A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications
Abstract
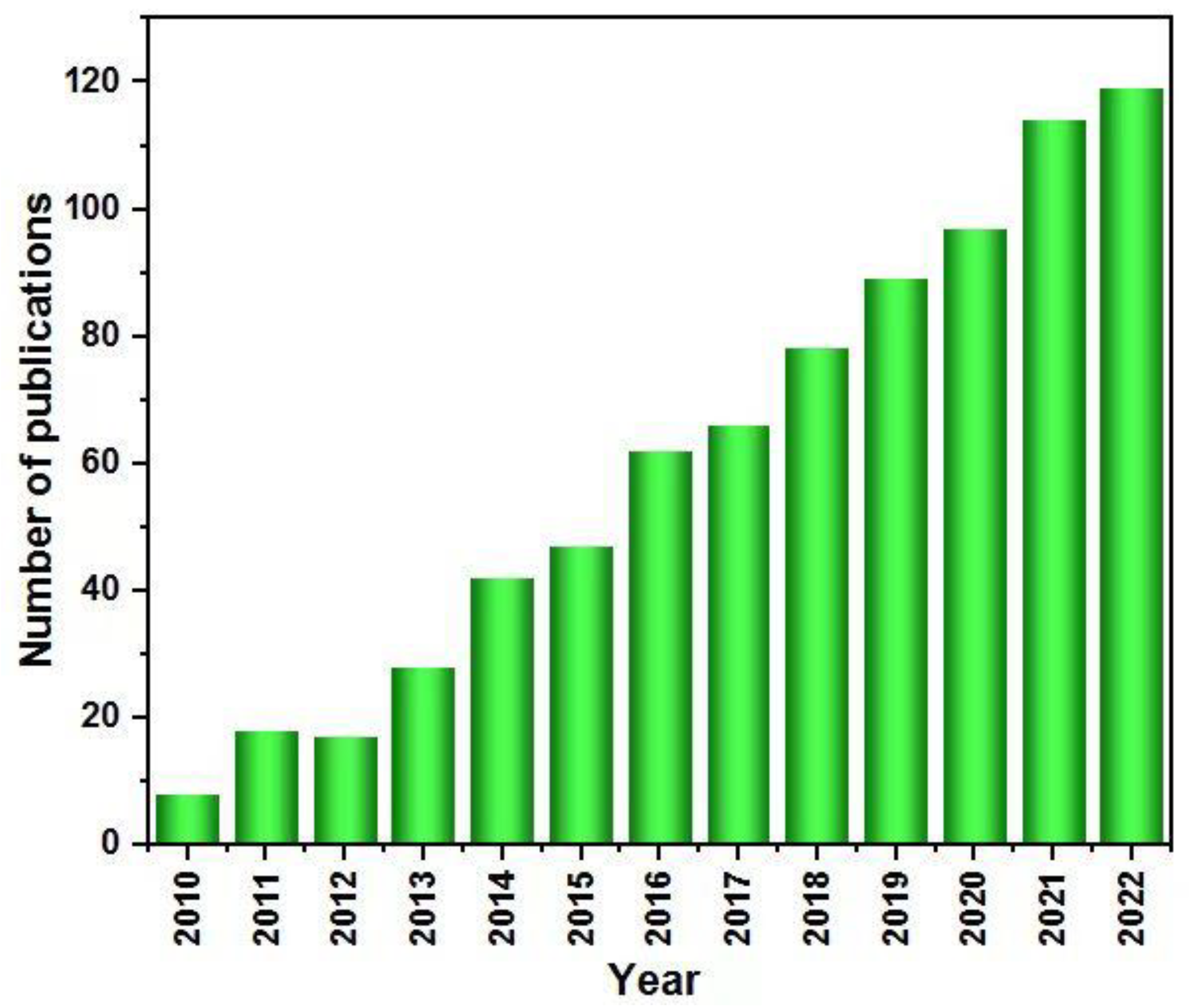
1. Introduction
2. Synthetic Methods
2.1. Preparation Methods
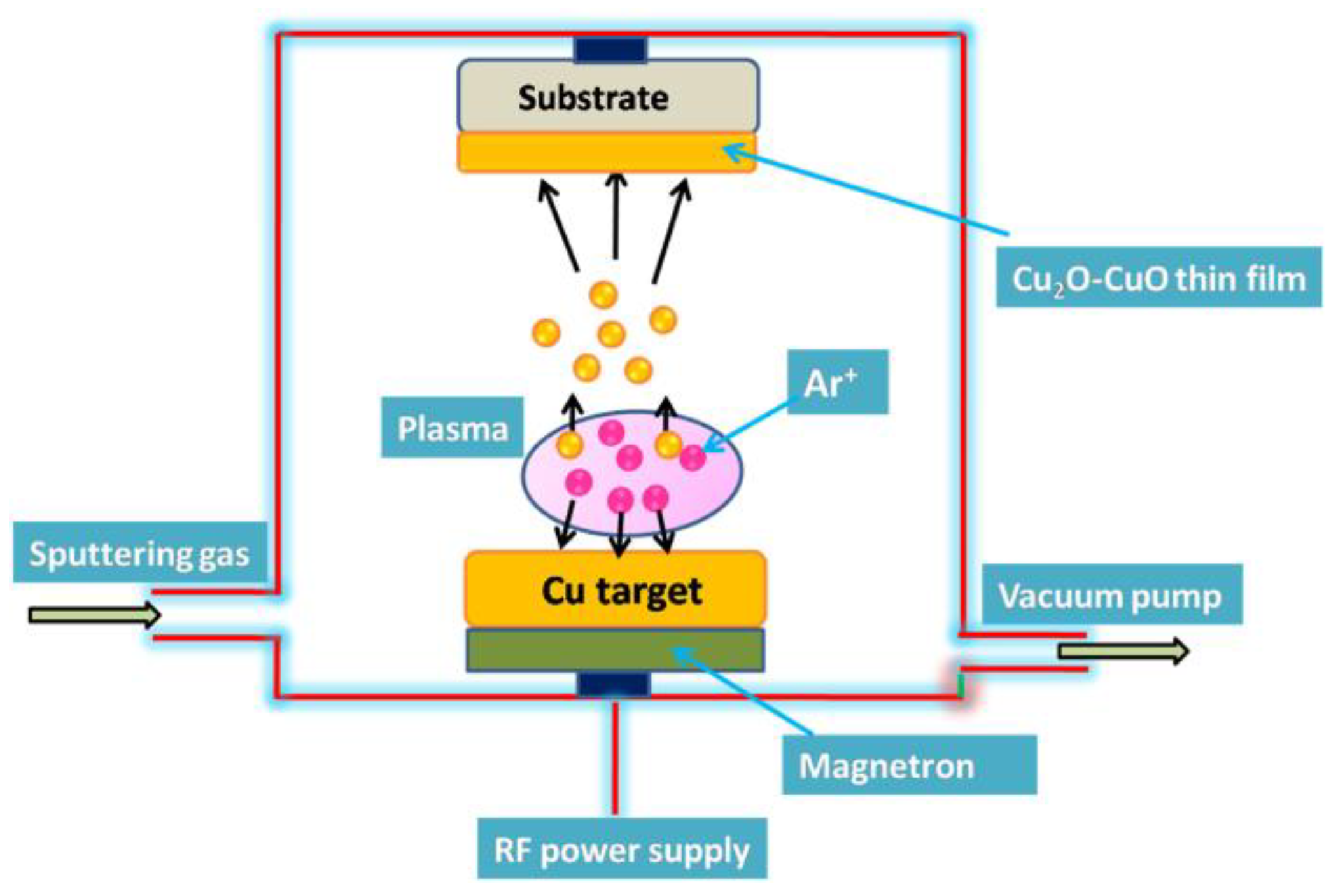
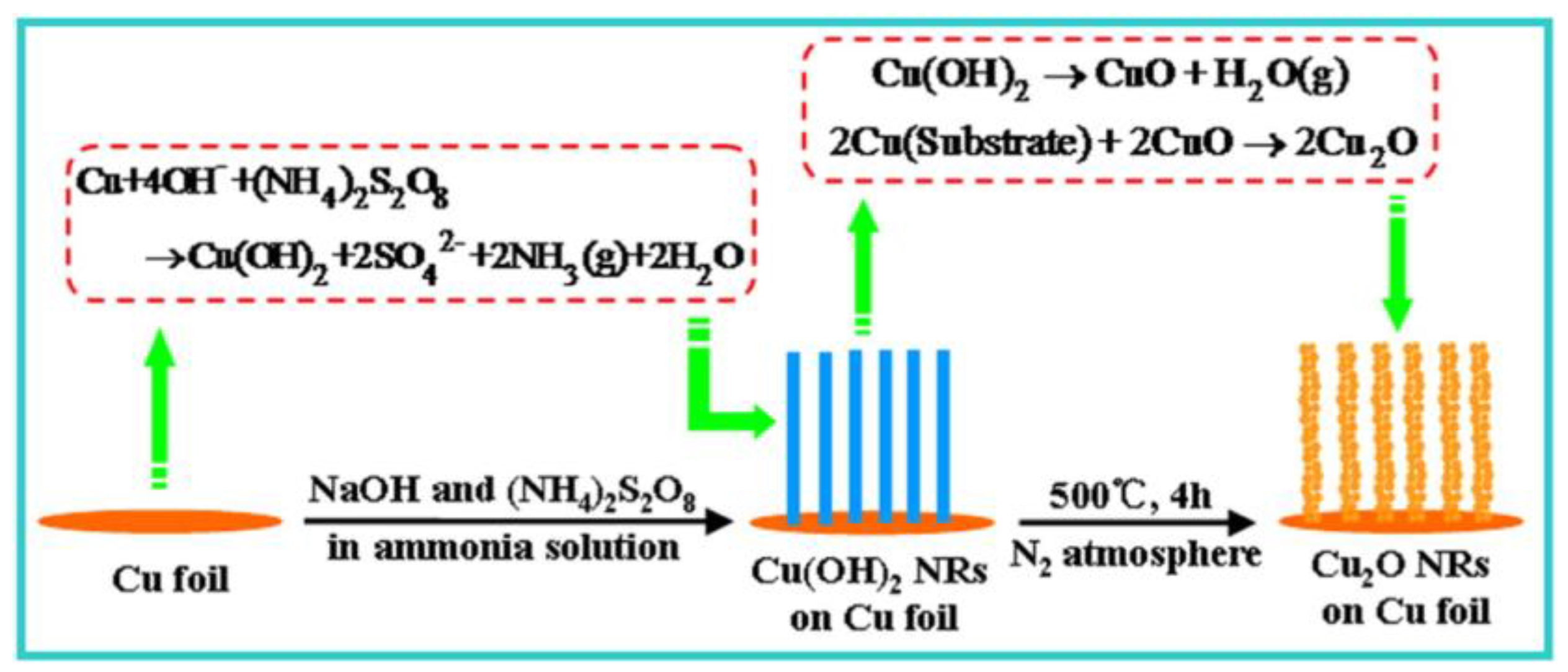
2.2. Other Methods
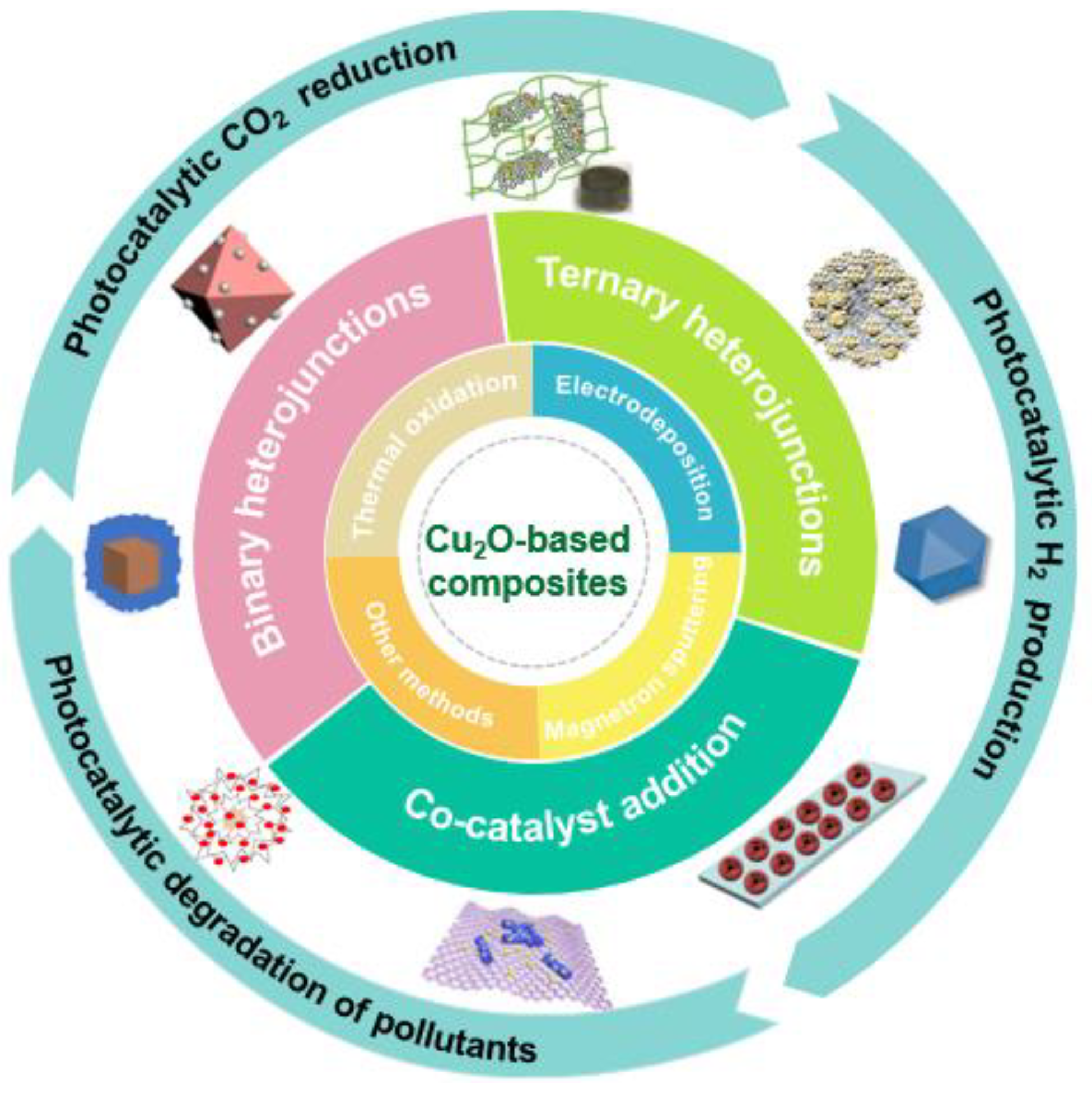
3. Modification Strategies
3.1. Binary Cu2O-Based Heterojunctions
3.1.1. Cu2O/Noble Metal Heterojunction
3.1.2. Cu2O/Graphene (GO) Heterojunction
3.2. Ternary Cu2O-Based Heterojunctions
3.3. Co-Catalyst Addition
4. Photocatalytic Applications
4.1. Photocatalytic CO2 Reduction
4.2. Photocatalytic H2 Production
4.3. Photocatalytic Degradation of Pollutants
| Photocatalyst | Synthesis Method | Morphology and Structure | Size | Bandgap (Eg) | Light Resource | Targe Pollutant/Concentration/Volume | Efficiency | Cycle | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Ag-Cu2O | Electrochemical deposition and redox reaction | Composite film | — | 2.02 eV | 500 W halogen lamp | MB/30 mg∙L−1/50 mL | 92% | 3 | [119] |
| Cr-doped Cu2O | Hydrothermal method | Octahedrons | 800–1200 nm | 2.06 eV | 500 W tungsten halogen lamp (400–1100 nm) | LVX/40 mg∙L−1/50 mL | 79.6–72.4% | 1–8 | [120] |
| BiOCl/Cu2O | Solvothermal method | Spherical shape | 3–5 μm | 2.00 eV | 500 W Xenon lamp | Moxifloxacin/20 mg∙L−1/50 mL | 72.3% | 5 | [121] |
