Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries
Abstract
:1. Introduction
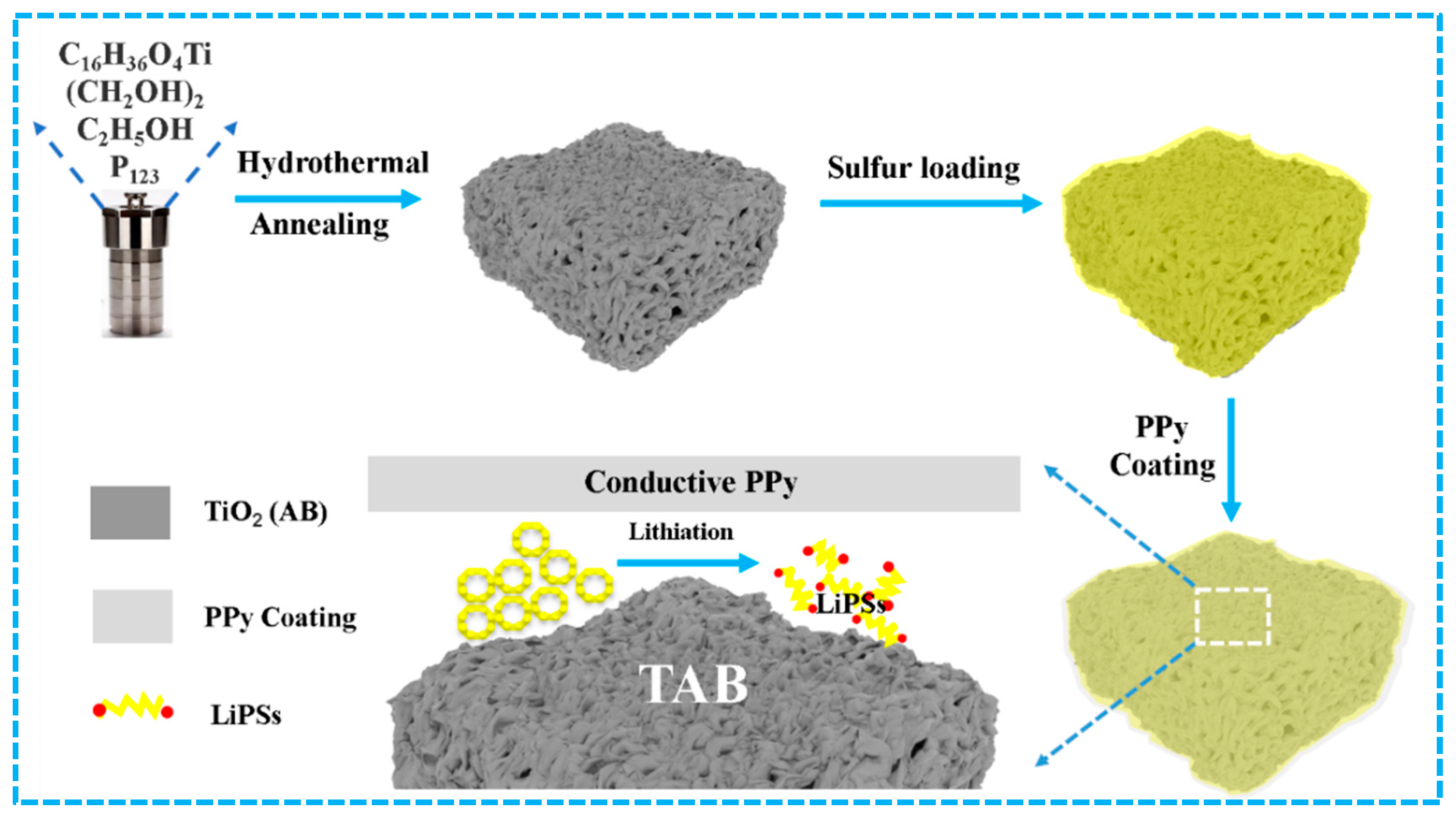
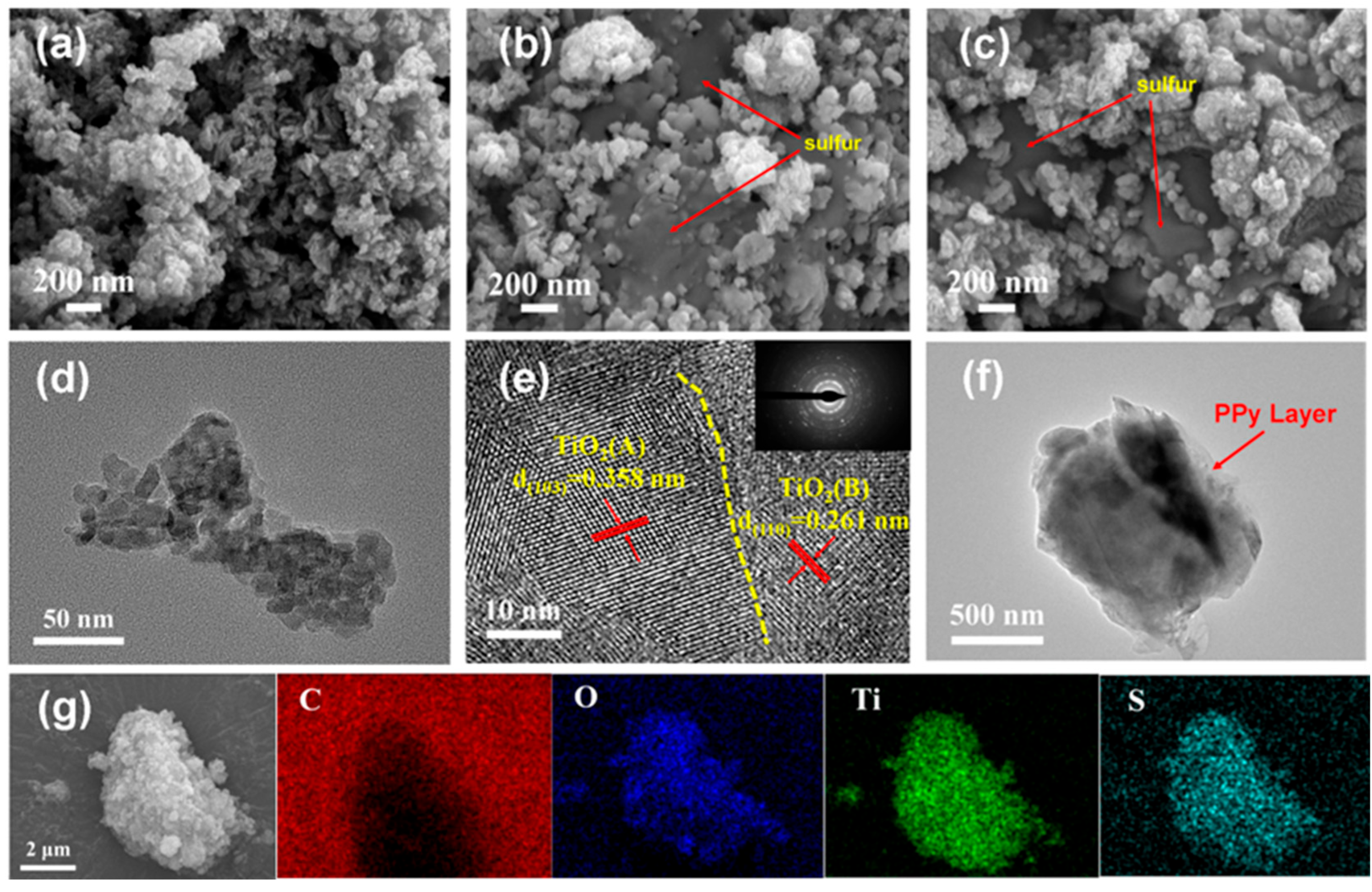
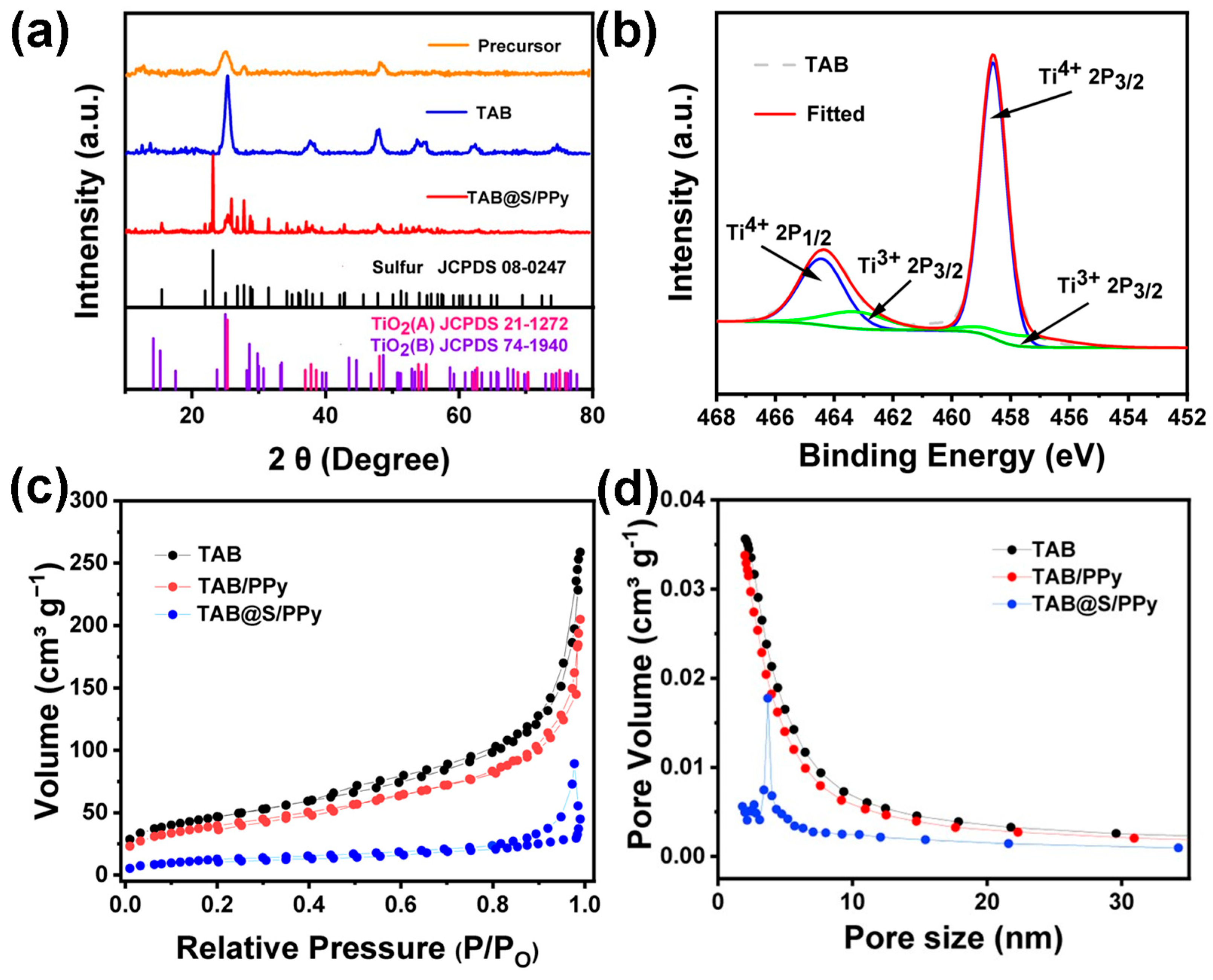
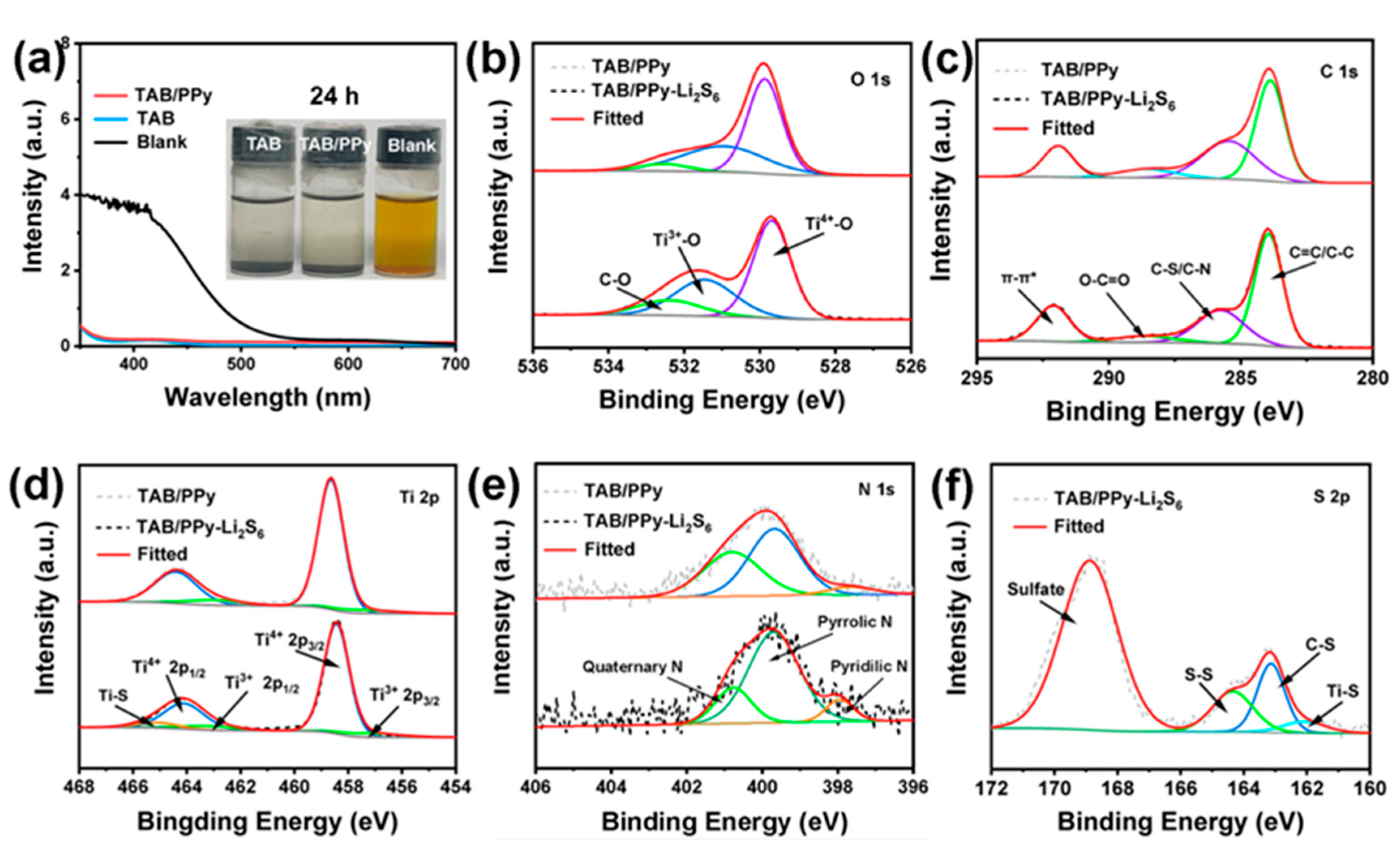
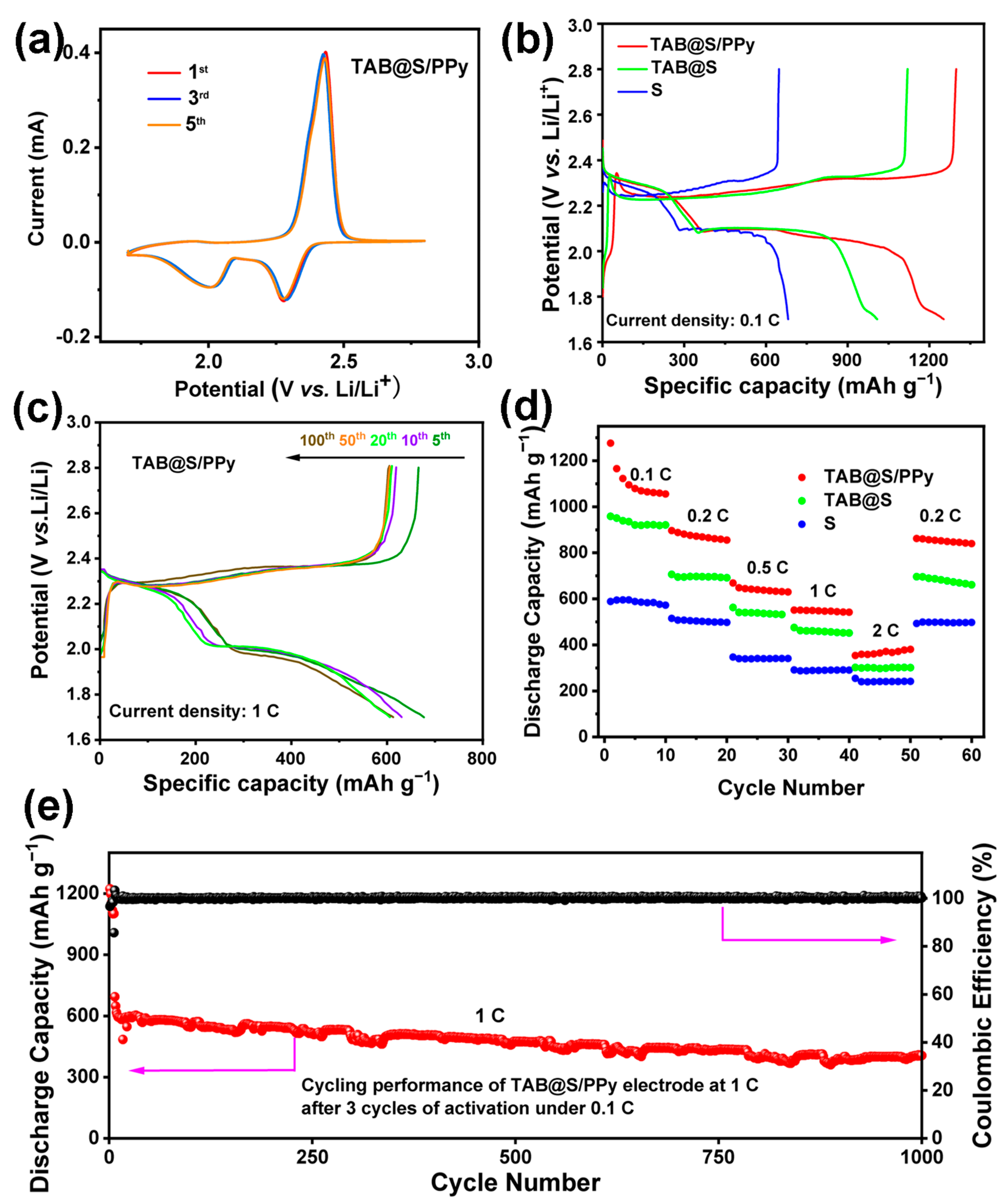
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Anatase/Bronze TiO2 (TAB)
3.2. Preparation of TAB@S
3.3. Preparation of TAB@S/PPy
3.4. Visible Polysulfide Adsorption Experiment
3.5. Material Characterization
3.6. Cell Assembly and Electrochemical Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manthiram, A.; Chung, S.H.; Zu, C.X. Lithium-Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium-Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fu, Z.-H.; Chen, X.; Zhang, Q. A review on theoretical models for lithium-sulfur battery cathodes. InfoMat 2022, 4, e12304. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Xiong, X.; Chen, G.; Huang, M.; Wang, J.-H.; Liu, Y.; Liu, M.; Huang, K. A robust sulfur host with dual lithium polysulfide immobilization mechanism for long cycle life and high capacity Li-S batteries. Energy Stor. Mater. 2019, 16, 344–353. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.J.; Liu, B.; Li, Y.H.; Shen, S.H.; Deng, S.J.; Lu, C.W.; Zhang, W.K.; Xia, Y.; Pan, G.X.; et al. Electrode Design for Lithium-Sulfur Batteries: Problems and Solutions. Adv. Funct. Mater. 2020, 30, 1910375. [Google Scholar] [CrossRef]
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of Multifunctional Separators: Stabilizing the Cathode and the Anode for Alkali (Li, Na, and K) Metal-Sulfur and Selenium Batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef]
- Yuan, N.; Deng, Y.R.; Wang, S.H.; Gao, L.; Yang, J.L.; Zou, N.C.; Liu, B.X.; Zhang, J.Q.; Liu, R.P.; Zhang, L. Towards superior lithium-sulfur batteries with metal-organic frameworks and their derivatives. Tungsten 2022, 4, 269–283. [Google Scholar] [CrossRef]
- Chen, W.; Lei, T.; Wu, C.; Deng, M.; Gong, C.; Hu, K.; Ma, Y.; Dai, L.; Lv, W.; He, W.; et al. Designing Safe Electrolyte Systems for a High-Stability Lithium-Sulfur Battery. Adv. Energy Mater. 2018, 8, 1702348. [Google Scholar] [CrossRef]
