Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications
Abstract
1. Introduction
1.1. History
1.2. Different Properties of Nanoparticles
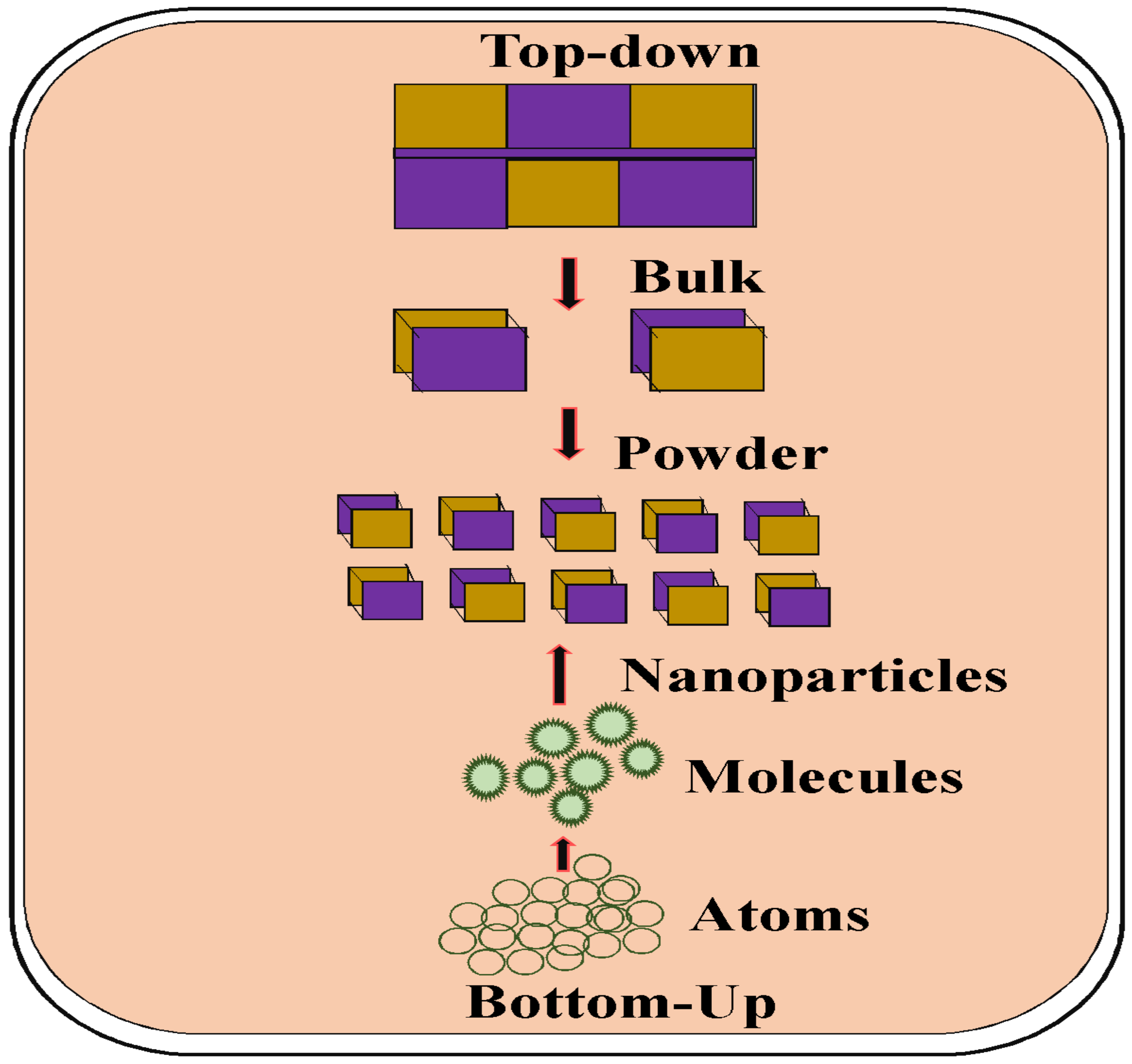
1.3. Several Approaches for Nanoparticle Synthesis
1.4. Antibacterial and Antiviral Properties, the Importance of Zeta Potential, and Mode of Action of Nanoparticles
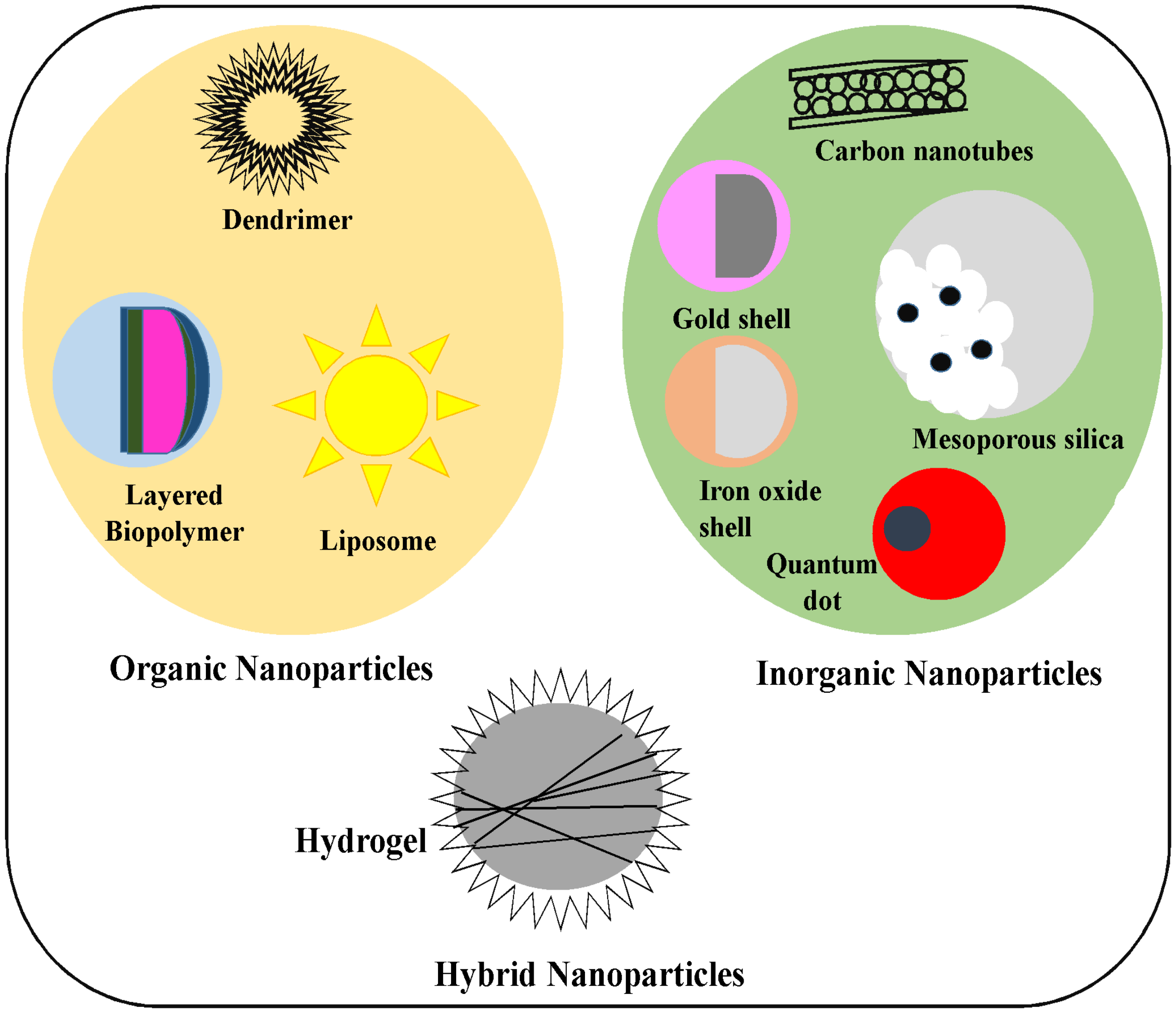
2. Different Types of Nanoparticles
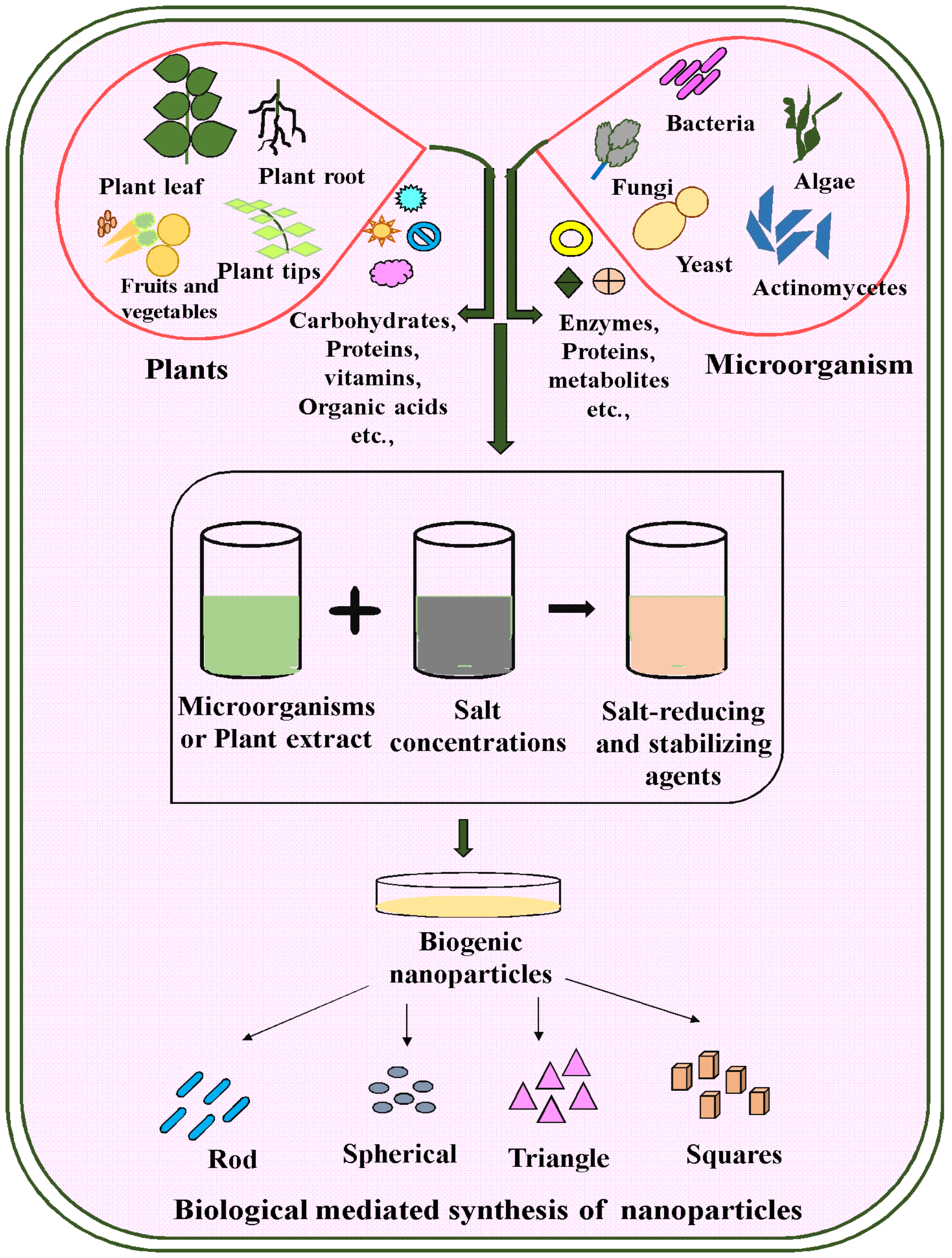
3. Various Biosources Are Used to Synthesize Nanoparticles
3.1. Green Synthesis of Nanoparticles
3.2. Plant-Based Synthesis of Nanoparticles
3.3. Amalgamation of Nanoparticles Using Marine Algae
3.4. Bacteria-Mediated Synthesis of Nanoparticles
3.5. Fungi-Mediated Synthesis of Nanoparticles
3.6. Actinomycetes-Mediated Synthesis of Nanoparticles
3.7. Yeast-Mediated Synthesis of Nanoparticles
| Yeast | Type of Nanoparticles | Size of Nanoparticles | Biological Activities | Reference |
|---|---|---|---|---|
| Saccharomyces cerevisiae | Selenium | 30–100 nm | Antimicrobial | [93] |
| Saccharomyces cerevisiae | Silver | 100 nm | Antibacterial | [94] |
| Saccharomyces cerevisiae | Palladium | 10–100 nm | Photocatalytic activity | [95] |
4. Biomedical Applications of Nanoparticles
4.1. Antibacterial Activity
4.2. Fungicidal Activity
4.3. Anti-Plasmodial Activity
4.4. Antiviral Activity
4.5. Anti-Inflammatory Activity
4.6. Antidiabetic Activity
4.7. Antioxidant Activity
4.8. Anticancer Therapy
4.9. Bio-Sensing Applications
4.10. Other Medical Applications
5. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Emami Moghaddam, S.A.; Ghadam, P.; Rahimzadeh, F. Biosynthesis of cadmium sulfide nanoparticles using aqueous extract of Lactobacillus acidophilus along with its improvement by response surface methodology. J. Clean. Prod. 2022, 356, 131848. [Google Scholar] [CrossRef]
- Xiang, H.; Meng, J.; Shao, W.; Zeng, D.; Ji, J.; Wang, P.; Zhou, X.; Qi, P.; Liu, L.; Yang, S. Plant protein-based self-assembling core–shell nanocarrier for effectively controlling plant viruses: Evidence for nanoparticle delivery behavior, plant growth promotion, and plant resistance induction. Chem. Eng. J. 2023, 464, 142432. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, M.K.; Devi, R.U.; Raja, K.P.; Krishna, K.B. Synthesis of Phyto Based Metal Nanoparticles: A Green Approach. J. Pharm. Res. Int. 2022, 34, 20–32. [Google Scholar] [CrossRef]
- Mubarik, N.; Gulelala, G.; Iqbal, S.; Shahmeel, M.; Hussain, A.A.; Razzaq, K.; Akram, M.N. Different Methods, Novel Tools towards the Synthesis of Nanoparticles and Applications in Engineering, Chemical, Physical Sciences and Technology. Sch. Bull. 2022, 8, 71–74. [Google Scholar] [CrossRef]
- Malik, M.; Aamir Iqbal, M.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Taghavizadeh Yazdi, M.E.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Kamaraj, C.; Gandhi, P.R.; Chandra Satish Kumar, R.; Balasubramani, G.; Malafaia, G. Biosynthesis and extrinsic toxicity of copper oxide nanoparticles against cattle parasites: An eco-friendly approach. Environ. Res. 2022, 214, 114009. [Google Scholar] [CrossRef]
- Krukowski, S.; Lysenko, N.; Kolodziejski, W. Synthesis and characterization of nanocrystalline composites containing calcium hydroxyapatite and glycine. J. Solid State Chem. 2018, 264, 59–67. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Arshad, M.K.M.; Poopalan, P.; Perumal, V. Nanoparticle synthetic methods: Strength and limitations. In Nanoparticles in Analytical and Medical Devices; Gopinath, S.C.B., Gang, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–43. ISBN 9780128211632. [Google Scholar]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, 274–282. [Google Scholar] [CrossRef]
- Qi, P.; Wang, N.; Zhang, T.; Feng, Y.; Zhou, X.; Zeng, D.; Meng, J.; Liu, L.; Jin, L.; Yang, S. Anti-Virulence Strategy of Novel Dehydroabietic Acid Derivatives: Design, Synthesis, and Antibacterial Evaluation. Int. J. Mol. Sci. 2023, 24, 2897. [Google Scholar] [CrossRef] [PubMed]