| C-dots/Cu2O/SrTiO3 | Hydrothermal and two-step method | Chocolate ball with sesame on the surface | ~2.16 μm | EgSrTiO3: 3.19 eV; EgCu2O: 2.10 eV | 500 W Xenon lamp (λ > 420 nm) | CTC.HCl/15 mg∙L−1/50 mL | 92.6% | 4 | [122] |
| CuO-Cu2O | Chemical–thermal oxidation | Nanorods | 60 nm | 1.90 eV | 150 W metal halide lamp (λ > 400 nm) | MB/5 mg∙L−1/50 mL | 80% | 3 | [123] |
| Cotton fabrics/Cu2O-NC | Impregnation and HH reduction | Octahedron Cu2O attached to cotton fibers | 20–40 nm of diameter of Cu2O | EgCu2O: 2.20 eV | 350 W Xenon lamp (λ > 400 nm) | MB/200 ppm/200 mL | 98.32–85% | 1–5 | [124] |
| Cu2O@HKUST-1 | In-situ converted strategy | Octahedron structure | — | EgCu2O: 1.95 eV; EgHKUST-1: 2.59 eV | Tungsten lamp (>420 nm, 500 W) | TC-HCl/20 mg∙L−1/100 mL | 93.40–90.02% | 1–4 | [125] |
| Fe3O4/Cu2O-Ag | Solvothermal and liquid deposition methods | Double six peak structure | ~5 nm | 2.23 eV | — | PAHs/5 mg∙L−1/100 mL | 95–90% | 1–8 | [126] |
| Cu2O/ZnO@PET | Electroless template deposition | Rectangular-shaped | ~13 ± 4.5 nm | 3.2–3.4 eV | Ultra-Vitalux 300W | Czm/1.0 mg∙L−1/100 mL | 98–26% | 1–6 | [127] |
| Cu2O-Au-TiO2 | Two-step photocatalytic deposition | Core–shell structure | ~50 nm | 1.4–1.7 eV | Xenon lamp (λ > 422 nm) | Cr(Ⅵ)/10 mg∙L−1/50 mL | 100% (3h) | 3 | [128] |