- Shi, C.; Huang, J.; Tang, Y.; Cen, Z.; Wang, Z.; Liu, S.; Fu, R. A hierarchical porous carbon aerogel embedded with small-sized TiO2 nanoparticles for high-performance Li-S batteries. Carbon 2023, 202, 59–65. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, T.; Li, Y.; Chen, W.; Hu, Y.; Huang, J.; Chu, J.; Yan, C.; Wu, C.; Yang, C. Entrapment of polysulfides by a BiFeO3/TiO2 heterogeneous structure on separator for high-performance Li-S batteries. J. Power Sources 2023, 556, 232501. [Google Scholar] [CrossRef]
- Wang, W.; Xi, K.; Li, B.; Li, H.; Liu, S.; Wang, J.; Zhao, H.; Li, H.; Abdelkader, A.M.; Gao, X.; et al. A Sustainable Multipurpose Separator Directed Against the Shuttle Effect of Polysulfides for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2200160. [Google Scholar] [CrossRef]
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164249. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, W.; Si, Y.; Wang, D.; Fu, Y.; Manthiram, A. Artificial dual solid-electrolyte interfaces based on in situ organothiol transformation in lithium sulfur battery. Nat. Commun. 2021, 12, 3031. [Google Scholar] [CrossRef]
- Han, Z.; Ren, H.-R.; Huang, Z.; Zhang, Y.; Gu, S.; Zhang, C.; Liu, W.; Yang, J.; Zhou, G.; Yang, Q.-H.; et al. A Permselective Coating Protects Lithium Anode toward a Practical Lithium-Sulfur Battery. ACS Nano 2023, 17, 4453–4462. [Google Scholar] [CrossRef]
- Castillo, J.; Antonio Coca-Clemente, J.; Rikarte, J.; Saenz de Buruaga, A.; Santiago, A.; Li, C. Recent progress on lithium anode protection for lithium-sulfur batteries: Review and perspective. APL Mater. 2023, 11, 010901. [Google Scholar] [CrossRef]
- Yan, Y.; Qin, H.; Wei, Y.; Yang, R.; Xu, Y.; Chen, L.; Li, Q.; Shi, M. A Mild Strategy to Strengthen Three Dimensional Graphene Aerogel for Supporting Sulfur as a Free-standing Cathode in Lithium-Sulfur Batteries. Bull. Korean Chem. Soc. 2018, 39, 605–610. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.; Zhang, L.; Niu, Z. Large-scale fabrication of reduced graphene oxide-sulfur composite films for flexible lithium-sulfur batteries. J. Energy Chem. 2019, 38, 199–206. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.J.; Zhai, X.L.; Wang, F.; Ren, X.Y.; Xiong, Y.; Akiyoshi, O.; Pan, K.M.; Ren, F.Z.; Wei, S.Z. Graphene-based interlayer for high-performance lithium-sulfur batteries: A review. Mater. Des. 2021, 211, 110171. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Luo, Y.; Kong, W.; Wu, Y.; Zhang, L.; Jiang, K.; Li, Q.; Zhang, Y.; Wang, J.; et al. Sulfur Embedded in a Mesoporous Carbon Nanotube Network as a Binder-Free Electrode for High-Performance Lithium Sulfur Batteries. ACS Nano 2016, 10, 1300–1308. [Google Scholar] [CrossRef]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur Vapor-Infiltrated 3D Carbon Nanotube Foam for Binder-Free High Areal Capacity Lithium-Sulfur Battery Composite Cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ticey, J.; Oleshko, V.; Zhu, Y.; Zhao, X.; Wang, C.; Cumings, J.; Qi, Y. Carbon-Nanotube-Encapsulated-Sulfur Cathodes for Lithium-Sulfur Batteries: Integrated Computational Design and Experimental Validation. Nano Lett. 2022, 22, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, Y.; Li, J.; Yang, K.; Liang, Z.; Chen, Y.; Zhao, C.; Zhang, Z.; Mai, K. A general dissolution-recrystallization strategy to achieve sulfur-encapsulated carbon for an advanced lithium-sulfur battery. J. Mater. Chem. A 2018, 6, 11664–11669. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, M.; Liu, Y.; Ahn, H.-J.; Kim, K.-W.; Cho, K.-K.; Ahn, J.-H. Root-like porous carbon nanofibers with high sulfur loading enabling superior areal capacity of lithium sulfur batteries. Carbon 2018, 128, 138–146. [Google Scholar] [CrossRef]
- Chen, M.Q.; Su, Z.; Jiang, K.; Pan, Y.K.; Zhang, Y.Y.; Long, D.H. Promoting sulfur immobilization by a hierarchical morphology of hollow carbon nanosphere clusters for high-stability Li-S battery. J. Mater. Chem. A 2019, 7, 6250–6258. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Z.; Huang, S.; Chen, L.; Liang, Y.; Mai, W.; Zhong, H.; Fu, R.; Wu, D. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nat. Commun. 2015, 6, 8221. [Google Scholar] [CrossRef]
- Kim, D.K.; Byun, J.S.; Moon, S.; Choi, J.; Chang, J.H.; Suk, J. Molten salts approach of metal-organic framework-derived nitrogen-doped porous carbon as sulfur host for lithium-sulfur batteries. Chem. Eng. J. 2022, 441, 135945. [Google Scholar] [CrossRef]