- Sobczak-Kupiec, A.; Pluta, K.; Drabczyk, A.; Włoś, M.; Tyliszczak, B. Synthesis and characterization of ceramic—Polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram. Int. 2018, 44, 13630–13638. [Google Scholar] [CrossRef]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 2200356. [Google Scholar] [CrossRef]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic Biohybrid Microrobot Multimers Based on Chlorella Cells for Enhanced Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Nalawade, S.A.; Shinde, B.; Chaudhari, S.; Badhe, M.S.; Kadam, V.K.; Chaskar, M.G.; Pingale, S.S. A review on biosynthesis and applications of various nanoparticles using extracts of medicinal plant Tribulus terrestris. Mater. Today Proc. 2022, 73, 427–430. [Google Scholar] [CrossRef]
- Maillard, A.P.V.F.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta potential beyond materials science: Applications to bacterial systems and to the development of novel antimicrobials. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Hussain, F.S.; Abro, N.Q.; Ahmed, N.; Memon, S.Q.; Memon, N. Nano-antivirals: A comprehensive review. Front. Nanotechnol. 2022, 4, 1064615. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Choudhary, S.; Kumar, R.; Dalal, U.; Tomar, S.; Reddy, S.N. Green synthesis of nanometal impregnated biomass—Antiviral potential. Mater. Sci. Eng. C 2020, 112, 110934. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; DeLong, R.K. Nanoscale Interaction Mechanisms of Antiviral Activity. ACS Pharmacol. Transl. Sci. 2023, 6, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Chapter 14—Nanoparticles in Antiviral Therapy. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–410. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Jangid, P.; Inbaraj, M.P. Applications of nanomaterials in wastewater treatment. Mater. Today Proc. 2021, 43, 2877–2881. [Google Scholar] [CrossRef]
- García, M.; Forbe, T.; Gonzalez, E. Potenciais aplicações de nanotecnologia no setor agro-alimentar. Food Sci. Technol. 2010, 30, 573–581. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Drexler, K.E. Molecular engineering: An approach to the development of general capabilities for molecular manipulation. Proc. Natl. Acad. Sci. USA 1981, 78, 5275–5278. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Bhuvaneshwari, V.K.; Ayyadurai, G.K.; Jayaprakash, R.; Gopinath, K.; Nicoletti, M.; Alarifi, S.; Govindarajan, M. Green synthesis of zinc oxide nanoparticles using Anoectochilus elatus, and their biomedical applications. Saudi J. Biol. Sci. 2022, 29, 2270–2279. [Google Scholar] [CrossRef]
- Alrajhi, A.H.; Ahmed, N.M.; Al Shafouri, M.; Almessiere, M.A.; ahmed Mohammed Al-Ghamdi, A. Green synthesis of zinc oxide nanoparticles using salvia officials extract. Mater. Sci. Semicond. Process. 2021, 125, 105641. [Google Scholar] [CrossRef]
- Rana, A.; Kumari, N.; Tyagi, M.; Jagadevan, S. Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics. Chem. Eng. J. 2018, 347, 91–100. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef]
- Mody, V.; Siwale, R.; Singh, A.; Mody, H. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.; Haider, A.; Siddique, M.I.; Zeb, A.; Jamal, S.B.; Khalil, A.A.K.; Naeem, M. Nanomaterials for chronic inflammatory diseases: The current status and future prospects. Appl. Nanosci. 2022, 12, 3097–3110. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open 2022, 5, 100080. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Shakeel Iqubal, S.M.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.S.; Yaqoob, S.; Gul, M.M. Dynamic green synthesis of iron oxide and manganese oxide nanoparticles and their cogent antimicrobial, environmental and electrical applications. Rev. Inorg. Chem. 2022, 42, 239–263. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Singh, V.N.; Shamsi, S.F.; Fatma, A.; Mehta, B.R. Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A Novel Approach Towards Weed Utilization. Biotechnol. Res. Int. 2011, 2011, 454090. [Google Scholar] [CrossRef] [PubMed]
- Anju Varghese, R.; Anandhi, P.; Arunadevi, R.; Boovisha, A.; Sounthari, P.; Saranya, J.; Parameswari, K.; Chitra, S. Satin leaf (Chrysophyllum oliviforme) extract mediated green synthesis of silver nanoparticles: Antioxidant and anticancer activities. J. Pharm. Sci. Res. 2015, 7, 266–273. [Google Scholar]
- Aisida, S.O.; Ugwu, K.; Akpa, P.A.; Nwanya, A.C.; Nwankwo, U.; Botha, S.S.; Ejikeme, P.M.; Ahmad, I.; Maaza, M.; Ezema, F.I. Biosynthesis of silver nanoparticles using bitter leave (Veronica amygdalina) for antibacterial activities. Surf. Interfaces 2019, 17, 100359. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Annamalai, A.; Babu, S.T.; Jose, N.A.; Sudha, D.; Lyza, C.V. Biosynthesis and characterization of silver and gold nanoparticles using aqueous leaf extraction of Phyllanthus amarus Schum. & Thonn. World Appl. Sci. J. 2011, 13, 1833–1840. [Google Scholar]