| Cu2O/N-CQD/ZIF-8 | Reduction precipitation | Spherical structure | ~80–100 nm | 2.6 eV, | 300 W Xenon lamp (λ > 420 nm) | Cr(Ⅵ)/20 mg∙L−1/50 mL | 98.99–97.13% | 1–5 | [129] |
| Cu2O/rGO/BiOBr | Two-step strategy | Hierarchical microspheres | 500 nm–1 μm | EgBiOBr: 2.7 eV; EgCu2O: 1.9 eV | 300 W Xenon lamp (λ ≥ 420 nm) | Cr(Ⅵ)/20 mg∙L−1/50 mL | 100% (40 min) | 5 | [130] |
| Cu-TiO2-Cu2O | Photodeposition | The triple junction structure | ~20 nm | — | 300 W Xenon lamp (200–2400 nm) | 2,4,5-T/50 ppm/100 mL | 93% | 3 | [131] |
| Ag-Cu2O/rGO | Two-step reduction process | Spherical AgNPs deposited on the Cu2O situated on the surface of rGO sheets | ~60 nm | — | 60 W tungsten filament lamp (500–700 nm, 0.24 W/cm2) | MO/40 mg∙L−1/50 mL Phenol/20 mg∙L−1/50 mL | 90% (60min); Rate constant of phenol degradation: 0.09732 | 3 | [132] |
4.4. DFT Study Applied in the Photocatalysis
5. Conclusions
- Currently, most Cu2O-based composites and sacrificial agents are synthesized from noble metal materials, which have high costs and significantly limit their large-scale applications. The development of non-precious metal catalysts, such as graphene, is vital to future development. More importantly, the catalytic efficiency of most Cu2O-based composites is very low, and the catalytic performance needs to be improved to meet the requirements of practical applications.
