Abstract
Naphtho[2,3-b]furan-4,9-dione is an important privileged structural motif which is present in natural products, drugs, and drug candidates. Herein, visible-light-mediated [3+2] cycloaddition reaction for the synthesis of naphtho[2,3-b]furan-4,9-diones and dihydronaphtho[2,3-b]furan-4,9-diones has been developed. Under environmentally friendly conditions, a variety of title compounds were delivered in good yields. This new protocol shows excellent regioselectivity and remarkable functional group tolerance. This approach provides a powerful, green, efficient, and facile means to expand the structural diversity of naphtho[2,3-b]furan-4,9-diones and dihydronaph-tho[2,3-b]furan-4,9-diones as promising scaffolds for novel drug discovery.
1. Introduction
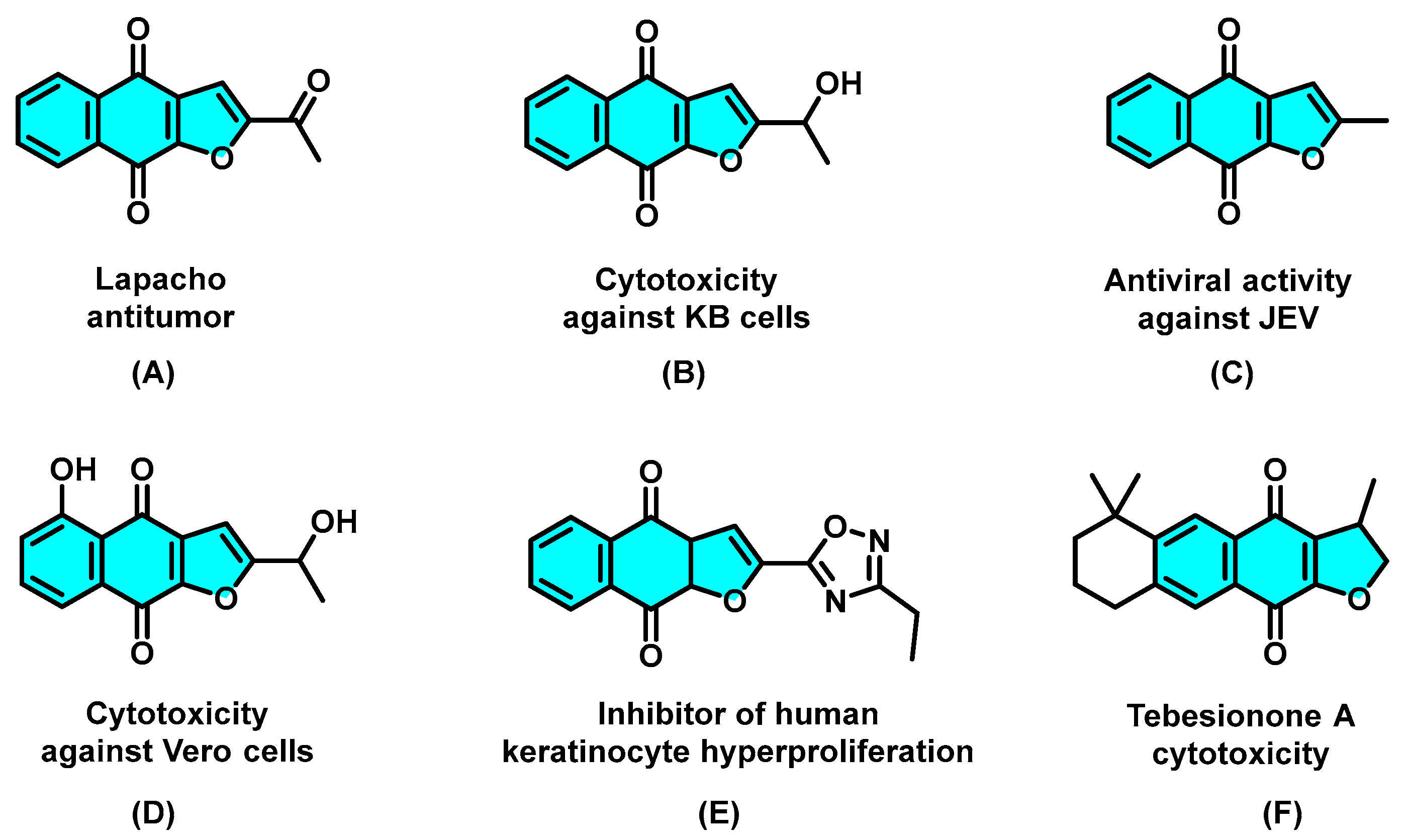
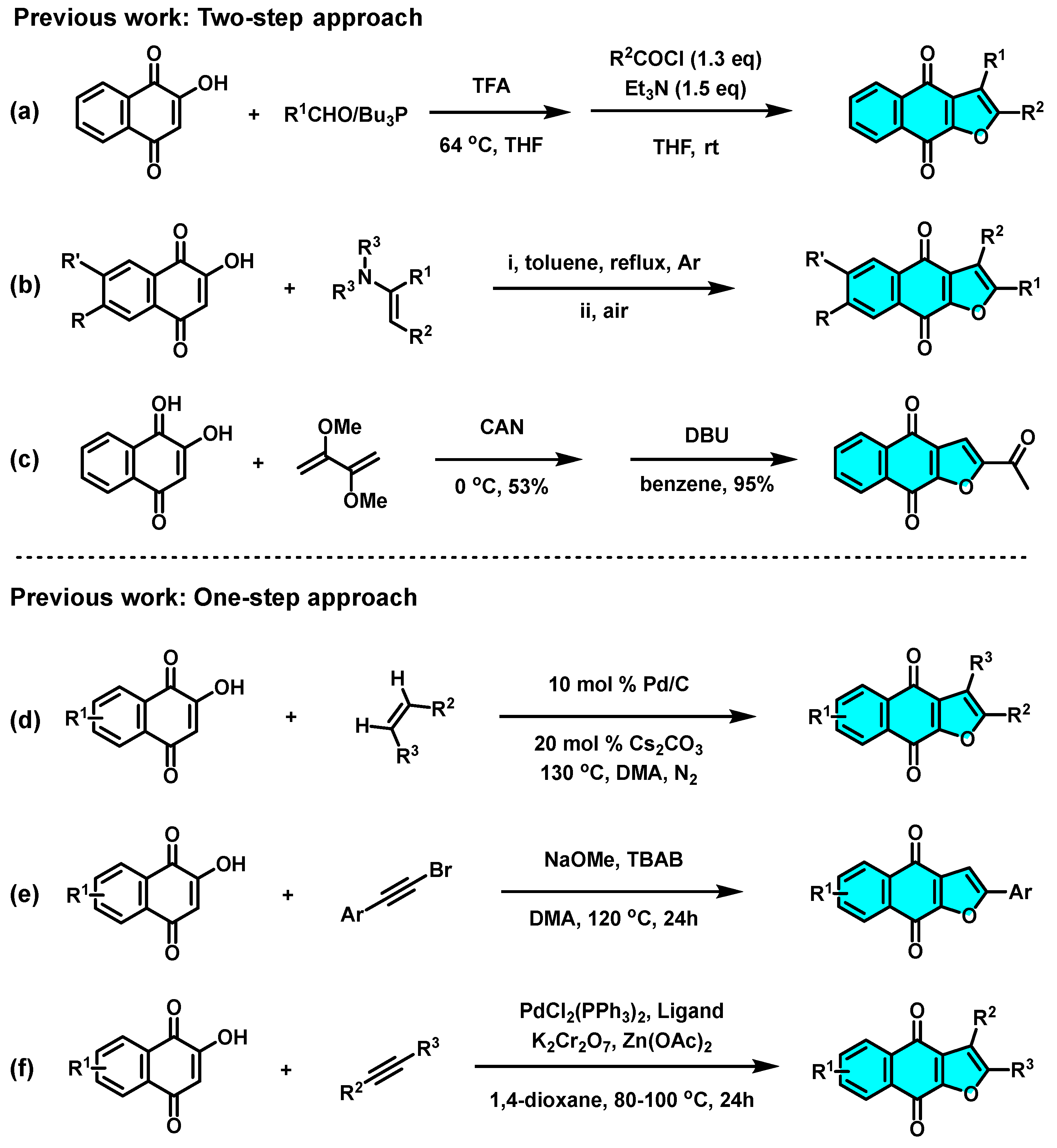
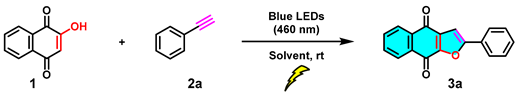
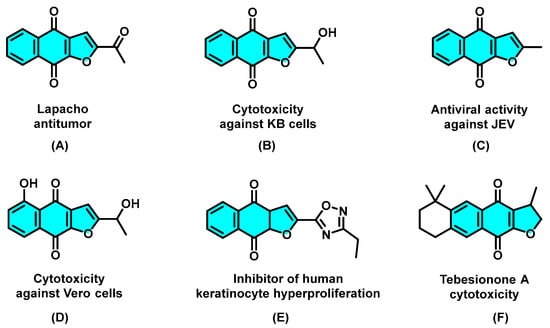
As an important privileged structural motif, many naturally occurring naphtho[2,3-b]furan-4,9-diones and synthetic analogs have been widely found to exhibit versatile biological activities [1,2,3,4,5,6,7,8,9], in particular, antitumor [10], cytotoxic activity toward KB [11], antiviral activity against the Japanese encephalitis virus [12], and Vero cells [13], an inhibitor of human keratinocyte hyperproliferation [14], and other cytotoxic activities [15] (A−F, Scheme 1). Because of the importance of furonaphthoquinones to pharmaceutical research and drug discovery, considerable efforts have been focused on the synthetic approaches of naphtho[2,3-b]furan-4,9-dione ring system. In recent years, different methodologies for the synthesis of furonaphthoquinones have been reported (Scheme 2). Predominantly starting from 2-hydroxy-1,4-naphthoquinones, the two-step procedures, such as multi-component reaction (Scheme 2a) [16], thermal cyclization with enamines (Scheme 2b) [17], and CAN-mediated oxidative cycloaddition with enol ether (Scheme 2c), [18] and one-step cascade approaches, such as transition-metal (Scheme 2d) [19] or strong-base (Scheme 2e) [20] or strong oxidant (Scheme 2f) [21] promoted thermal cyclization methods have been developed. In addition, other multifarious methods, such as Friedel−Crafts acylation/oxidation [22] and bromine-mediated intramolecular cyclization [23], have also been developed. Although significant progress has been made in the synthesis of naphtho[2,3-b]furan-4,9-diones, novel green synthetic approaches with milder reaction conditions and enhanced reaction efficiency are still desirable.