- Arunachalam, K.D.; Annamalai, S.K. Chrysopogon zizanioides aqueous extract mediated synthesis, characterization of crystalline silver and gold nanoparticles for biomedical applications. Int. J. Nanomed. 2013, 8, 2375–2384. [Google Scholar] [CrossRef]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: Catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef]
- Hao, R.; Li, D.; Zhang, J.; Jiao, T. Green synthesis of iron nanoparticles using green tea and its removal of hexavalent chromium. Nanomaterials 2021, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, D. Characterization of Green Synthesized Antibacterial Silver Nanoparticles from Amaranthus spinosus L. Extract. Bionanoscience 2022, 12, 502–511. [Google Scholar] [CrossRef]
- Sudha, K.G.; Ali, S.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Rajeshkumar, M.P. Eco-friendly synthesis of ZnO nanorods using Cycas pschannae plant extract with excellent photocatalytic, antioxidant, and anticancer nanomedicine for lung cancer treatment. Appl. Organomet. Chem. 2020, 34, e5511. [Google Scholar] [CrossRef]
- Sudha, K.G.; Ali, S.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Gorshenkov, M.V.; Rajeshkumar, M.P. Cyrtrandroemia nicobarica-Synthesized ZnO NRs: A New Tool in Cancer Treatment. JOM 2021, 73, 364–372. [Google Scholar] [CrossRef]
- Govindaraj Sudha, K.; Ali, S.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Gorshenkov, M.V.; Velmurugan, T.; Prasanna Rajeshkumar, M. An eco-friendly production of ZnO NRs using Knema andamanica (Warb) extracts for photocatalytic and anticancer applications. Inorg. Chem. Commun. 2021, 134, 109030. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Rajeshkumar, M.P. Green synthesis of stable antioxidant, anticancer and photocatalytic activity of zinc oxide nanorods from Leea asiatica leaf. J. Biotechnol. 2021, 329, 65–79. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Gorshenkov, M.V.; Rajeshkumar, M.P. Novel Leea grandifolia leaves mediated synthesis of ZnO nanorods for photocatalytic and anticancer applications. Appl. Organomet. Chem. 2021, 35, e6239. [Google Scholar] [CrossRef]
- Ali, S.; Govindaraj Sudha, K.; Karunakaran, G.; Kowsalya, M.; Kolesnikov, E.; Gorshenkov, M.V.; Velmurugan, T.; Rajeshkumar, M.P. Anticancer and photocatalytic activities of zinc oxide nanorods synthesized from Manilkara littoralis leaf extract. Mater. Chem. Phys. 2022, 277, 125541. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii grevilli and their Antibacterial effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Patric Raja, D.; Rathi, J.M.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopestic. 2012, 5, 119–128. [Google Scholar]
- Prasad, T.N.V.K.V.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Stalin Dhas, T.; Ganesh Kumar, V.; Karthick, V.; Jini Angel, K.; Govindaraju, K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Use of Bacteria and Microalgae in Synthesis of Nanoparticles. Chem. J. Mold. 2021, 7, 32–38. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Bacteria in Heavy Metal Remediation and Nanoparticle Biosynthesis. ACS Sustain. Chem. Eng. 2020, 8, 5395–5409. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol. Prog. 2008, 24, 476–480. [Google Scholar] [CrossRef]
- Selvarajan, E.; Mohanasrinivasan, V. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater. Lett. 2013, 112, 180–182. [Google Scholar] [CrossRef]
- Syed, B.; Prasad, N.; Dhananjaya, B.L.; Yallappa, S.; Satish, S. Synthesis of silver nanoparticles by endosymbiont Pseudomonas fluorescens CA 417 and their bactericidal activity. Enzym. Microb. Technol. 2016, 95, 128–136. [Google Scholar] [CrossRef]
- Kathiresan, K.; Alikunhi, N.M.; Pathmanaban, S.M.; Nabikhan, A.; Kandasamy, S. Analysis of antimicrobial silver nanoparticles synthesized by coastal strains of escherichia coli and aspergillus niger. Can. J. Microbiol. 2010, 56, 1050–1059. [Google Scholar] [CrossRef]