- Although many experimental studies on the photocatalysis of Cu2O-based composites are introduced in this paper, these works are still in their infancy. In addition, the large-scale production of high-quality Cu2O-based photocatalysts faces numerous difficulties, considering the secondary hazards of nanomaterials. Therefore, it is urgent that we further study the photocatalytic mechanism of Cu2O-based composites from the above perspectives, and promote the industrial application process of Cu2O-based composite catalysts.
- The photocorrosion of Cu2O still deserves attention. Although the current method of constructing heterojunctions to suppress photocorrosion has achieved certain results, the photocorrosion phenomenon of Cu2O still exists, and affects its long-term use. Establishing a core–shell structure is a good governance measure but, when synthesizing photocatalysts, it is necessary to carefully handle the thickness of the shell layer, to ensure sufficient absorption of light by the Cu2O.
- The structure of the catalyst determines the catalytic activity, while the catalytic microstructure and mechanism of Cu2O-based composites is still unclear. Theoretical calculations should be introduced when designing a Cu2O-based photocatalyst, and studying the mechanism of improving photocatalytic activity at the molecular level. In future research, DFT simulations and experiments are needed, to reveal the relationship between the establishment of the microstructure and the catalytic activity of photocatalysts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ju, L.; Tang, X.; Zhang, Y.; Li, X.; Cui, X.; Yang, G. Single Selenium Atomic Vacancy Enabled Efficient Visible-Light-Response Photocatalytic NO Reduction to NH3 on Janus WSSe Monolayer. Molecules 2023, 28, 2959. [Google Scholar] [CrossRef]
- Duic, N.; Guzovic, Z.; Vyatcheslav, K.; Klemes, J.J.; Mathiessen, B.V.; Yan, J.Y. Sustainable development of energy, water and environment systems. Appl. Energ. 2013, 101, 3–5. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Li, J.; Dong, H.; Yang, S.; Gao, Y.; Liu, W. Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction. Molecules 2023, 28, 4602. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Wang, D.W. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; Garcia, H. Photocatalytic CO2 reduction to C2+ products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Ref. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photoch. Photobio. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ma, D.G.; Zhai, S.; Wang, Y.; Liu, A.A.; Chen, C.C. TiO2 photocatalysis for transfer hydrogenation. Molecules 2019, 24, 330. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X.M. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloy Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Liu, X.Q.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.H.; Zhao, S.Q.; Li, Z.; Lin, Z.Q. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Liu, Y.P.; Shen, S.J.; Zhang, J.T.; Zhong, W.W.; Huang, X.H. Cu2−xSe/CdS composite photocatalyst with enhanced visible light photocatalysis activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xiao, J.T.; Huang, X.S.; Mei, P.; Wang, H.H. Boosting photocatalytic efficiency of MoS2/CdS by modulating morphology. Environ. Sci. Pollut. Res. 2022, 29, 73282–73291. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.X.; Jin, H.M.; Wu, Z.K.; Guo, Y.N.; Shang, Q.K. Selective photocatalytic oxidation of aromatic alcohols using B-g-C3N4/Bi2WO6 composites. Sep. Purif. Technol. 2023, 317, 123915. [Google Scholar] [CrossRef]
- Qin, N.B.; Zhang, S.F.; He, J.Y.; Long, F.; Wang, L.L. In situ synthesis of BiVO4/BiOBr microsphere heterojunction with enhanced photocatalytic performance. J. Alloy Compd. 2022, 927, 166661. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Xiong, Z.G.; Wang, H.T.; Liao, G.D.; Bai, S.S.; Zou, J.; Wu, P.X.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Sekkat, A.; Bellet, D.; Chichignoud, G.; Munoz-Rojas, D.; Kaminski-Cachopo, A. Unveiling key limitations of ZnO/Cu2O all-oxide solar cells through numerical simulations. ACS Appl. Energy Mater. 2022, 5, 5423–5433. [Google Scholar] [CrossRef]
- Wu, L.K.; Ma, P.D.; Zhang, C.H.; Yi, X.K.; Hao, Q.L.; Dou, B.J.; Bin, F. Effects of Cu2O morphology on the performance of CO self-sustained catalytic combustion. Appl. Catal. A-Gen. 2023, 652, 119034. [Google Scholar] [CrossRef]
- Li, J.W.; Sun, Z.L.; He, M.Z.; Gao, D.; Li, Y.T.; Ma, J.J. Simple synthesis of Ag nanoparticles/Cu2O cube photocatalyst at room temperature: Efficient electron transfer improves photocatalytic performance. Inorg. Chem. Commun. 2022, 138, 109200. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.F.; Zhou, J.H. MOF-derived Cu@Cu2O heterogeneous electrocatalyst with moderate intermediates adsorption for highly selective reduction of CO2 to methanol. Chem. Eng. J. 2022, 431, 134171. [Google Scholar] [CrossRef]
- Wang, N.; Tao, W.; Gong, X.Q.; Zhao, L.P.; Wang, T.S.; Zhao, L.J.; Liu, F.M.; Liu, X.M.; Sun, P.; Lu, G.Y. Highly sensitive and selective NO2 gas sensor fabricated from Cu2O-CuO microflowers. Sens. Actuators B-Chem. 2022, 362, 131803. [Google Scholar] [CrossRef]
- Li, J.W.; He, M.Z.; Yan, J.K.; Liu, J.H.; Zhang, J.X.; Ma, J.J. Room temperature engineering crystal facet of Cu2O for photocatalytic degradation of methyl orange. Nanomaterials 2022, 12, 1697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Liu, M.M.; Chen, J.L.; Fang, S.M.; Zhou, P.P. Recent advances in Cu2O-based composites for photocatalysis: A review. Dalton Trans. 2021, 50, 4091–4111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Guo, R.T.; Zhang, Z.R.; Wu, T.; Pan, W.G. Converting CO2 into Value-Added Products by Cu2O-Based Catalysts: From Photocatalysis, Electrocatalysis to Photoelectrocatalysis. Small 2023, 19, 2207875. [Google Scholar] [CrossRef]