- Ren, Y.X.; Zeng, L.; Jiang, H.R.; Ruan, W.Q.; Chen, Q.; Zhao, T.S. Rational design of spontaneous reactions for protecting porous lithium electrodes in lithium-sulfur batteries. Nat. Commun. 2019, 10, 3249. [Google Scholar] [CrossRef]
- Huang, X.; Tang, J.; Luo, B.; Knibbe, R.; Lin, T.; Hu, H.; Rana, M.; Hu, Y.; Zhu, X.; Gu, Q.; et al. Sandwich-Like Ultrathin TiS2 Nanosheets Confined within N, S Codoped Porous Carbon as an Effective Polysulfide Promoter in Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1901872. [Google Scholar] [CrossRef]
- Kiai, M.S.; Mansoor, M.; Ponnada, S.; Gorle, D.B.; Aslfattahi, N.; Sharma, R.K. Integration of PDAAQ and Non-stoichiometric MgO as Host Cathode Materials for Lithium-Sulfur Batteries with Superior Cycle Stability: Density Functional Theory Calculations and Experimental Validations. Energy Fuels 2022, 36, 15199–15209. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium-sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Xu, J.; Guo, Z. Advances in Polar Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707520. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsiang, H.-I.; Chung, S.-H. Investigation and Design of High-Loading Sulfur Cathodes with a High-Performance Polysulfide Adsorbent for Electrochemically Stable Lithium-Sulfur Batteries. ACS Sustain. Chem. Eng. 2022, 10, 9254–9264. [Google Scholar] [CrossRef]
- Chen, Y.; Choi, S.; Su, D.; Gao, X.; Wang, G. Self-standing sulfur cathodes enabled by 3D hierarchically porous titanium monoxide-graphene composite film for high-performance lithium-sulfur batteries. Nano Energy 2018, 47, 331–339. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Liu, S.; Li, G.-R.; Yan, T.-Y.; Gao, X.-P. High Volumetric Energy Density Sulfur Cathode with Heavy and Catalytic Metal Oxide Host for Lithium-Sulfur Battery. Adv. Sci. 2020, 7, 1903693. [Google Scholar] [CrossRef]
- Chu, H.; Noh, H.; Kim, Y.-J.; Yuk, S.; Lee, J.-H.; Lee, J.; Kwack, H.; Kim, Y.; Yang, D.-K.; Kim, H.-T. Achieving three-dimensional lithium sulfide growth in lithium-sulfur batteries using high-donor-number anions. Nat. Commun. 2019, 10, 188. [Google Scholar] [CrossRef]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D Metal Carbides and Nitrides (MXenes) as High-Performance Electrode Materials for Lithium-Based Batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, H.W.; Luo, L.Q.; Li, H.; He, J.Y.; Zu, H.L.; Liu, L.; Liu, H.; Wang, F.Y.; Song, J.J. Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2021, 9, 2205–2213. [Google Scholar] [CrossRef]