- Yusof, H.M.; Rahman, N.A.; Mohamad, R.; Zaidan, U.H. Microbial mediated synthesis of silver nanoparticles by lactobacillus plantarum ta4 and its antibacterial and antioxidant activity. Appl. Sci. 2020, 10, 6973. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.A.; Bhaskara Rao, K.V. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- Vishnu Kirthi, A.; Abdul Rahuman, A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Jayaseelan, C.; Elango, G.; Abduz Zahir, A.; Kamaraj, C.; Bagavan, A. Biosynthesis of titanium dioxide nanoparticles using bacterium Bacillus subtilis. Mater. Lett. 2011, 65, 2745–2747. [Google Scholar] [CrossRef]
- Dehnad, A.; Hamedi, J.; Derakhshan-Khadivi, F.; Abusov, R. Green synthesis of gold nanoparticles by a metal resistant arthrobacter nitroguajacolicus isolated from gold mine. IEEE Trans. Nanobiosci. 2015, 14, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M. Biosynthesis of iron oxide nanoparticles by cytoplasmic extracts of bacteria lactobacillus casei. Asian J. Green Chem. 2018, 2, 181–188. [Google Scholar]
- Pugazhenthiran, N.; Anandan, S.; Kathiravan, G.; Udaya Prakash, N.K.; Crawford, S.; Ashokkumar, M. Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanoparticle Res. 2009, 11, 1811–1815. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Heinrich Factors Affecting the Geometry of Silver Nanoparticles Synthesis in Chrysosporium tropicum and Fusarium oxysporum. Am. J. Nanotechnol. 2011, 2, 112–121. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.D.; Elisa Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mukherjee, T.; Chakraborty, S.; Das, T.K. Biosynthesis, characterisation & antifungal activity of silver nanoparticles synthesized by the fungus Aspergillus foetidus MTCC8876. Dig. J. Nanomater. Biostructures 2012, 8, 197–205. [Google Scholar]
- Pavani, K.V.; Kumar, N.S. Adsorption of Iron and Synthesis of Iron Nanoparticles by Aspergillus Species Kvp 12. Am. J. Nanomater. 2013, 1, 24–26. [Google Scholar]
- Narasimha, G.; Khadri, H.; Alzohairy, M. Antiviral properties of silver nanoparticles synthesized by Aspergillus sps. Pharm. Lett. 2012, 4, 649–651. [Google Scholar]
- Chandrappa, C.P.; Govindappa, M.; Chandrasekar, N.; Sarkar, S.; Ooha, S.; Channabasava, R. Endophytic synthesis of silver chloride nanoparticles from Penicillium sp. of Calophyllum apetalum. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025016. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Mohmed, A.; Hassan, S.; Fouda, A.; Elgamal, M.; Salem, S. Extracellular Biosynthesis of Silver Nanoparticles Using Aspergillus sp. and Evaluation of their Antibacterial and Cytotoxicity. J. Appl. Life Sci. Int. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Channabasava; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Du, L.; Xian, L.; Feng, J.X. Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J. Nanoparticle Res. 2011, 13, 921–930. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Narasimha, G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf. B Biointerfaces 2010, 81, 430–433. [Google Scholar] [CrossRef]
- Ranjitha, V.R.; Rai, V.R. Actinomycetes mediated synthesis of gold nanoparticles from the culture supernatant of Streptomyces griseoruber with special reference to catalytic activity. 3 Biotech 2017, 7, 299. [Google Scholar] [CrossRef]
- Manimaran, M.; Kannabiran, K. Actinomycetes-mediated biogenic synthesis of metal and metal oxide nanoparticles: Progress and challenges. Lett. Appl. Microbiol. 2017, 64, 401–408. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Hariharan, H.; Al-Harbi, N.A.; Karuppiah, P.; Rajaram, S.K. Microbial synthesis of selinium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515. [Google Scholar]
- Olobayotan, I.; Akin-Osanaiye, B. Biosynthesis of silver nanoparticles using baker’s yeast, Saccharomyces cerevisiae and its antibacterial activities. Access Microbiol. 2019, 1, 526. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Biosynthesis of palladium nanoparticles using Saccharomyces cerevisiae extract and its photocatalytic degradation behaviour. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025018. [Google Scholar] [CrossRef]
- Antony, J.J.; Sivalingam, P.; Siva, D.; Kamalakkannan, S.; Anbarasu, K.; Sukirtha, R.; Krishnan, M.; Achiraman, S. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B Biointerfaces 2011, 88, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Seckin, H.; Tiri, R.N.E.; Meydan, I.; Aygun, A.; Gunduz, M.K.; Sen, F. An environmental approach for the photodegradation of toxic pollutants from wastewater using Pt–Pd nanoparticles: Antioxidant, antibacterial and lipid peroxidation inhibition applications. Environ. Res. 2022, 208, 112708. [Google Scholar] [CrossRef]