Scheme 1.
Representative biologically active naphtho[2,3-b]furan-4,9-diones.
Scheme 2.
Synthetic approaches starting from 2-hydroxy-1,4-naphthoquinones: (a) multi-component reaction, (b) thermal cyclization with enamines, (c) CAN-mediated oxidative cycloaddition with enol ether, (d) transition-metal promoted thermal cyclization, (e) strong-base promoted thermal cyclization, (f) strong oxidantpromoted thermal cyclization.
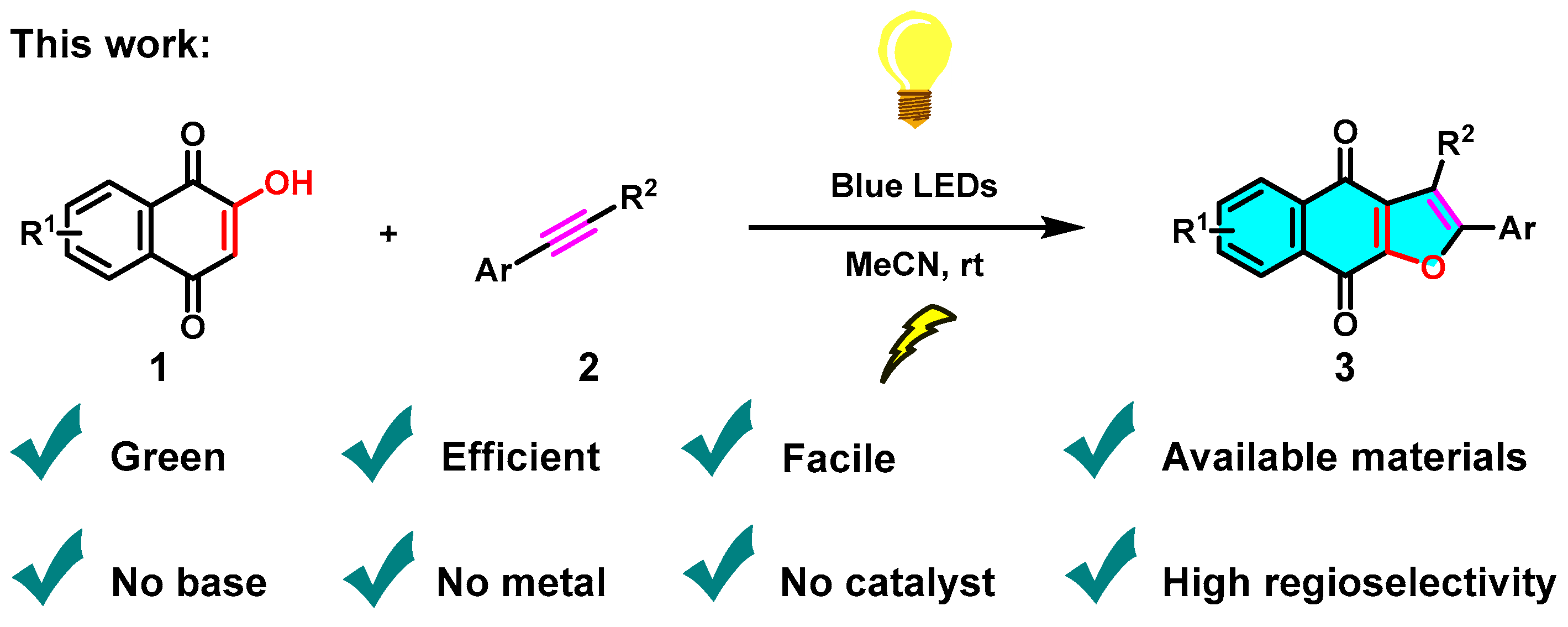
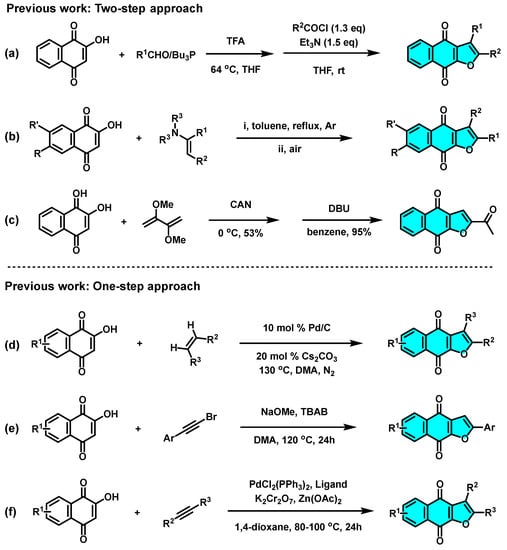
To supplement our initial research in the synthesis of heterocyclic compounds by green MCR approaches and photochemical protocols [24,25,26,27], herein, we report a concise, efficient and green synthetic approach that affords naphtho[2,3-b]furan-4,9-diones via the visible-light-mediated [3+2] cycloaddition reaction of 2-hydroxy-1,4-naphthoquinones (1) and phenylacetylenes (2) under irradiation of blue LEDs (460 nm) in the absence of any bases, metals, ligands, or other catalysts (Scheme 3).
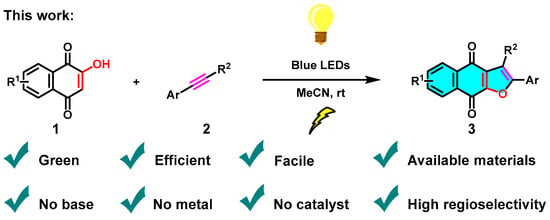
Scheme 3.
Visible-light-mediated green synthesis of naphtho[2,3-b]furan-4,9-diones.
2. Results and Discussion
In the pilot experiment, 2-hydroxy-1,4-naphthoquinone (1) was selected as the model substrate to react with phenylacetylene (2a) at ambient temperature under irradiation of blue LEDs (460 nm). Product 3a was obtained in 58% yield without any catalyst after 6 h irradiation in DCM (Table 1, entry 2). Furthermore, the use of different solvents, including acetone, THF, and toluene, failed to give better results than DCM (Table 1, entries 4–12, respectively). However, the yield was improved to 75% when a solvent of MeCN was used with irradiation for 6 h. (Table 1, entry 14). Consequently, the optimized reaction conditions turned out to be using MeCN as solvent under blue LEDs (460 nm) irradiation at ambient temperature for 6 h.

Table 1.
Optimization of the reaction conditions a.
