Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products
Abstract
:1. Introduction
2. Results
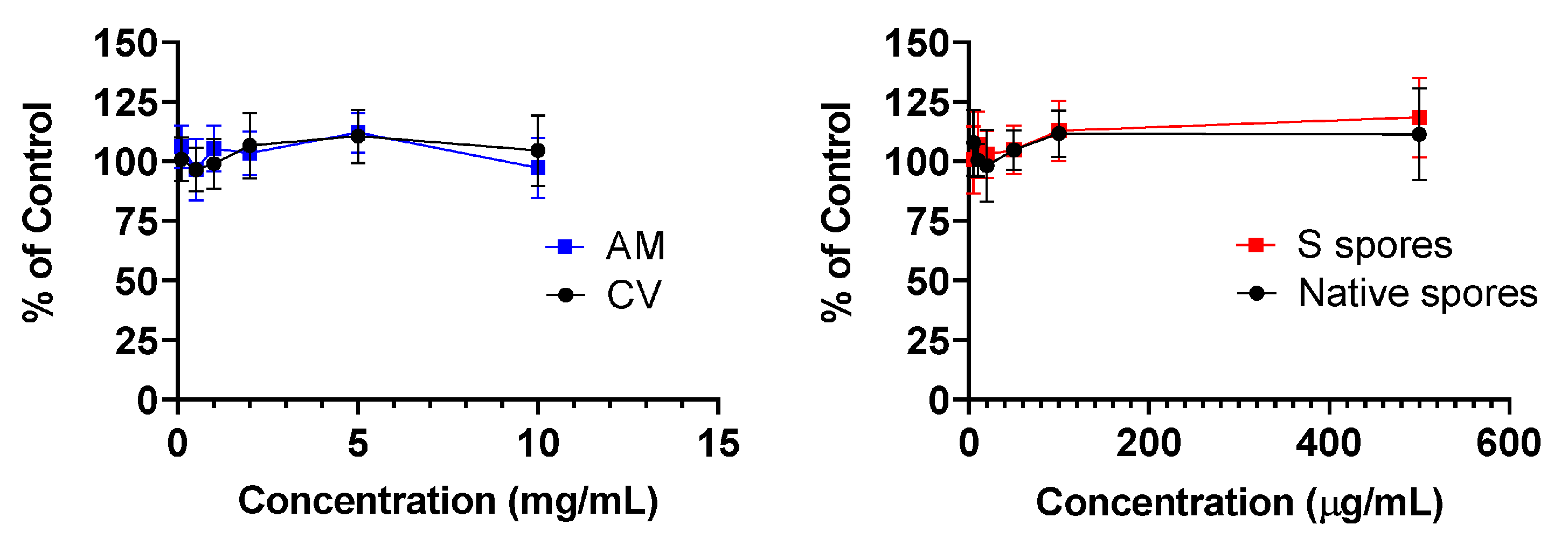
2.1. MTT Cytotoxicity Test
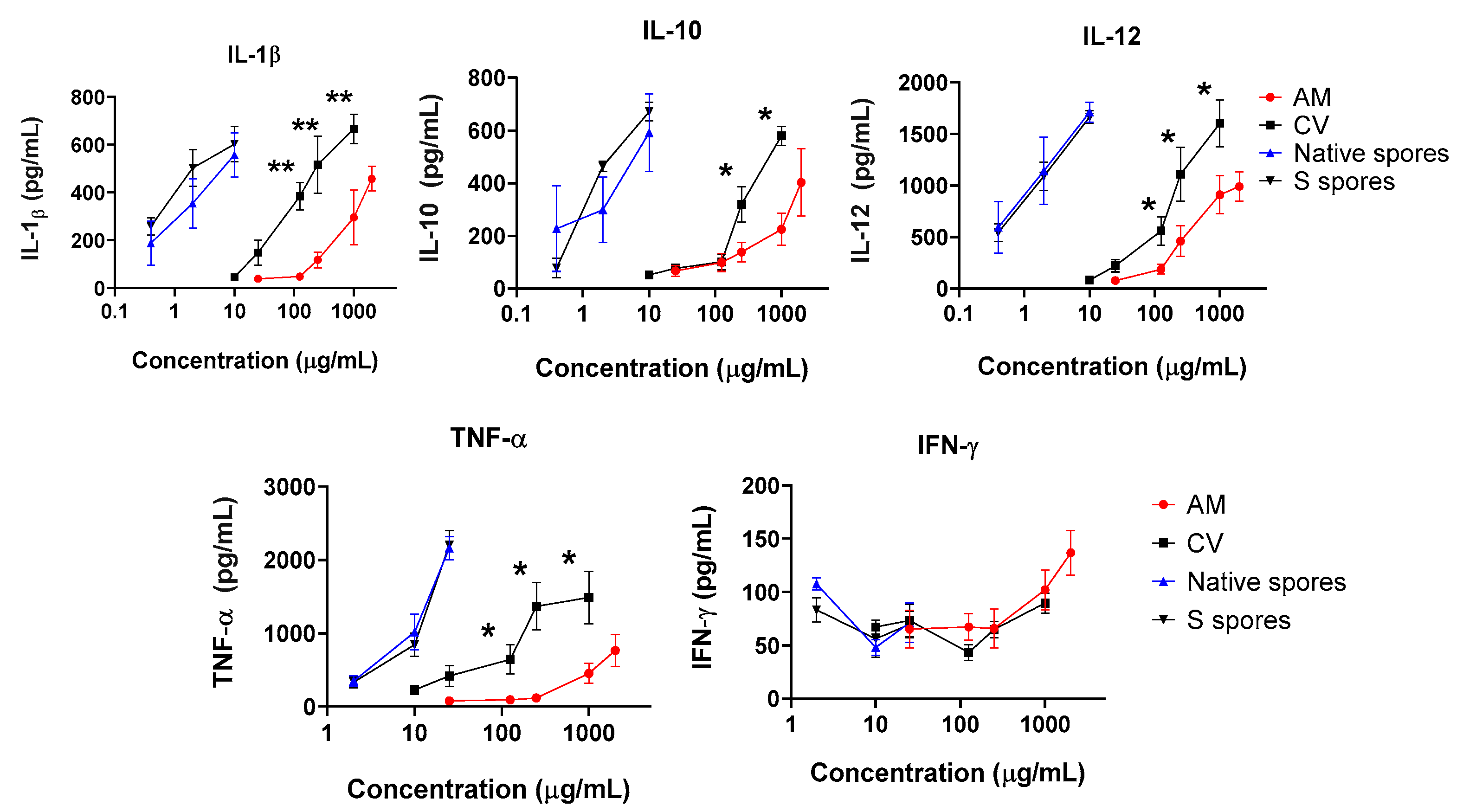
2.2. Co-Culture of Human Monocyte-Derived Macrophages with Intestine HT-29 Cells and Dendritic Cells (DC)
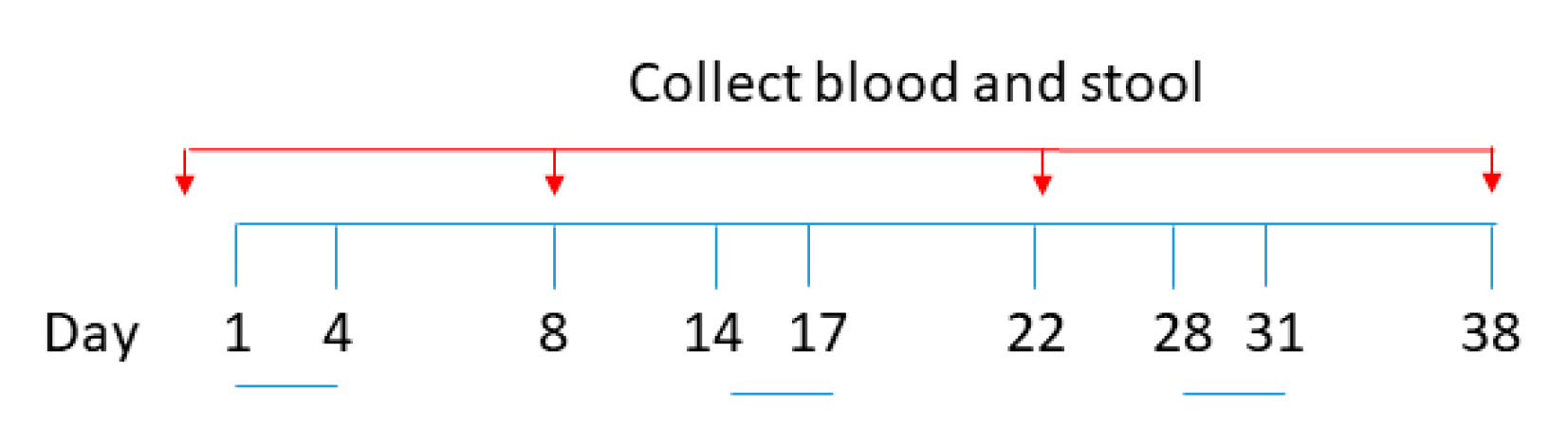
2.3. Oral Vaccination of B. subtilis Spores, AM, and CV
2.4. Toxicity Studies
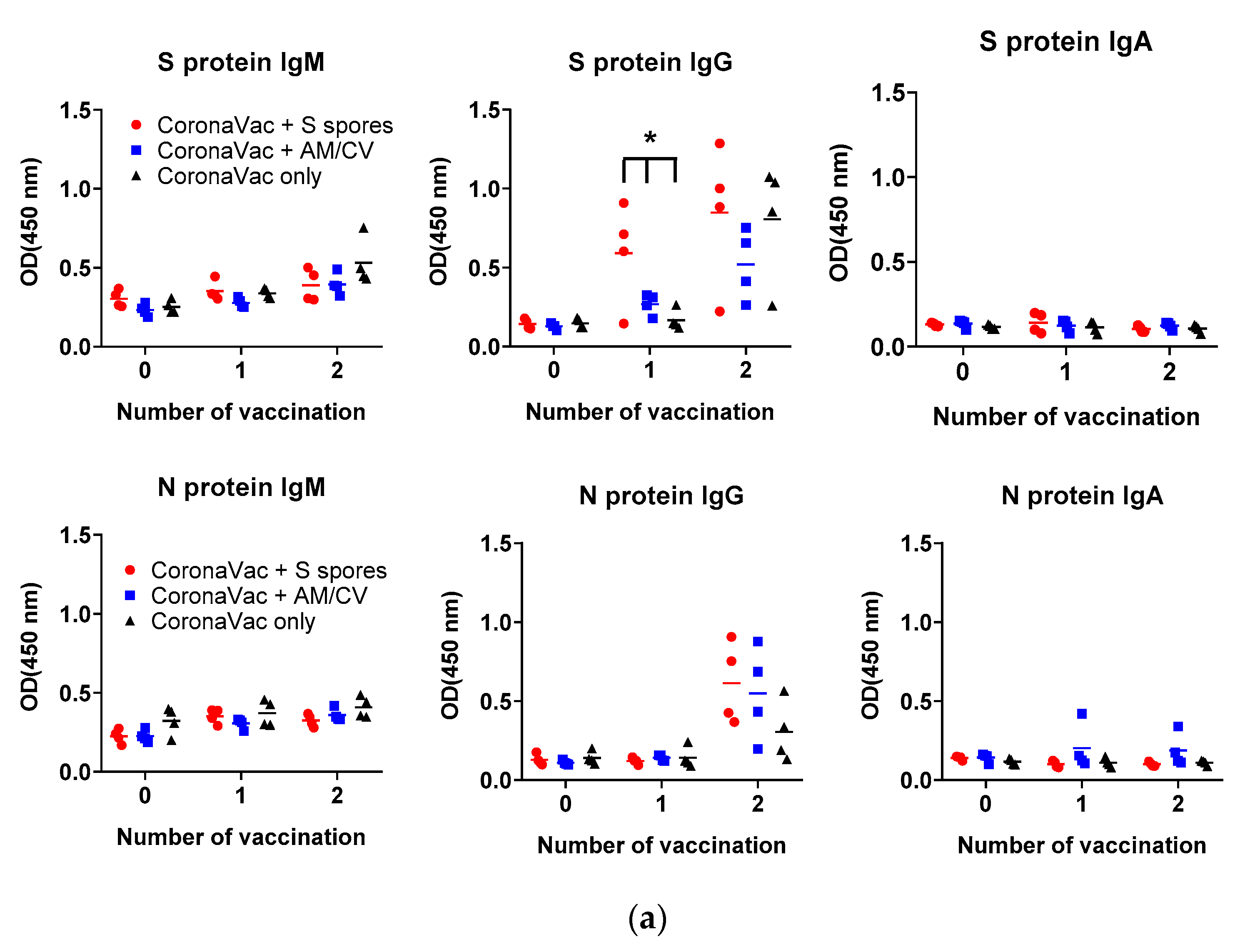
2.5. Adjuvant Effects of B. subtilis Spores, AM/CV on CoronaVac Vaccine
3. Discussion
4. Materials and Methods
4.1. Preparation of B. subtilis Spores (S Spores) Expressing the Ancestral SARS-CoV-2 RBD of S Protein
4.2. Preparation of the Extract of AM and CV
4.3. 2.3 3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) Assay
4.4. Co-Culture of Human Monocyte Derived Macrophages with Intestinal HT-29 Cells and Monocyte Derived Dendritic Cells (DC) Culture
4.5. Immunization Regimens
4.6. Adjuvant Effects of B. subtilis Spores, AM, and CV on CoronaVac Vaccine
4.7. Neutralization Assay
4.8. Statistical Analyses
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A


References
- Callaway, E. COVID ‘variant soup’ is making winter surges hard to predict. Nature 2022, 611, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.; Evans, J.P.; Zou, X.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Lozanski, G.; Mallampalli, R.K.; Saif, L.J.; et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 Subvariants. N. Engl. J. Med. 2022, 386, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Fonager, J.; Bennedbaek, M.; Bager, P.; Wohlfahrt, J.; Ellegaard, K.M.; Ingham, A.C.; Edslev, S.M.; Stegger, M.; Sieber, R.N.; Lassauniere, R.; et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Eurosurveillance 2022, 27, 2200181. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.G.; Cerovic, V.; Hobson, P.S.; Klavinskis, L.S. Bacillus subtilis spores: A novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur. J. Immunol. 2007, 37, 1538–1547. [Google Scholar] [CrossRef]