- Jaybhaye, S.V. Antimicrobial Activity of Silver Nanoparticles Synthesized from Waste Vegetable Fibers. Mater. Today Proc. 2015, 2, 4323–4327. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Silver nanocrystallites: Biofabrication using shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef]
- Ul’berg, Z.R.; Podol’skaya, V.I.; Voitenko, E.Y.; Grishchenko, N.I.; Yakubenko, L.N. Formation and biological activity of preparations based on microorganisms and colloidal silver. Colloid J. 2010, 72, 66–73. [Google Scholar] [CrossRef]
- Wongkamhaeng, K.; Wang, J.; Banas, J.A.; Dawson, D.V.; Holloway, J.A.; Haes, A.J.; Denry, I. Antimicrobial efficacy of platinum-doped silver nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3393–3401. [Google Scholar] [CrossRef]
- Hossain, M.J.; Rahman, M.S.; Khatun, M.; Nandi, N.C.; Akhter, S. Purification of poly(vinylpyrrolidone) stabilized platinum nanoparticles by hemodialyzer. Nano-Struct. Nano-Objects 2017, 10, 105–111. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Gaikwad, S.; Tiwari, V.; Yadav, A.; Ingle, A.; Rai, M. Biofabrication of Silver Nanoparticles by Opuntia ficus-indica: In vitro Antibacterial Activity and Study of the Mechanism Involved in the Synthesis. Curr. Nanosci. 2010, 6, 370–375. [Google Scholar] [CrossRef]
- Mohammed Fayaz, A.; Balaji, K.; Girilal, M.; Kalaichelvan, P.T.; Venkatesan, R. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J. Agric. Food Chem. 2009, 57, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sarkar, J.; Chattopadhyay, D.; Patra, S.; Chakraborty, A.; Acharya, K. Production Of Silver Nanoparticles By A Phytopathogenic Fungus Bipolaris Nodulosa And Its Antimicrobial Activity. Dig. J. Nanomater. Biostructures 2010, 5, 887–895. [Google Scholar]
- Musa, S.F.; Yeat, T.S.; Kamal, L.Z.M.; Tabana, Y.M.; Ahmed, M.A.; El Ouweini, A.; Lim, V.; Keong, L.C.; Sandai, D. Pleurotus sajor-caju can be used to synthesize silver nanoparticles with antifungal activity against Candida albicans. J. Sci. Food Agric. 2018, 98, 1197–1207. [Google Scholar] [CrossRef]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int. J. Nanomed. 2019, 14, 3517–3524. [Google Scholar] [CrossRef]
- Galanski, M.; Jakupec, M.; Keppler, B. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Gnanadesigan, M.; Anand, M.; Ravikumar, S.; Maruthupandy, M.; Vijayakumar, V.; Selvam, S.; Dhineshkumar, M.; Kumaraguru, A.K. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac. J. Trop. Med. 2011, 4, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V.; et al. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Oxford, J.S. Drug resistance and antiviral agents. J. Antimicrob. Chemother. 1976, 2, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.; Kullappan, M.; Patil, S.; Alzahrani, K.; Banjer, H.; Qashqari, F.I.; Raj, A.T.; Bhandi, S.; Veeraraghavan, V.; Jayaraman, S.; et al. Plant-Derived Antiviral Compounds as Potential Entry Inhibitors against Spike Protein of SARS-CoV-2 Wild-Type and Delta Variant: An Integrative In SilicoApproach. Molecules 2022, 27, 1773. [Google Scholar] [CrossRef]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.R.S. Influenza virus-induced lung injury: Pathogenesis and implications for treatment. Eur. Respir. J. 2015, 45, 1463–1478. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-directed antiviral therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Bold, B.E.; Urnukhsaikhan, E.; Mishig-Ochir, T. Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Front. Chem. 2022, 10, 972534. [Google Scholar] [CrossRef]
- Lopez-Miranda, J.L.; Molina, G.A.; González-Reyna, M.A.; España-Sánchez, B.L.; Esparza, R.; Silva, R.; Estévez, M. Antibacterial and Anti-Inflammatory Properties of ZnO Nanoparticles Synthesized by a Green Method Using Sargassum Extracts. Int. J. Mol. Sci. 2023, 24, 1474. [Google Scholar] [CrossRef]
- Daisy, P.; Saipriya, K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Swarnalatha, L.; Rachel, C.; Ranjan, S.; Baradwaj, P. Evaluation of invitro antidiabetic activity of Sphaeranthus amaranthoides silver nanoparticles. Int. J. Nanomater. Biostruct. 2012, 2, 25–29. [Google Scholar]
- Manikanth, G.; Viswambharan, A.I.; Mathad, P. Novel compounds from Premna herbacea Roxb. With antidiabetic potential. Pak. J. Pharm. Sci. 2020, 33, 1971–1979. [Google Scholar] [PubMed]