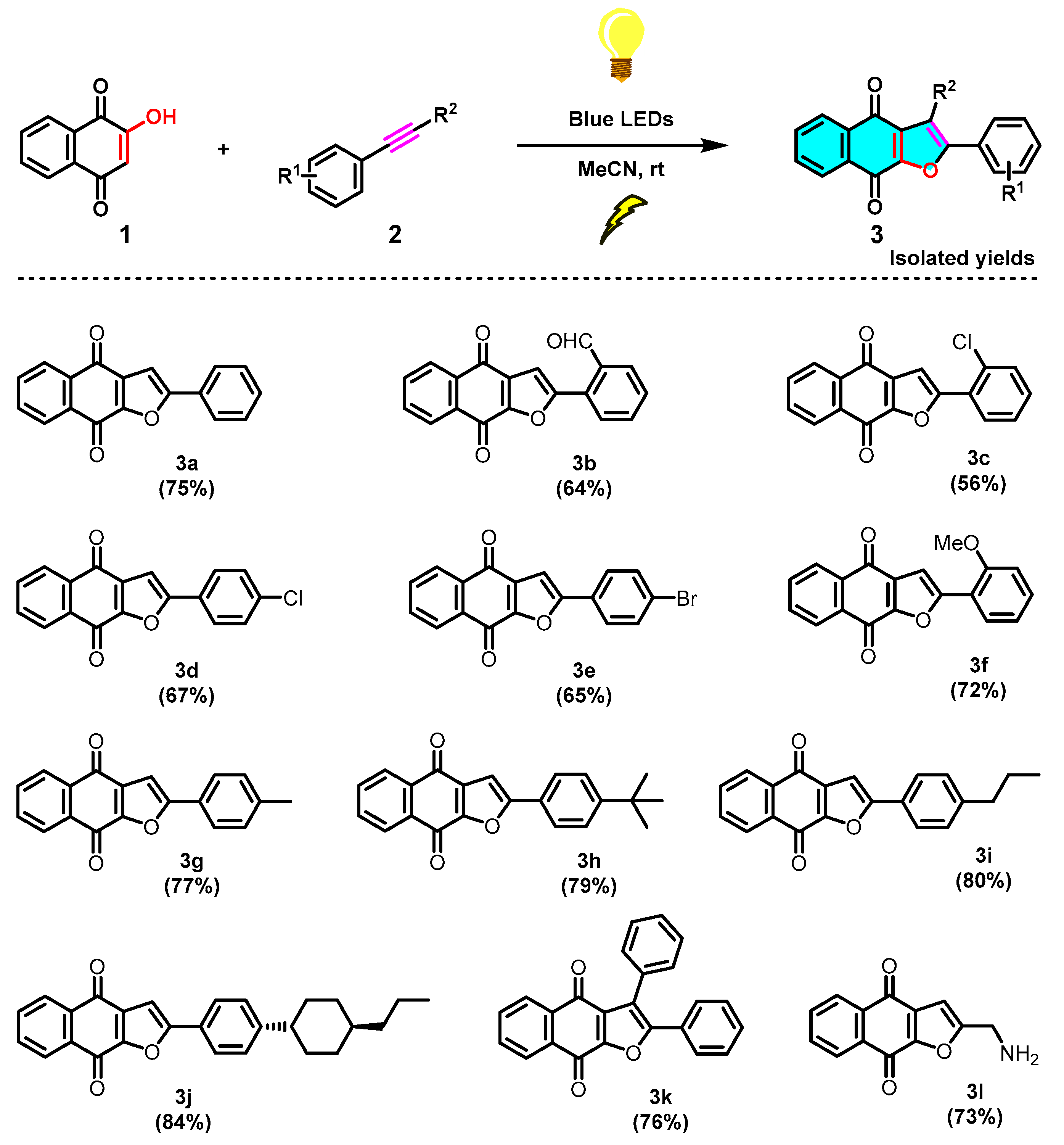
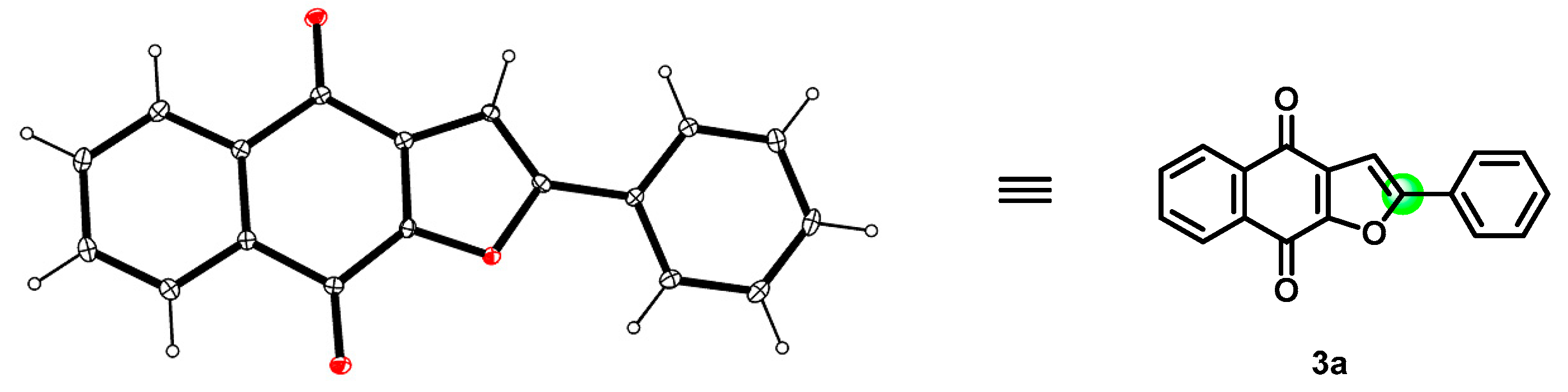
The structure of product 3a was confirmed firstly by means of proton and carbon NMR spectra. It was noted that the naphtho[2,3-b]furan-4,9-dione 3a was obtained with complete regioselectivity, with only 2-phenylnaphtho[2,3-b]furan-4,9-dione was obtained and no isomer 3-phenylnaphtho[2,3-b]furan-4,9-dione being detectable or isolable (Scheme 3). Furthermore, the configuration of the main compound 3a was unambiguously established by single-crystal X-ray diffraction analysis (Figure 1), which indicated that the phenyl group is at a C-2 position (green) instead of a C-3 position. With the optimized conditions in hand (Table 1, entry 14), a series of substituted phenylacetylenes 2a−2k were evaluated (Scheme 4). It was noticed that the analogous reactions of formyl and various halogens substituted phenylacetylenes (2b–2e) with 2-hydroxy-1,4-naphthoquinone (1) successfully generated the corresponding products 3b-3e in moderate yields, respectively. Subsequently, we evaluated various alkyls substituted phenylacetylenes (2g–2j), such as Me, tBu, and cyclohexyl, all of which favourably delivered the corresponding products 3g–3j in good to very good yields, not showing significant differences in yield. Relatively speaking, an electron-donating group on the benzene ring, as in the case of 3j, is more favorable than the electron-withdrawing groups (CHO and halogens) of 3b–3e. Furthermore, other two phenylacetylene derivatives, diphenylacetylene and propargylamine (2k and 2l), were evaluated too, and corresponding naphtho[2,3-b]furan-4,9-diones 3k and 3l were obtained swimmingly, as predicted, in good yields.
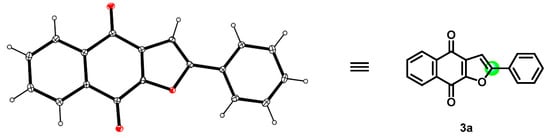
Figure 1.
X-ray structure of compound 3a (CCDC-2264554).
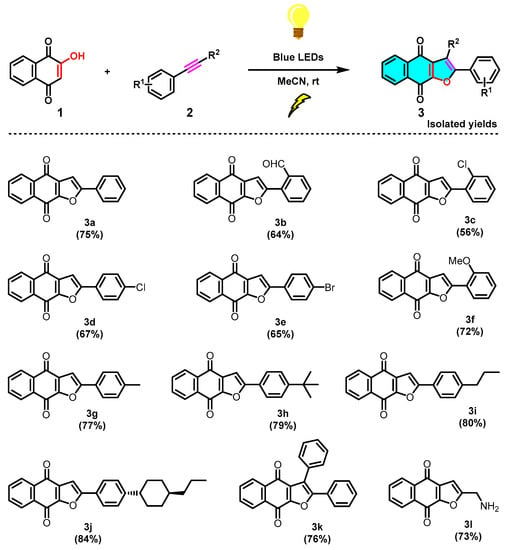
Scheme 4.
Scope of the photochemical synthesis of naphtho[2,3-b]furan-4,9-diones (3).