- de Souza, R.D.; Batista, M.T.; Luiz, W.B.; Cavalcante, R.C.M.; Amorim, J.H.; Bizerra, R.S.P.; Martins, E.G.; Ferreira, L.C.D. Bacillus subtilis Spores as Vaccine Adjuvants: Further Insights into the Mechanisms of Action. PLoS ONE 2014, 9, e87454. [Google Scholar] [CrossRef]
- Tavares Batista, M.; Souza, R.D.; Paccez, J.D.; Luiz, W.B.; Ferreira, E.L.; Cavalcante, R.C.; Ferreira, R.C.; Ferreira, L.C. Gut adhesive Bacillus subtilis spores as a platform for mucosal delivery of antigens. Infect. Immun. 2014, 82, 1414–1423. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.C.; Lai, N.C.; Wu, K.C.; Choi, M.C.; Ma, C.H.; Lin, J.; Kuok, C.N.; Leong, W.L.; Lam, W.K.; Hamied, Y.K.; et al. Safety and Immunogenicity of Inactivated Bacillus subtilis Spores as a Heterologous Antibody Booster for COVID-19 Vaccines. Vaccines 2022, 10, 1014. [Google Scholar] [CrossRef]
- Vetrakova, A.; Chovanova, R.K.; Rechtorikova, R.; Krajcikova, D.; Barak, I. Bacillus subtilis spores displaying RBD domain of SARS-CoV-2 spike protein. Comput. Struct. Biotechnol. J. 2023, 21, 1550–1556. [Google Scholar] [CrossRef]
- Hong, F.; Xiao, W.; Ragupathi, G.; Lau, C.B.; Leung, P.C.; Yeung, K.S.; George, C.; Cassileth, B.; Kennelly, E.; Livingston, P.O. The known immunologically active components of Astragalus account for only a small proportion of the immunological adjuvant activity when combined with conjugate vaccines. Planta Med. 2011, 77, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Ragupathi, G.; Yeung, K.S.; Leung, P.C.; Lee, M.; Lau, C.B.; Vickers, A.; Hood, C.; Deng, G.; Cheung, N.K.; Cassileth, B.; et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine 2008, 26, 4860–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliza, W.L.Y.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Chan, B.C.L.; Yu, H.; Lau, I.Y.K.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, C.B.S.; Xie, M.Y.; Fung, K.P.; et al. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [PubMed]