- Pickup, J.C.; Zhi, Z.L.; Khan, F.; Saxl, T.; Birch, D.J.S. Nanomedicine and its potential in diabetes research and practice. Diabetes. Metab. Res. Rev. 2008, 24, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.; Khan, F.; Abbassi, R.; Rusli, R. Improved DEMATEL methodology for effective safety management decision-making. Saf. Sci. 2020, 127, 104705. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Javadi, F.; Taghavizadeh Yazdi, M.E.; Baghani, M.; Es-Haghi, A. Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Mater. Res. Express 2019, 6, 065408. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A. Biosynthesis of metal and metal oxide nanoparticles using various parts of plants for antibacterial, antifungal and anticancer activity: A review. J. Indian Chem. Soc. 2023, 100, 100987. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Kolesnikov, E.; Dmitry, A.; Ishteev, A.; Gusev, A.; Kuznetsov, D. Floral Biosynthesis of Mn3O4 and Fe2O3 Nanoparticles Using Chaenomeles sp. Flower Extracts for Efficient Medicinal Applications. JOM 2017, 69, 1325–1333. [Google Scholar] [CrossRef]
- Kumar, G.S.; Rajendran, S.; Karthi, S.; Govindan, R.; Girija, E.K.; Karunakaran, G.; Kuznetsov, D. Green synthesis and antibacterial activity of hydroxyapatite nanorods for orthopedic applications. MRS Commun. 2017, 7, 183–188. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Venkatesh, M.; Suresh Kumar, G.; Kolesnikov, E.; Dmitry, A.; Gusev, A.; Kuznetsov, D. Hydrangea paniculata flower extract-mediated green synthesis of MgNPs and AgNPs for health care applications. Powder Technol. 2017, 305, 488–494. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Kolesnikov, E.; Mandal, A.R.; Kuznetsov, D. Allamanda cathartica flower’s aqueous extract-mediated green synthesis of silver nanoparticles with excellent antioxidant and antibacterial potential for biomedical application. MRS Commun. 2016, 6, 41–46. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Kumar, G.S.; Kolesnikov, E. Hylotelephium telephium Flower Extract-Mediated Biosynthesis of CuO and ZnO Nanoparticles with Promising Antioxidant and Antibacterial Properties for Healthcare Applications. JOM 2020, 72, 1264–1272. [Google Scholar] [CrossRef]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Sidhu, C.; Pinnaka, A.K.; Choudhury, A.R. Extracellular polysaccharide production by a novel osmotolerant marine strain of Alteromonas macleodii and its application towards biomineralization of silver. PLoS ONE 2014, 9, e98798. [Google Scholar] [CrossRef]
- Gahlawat, G.; Shikha, S.; Chaddha, B.S.; Chaudhuri, S.R.; Mayilraj, S.; Choudhury, A.R. Microbial glycolipoprotein-capped silver nanoparticles as emerging antibacterial agents against cholera. Microb. Cell Fact. 2016, 15, 25. [Google Scholar] [CrossRef]
- Naik, M.M.; Prabhu, M.S.; Samant, S.N.; Naik, P.M.; Shirodkar, S. Synergistic action of silver nanoparticles synthesized from silver resistant estuarine Pseudomonas aeruginosa strain SN5 with antibiotics against antibiotic resistant bacterial human pathogens. Thalassas 2017, 33, 73–80. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Torres, J.A.L.; Kolesnikov, E.; Kuznetsov, D. Rapid Biosynthesis of AgNPs Using Soil Bacterium Azotobacter vinelandii with Promising Antioxidant and Antibacterial Activities for Biomedical Applications. JOM 2017, 69, 1206–1212. [Google Scholar] [CrossRef]
- Karunakaran, G.; Jagathambal, M.; Gusev, A.; Van Minh, N.; Kolesnikov, E.; Mandal, A.R.; Kuznetsov, D. Nitrobacter sp. extract mediated biosynthesis of Ag2O NPs with excellent antioxidant and antibacterial potential for biomedical application. IET Nanobiotechnol. 2016, 10, 425–430. [Google Scholar] [CrossRef]
- Ganachari, S.V.; Bhat, R.; Deshpande, R.; Venkataraman, A. Extracellular Biosynthesis of Silver Nanoparticles Using Fungi Penicillium diversum and Their Antimicrobial Activity Studies. Bionanoscience 2012, 2, 316–321. [Google Scholar] [CrossRef]
- El Domany, E.B.; Essam, T.M.; Ahmed, A.E.; Farghali, A.A. Biosynthesis physico-chemical optimization of gold nanoparticles as anti-cancer and synergetic antimicrobial activity using Pleurotus ostreatus fungus. J. Appl. Pharm. Sci. 2018, 8, 119–128. [Google Scholar] [CrossRef]