- Qiu, W.L.; Li, G.R.; Luo, D.; Zhang, Y.G.; Zhao, Y.; Zhou, G.F.; Shui, L.L.; Wang, X.; Chen, Z.W. Hierarchical Micro-Nanoclusters of Bimetallic Layered Hydroxide Polyhedrons as Advanced Sulfur Reservoir for High-Performance Lithium-Sulfur Batteries. Adv. Sci. 2021, 8, 2003400. [Google Scholar] [CrossRef]
- Wei, H.; Liu, J.; Liu, Y.; Wang, L.; Li, L.; Wang, F.; Ren, X.; Ren, F. Hollow Co-Fe LDH as an effective adsorption/catalytic bifunctional sulfur host for high-performance Lithium-Sulfur batteries. Compos. Commun. 2021, 28, 100973. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium-sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and Catalytic Conversion of Polysulfides by In Situ Built TiO2-MXene Heterostructures for Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Wang, T.; Lang, X.; Li, L.; Yao, C.; Liu, J.; Shi, R.; Cai, K. An excellent adsorptive TiO2@yV(2)O(5) (y = 0.025−0.045) bifunctional composite endowing high sulfur loading as cathode material for lithium-sulfur batteries. J. Alloys Compd. 2022, 902, 162650. [Google Scholar] [CrossRef]
- Liu, G.L.; Wu, H.H.; Meng, Q.Q.; Zhang, T.; Sun, D.; Jin, X.Y.; Guo, D.L.; Wu, N.T.; Liu, X.M.; Kim, J.K. Role of the anatase/TiO2(B) heterointerface for ultrastable high-rate lithium and sodium energy storage performance. Nanoscale Horiz. 2020, 5, 150–162. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.M.; Zhang, J.; Wu, N.T.; Liu, G.L.; Chen, H.P.; Yuan, C.Z.; Liu, X.M. Optimizing electronic structure of porous Ni/MoO2 heterostructure to boost alkaline hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 627, 862–871. [Google Scholar] [CrossRef]
- Cai, K.; Wang, T.; Wang, Z.; Wang, J.; Li, L.; Yao, C.; Lang, X. A cocklebur-like sulfur host with the TiO2-VOx heterostructure efficiently implementing one-step adsorption-diffusion-conversion towards long-life Li-S batteries. Compos. Part B Eng. 2023, 249, 110410. [Google Scholar] [CrossRef]