- Han, X.Q.; Chung Lap Chan, B.; Dong, C.X.; Yang, Y.H.; Ko, C.H.; Gar-Lee Yue, G.; Chen, D.; Wong, C.K.; Bik-San Lau, C.; Tu, P.F.; et al. Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of Ganoderma sinense. J. Agric. Food Chem. 2012, 60, 4276–4281. [Google Scholar] [CrossRef]
- Han, X.Q.; Chan, B.C.; Yu, H.; Yang, Y.H.; Hu, S.Q.; Ko, C.H.; Dong, C.X.; Wong, C.K.; Shaw, P.C.; Fung, K.P.; et al. Structural characterization and immuno-modulating activities of a polysaccharide from Ganoderma sinense. Int. J. Biol. Macromol. 2012, 51, 597–603. [Google Scholar] [CrossRef]
- Han, X.Q.; Yue, G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K.; et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE 2014, 9, e100380. [Google Scholar]
- Topfer, E.; Boraschi, D.; Italiani, P. Innate Immune Memory: The Latest Frontier of Adjuvanticity. J. Immunol. Res. 2015, 2015, 478408. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Dong, H.; Huang, Y.; Yao, S.; Liang, B.; Xie, Y.; Long, Y.; Mai, J.; Gong, S. Recombinant Bacillus subtilis spores expressing cholera toxin B subunit and Helicobacter pylori urease B confer protection against H. pylori in mice. J. Med. Microbiol. 2017, 66, 83–89. [Google Scholar] [CrossRef]
- Potocki, W.; Negri, A.; Peszynska-Sularz, G.; Hinc, K.; Obuchowski, M.; Iwanicki, A. IL-1 Fragment Modulates Immune Response Elicited by Recombinant Bacillus subtilis Spores Presenting an Antigen/Adjuvant Chimeric Protein. Mol. Biotechnol. 2018, 60, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Knoll, R.; Schultze, J.L.; Schulte-Schrepping, J. Monocytes and Macrophages in COVID-19. Front. Immunol. 2021, 12, 720109. [Google Scholar]