- Murugan, K.; Samidoss, C.M.; Panneerselvam, C.; Higuchi, A.; Roni, M.; Suresh, U.; Chandramohan, B.; Subramaniam, J.; Madhiyazhagan, P.; Dinesh, D.; et al. Seaweed-synthesized silver nanoparticles: An eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol. Res. 2015, 114, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Sherif, M.H.; Malarkodi, C.; Ponnanikajamideen, M.; Arasu, M.V.; Al-Dhabi, N.A.; Roopan, S.M. Cytotoxicity behaviour of response surface model optimized gold nanoparticles by utilizing fucoidan extracted from padina tetrastromatica. J. Mol. Struct. 2021, 1228, 129440. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Abdoli, M.; Mohammadi, G.; Mansouri, K.; Khaledian, S.; Taran, T.; Martinez, F. A review on anticancer, antibacterial and photo catalytic activity of various nanoparticles synthesized by probiotics. J. Biotechnol. 2022, 354, 63–71. [Google Scholar] [CrossRef]
- Michael, H.; Alan, L.G.; Serge, C. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, G.; Qian, Q.; Cui, D. Chloroplasts-mediated biosynthesis of nanoscale Au-Ag alloy for 2-butanone assay based on electrochemical sensor. Nanoscale Res. Lett. 2012, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Mashitah, M.Y.; Maniam, G.P.; Govindan, N. Biosynthesized gold nanoparticle developed as a tool for detection of HCG hormone in pregnant women urine sample. Asian Pac. J. Trop. Dis. 2014, 4, 237. [Google Scholar] [CrossRef]
- Elgamouz, A.; Idriss, H.; Nassab, C.; Bihi, A.; Bajou, K.; Hasan, K.; Abu Haija, M.; Patole, S.P. Green Synthesis, Characterization, Antimicrobial, Anti-Cancer, and Optimization of Colorimetric Sensing of Hydrogen Peroxide of Algae Extract Capped Silver Nanoparticles. Nanomaterials 2020, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.F.; Hassan, H.H.A.M. Antimicrobial and antitumor activity of platinum and palladium complexes of novel spherical aramides nanoparticles containing flexibilizing linkages: Structure-property relationship. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 232–245. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, D.; Wu, L.; Li, J.; Zhao, B.; Zhang, S.; He, R.; Xiao, L.; Zoya, I.; Yu, L.; et al. Gold-nanoparticle-based multistage drug delivery system for antitumor therapy. Drug Deliv. 2022, 29, 3186–3196. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the composition of AuPt bimetallic nanoparticles for antibacterial application. Angew. Chem. Int. Ed. 2014, 53, 8127–8131. [Google Scholar] [CrossRef]
- Gopal, J.; Hasan, N.; Manikandan, M.; Wu, H.F. Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci. Rep. 2013, 3, 1260. [Google Scholar] [CrossRef]

| Plant Name | Type of Nanoparticles | Size of Nanoparticles | Biological Activities | Reference |
|---|---|---|---|---|
| Desmodium triflorum. | Silver | 5–20 nm | Antimicrobial | [46] |
| Chrysophyllum oliviforme | Silver | 20–50 nm | Antioxidant Anticancer | [47] |
| Veronica amygdalina | Silver | 2–18 nm | Antibacterial | [48] |
| Cinnamon zeylanicum | Silver | 8–12 nm | Antibacterial | [49] |
| Phyllanthus amarus | Silver and Gold | 25 & 50 nm | Antibacterial | [50] |
| Chrysopogon zizanioides | Silver and Gold | 20 & 50 nm | Antibacterial, Antioxidant | [51] |
| Camellia sinensis | Palladium | 5–20 nm | Catalytic | [52] |
| Green tea | Iron | 50–80 nm | Removal of Hexavalent Chromium | [53] |
| Ocimum sanctum | Silver | 10–17 nm | Antibacterial | [54] |
| Amaranthus spinosus | Silver | 10–50 nm | Antibacterial | [55] |
| Cycas pschannae | ZnO NRs | 50–100 nm | Antibacterial | [56] |
| Cyrtrandroemia nicobarica | ZnO NRs | 20–200 nm | Antioxidant | [57] |
| Knema andamanica | ZnO NRs | 20–200 nm | Antibacterial | [58] |
| Leea asiatica | ZnO NRs | 20–200 nm | Antioxidant | [59] |
| Leea grandifolia | ZnO NRs | 50–100 nm | Antibacterial | [60] |
| Manilkara littoralis | ZnO NRs | 50–100 nm | Antioxidant | [61] |
| Marine Algae | Type of Nanoparticles | Size of Nanoparticles | Biological Activities | Reference |
|---|---|---|---|---|
| Sargassum wightii | Gold | 8–12 nm | Antibacterial | [62] |
| Sargassum wightii | Silver | 6.20 nm | Fabric | [63] |
| Ulva fasciata | Silver | 4–10 nm | Antifungal | [64] |
| Cystophora moniliforms | Silver | 27–35 nm | Antibacterial | [65] |
| Caulerpa racemosa | Silver | 5–25 nm | Antibacterial | [66] |
| Bacteria Species | Type of Nanoparticles | Size of Nanoparticles | Biological Activities | Reference |
|---|---|---|---|---|
| Lactobacillus plantarum | Zinc oxide | 7 nm | Wound healing | [70] |