- Xue, P.; Zhu, K.; Gong, W.; Pu, J.; Li, X.; Guo, C.; Wu, L.; Wang, R.; Li, H.; Sun, J.; et al. “One Stone Two Birds” Design for Dual-Functional TiO2-TiN Heterostructures Enabled Dendrite-Free and Kinetics-Enhanced Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2200308. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Li, L.; Zhou, H.; Wang, M.; Li, L.; Geng, X.; An, B.; Sun, C. A rational design of titanium-based heterostructures as electrocatalyst for boosted conversion kinetics of polysulfides in Li-S batteries. J. Colloid Interface Sci. 2023, 633, 432–440. [Google Scholar] [CrossRef]
- Hao, Q.; Cui, G.; Zhang, Y.; Li, J.; Zhang, Z. Novel MoSe2/MoO2 heterostructure as an effective sulfur host for high-performance lithium/sulfur batteries. Chem. Eng. J. 2020, 381, 122672. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.Y.; Wang, P.; Wang, S.M.; Feng, M. Synthesis of MOF-derived nitrogen-doped carbon microtubules via template self-consumption. Rare Met. 2022, 41, 2582–2587. [Google Scholar] [CrossRef]
- Di, T.; Zhang, J.; Cheng, B.; Yu, J.; Xu, J. Hierarchically nanostructured porous TiO2(B) with superior photocatalytic CO2 reduction activity. Sci. China-Chem. 2018, 61, 344–350. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.H.; Wei, H.J.; Zhai, X.L.; Wang, F.; Zhou, Y.Z.; Tao, F.; Zhai, P.H.; Liu, W.; Liu, Y. Recent advances and perspectives in conductive-polymer-based composites as cathode materials for high-performance lithium-sulfur batteries. Sustain. Energ. Fuels 2022, 6, 2901–2923. [Google Scholar] [CrossRef]
- Sui, Y.L.; Zeng, J.; Shi, Z.H.; Lu, S.X.; Zhang, X.P.; Wang, B.J.; Zhong, S.K.; Wu, L. Superior sodium storage in anatase/bronze TiO2 nanofibers through surface phosphorylation. Appl. Surf. Sci. 2022, 606, 154982. [Google Scholar] [CrossRef]
- Wu, T.; Sun, G.R.; Lu, W.; Zhao, L.P.; Mauger, A.; Julien, C.M.; Sun, L.Q.; Xie, H.M.; Liu, J.H. A polypyrrole/black-TiO2/S double-shelled composite fixing polysulfides for lithium-sulfur batteries. Electrochim. Acta 2020, 353, 11. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Current Status and Future Prospects of Metal-Sulfur Batteries. Adv. Mater. 2019, 31, 1901125. [Google Scholar] [CrossRef]