- van der Meer, J.W.M.; Joosten, L.A.B.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar]
- Gyssens, I.C.; Netea, M.G. Heterologous effects of vaccination and trained immunity. Clin. Microbiol. Infect. 2019, 25, 1457–1458. [Google Scholar] [PubMed] [Green Version]
- Jensen, J.; Warner, T.; Balish, E. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: Role of polymorphonuclear leukocytes and macrophages. J. Infect. Dis. 1993, 167, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334. [Google Scholar]
- Perez-Hernandez, C.A.; Kern, C.C.; Butkeviciute, E.; McCarthy, E.; Dockrell, H.M.; Moreno-Altamirano, M.M.B.; Aguilar-Lopez, B.A.; Bhosale, G.; Wang, H.; Gems, D.; et al. Mitochondrial Signature in Human Monocytes and Resistance to Infection in C. elegans During Fumarate-Induced Innate Immune Training. Front. Immunol. 2020, 11, 1715. [Google Scholar] [CrossRef]
- Pellon, A.; Nasab, S.D.S.; Moyes, D.L. New Insights in Candida albicans Innate Immunity at the Mucosa: Toxins, Epithelium, Metabolism, and Beyond. Front. Cell. Infect. Microbiol. 2020, 10, 81. [Google Scholar]
- Wang, X.; Guan, F.; Miller, H.; Byazrova, M.G.; Cndotti, F.; Benlagha, K.; Camara, N.O.S.; Lei, J.; Filatov, A.; Liu, C. The role of dendritic cells in COVID-19 infection. Emerg. Microbes Infect. 2023, 12, 2195019. [Google Scholar] [CrossRef]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [PubMed] [Green Version]
- Mou, C.; Zhu, L.; Xing, X.; Lin, J.; Yang, Q. Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antivir. Res. 2016, 131, 74–84. [Google Scholar]
- Hu, B.; Li, C.; Lu, H.; Zhu, Z.; Du, S.; Ye, M.; Tan, L.; Ren, D.; Han, J.; Kan, S.; et al. Immune responses to the oral administration of recombinant Bacillus subtilis expressing multi-epitopes of foot-and-mouth disease virus and a cholera toxin B subunit. J. Virol. Methods 2011, 171, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, Y.; Hu, L.P.; Kai, W.; Zhu, R. Immunization of mice against alpha, beta, and epsilon toxins of Clostridium perfringens using recombinant rCpa-b-x expressed by Bacillus subtilis. Mol. Immunol. 2020, 123, 88–96. [Google Scholar]