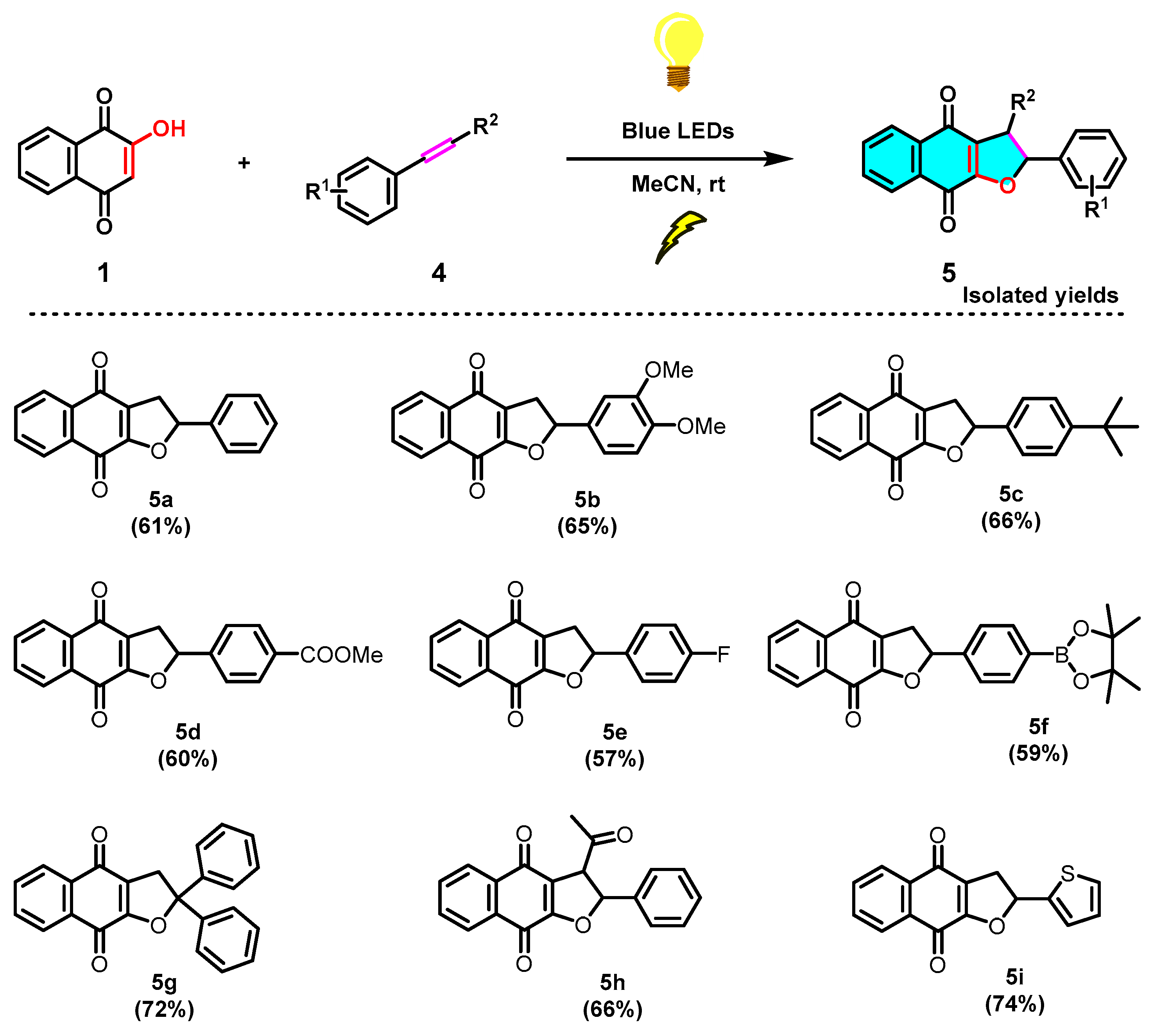
Next, to further examine the feasibility of this reaction, we examined the variation of the styrene component toward the formation of dihydronaphtho[2,3-b]furan-4,9-diones (5) (Scheme 5). With the above optimized conditions in hand (Table 1, entry 14), a series of substituted styrenes 4a–4h were evaluated (Scheme 5). It was observed that the analogous reactions of EDG or EWG substituted styrenes with 2-hydroxy-1,4-naphthoquinone (1) smoothly produced the corresponding products 5a–5h in moderate yields, respectively. Thereafter, we evaluated a heterocyclic olefin 2-vinylthiophene (4i), which was also found to be effective, as demonstrated in the successful installation of 5i with a 74% yield. Overall, different substituted phenylacetylene derivatives (2) and styrene derivatives (4) reacted with 2-hydroxy-1,4-naphthoquinone (1) and generated corresponding cycloaddition products naphtho[2,3-b]furan-4,9-diones (3) and dihydronaphtho[2,3-b]furan-4,9-diones (5), not showing significant differences in yield.
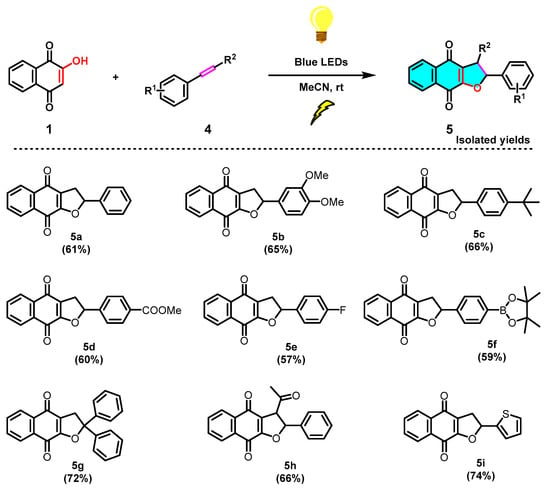
Scheme 5.
Scope of the photochemical synthesis of dihydronaphtho[2,3-b]furan-4,9-diones (5).
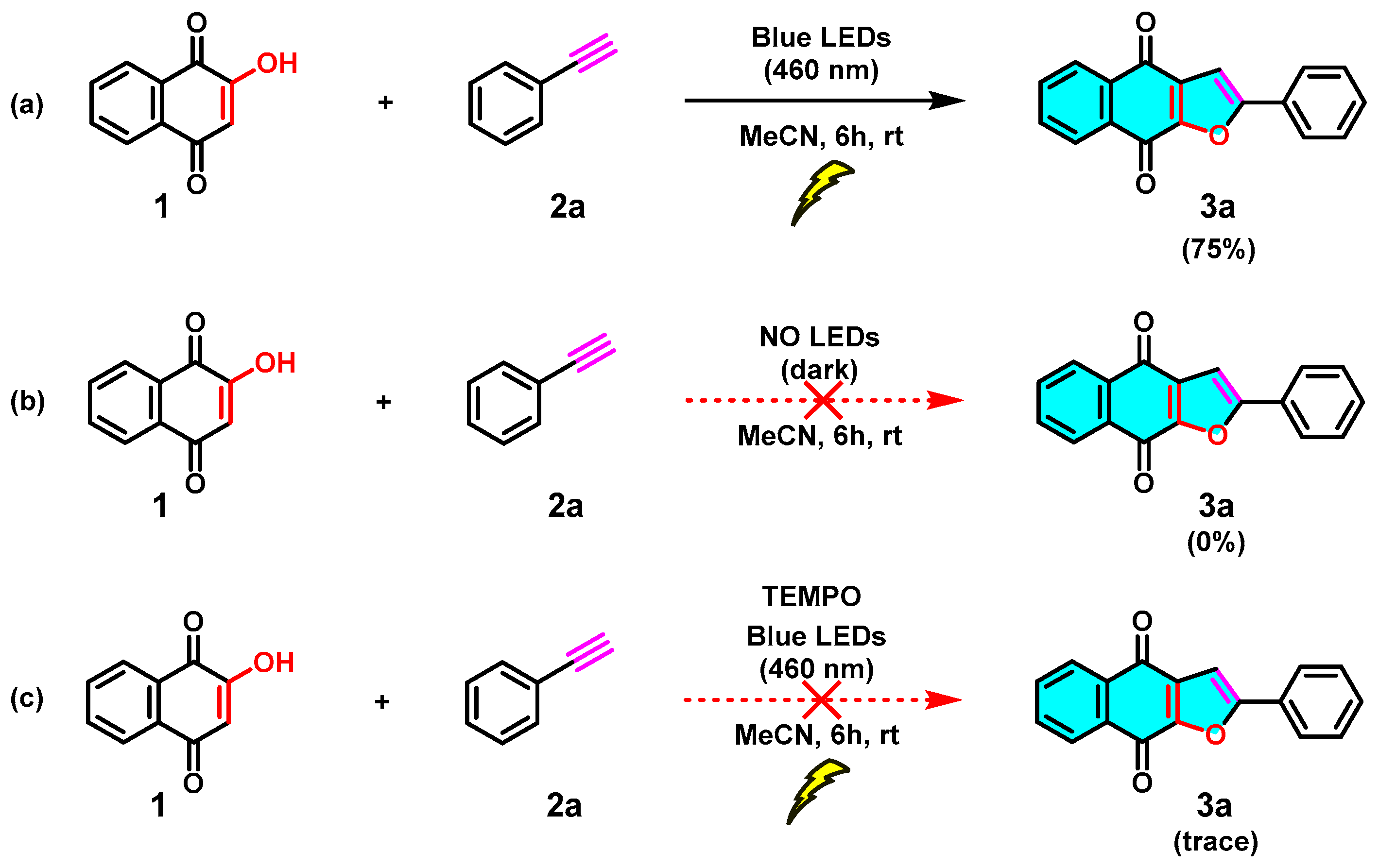
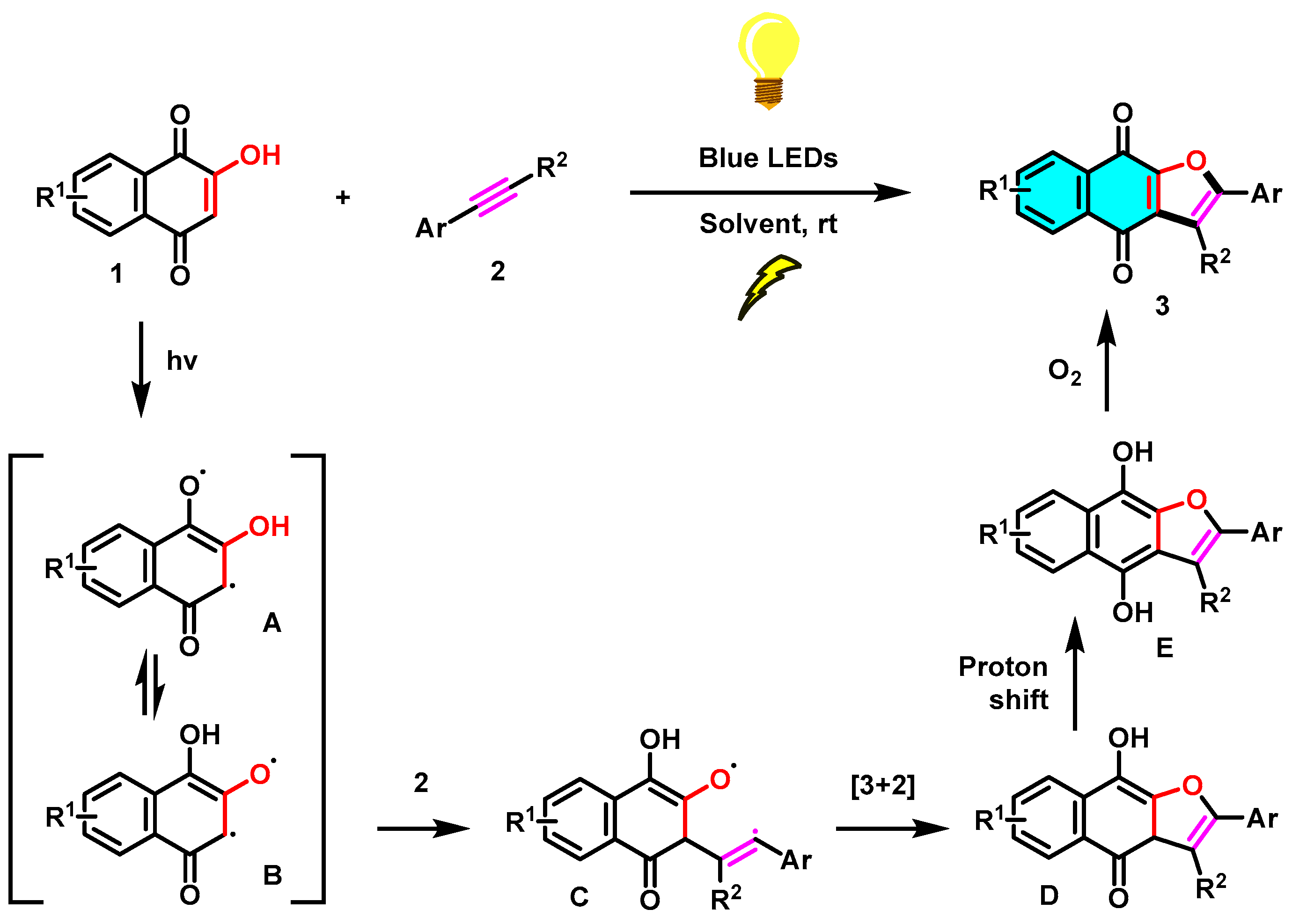
To reveal the mechanism of this visible-light-mediated [3+2] cycloaddition reaction, several control experiments were performed (Scheme 6). As expected, no reaction occurs in the absence of light (Scheme 6b), which highlights the fact that the [3+2] cycloaddition reaction needs to be mediated by visible light. Furthermore, in reaction c of Scheme 6, TEMPO was added as a radical scavenger under standard conditions, and a trace amount of the target product 3a was observed. Based on the control experimental results and the reported literature [28], a possible mechanism for this blue visible-light photocatalyzed [3+2] cycloaddition reaction was proposed, as shown in Scheme 7. First, the irradiation of 1 in MeCN generates tautomeric excited triplets (A) and (B), which react with an alkyne (2) to give a 1,5-biradical intermediate (C). Subsequently, an intramolecular [3+2] cyclization of the intermediate C gives hydroquinone intermediate (D). Upon 1,3-hydrogen transfer, the hydroquinone intermediate (E) is formed, and then, naphtho[2,3-b]furan-4,9-diones (3) is produced by air oxidation of the hydroquinone by oxygen in the air. Similarly, the [3+2] cycloaddition reaction of 2-hydroxy-1,4-naphthoquinone (1) with alkenes (4) leading to product dihydronaphtho[2,3-b]furan-4,9-diones (5) may proceed in a manner parallel to the [3+2] cycloaddition of alkynes and may also involve biradical intermediates.
