicable | 417 | Upregulated in foragers’ head compared to nurses; Upregulated in mated queen spermathecal fluid and spermatheca of the mated queen | Source of energy for stored sperm; Worker honeybees temporal polytheism and phenotypic plasticity’s regulation | [53,56] |
| MRJP7 | Not applicable | 426 | Upregulated in nurse bee’s head compared to foragers | Worker honeybees’ temporal polytheism and phenotypic plasticity’s regulation | [56] |
| MRJP8 | Not applicable | 400 | Evenly expressed in head, thorax, abdomen of all groups | Honeybee worker’s defense; queen’s long life span maintenance | [59,60] |
| MRJP9 | Not applicable | 403 | Upregulated in virgin queens and workers bees | General philological role | [45] |
| Target Condition | Dosage (mg/Day) | Duration | Participants Number | Gender | Study Type | Reference |
|---|---|---|---|---|---|---|
| Menopausal symptoms | 1000 mg | 8 weeks | 200 | Women | Double-blind | [79] |
| Sub-fertility | 5000 mg | 8 weeks | 27 | Men | Non-randomized | [123] |
| Type 2 diabetes mellitus | 1000 mg | 8 weeks | 50 | Men, women | Double-blind randomized | [124] |
| Hemodialysis | 3600 mg | 24 months | 270 | Men, women | Double-blind randomized | [125] |
| Multiple sclerosis | 500 mg | 3 weeks | 100 | Women | Double-blind randomized | [126] |
| Dry mouth sensation | 800 mg | 12 weeks | 14 | Men, women | Double-blind randomized | [127] |
| Traumatic brain injury | 3000 mg | 2 weeks | 61 | Men, women | Double-blind randomized | [128] |
| Infertility | 100 mg | 8 weeks | 100 | Men | Quasi-experimental | [129] |
| Genitourinary syndrome | 1000 mg | 8 weeks | 192 | Women | Double-blind randomized | [130] |
| Metastatic renal cell carcinoma | 900 mg | 13 weeks | 33 | Men, women | Double-blind randomized | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botezan, S.; Baci, G.-M.; Bagameri, L.; Pașca, C.; Dezmirean, D.S. Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules 2023, 28, 1510. https://doi.org/10.3390/molecules28031510
Botezan S, Baci G-M, Bagameri L, Pașca C, Dezmirean DS. Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules. 2023; 28(3):1510. https://doi.org/10.3390/molecules28031510
Chicago/Turabian StyleBotezan, Sara, Gabriela-Maria Baci, Lilla Bagameri, Claudia Pașca, and Daniel Severus Dezmirean. 2023. "Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects" Molecules 28, no. 3: 1510. https://doi.org/10.3390/molecules28031510
APA StyleBotezan, S., Baci, G.-M., Bagameri, L., Pașca, C., & Dezmirean, D. S. (2023). Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules, 28(3), 1510. https://doi.org/10.3390/molecules28031510