- Jiang, B.; Li, Z.; Ou, B.; Duan, Q.; Zhu, G. Targeting ideal oral vaccine vectors based on probiotics: A systematical view. Appl. Microbiol. Biotechnol. 2019, 103, 3941–3953. [Google Scholar] [PubMed]
- Lei, Z.; Zhu, L.; Pan, P.; Ruan, Z.; Gu, Y.; Xia, X.; Wang, S.; Ge, W.; Yao, Y.; Luo, F.; et al. A vaccine delivery system promotes strong immune responses against SARS-CoV-2 variants. J. Med. Virol. 2023, 95, e28475. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Z.J.; Zhang, Y.K.; Sun, H.J. Mechanism and potential treatments for gastrointestinal dysfunction in patients with COVID-19. World J. Gastroenterol. 2022, 28, 6811–6826. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [PubMed]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10, 564. [Google Scholar] [CrossRef]
- Alam, S.; Sadiqi, S.; Sabir, M.; Nisa, S.; Ahmad, S.; Abbasi, S.W. Bacillus species; a potential source of anti-SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 5748–5758. [Google Scholar]
- Commercial Press. Chinese Pharmacopoeia; Commercial Press: Shanghai, China, 2015. [Google Scholar]
- Tani, T.; Shimizu, T.; Tani, M.; Shoji, H.; Endo, Y. Anti-endotoxin Properties of Polymyxin B-immobilized Fibers. Adv. Exp. Med. Biol. 2019, 1145, 321–341. [Google Scholar]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.K.; Wang, H.; et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine 2022, 77, 103904. [Google Scholar] [PubMed]