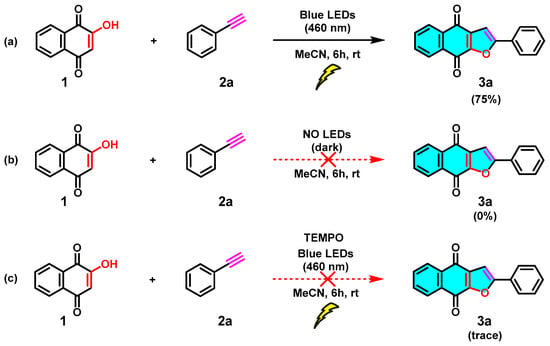
Scheme 6.
Control experiments for the photocatalyzed [3+2] cycloaddition reaction: (a) experiment was performed under standard conditions, (b) experiment was performed in the absence of light, (c) experiment was performed in the presence of TEMPO.
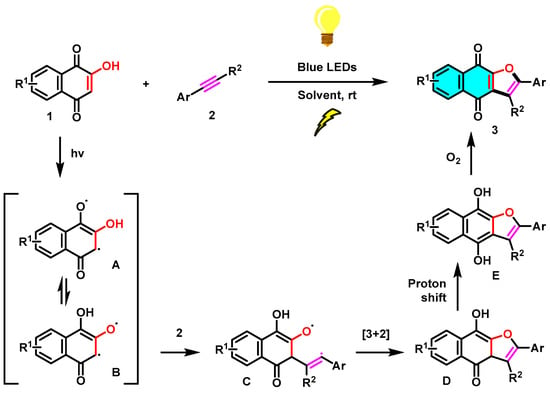
Scheme 7.
Proposed mechanism for the photocatalyzed [3+2] cycloaddition reaction.
3. Materials and Methods
3.1. General Information
All starting materials were purchased from available commercial suppliers and used without further purification. Thin layer chromatography (TLC) was performed on silica gel GF254 plates. Melting points were obtained by using an XT-5A digital melting-point apparatus and were uncorrected. The NMR spectra were recorded on a Bruker Avance 400 spectrometer at 400 MHz (1H NMR) and 100 MHz (13C NMR). HRMS analyses were carried out on a Thermo Fisher Q-Exactive mass spectrometer, which was operated in electrospray ionization (ESI) mode.
3.2. General Procedure for the Synthesis of Naphtho[2,3-b]furan-4,9-diones (3)
In a 25 mL tube, 2-hydroxy-1,4-naphthoquinone 1 (1.0 mmol) and alkyne 2 (1.0 mmol) were dissolved in 20 mL of acetonitrile. The reaction mixture was under irradiation of visible blue LEDs (460 nm) for 6.0 h. After completion (by TLC), the reaction mixture was evaporated to dryness in a vacuo. The residue was purified by medium-pressure chromatography (silica gel) using a mixed solvent of hexane and ethyl acetate (10–50% EA). The products were characterized by 1H NMR, 13C NMR, and HRMS spectroscopy.
2-Phenylnaphtho[2,3-b]furan-4,9-dione (3a). Yellow solid, yield 75%, m.p. 244–247 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.12 (s, 1H, Ar-H), 7.37–7.43 (m, 3H, Ar-H), 7.67–7.70 (m, 2H, Ar-H), 7.81–7.84 (m, 2H, Ar-H), 8.11–8.18 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 102.9, 125.6, 126.9, 127.0, 128.3, 130.3, 132.4, 132.9, 133.1, 133.6, 134.0, 151.6, 160.4, 173.1, 180.8; HRMS (ESI), m/z calcd 275.0803 for C18H11O3 [M + H]+, found 275.0807.
2-(4,9-Dioxo-4,9-dihydronaphtho[2,3-b]furan-2-yl)benzaldehyde (3b). Faint yellow solid, yield 64%, m.p. 188–191 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.28 (s, 1H, Ar-H), 7.64–7.68 (m, 1H, Ar-H), 7.74–7.82 (m, 3H, Ar-H), 7.89 (d, 1H, J = 7.6 Hz, Ar-H), 8.10 (dd, 1H, J = 7.6 Hz, 0.8 Hz, Ar-H), 8.24–8.29 (m, 2H, Ar-H), 10.44 (s, 1H, CHO); 13C NMR (100 MHz, CDCl3): δ (ppm) 108.8, 127.1, 127.2, 129.4, 129.8, 130.4, 132.6, 132.6, 133.0, 133.9, 134.0, 134.1, 134.2, 152.8, 158.2, 173.3, 180.5, 190.6; HRMS (ESI), m/z calcd 303.0652 for C19H11O4 [M + H]+, found 303.0655.