- Wu, S.L.; Shi, J.Y.; Nie, X.L.; Yu, Z.F.; Huang, F.L. Multi-duties for one post: Biodegradable bacterial cellulose-based separator for lithium sulfur batteries. Carbohydr. Polym. 2022, 285, 119201. [Google Scholar] [CrossRef]
- Qin, X.Y.; Wu, J.X.; Xu, Z.L.; Chong, W.G.; Huang, J.Q.; Liang, G.M.; Li, B.H.; Kang, F.Y.; Kim, J.K. Electrosprayed multiscale porous carbon microspheres as sulfur hosts for long-life lithium-sulfur batteries. Carbon 2019, 141, 16–24. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sokolov, A.A.; Podgorbunsky, A.B.; Ustinov, A.Y.; Mayorov, V.Y.; Kuryavyi, V.G.; Sinebryukhov, S.L. Vanadium-doped TiO2-B/anatase mesoporous nanotubes with improved rate and cycle performance for rechargeable lithium and C sodium batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Fang, M.M.; Chen, Z.M.; Liu, Y.; Quan, J.P.; Yang, C.; Zhu, L.C.; Xu, Q.B.; Xu, Q. Design and synthesis of novel sandwich-type C@TiO2@C hollow microspheres as efficient sulfur hosts for advanced lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 1630–1638. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.Y.; Yang, P.; Zhang, H.P.; Du, C.; Xu, J.M.; Song, K.X. S@TiO2 nanospheres loaded on PPy matrix for enhanced lithium-sulfur batteries. J. Alloys Compd. 2019, 783, 279–285. [Google Scholar] [CrossRef]
- Ansari, Y.; Zhang, S.; Wen, B.; Fan, F.; Chiang, Y.-M. Stabilizing Li-S Battery Through Multilayer Encapsulation of Sulfur. Adv. Energy Mater. 2019, 9, 1802213. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, C.; Hua, J.; Hong, X.; Sun, C.; Li, H.-W.; Xu, X.; Mai, L. Engineering Oxygen Vacancies in a Polysulfide-Blocking Layer with Enhanced Catalytic Ability. Adv. Mater. 2020, 32, 1907444. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.H.; Zhai, P.B.; He, Q.Q.; Wang, L.; Chen, Q.; Gu, X.K.; Yang, Z.-L.; Gong, Y.J. In-situ constructed three-dimensional MoS2-MoN heterostructure as the cathode of lithium-sulfur battery. Rare Met. 2022, 41, 1743–1752. [Google Scholar] [CrossRef]
- Song, H.S.; Yuan, H.L.; Chen, H.Z.; Tang, A.P.; Xu, G.R.; Liu, L.H.; Zhang, Z.; Kuang, Q.J. Synthesis of TiO2/S@PPy composite for chemisorption of polysulfides in high performance Li-S batteries. J. Solid State Electrochem. 2020, 24, 997–1006. [Google Scholar] [CrossRef]