| Pseudomonas Fluorescens | Silver | 20–30 nm | Antibacterial | [71] |
| Escherichia coli | Silver | 35 nm | Antibacterial | [72] |
| Lactobacillus plantarum | Silver | 4.7–24.3 nm | Antibacterial and Antioxidant Activity | [73] |
| Streptomyces sp | Silver | 5–3.9 nm. | Antiparasitic activity | [74] |
| Bacillus subtilis | Titanium dioxide | 66–77 nm | Antibacterial | [75] |
| Rhodopseudomonas Capsulate | Gold | 50–60 nm | Bioreduction | [69] |
| Arthrobacter nitroguajacolicus | Gold | 40 nm | Antibacterial | [76] |
| Lactobacillus Fermentum, | Iron Oxide | 10–15 nm | Antibacterial | [77] |
| Bacillus sp. | Silver | 5–15 nm | Antibacterial | [78] |
| Fungi Name and Species | Type of Nanoparticles | Size of Nanoparticles | Biological Activities | Reference |
|---|---|---|---|---|
| Aspergillus foetidus | Silver | 20–40 nm | Antifungal | [81] |
| Aspergillus sps | Iron | 50–200 nm | Iron absorption | [82] |
| Aspergillus sps | Silver | 30–210 nm | Antiviral | [83] |
| Calophyllum apetalum | Silver chloride | 100 nm | Treating rheumatism and leprosy | [84] |
| Aspergillus Niger | Silver | 20 nm | Antibacterial | [85] |
| Aspergillus sp | Silver | 3–40 nm | Antibacterial and Anticancer | [86] |
| Glycosmis mauritiana | Silver | 65 nm | antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity | [87] |
| Chrysosporium tropicum | Silver | 20–50 nm | Drug formation and diseases diagnosis | [79] |
| Fusarium oxysporum | Silver | 20–50 nm | Drug formation and diseases diagnosis | [80] |
| Penicillium sp. | Gold | 50 nm | Extracellular synthesis | [88] |
| Source | Source Name | Nanoparticles | Biological Activity | References |
|---|---|---|---|---|
| Plant | Cissus arnotiana | Cu | Antimicrobial and antioxidant properties | [130] |
| Taraxacum laevigatum | Pt | Antimicrobial activity | [130] | |
| Filicium decipiens | Pd | Antimicrobial activity | [130] | |
| Elettaria Cardamomum | Au | Antimicrobial activity | [130] | |
| Trigonella foenum-graecum | TiO2 | Antimicrobial activity | [130] | |
| Chaenomeles sp | Fe2O3 | Antibacterial activity | [131] | |
| Azardirachta indica Coccinia grandis | CaNPs | Antibacterial activity | [132] | |
| Hydrangea paniculata | Mg and Ag | Health care application | [133] | |
| Allamanda cathartica | AgNPs | Antioxidant and Antibacterial activity | [134] | |
| Hylotelephium telephium | CuO and ZnO | Antioxidant and Antibacterial activity | [135] | |
| Bacteria | Bacillus cereus | Ag | Antibacterial activity | [136] |
| Alteromonas macleodii | Ag | Antibacterial activity | [137] | |
| Deinococcus radiodurans | Ag | Antibacterial activity, anti-biofouling agent and anticancer activity | [138] | |
| Pseudomonas aeruginosa | Ag | Antibacterial activity | [139] | |
| Bacillus brevis | Ag | Antibacterial activity against multi-drug resistant bacteria | [140] | |
| Azotobacter vinelandii | Ag | Antioxidant and Antibacterial activity | [141] | |
| Nitrobacter sp | Ag2O | Antioxidant and Antibacterial activity | [142] | |
| Fungi | Penicillium diversum | Ag | Antimicrobial activity | [143] |
| Aspergillus foetidus | Ag | Antifungal activity | [126] | |
| Pleurotus ostreatus | Au | Antimicrobial activity | [144] | |
| Algae | Ulva lactuca | Ag | Antiplasmodial activity | [145] |
| Chlorella vulgaris | Au | Anti-pathogenic activity | [146] | |
| Galaxaura elongata | Ag | Antibacterial activity | [147] | |
| Padina tetrastromatica | Au | Antibacterial activity | [148] | |
| Sargassum muticum | ZnO | Anti-angiogenesis and antiapoptotic activity | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunakaran, G.; Sudha, K.G.; Ali, S.; Cho, E.-B. Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules 2023, 28, 4527. https://doi.org/10.3390/molecules28114527
Karunakaran G, Sudha KG, Ali S, Cho E-B. Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules. 2023; 28(11):4527. https://doi.org/10.3390/molecules28114527
Chicago/Turabian StyleKarunakaran, Gopalu, Kattakgoundar Govindaraj Sudha, Saheb Ali, and Eun-Bum Cho. 2023. "Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications" Molecules 28, no. 11: 4527. https://doi.org/10.3390/molecules28114527
APA StyleKarunakaran, G., Sudha, K. G., Ali, S., & Cho, E.-B. (2023). Biosynthesis of Nanoparticles from Various Biological Sources and Its Biomedical Applications. Molecules, 28(11), 4527. https://doi.org/10.3390/molecules28114527