2-(2-Chlorophenyl)naphtho[2,3-b]furan-4,9-dione (3c). Orange-yellow, yield 56%, m.p. 191–194 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.36–7.44 (m, 2H, Ar-H), 7.51–7.54 (m, 1H, Ar-H), 7.68 (s, 1H, Ar-H), 7.76–7.78 (m, 2H, Ar-H), 8.09 (dd, 1H, J = 8.0 Hz, 2.0 Hz, Ar-H), 8.21–8.26 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 108.5, 126.9, 127.1, 127.3, 129.3, 130.7, 131.9, 132.0, 132.8, 133.2, 133.8, 134.0, 151.3, 156.4, 173.2, 180.7; HRMS (ESI), m/z calcd 309.0313 for C18H10ClO3 [M + H]+, found 309.0317.
2-(4-Chlorophenyl)naphtho[2,3-b]furan-4,9-dione (3d). Orange solid, yield 67%, m.p. 209–212 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.19 (s, 1H, Ar-H), 7.62 (d, 2H, J = 8.4 Hz, Ar-H), 7.74–7.78 (m, 4H, Ar-H), 8.18–8.25 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 103.5, 124.7, 126.9, 126.9, 127.0, 127.2, 132.3, 132.4, 132.8, 133.1, 133.7, 134.1, 151.7, 159.2, 173.0, 180.6; HRMS (ESI), m/z calcd 309.0313 for C18H10ClO3 [M + H]+, found 309.0316.
2-(4-Bromophenyl)naphtho[2,3-b]furan-4,9-dione (3e). Yellow solid, yield 65%, m.p. 264–266 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.12 (s, 1H, Ar-H), 7.37–7.43 (m, 3H, Ar-H), 7.67–7.70 (m, 2H, Ar-H), 7.81–7.84 (m, 2H, Ar-H), 8.11–8.18 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 55.6, 107.7, 111.2, 117.3, 121.0, 126.8, 126.9, 127.5, 131.2, 132.6, 133.0, 133.2, 133.5, 133.9, 150.5, 157.0, 173.0, 181.2; HRMS (ESI), m/z calcd 352.9808 for C18H10BrO3 [M + H]+, found 352.9810.
2-(2-Methoxyphenyl)naphtho[2,3-b]furan-4,9-dione (3f). Yellow solid, yield 72%, m.p. 207–209 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 4.03 (s, 3H, OCH3), 7.05 (d, 1H, J = 8.4 Hz, Ar-H), 7.12 (t, 1H, J = 7.2 Hz, Ar-H), 7.41–7.45 (m, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.75–7.78 (m, 2H, Ar-H), 8.13–8.27 (m, 3H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 103.4, 124.7, 126.9, 127.0, 127.2, 132.3, 132.4, 132.8, 133.1, 133.6, 134.1, 151.7, 159.2, 173.1, 180.6; HRMS (ESI), m/z calcd 305.0808 for C19H13O4 [M + H]+, found 305.0812.
2-(p-Tolyl)naphtho[2,3-b]furan-4,9-dione (3g). Faint yellow solid, yield 77%, m.p. 260–263 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 2.39 (s, 3H, CH3), 7.11 (s, 1H, Ar-H), 7.27 (d, 2H, J = 7.6 Hz, Ar-H), 7.72–7.77 (m, 4H, Ar-H), 8.16–8.21 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 21.5, 102.3, 125.5, 126.8, 126.9, 129.8, 132.5, 132.9, 133.1, 133.5, 133.9, 134.7, 160.6, 172.9, 180.9; HRMS (ESI), m/z calcd 289.0859 for C19H13O3 [M + H]+, found 289.0861.
2-(4-(Tert-butyl)phenyl)naphtho[2,3-b]furan-4,9-dione (3h). Faint yellow solid, yield 79%, m.p. 180–182 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 1.36 (s, 9H, C(CH3)3), 7.15 (s, 1H, Ar-H), 7.50–7.52 (m, 2H, Ar-H), 7.73–7.84 (m, 4H, Ar-H), 8.18–8.25 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 31.2, 35.0, 102.4, 125.4, 125.6, 126.1, 126.9, 126.9, 132.6, 133.0, 133.1, 133.5, 133.9, 151.4, 153.9, 160.9, 173.0, 180.9; HRMS (ESI), m/z calcd 331.1329 for C22H19O3 [M + H]+, found 331.1333.
2-(4-Propylphenyl)naphtho[2,3-b]furan-4,9-dione (3i). Yellow solid, yield 80%, m.p. 166–169 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 0.99 (t, 3H, J = 7.6 Hz, CH3), 1.61–1.73 (m, 2H, CH2), 2.66 (t, 2H, J = 7.6 Hz, CH2), 7.16 (s, 1H, Ar-H), 7.31 (d, 2H, J = 8.0 Hz, Ar-H), 7.75–7.84 (m, 4H, Ar-H), 8.20–8.27 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 13.8, 24.3, 38.0, 102.3, 125.6, 126.8, 126.9, 126.9, 129.2, 132.6, 132.9, 145.5, 160.8, 172.9, 180.9; HRMS (ESI), m/z calcd 317.1172 for C21H17O3 [M + H]+, found 317.1175.
2-(4-((1s,4r)-4-Propylcyclohexyl)phenyl)naphtho[2,3-b]furan-4,9-dione (3j). Yellow solid, yield 84%, m.p. 179–182 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 0.91 (t, 3H, J = 7.6 Hz, CH3), 1.05–1.08 (m, 2H, CH2), 1.22–1.25 (m, 2H, CH2), 1.32–1.37 (m, 3H, CH2, CH), 1.46–1.50 (m, 2H, CH2), 1.88–1.93 (m, 4H, CH2), 2.50–2.56 (m, 1H, CH), 7.14 (s, 1H, Ar-H), 7.33 (d, 2H, J = 8.0 Hz, Ar-H), 7.74–7.76 (m, 2H, Ar-H), 7.81 (d, 2H, J = 8.0 Hz, Ar-H), 8.18–8.25 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 14.4, 20.0, 33.4, 33.4, 37.0, 39.7, 44.6, 102.3, 125.6, 125.9, 126.9, 126.9, 127.6, 132.6, 133.0, 133.1, 133.5, 133.9, 150.7, 151.3, 160.8, 172.9, 180.9; HRMS (ESI), m/z calcd 399.1955 for C27H27O3 [M + H]+, found 399.1957.
2,3-Diphenylnaphtho[2,3-b]furan-4,9-dione (3k). Orange solid, yield 76%, m.p. 262–265 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 7.37–7.43 (m, 3H, Ar-H), 7.35–7.43 (m, 8H, Ar-H), 7.59–7.63 (m, 1H, Ar-H), 7.77 (d, 1H, J = 7.6 Hz, Ar-H), 8.00 (dd, 1H, J = 8.0 Hz, 0.4 Hz, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 121.8, 121.8, 122.4, 126.6, 128.4, 128.5, 128.6, 128.7, 128.9, 129.0, 129.1, 129.9, 130.2, 130.2, 130.4, 135.4, 151.5, 159.2, 174.5, 180.6; HRMS (ESI), m/z calcd 351.1016 for C24H15O3 [M + H]+, found 351.1019.
2-(Aminomethyl)naphtho[2,3-b]furan-4,9-dione (3l). Faint yellow solid, yield 73%, m.p. 217–219 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.71 (d, 2H, J = 2.4 Hz, CH2), 5.47 (s, 1H, Ar-H), 7.56 (t, 1H, J = 7.2 Hz, Ar-H), 7.67 (t, 1H, J = 7.6 Hz, Ar-H), 7.80 (d, 1H, J = 7.6 Hz, Ar-H), 7.85 (d, 1H, J = 7.6 Hz, Ar-H),; 13C NMR (100 MHz, CDCl3): δ (ppm) 29.1, 77.6, 107.6, 125.3, 125.5, 130.9, 132.1, 133.8, 135.8, 181.3; HRMS (ESI), m/z calcd 228.0655 for C13H10NO3 [M + H]+, found 228.0655.
3.3. General Procedure for the Synthesis of Dihydronaphtho[2,3-b]furan-4,9-diones (5)
In a 25 mL tube, 2-hydroxy-1,4-naphthoquinone 1 (1.0 mmol) and alkenes 4 (1.0 mmol) were dissolved in 20 mL of acetonitrile. The reaction mixture was under irradiation of visible blue LEDs (460 nm) for 6.0 h. After completion (by TLC), the reaction mixture was evaporated to dryness in vacuo. The residue was purified by medium-pressure chromatography (silica gel) using a mixed solvent of hexane and ethyl acetate (10–50% EA). The products were characterized by 1H NMR, 13C NMR, and HRMS spectroscopy.
2-Phenyl-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5a). Yellow solid, yield 61%, m.p. 171–173 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.27 (dd, 1H, J = 16.8 Hz, 8.4 Hz, CH2); 3.67 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 6.00 (dd, 1H, J = 10.8 Hz, 8.8 Hz, CH); 7.39–7.41 (m, 4H, Ar-H), 7.46 (s, 1H, Ar-H), 7.69–7.74 (m, 2H, Ar-H), 8.08–8.12 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 35.3, 86.8, 123.9, 126.0, 126.1, 126.4, 128.9, 131.6, 133.1, 133.1, 134.2, 139.5, 159.9, 177.7, 182.2; HRMS (ESI), m/z calcd 277.0859 for C18H13O3 [M + H]+, found 277.0861.