- Ribeiro, L.A.; Azevedo, V.; Le Loir, Y.; Oliveira, S.C.; Dieye, Y.; Piard, J.C.; Gruss, A.; Langella, P. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: A first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 2002, 68, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Sun, C.; Ma, J.; Yu, C.; Kong, D.; Chen, M.; Liu, X.; Zhao, D.; Gao, S.; Kou, S.; et al. A Bivalent COVID-19 Vaccine Based on Alpha and Beta Variants Elicits Potent and Broad Immune Responses in Mice against SARS-CoV-2 Variants. Vaccines 2022, 10, 702. [Google Scholar] [CrossRef]
- Castro, J.T.; Azevedo, P.; Fumagalli, M.J.; Hojo-Souza, N.S.; Salazar, N.; Almeida, G.G.; Oliveira, L.I.; Faustino, L.; Antonelli, L.R.; Marcal, T.G.; et al. Promotion of neutralizing antibody-independent immunity to wild-type and SARS-CoV-2 variants of concern using an RBD-Nucleocapsid fusion protein. Nat. Commun. 2022, 13, 4831. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, B.C.-L.; Li, P.; Tsang, M.S.-M.; Sung, J.C.-C.; Kwong, K.W.-Y.; Zheng, T.; Hon, S.S.-M.; Lau, C.-P.; Cheng, W.; Chen, F.; et al. Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products. Molecules 2023, 28, 4996. https://doi.org/10.3390/molecules28134996
Chan BC-L, Li P, Tsang MS-M, Sung JC-C, Kwong KW-Y, Zheng T, Hon SS-M, Lau C-P, Cheng W, Chen F, et al. Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products. Molecules. 2023; 28(13):4996. https://doi.org/10.3390/molecules28134996
Chicago/Turabian StyleChan, Ben Chung-Lap, Peiting Li, Miranda Sin-Man Tsang, Johnny Chun-Chau Sung, Keith Wai-Yeung Kwong, Tao Zheng, Sharon Sze-Man Hon, Ching-Po Lau, Wen Cheng, Fang Chen, and et al. 2023. "Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products" Molecules 28, no. 13: 4996. https://doi.org/10.3390/molecules28134996
APA StyleChan, B. C. -L., Li, P., Tsang, M. S. -M., Sung, J. C. -C., Kwong, K. W. -Y., Zheng, T., Hon, S. S. -M., Lau, C. -P., Cheng, W., Chen, F., Lau, C. B. -S., Leung, P. -C., & Wong, C. -K. (2023). Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products. Molecules, 28(13), 4996. https://doi.org/10.3390/molecules28134996