- Yuan, C.; Zhu, S.; Cao, H.; Hou, L.; Lin, J. Hierarchical sulfur-impregnated hydrogenated TiO2 mesoporous spheres comprising anatase nanosheets with highly exposed (001) facets for advanced Li-S batteries. Nanotechnology 2016, 27, 045403. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, W.; Chen, G.Z.; Cairns, E.J. Polypyrrole/TiO2 nanotube arrays with coaxial heterogeneous structure as sulfur hosts for lithium sulfur batteries. J. Power Sources 2016, 327, 447–456. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhong, W.T.; Li, Y.S.; Deng, Q.; Ou, X.; Pan, Q.C.; Wang, X.W.; Xiong, X.H.; Yang, C.H.; Liu, M.L. Rational Design of TiO-TiO2 Heterostructure/Polypyrrole as a Multifunctional Sulfur Host for Advanced Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 5055–5063. [Google Scholar] [CrossRef]
- Tan, J.; Ye, M.; Shen, J. Deciphering the role of LiNO3 additives in Li-S batteries. Mater. Horiz. 2022, 9, 2325–2334. [Google Scholar] [CrossRef]
- Guo, J.; Pei, H.; Dou, Y.; Zhao, S.; Shao, G.; Liu, J. Rational Designs for Lithium-Sulfur Batteries with Low Electrolyte/Sulfur Ratio. Adv. Funct. Mater. 2021, 31, 2010499. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, Y.; Li, T.; Liang, L.; Wen, S.; Zhang, Y.; Liu, G.; Ren, F.; Wang, G. Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries. Molecules 2023, 28, 4286. https://doi.org/10.3390/molecules28114286
Liu J, Liu Y, Li T, Liang L, Wen S, Zhang Y, Liu G, Ren F, Wang G. Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries. Molecules. 2023; 28(11):4286. https://doi.org/10.3390/molecules28114286
Chicago/Turabian StyleLiu, Jing, Yong Liu, Tengfei Li, Longlong Liang, Sifan Wen, Yue Zhang, Guilong Liu, Fengzhang Ren, and Guangxin Wang. 2023. "Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries" Molecules 28, no. 11: 4286. https://doi.org/10.3390/molecules28114286
APA StyleLiu, J., Liu, Y., Li, T., Liang, L., Wen, S., Zhang, Y., Liu, G., Ren, F., & Wang, G. (2023). Efficient Regulation of Polysulfides by Anatase/Bronze TiO2 Heterostructure/Polypyrrole Composites for High-Performance Lithium-Sulfur Batteries. Molecules, 28(11), 4286. https://doi.org/10.3390/molecules28114286