2-(3,4-Dimethoxyphenyl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5b). Faint yellow solid, yield 65%, m.p. 177–179 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.28 (dd, 1H, J = 17.2 Hz, 9.2 Hz, CH2); 3.62 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 3.88 (s, 6H, OCH3); 5.94 (dd, 1H, J = 8.4 Hz, 7.2 Hz, CH); 6.87 (d, 1H, J = 8.0 Hz, Ar-H); 6.92–6.98 (m, 2H, Ar-H), 7.68–7.73 (m, 2H, Ar-H), 8.07–8.09 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 34.8, 56.0, 56.0, 87.1, 109.5, 111.2, 119.0, 123.9, 126.0, 126.3, 131.6, 133.0, 133.1, 134.2, 149.4, 149.7, 159.7, 177.7, 182.2; HRMS (ESI), m/z calcd 336.0992 for C20H16O5 [M + H]+, found 336.0995.
2-(4-(Tert-butyl)phenyl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5c). Yellow solid, yield 66%, m.p. 166–169 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 1.32 (s, 9H, C(CH3)3), 3.30 (dd, 1H, J = 17.2 Hz, 8.8 Hz, CH2); 3.64 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 5.98 (dd, 1H, J = 10.4 Hz, 8.4 Hz, CH), 7.34 (d, 2H, J = 8.4 Hz, Ar-H), 7.42 (d, 2H, J = 8.4 Hz, Ar-H), 7.69–7.75 (m, 2H, Ar-H), 8.01–8.12 (m, 2H, Ar-H),; 13C NMR (100 MHz, CDCl3): δ (ppm) 29.7, 31.3, 34.9, 86.8, 123.9, 124.0, 125.8, 125.9, 126.1, 126.4, 127.8, 131.7, 133.0, 134.2, 136.4, 152.2, 159.9, 182.2; HRMS (ESI), m/z calcd 333.1485 for C22H21O3 [M + H]+, found 333.1488.
Methyl 4-(4,9-dioxo-2,3,4,9-tetrahydronaphtho[2,3-b]furan-2-yl)benzoate (5d). Yellow solid, yield 60%, m.p. 168–171 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.22 (dd, 1H, J = 17.2 Hz, 8.8 Hz, CH2); 3.72 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 3.92 (s, 3H, OCH3), 6.05 (dd, 1H, J = 10.8 Hz, 8.4 Hz, CH); 7.48 (d, 2H, J = 8.4 Hz, Ar-H), 7.68–7.76 (m, 2H, Ar-H), 8.06–8.11 (m, 4H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 35.5, 52.2, 85.8, 123.7, 125.7, 126.1, 126.4, 130.2, 131.6, 133.0, 133.2, 134.3, 144.5, 159.7, 166.5, 177.5, 182.0; HRMS (ESI), m/z calcd 334.0841 for C20H14O5 [M + H]+, found 334.0844.
2-(4-Fluorophenyl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5e). Faint yellow solid, yield 57%, m.p. 175–177 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.23 (dd, 1H, J = 17.2 Hz, 8.4 Hz, CH2); 3.66 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 5.98 (dd, 1H, J = 10.4 Hz, 8.8 Hz, CH); 7.06–7.09 (m, 2H, Ar-H), 7.37–7.41 (m, 2H, Ar-H), 7.70–7.76 (m, 2H, Ar-H), 8.08–8.11 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 35.3, 86.1, 115.8, 116.0, 123.7, 126.1, 126.4, 127.9, 131.6, 133.0, 133.1, 134.3, 159.7, 177.7, 182.1; HRMS (ESI), m/z calcd 295.0765 for C18H12FO3 [M + H]+, found 295.0769.
2-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5f). Yellow solid, yield 59%, m.p. 170–172 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 1.35 (s, 12H, CH3), 3.23 (dd, 1H, J = 17.2 Hz, 8.8 Hz, CH2); 3.67 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 6.01 (dd, 1H, J = 10.8 Hz, 8.8 Hz, CH); 7.48 (d, 2H, J = 8.4 Hz, Ar-H), 7.69–7.75 (m, 2H, Ar-H), 7.84 (d, 2H, J = 8.4 Hz, Ar-H), 8.08–8.12 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 24.9, 35.4, 84.0, 86.6, 123.9, 125.0, 126.1, 126.4, 131.6, 133.1, 133.1, 134.2, 135.4, 142.5, 159.9, 177.7, 182.2; HRMS (ESI), m/z calcd 403.1711 for C24H24BO5 [M + H]+, found 403.1713.
2,2-Diphenyl-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5g). Faint yellow solid, yield 72%, m.p. 171–173 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.92 (s, 2H, CH2), 7.28–7.36 (m, 6H, Ar-H), 7.45–7.48 (m, 4H, Ar-H), 7.64–7.68 (m, 2H, Ar-H), 8.02–8.09 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 41.6, 96.4, 123.7, 125.8, 126.0, 126.4, 128.2, 128.6, 131.7, 133.0, 134.1, 143.6, 158.6, 177.6, 182.2; HRMS (ESI), m/z calcd 353.1172 for C24H17O3 [M + H]+, found 353.1175.
3-Acetyl-2-phenyl-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5h). Yellow solid, yield 66%, m.p. 195–197 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 2.54 (s, 3H, CH3), 4.52 (d, 1H, J = 7.2 Hz, CH); 6.27 (d, 1H, J = 6.8 Hz, CH); 7.31–7.36 (m, 2H, Ar-H), 7.37–7.40 (m, 3H, Ar-H), 7.72–7.76 (m, 2H, Ar-H), 8.07–8.13 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 31.0, 60.9, 88.8, 122.0, 126.0, 125.6, 126.3, 126.6, 129.1, 129.3, 131.5, 132.9, 133.4, 134.5, 138.5, 160.4, 177.5, 181.7; HRMS (ESI), m/z calcd 319.0965 for C20H15O4 [M + H]+, found 319.0968.
2-(Thiophen-2-yl)-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (5i). Orange solid, yield 74%, m.p. 176–179 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.42 (dd, 1H, J = 17.6 Hz, 8.8 Hz, CH2); 3.67 (dd, 1H, J = 17.2 Hz, 10.8 Hz, CH2); 6.00 (dd, 1H, J = 10.0 Hz, 8.4 Hz, CH); 7.01–7.04 (m, 1H, Ar-H), 7.19–7.20 (m, 1H, Ar-H), 7.36–7.38 (m, 1H, Ar-H), 7.68–7.75 (m, 2H, Ar-H), 8.07–8.10 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm) 35.3, 82.5, 123.6, 126.1, 126.4, 127.0, 127.1, 131.6, 133.0, 133.1, 134.2, 141.7, 159.1, 177.6, 182.1; HRMS (ESI), m/z calcd 283.0423 for C16H11O3S [M + H]+, found 283.0425.
The results of the X-ray diffraction analysis for compounds 3a and 5d were deposited with the Cambridge Crystallographic Data Centre (CCDC 2264554 and 2264555).
4. Conclusions
In conclusion, visible-light-mediated [3+2] cycloaddition reactions of 2-hydroxy-1,4-naphthoquinones and alkynes and alkenes under irradiation of blue LEDs (460 nm) in the absence of any bases, metals, ligands, or other catalysts have been demonstrated. Under environmentally friendly conditions, a variety of naphtho[2,3-b]furan-4,9-diones and dihydronaphtho[2,3-b]furan-4,9-diones were delivered within 6 h in comparable or sometimes even (slightly) better yields than those presented in the literature. This green and efficient protocol shows excellent regioselectivity and remarkable functional group tolerance. This work provides a powerful, green, efficient, and facile means to expand the structural diversity of naphtho[2,3-b]furan-4,9-diones and dihydronaphtho[2,3-b]furan-4,9-diones as promising scaffolds for novel drug discovery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28124751/s1, including 1H, 13C NMR, and ORTEP spectra of [3+2] cycloaddition products naphtho[2,3-b]furan-4,9-diones (3) and dihydronaphtho[2,3-b]furan-4,9-diones (5).
Author Contributions
Conceptualization, H.T. and B.W.; methodology, Z.Q., Y.Y., X.Z., Y.X. and J.H.; software, H.T.; validation, Z.X., D.T. and Z.C.; formal analysis, H.T.; investigation, Z.Q., Y.Y., X.Z., Y.X. and J.H.; resources, Z.X., D.T. and Z.C.; data curation, H.T.; writing—original draft preparation, H.T.; writing—review and editing, H.T.; visualization, H.T.; supervision, Z.X., D.T. and Z.C.; project administration, Z.X., D.T. and Z.C.; funding acquisition, H.T., Z.X., D.T. and Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Research Program of Chongqing Municipal Education Commission (No. KJQN202201326) and the Science and Technology Research Program of Chongqing University of Arts and Sciences (No. P2020XY10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained in the article tables and Supplementary Materials.