- Yu, W.B.; Yi, M.; Fu, H.H.; Pei, M.J.; Liu, Y.; Xu, B.M.; Zhang, J. Dandelion-Like Nanostructured Cu/Cu2O Heterojunctions with Fast Diffusion Channels Enabling Rapid Photocatalytic Pollutant Removal. ACS Appl. Nano Mater. 2023, 6, 2928–2941. [Google Scholar] [CrossRef]
- Stareck, J.E. Decorating Metals. U.S. Patents 2,081,121A, 18 May 1937. [Google Scholar]
- Zhou, Q.Q.; Chen, Y.X.; Shi, H.Y.; Chen, R.; Ji, M.H.; Li, K.X.; Wang, H.L.; Jiang, X.; Lu, C.Z. The construction of p/n-Cu2O heterojunction catalysts for efficient CO2 photoelectric reduction. Catalysts 2023, 13, 857. [Google Scholar] [CrossRef]
- Sahu, K.; Bisht, A.; Khan, S.A.; Sulania, I.; Singhal, R.; Pandey, A.; Mohapatra, S. Thickness dependent optical, structural, morphological, photocatalytic and catalytic properties of radio frequency magnetron sputtered nanostructured Cu2O-CuO thin films. Ceram. Int. 2020, 46, 14902–14912. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Synthesis of Cu2O nanoboxes, nanocubes and nanospheres by polyol process and their adsorption characteristic. Mater. Res. Bull. 2008, 43, 3047–3053. [Google Scholar] [CrossRef]
- Ma, L.L.; Li, J.L.; Sun, H.Z.; Qiu, M.Q.; Wang, J.B.; Chen, J.Y.; Yu, Y. Self-assembled Cu2O flowerlike architecture: Polyol synthesis, photocatalytic activity and stability under simulated solar light. Mater. Res. Bull. 2010, 45, 961–968. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Wang, X.; Yang, X.; Lu, L. A convenient method for preparing shape-controlled nanocrystalline Cu2O in a polyol or water/polyol system. Powder Technol. 2008, 181, 249–254. [Google Scholar] [CrossRef]
- Huang, X.W.; Liu, Z.J.; Zheng, Y.F. Synthesis of Cu2O nanobelts via surfactant-assisted polyol method. Chin. Chem. Lett. 2011, 22, 879–882. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, T.; Gu, Q.; Cheng, G.; Zheng, R. Shape control mechanism of cuprous oxide nanoparticles in aqueous colloidal solutions. Powder Technol. 2012, 227, 35–42. [Google Scholar] [CrossRef]
- Shin, H.S.; Song, J.Y.; Yu, J. Template-assisted electrochemical synthesis of cuprous oxide nanowires. Mater. Lett. 2009, 63, 397–399. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Leoni, M.; Scardi, P.; Garnier, E.; Mittiga, A. Synthesis, characterisation and stability of Cu2O nanoparticles produced via reverse micelles microemulsion. Mater. Chem. Phys. 2010, 122, 602–608. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Liang, W.S.; Satti, A.; Nikitin, K. Fabrication and microstructure of Cu2O nanocubes. J. Cryst. Growth 2010, 312, 1605–1609. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Z. Solution-phase synthesis of smaller cuprous oxide nanocubes. Mater. Res. Bull. 2008, 43, 1583–1589. [Google Scholar] [CrossRef]
- Zhu, J.; Bi, H.; Wang, Y.; Wang, X.; Yang, X.; Lu, L. Solution-phase synthesis of Cu2O cubes using CuO as a precursor. Mater. Lett. 2008, 62, 2081–2083. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z. One-pot growth of Cu2O concave octahedron microcrystal in alkaline solution. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2009, 162, 82–86. [Google Scholar] [CrossRef]
- Liang, Z.H.; Zhu, Y.J. Synthesis of uniformly sized Cu2O crystals with star-like and flower-like morphologies. Mater. Lett. 2005, 59, 2423–2425. [Google Scholar] [CrossRef]
- Yang, Y.M.; Wang, K.; Yang, Z.H.; Zhang, Y.M.; Gu, H.Y.; Zhang, W.X.; Li, E.R.; Zhou, C. An efficient route to Cu2O nanorod array film for high-performance Li-ion batteries. Thin Solid Films 2016, 608, 79–87. [Google Scholar] [CrossRef]
- Luo, Y.; Tu, Y.; Ren, Q.; Dai, X.; Xing, L.; Li, J. Surfactant-free fabrication of Cu2O nanosheets from Cu colloids and their tunable optical properties. J. Solid State Chem. 2009, 182, 182–186. [Google Scholar] [CrossRef]
- Wang, H.; He, S.; Yu, S.; Shi, T.; Jiang, S. Template-free synthesis of Cu2O hollow nanospheres and their conversion into Cuhollow nanospheres. Powder Technol. 2009, 193, 182–186. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, Y.; Fu, W.; Yang, H.; Zhao, Q.; Sun, P.; Ma, D.; Yuan, M.; Li, Y.; Zou, G. Low-temperature template-free synthesis of Cu2O hollow spheres. J. Cryst. Growth 2009, 311, 2285–2290. [Google Scholar] [CrossRef]
- Wei, M.; Huo, J. Preparation of Cu2O nanorods by a simple solvothermal method. Mater. Chem. Phys. 2010, 121, 291–294. [Google Scholar] [CrossRef]
- Wei, M.; Lun, N.; Ma, X.; Wen, S. A simple solvothermal reduction route to copper and cuprous oxide. Mater. Lett. 2007, 61, 2147–2150. [Google Scholar] [CrossRef]
- Lim, Y.F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol-gel deposited Cu2O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Chen, G.; Zhang, H.; Chen, Y. Synthesis of Cu2O nano-whiskers by a novel wet-chemical route. Mater. Lett. 2008, 62, 886–888. [Google Scholar] [CrossRef]
- Susman, M.D.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Chemical Deposition of Cu2O Nanocrystals with Precise Morphology Control. ACS Nano 2014, 8, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, B.; Mehta, B.R. Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Films 2001, 396, 90–96. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F. Characterization of copper oxide thin films deposited by the thermal evaporation of cuprous oxide (Cu2O). Vacuum 2008, 82, 623–629. [Google Scholar] [CrossRef]