Acknowledgments
We sincerely thank Jia Xu for her valuable assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of experimental data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of compounds 3 and 5 are available from the authors.
References
- Molfetta, F.A.; Bruni, A.T.; Honório, K.M.; da Silva, A.B.F. A structure–activity relationship study of quinone compounds with trypanocidal activity. Eur. J. Med. Chem. 2005, 40, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, R.; Ninomiya, M.; Tanaka, K.; Watanabe, K.; Koketsu, M. Synthesis and Cytotoxicity on Human Leukemia Cells of Furonaphthoquinones Isolated from Tabebuia Plants. Chem. Pharm. Bull. 2013, 61, 670–673. [Google Scholar] [CrossRef]
- Desmond, J.C.; Kawabata, H.; Mueller-Tidow, C.; Simamura, E.; Heber, D.; Hirai, K.-I.; Phillip Koeffler, H. The synthetic furanonaphthoquinone induces growth arrest, apoptosis and differentiation in a variety of leukaemias and multiple myeloma cells. Br. J. Haematol. 2005, 131, 520–529. [Google Scholar] [CrossRef]
- Müller, K.; Sellmer, A.; Wiegrebe, W. Potential Antipsoriatic Agents: Lapacho Compounds as Potent Inhibitors of HaCaT Cell Growth. J. Nat. Prod. 1999, 62, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Kaneko, M.; Tokuda, H.; Nishimura, K.; Kumeda, Y.; Iida, A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg. Med. Chem. 2009, 17, 6286–6291. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Yang, X.; Gao, Q.; Qu, Y.; Wu, L. Design, synthesis, and biological evaluation of novel napabucasin-melatonin hybrids as potent STAT3 inhibitors. Bioorganic Chem. 2023, 136, 106541. [Google Scholar] [CrossRef]
- Fodor, E.; Maftei, C.-V.; Freytag, M.; Franz, M.; Bannenberg, T.; Neda, I. Clarification of stereochemistry aspects for N-hydroxy-5-norbornene-2,3-dicarboximide derivatives and elucidation of them by experimental and theoretical investigations, including the synthesis of N, N'-bis-(5-exo-norbornene-2,3-dicarboxyimidyl) carbonate. Rev. Roum. Chim. 2018, 63, 245–255. [Google Scholar]
- Neda, I.; Sakhaii, P.; Waßmann, A.; Niemeyer, U.; Günther, E.; Engel, J. A Practical Synthesis of Benzyl α- and Allyl β-d-Glucopyranosides Regioselectively Substituted with (CH2)3OH Groups: Stereocontrolled β-Galactosidation by Cation π-Interaction. Synthesis 1999, 1999, 1625–1632. [Google Scholar] [CrossRef]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72, 1185–1199. [Google Scholar] [CrossRef]
- Gach, K.; Modranka, J.; Szymański, J.; Pomorska, D.; Krajewska, U.; Mirowski, M.; Janecki, T.; Janecka, A. Anticancer properties of new synthetic hybrid molecules combining naphtho[2,3-b]furan-4,9-dione or benzo[f]indole-4,9-dione motif with phosphonate subunit. Eur. J. Med. Chem. 2016, 120, 51–63. [Google Scholar] [CrossRef]
- Ogawa, M.; Koyanagi, J.; Sugaya, A.; Tsuda, T.; Ohguchi, H.; Nakayama, K.; Yamamoto, K.; Tanaka, A. Cytotoxic Activity toward KB Cells of 2-Substituted Naphtho[2,3-b]furan-4,9-diones and Their Related Compounds. Biosci. Biotechnol. Biochem. 2006, 70, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Takegami, T.; Simamura, E.; Hirai, K.; Koyama, J. Inhibitory effect of furanonaphthoquinone derivatives on the replication of Japanese encephalitis virus. Antiviral. Res. 1998, 37, 37–45. [Google Scholar] [CrossRef]
- Heltzel, C.E.; Gunatilaka, A.A.L.; Glass, T.E.; Kingston, D.G.I.; Hoffmann, G.; Johnson, R.K. Bioactive Furanonaphthoquinones from Crescentia cujete. J. Nat. Prod. 1993, 56, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, A.; Vortherms, S.; Bannwitz, S.; Tentrop, J.; Prinz, H.; Müller, K. Synthesis and Structure–Activity Relationships of Lapacho Analogues. 1. Suppression of Human Keratinocyte Hyperproliferation by 2-Substituted Naphtho[2,3-b]furan-4,9-diones, Activation by Enzymatic One- and Two-Electron Reduction, and Intracellular Generation of Superoxide. J. Med. Chem. 2012, 55, 7273–7284. [Google Scholar]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Jang, Y.-J.; Lee, C.-J.; Lee, Y.-T.; Lin, W. A versatile and practical method for regioselective synthesis of polysubstituted furanonaphthoquinones. Org. Biomol. Chem. 2013, 11, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tanaka, K.; Uneda, T.; Maeda, K.; Morikawa, O.; Konishi, H. A Direct One-Pot Preparation of Naphtho[2,3-b]furan-4,9-diones from 2-Hydroxy-1,4-naphthoquinones and Enamines. Synthesis 1998, 1998, 1243–1245. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, B.S.; Kim, D.H. Ceric Ammonium Nitrate (CAN)-Mediated Oxidative Cycloaddition of 1,3-Dicarbonyls to Conjugated Compounds. Efficient Synthesis of Dihydrofurans, Dihydrofurocoumarins, Dihydrofuroquinolinones, Dihydrofurophenalenones, and Furonaphthoquinone Natural Products. Tetrahedron 2000, 56, 8845–8853. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, M.; Zhang, C.; Yuan, Y.; Liu, R. Naphtho[2,3-b]furan-4,9-dione synthesis via palladium-catalyzed reverse hydrogenolysis. Chem. Commun. 2019, 55, 2348–2351. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Xie, K.; Zhou, D.; Peng, J.; Fan, A.; Zhang, J.; Chen, C. Transition-Metal-Free One-Pot Synthesis of Naphthoquinonefuran Derivatives Through Sequential Nucleophilic Substitution–Nucleophilic Addition Reaction. J. Org. Chem. 2020, 85, 9313–9320. [Google Scholar] [CrossRef]
- Sun, P.; Yang, J.; Peng, J.; Mo, B.; Chen, X.; Li, X.; Chen, C. Palladium(II)-Catalyzed Oxidative Annulation of 2-Hydroxynaphthalene-1,4-diones and Internal Alkynes via C–H Functionalization. J. Org. Chem. 2020, 85, 6761–6769. [Google Scholar] [CrossRef] [PubMed]
- Perry, P.J.; Pavlidis, V.H.; Hadfield, J.A. Synthesis of cytotoxic furonaphthoquinones: Regiospecific synthesis of diodantunezone and 2-ethylfuronaphthoquinones. Tetrahedron 1997, 53, 3195–3204. [Google Scholar] [CrossRef]
- Kang, W.-B.; Sekiya, T.; Toru, T.; Ueno, Y. A new route to naphtho[2,3-b]furan-4,9-diones from thio-substituted 1,4-naphthoquinones. J. Chem. Soc. Perkin Trans. 1990, 1, 441–445. [Google Scholar] [CrossRef]
- Tan, H.-B.; Yu, Y.-H.; Qi, Z.-H.; Zhang, X.; Xu, Z.-G.; Tang, D.-Y.; Chen, Z.-Z.; Wang, B.-C.; Qu, X.-Y. Photochemical diastereoselective synthesis and spectral characterization of (E)-3-(2-benzoylstyryl)-4H-chromen-4-ones. J. Mol. Struct. 2023, 1283, 135308. [Google Scholar] [CrossRef]
- Tan, H.; Wang, Y. Facile Synthesis of Novel Hexahydroimidazo[1,2-a]pyridine Derivatives by One-pot, Multi-component Reaction under Ambient Conditions. ACS Comb. Sci. 2020, 22, 468–474. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Tang, Y.; Qi, Z.-H.; Yu, Y.-H.; Tan, H.-B.; Tang, D.-Y.; Xu, Z.-G.; Chen, Z.-Z. A four-component domino reaction for the synthesis of novel bridgehead nitrogen-containing pyrido[1,2-d][1,4]diazepines. New J. Chem. 2022, 46, 592–598. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, Z.-S.; Wang, R.; Zhang, W.; Qi, Z.-H.; Yu, Y.-H.; Tan, H.-B. Diastereoselective synthesis and spectral characterization of trans-4, 4a-dihydro-3H-benzo [4, 5] oxazolo [3, 2-a] pyridines. J. Mol. Struct. 2022, 1251, 131935. [Google Scholar] [CrossRef]
- Kobayashi, K.; Shimizu, H.; Sasaki, A.; Suginome, H. Photoinduced molecular transformations. 140. New one-step general synthesis of naphtho[2,3-b]furan-4,9-diones and their 2,3-dihydro derivatives by the regioselective [3+2] photoaddition of 2-hydroxy-1,4-naphthoquinones with various alkynes and alkenes: Application of the photoaddition to a two-step synthesis of maturinone. J. Org. Chem. 1993, 58, 4614–4618. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).