- Barreca, D.; Comini, E.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Chemical vapor deposition of copper oxide films and entangled quasi-1D nanoarchitectures as innovative gas sensors. Sens. Actuators B Chem. 2009, 141, 270–275. [Google Scholar] [CrossRef]
- Gomersall, D.E.; Flewitt, A.J. Plasma enhanced chemical vapor deposition of p-type Cu2O from metal organic precursors. J. Appl. Phys. 2022, 131, 215301. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Pan, L.; Zhao, F.-M.; Zou, J.-J.; Zhang, T.; Wang, L. Controllable sonochemical synthesis of Cu2O/Cu2(OH)3NO3 composites toward synergy of adsorption and photocatalysis. Appl. Catal. B Environ. 2015, 164, 234–240. [Google Scholar] [CrossRef]
- Ma, D.; Liu, H.; Yang, H.; Fu, W.; Zhang, Y.; Yuan, M.; Sun, P.; Zhou, X. High pressure hydrothermal synthesis of cuprous oxide microstructures of novel morphologies. Mater. Chem. Phys. 2009, 116, 458–463. [Google Scholar] [CrossRef]
- Valodkar, M.; Pal, A.; Thakore, S. Synthesis and characterization of cuprous oxide dendrites: New simplified green hydrothermal route. J. Alloys Compd. 2011, 509, 523–528. [Google Scholar] [CrossRef]
- Togashi, T.; Hitaka, H.; Ohara, S.; Naka, T.; Takami, S.; Adschiri, T. Controlled reduction of Cu2+ to Cu+ with an N,O-type chelate under hydrothermal conditions to produce Cu2O nanoparticles. Mater. Lett. 2010, 64, 1049–1051. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.; Yan, Y. One-step in-situ fabrication of silver-modified Cu2O crystals with enhanced visible photocatalytic activity. Micro-Nano Lett. 2016, 11, 363–365. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Zhang, Z.; Zhang, W.; Yan, X.; Wu, X.; Cheng, G.; Zheng, R. Novel Au/CuO Multi-shelled Porous heterostructures forenhanced efficiency photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 14415–14421. [Google Scholar] [CrossRef]
- Jin, J.Y.; Mei, H.; Wu, H.M.; Wang, S.F.; Xia, Q.H.; Ding, Y. Selective detection of dopamine based on Cu2O@Pt core-shell nanoparticles modified electrode in the presence of ascorbic acid and uric acid. J. Alloy Compd. 2016, 689, 174–181. [Google Scholar] [CrossRef]
- Kuo, M.Y.; Hsiao, C.F.; Chiu, Y.H.; Lai, T.H.; Fang, M.J.; Wu, J.Y.; Chen, J.W.; Wu, C.L.; Wei, K.H.; Lin, H.C.; et al. Au@Cu2O core@shell nanocrystals as dual-functional catalysts for sustainable environmental applications. Appl. Catal. B 2019, 242, 499–506. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, C.; Wang, W.; Zhang, M.; Hao, X.S. Chen. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 557, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, C.F.; Guo, C.Q.; Lu, Z.X.; Shi, Y.; Wang, Z.D. Synthesis of GO-modified Cu2O nanosphere and the photocatalytic mechanism of water splitting for hydrogen production. Int. J. Hydrogen Energy 2016, 7, 4007–4016. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M. A noble metal-free reduced graphene oxide-CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 2, 3407. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Li, Y. High Efficient Photocatalytic Degradation of p-Nitrophenol on a Unique Cu2O/TiO2 p-n Heterojunction Network Catalyst. Environ. Sci. technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef]
- Fu, J.; Cao, S.; Yu, J. Dual Z-scheme charge transfer in TiO2-Ag-Cu2O composite for enhanced photocatalytic hydrogen generation. J. Mater. 2015, 1, 124–133. [Google Scholar] [CrossRef]
- Yu, L.; Ba, X.; Qiu, M.; Li, Y.; Shuai, L.; Zhang, W.; Ren, Z.; Yu, Y. Visible-light driven CO2 reduction coupled with water oxidation on Cl-doped Cu2O nanorods. Nano Energy 2019, 60, 576–582. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Jing, D.W.; Guo, L.J.; Yao, X.D. In situ photochemical synthesis of Zn-Doped Cu2O hollow microcubes for high efficient photocatalytic H2 production. ACS Sustain. Chem. Eng. 2014, 2, 1446–1452. [Google Scholar] [CrossRef]
- Kalubowila, K.D.R.N.; Gunewardene, M.S.; Jayasingha, J.L.K.; Dissanayake, D.; Jayathilaka, C.; Jayasundara, J.M.D.; Gao, Y.; Jayanetti, J.K.D.S. Reduction-induced synthesis of reduced graphene oxide-wrapped Cu2O/Cu nanoparticles for photodegradation of methylene blue. ACS Appl. Nano Mater. 2021, 4, 2673–2681. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, L.; Su, W.; Xing, Y. Photocatalytic CO2 reduction over copper-based materials: A review. J. CO2 Util. 2022, 61, 102056. [Google Scholar] [CrossRef]
- Celaya, C.A.; Delesma, C.; Torres-Arellano, S.; Sebastian, P.J.; Muniz, J. Understanding CO2 conversion into hydrocarbons via a photoreductive process supported on the Cu2O(100), (110) and (111) surface facets: A first principles study. Fuel 2021, 306, 121643. [Google Scholar] [CrossRef]
- Wu, Y.A.; McNulty, I.; Liu, C.; Lau, K.C.; Liu, Q.; Paulikas, A.P.; Sun, C.J.; Cai, Z.H.; Guest, J.R.; Ren, Y.; et al. Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol. Nat. Energy 2019, 4, 957–968. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pokhriyal, M.; Upadhyayula, S.; Zhao, X.S. Modulating charge carrier dynamics among anisotropic crystal facets of Cu2O for enhanced CO2 photoreduction. J. Phys. Chem. C 2022, 126, 13094–13104. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Yi, J.; Zhou, D.C. Electronic structures of halogen-doped Cu2O based on DFT calculations. Chinese. Phys. B. 2014, 23, 017401. [Google Scholar] [CrossRef]
- Cheng, S.P.; Wei, L.W.; Wang, H.P. Photocatalytic reduction of CO2 to methanol by Cu2O/TiO2 heterojunctions. Sustainability 2022, 14, 374. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B-Environ. 2020, 268, 118380. [Google Scholar] [CrossRef]
- Song, Y.Y.; Zhao, X.J.; Feng, X.Y.; Chen, L.M.; Yuan, T.C.; Zhang, F.Q. Z-scheme Cu2O/Cu/Cu3V2O7(OH)2·2H2O heterostructures for efficient visible-light photocatalytic CO2 reduction. ACS Appl. Energy Mater. 2022, 5, 10542–10552. [Google Scholar] [CrossRef]
- Cui, L.K.; Hu, L.Q.; Shen, Q.Q.; Liu, X.G.; Jia, H.S.; Xue, J.B. Three-dimensional porous Cu2O with dendrite for efficient photocatalytic reduction of CO2 under visible light. Appl. Catal. B-Environ. 2022, 581, 152343. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Duan, Z.T.; Liang, R.X.; Lv, R.Q.; Wang, C.; Zhang, Z.X.; Wan, S.L.; Wang, S.; Xiong, H.F.; Ngaw, C.K. Shape-dependent performance of Cu/Cu2O for photocatalytic reduction of CO2. ChemSusChem 2022, 15, e202200216. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhao, X.; Chen, K.; Fan, Y.Y.; Wei, S.L.; Zhang, W.S.; Han, D.X.; Niu, L. Palladium-modified cuprous(i) oxide with {100} facets for photocatalytic CO2 reduction. Nanoscale 2021, 13, 2883–2890. [Google Scholar] [CrossRef]
- Zhao, X.X.; Sun, L.L.; Jin, X.; Xu, M.Y.; Yin, S.K.; Li, J.Z.; Li, X.; Shen, D.; Yan, Y.; Huo, P.W. Cu media constructed Z-scheme heterojunction of UiO-66-NH2/Cu2O/Cu for enhanced photocatalytic induction of CO2. Appl. Surf. Sci. 2021, 545, 148967. [Google Scholar] [CrossRef]
- Deng, Y.; Wan, C.A.; Li, C.; Wang, Y.Y.; Mu, X.Y.; Liu, W.; Huang, Y.P.; Wong, P.K.; Ye, L.Q. Synergy effect between facet and zero-valent copper for selectivity photocatalytic methane formation from CO2. ACS Catal. 2022, 12, 4526–4533. [Google Scholar] [CrossRef]
- Tang, Z.L.; He, W.J.; Wang, Y.L.; Wei, Y.C.; Yu, X.L.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B-Environ. 2022, 311, 121371. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Li, Y.F.; Yu, L.; Li, Z.P.; Xie, D.F.; Zhao, Y.Y.; Yu, Y. Facile in situ fabrication of Cu2O@Cu metal-semiconductor heterostructured nanorods for efficient visible-light driven CO2 reduction. Chem. Eng. J. 2020, 385, 123940. [Google Scholar] [CrossRef]
- Ali, S.; Lee, J.; Kim, H.; Hwang, Y.; Razzaq, A.; Jung, J.W.; Cho, C.H.; In, S.I. Sustained, photocatalytic CO2 reduction to CH4 in a continuous flow reactor by earth-abundant materials: Reduced titania-Cu2O Z-scheme heterostructures. Appl. Catal. B-Environ. 2020, 279, 119344. [Google Scholar] [CrossRef]
- Yao, S.; Sun, B.Q.; Zhang, P.; Tian, Z.Y.; Yin, H.Q.; Zhang, Z.M. Anchoring ultrafine Cu2O nanocluster on PCN for CO2 photoreduction in water vapor with much improved stability. Appl. Catal. B-Environ. 2022, 317, 121702. [Google Scholar] [CrossRef]
- Movahed, S.K.; Najinasab, A.; Nikbakht, R.; Dabiri, M. Visible light assisted photocatalytic reduction of CO2 to methanol using Fe3O4@N-C/Cu2O nanostructure photocatalyst. J. Photochem. Photobiol. A 2020, 401, 112763. [Google Scholar] [CrossRef]
- Liu, S.H.; Lu, J.S.; Pu, Y.C.; Fan, H.C. Enhanced photoreduction of CO2 into methanol by facet-dependent Cu2O/reduce graphene oxide. J. CO2 Util. 2019, 33, 171–178. [Google Scholar] [CrossRef]
- Li, H.T.; Deng, Y.D.; Liu, Y.D.; Zeng, X.; Wiley, D.; Huang, J. Carbon quantum dots and carbon layer double protected cuprous oxide for efficient visible light CO2 reduction. Chem. Commun. 2019, 55, 4419–4422. [Google Scholar] [CrossRef]
- Zeng, Z.P.; Yan, Y.B.; Chen, J.; Zan, P.; Tian, Q.H.; Chen, P. Boosting the photocatalytic ability of Cu2O nanowires for CO2 conversion by mxene quantum dots. Adv. Funct. Mater. 2018, 29, 1806500. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, S.J.; Cai, X.; Cheng, L.H.; Zhou, R.J.; Hou, T.T.; Li, Y.W. Photocatalytic CO2 reduction to HCOOH over core-shell Cu@Cu2O catalysts. Catal. Commun. 2022, 162, 106372. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, Y.N.; Tao, Y.; Li, T.; Zhang, Y.; Shang, H.; Song, J.X.; Li, G.S. CO2 reduction to formic acid via NH2-C@Cu2O photocatalyst in situ derived from amino modified Cu-MOF. J. CO2 Util. 2021, 54, 101781. [Google Scholar] [CrossRef]
- Dominguez-Arvizu, J.L.; Jimenez-Miramontes, J.A.; Hernandez-Majalca, B.C.; Valenzuela-Castro, G.E.; Gaxiola-Cebreros, F.A.; Salinas-Gutierrez, J.M.; Collins-Martinez, V.; Lopez-Ortiz, A. Study of NiFe2O4/Cu2O p-n heterojunctions for hydrogen production by photocatalytic water splitting with visible light. J. Mater. Res. Technol. 2023, 21, 4184–4199. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, H.Y.; Dong, Y.H.; Cui, H.; Sun, H.; Yin, S.Y. Fabrication of Cu2O/MTiO3 (M = Ca, Sr and Ba) p-n heterojunction for highly enhanced photocatalytic hydrogen generation. J. Alloy Compd. 2023, 930, 167333. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, M.M.; Chen, J.L.; Xie, K.F.; Fang, S.M. Dendritic branching Z-scheme Cu2O/TiO2 heterostructure photocatalysts for boosting H2 production. J. Phys. Chem. Solids 2021, 152, 109948. [Google Scholar] [CrossRef]
- Dai, B.L.; Li, Y.Y.; Xu, J.M.; Sun, C.; Li, S.J.; Zhao, W. Photocatalytic oxidation of tetracycline, reduction of hexavalent chromium and hydrogen evolution by Cu2O/g-C3N4 S-scheme photocatalyst: Performance and mechanism insight. Appl. Surf. Sci. 2022, 592, 153309. [Google Scholar] [CrossRef]
- Liu, Y.X.; Tan, H.; Wei, Y.A.; Liu, M.H.; Hong, J.X.; Gao, W.Q.; Zhao, S.Q.; Zhang, S.P.; Guo, S.J. Cu2O/2D COFs core/shell nanocubes with antiphotocorrosion ability for efficient evolution. ACS Nano 2023, 17, 5994–6001. [Google Scholar] [CrossRef]
- Muscetta, M.; Al Jitan, S.; Palmisano, G.; Andreozzi, R.; Marotta, R.; Cimino, S.; Di Somma, I. Visible light-driven photocatalytic hydrogen production using Cu2O/TiO2 composites prepared by facile mechanochemical synthesis. J. Environ. Chem. Eng. 2022, 10, 107735. [Google Scholar] [CrossRef]
- Chang, Y.C.; Syu, S.Y.; Lu, M.Y. Fabrication of In(OH)3-In2S3-Cu2O nanofiber for highly efficient photocatalytic hydrogen evolution under blue light LED excitation. Int. J. Hydrogen Energy 2023, 48, 9318–9332. [Google Scholar] [CrossRef]
- Lai, T.H.; Tsao, C.W.; Fang, M.J.; Wu, J.Y.; Chang, Y.P.; Chiu, Y.H.; Hsieh, P.Y.; Kuo, M.Y.; Chang, K.D.; Hsu, Y.J. Au@Cu2O core-shell and Au@Cu2Se yolk-shell nanocrystals as promising photocatalysts in photoelectrochemical water splitting and photocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2022, 14, 40771–40783. [Google Scholar] [CrossRef]
- Ibarra-Rodriguez, L.I.; Huerta-Flores, A.M.; Torres-Martinez, L.M. Development of Na2Ti6O13/CuO/Cu2O heterostructures for solar photocatalytic production of low-carbon fuels. Mater. Res. Bull. 2020, 122, 110679. [Google Scholar] [CrossRef]
- Dubale, A.A.; Ahmed, I.N.; Zhang, Y.J.; Yang, X.L.; Xie, M.H. A facile strategy for fabricating C@Cu2O/CuO composite for efficient photochemical hydrogen production with high external quantum efficiency. Appl. Surf. Sci. 2020, 534, 147582. [Google Scholar] [CrossRef]
- Fan, Z.B.; Zhang, X.J.; Li, Y.J.; Guo, X.; Jin, Z.L. Construct 3D NiCo-LDH/Cu2O p-n heterojunction via electrostatic self-assembly for enhanced photocatalytic hydrogen evolution. J. Ind. Eng. Chem. 2022, 110, 491–502. [Google Scholar] [CrossRef]
- Mohite, S.V.; Kim, S.; Lee, C.S.; Bae, J.; Kim, Y. Z-scheme heterojunction photocatalyst: Deep eutectic solvents-assisted synthesis of Cu2O nanocluster improved hydrogen production of TiO2. J. Alloy Compd. 2022, 928, 167168. [Google Scholar] [CrossRef]
- Qiu, P.; Xiong, J.Y.; Lu, M.J.; Liu, L.J.; Li, W.; Wen, Z.P.; Li, W.J.; Chen, R.; Cheng, G. Integrated p-n/Schottky junctions for efficient photocatalytic hydrogen evolution upon Cu@TiO2-Cu2O ternary hybrids with steering charge transfer. J. Colloid Interface Sci. 2022, 622, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Paniagua, D.K.; Torres-Arellano, S.; Martinez-Alonso, C.; Luevano-Hipolito, E.; Sebastian, P.J. Facile and green synthesis of Cu/Cu2O composite for photocatalytic H2 generation. Mater. Sci. Semicond. Process. 2023, 162, 107485. [Google Scholar] [CrossRef]
- Zhu, H.; Xi, M.Y.; Huang, G.P.; Qin, L.X.; Zhang, T.Y.; Kang, S.Z.; Li, X.Q. Cuprous oxide core-shell heterostructure facilely encapsulated by cadmium metal organic frameworks for enhanced photocatalytic hydrogen generation. J. Phys. Chem. Solids 2023, 181, 111476. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.J.; Chen, J.F.; Wang, Y.A.; Cheng, Z.Y.; Chen, X.Q.; Gao, X.; Guo, M.H. Porous spherical Cu2O supported by wood-based biochar skeleton for the adsorption-photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 611, 155744. [Google Scholar] [CrossRef]
- Sehrawat, P.; Rana, S.; Mehta, S.K.; Kansal, S.K. Optimal synthesis of MoS2/Cu2O nanocomposite to enhance photocatalytic performance towards indigo carmine dye degradation. Appl. Surf. Sci. 2022, 604, 154482. [Google Scholar] [CrossRef]
- Li, J.J.; Guo, C.P.; Li, L.H.; Gu, Y.J.; BoK-Hee, K.; Huang, J.L. Construction of Z-scheme WO3-Cu2O nanorods array heterojunction for efficient photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2022, 138, 109248. [Google Scholar] [CrossRef]
- Nie, J.K.; Yu, X.J.; Liu, Z.B.; Zhang, J.; Ma, Y.; Chen, Y.Y.; Ji, Q.G.; Zhao, N.N.; Chang, Z. Energy band reconstruction mechanism of Cl-doped Cu2O and photocatalytic degradation pathway for levofloxacin. J. Clean. Prod. 2022, 363, 132593. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.Q.; Li, H.; Guo, H.; Yang, Q.; Li, X.M. Tunning heterostructures interface of Cu2O@HKUST-1 for enhanced photocatalytic degradation of tetracycline hydrochloride. Sep. Purif. Technil. 2022, 303, 122106. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.Y.; Peng, J.F.; Peng, D.Y.; Liu, F.Y.; Xu, S.; Yang, Z.L. abrication of Cu2O/ZnTi-LDH p-n heterostructure by grafting Cu2O NPs onto the LDH host layers from Cu-doped ZnTi-LDH and insight into the photocatalytic mechanism. Compos. Part. B-Eng. 2023, 250, 110447. [Google Scholar] [CrossRef]
- Zhu, P.; Li, Y.; Ma, Y.; Ruan, X.X.; Zhang, Q.Z. Preparation of waste-based Cu-Cu2O/SiO2 photocatalyst from serpentine tailings and waste printed circuit boards and photoreduction of Cr(Ⅵ). Ceram. Int. 2023, 49, 12518–12528. [Google Scholar] [CrossRef]
- Alp, E. The facile synthesis of Cu2O-Cu hybrid cubes as efficient visible-light-driven photocatalysts for water remediation processes. Powder Technol. 2021, 394, 1111–1120. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Shawky, A. Cu-supported Cu2O nanoparticles: Optimized photodeposition enhances the visible light photodestruction of atrazine. J. Alloy Compd. 2021, 853, 157040. [Google Scholar] [CrossRef]
- Dubale, A.A.; Ahmed, I.N.; Chen, X.H.; Ding, C.; Hou, G.H.; Guan, R.F.; Meng, X.M.; Yang, X.L.; Xie, M.H. A highly stable metal-organic framework derived phosphorus doped carbon/Cu2O structure for efficient photocatalytic phenol degradation and hydrogen production. J. Mater. Chem. A 2019, 7, 6062–6079. [Google Scholar] [CrossRef]
- Yu, X.J.; Zhang, J.; Chen, Y.Y.; Ji, Q.G.; Wei, Y.C.; Niu, J.N.; Yu, Z.; Yao, B.H. Ag-Cu2O composite films with enhanced photocatalytic activities for methylene blue degradation: Analysis of the mechanism and the degradation pathways. J. Environ. Chem. Eng. 2021, 9, 106161. [Google Scholar] [CrossRef]
- Nie, J.K.; Yu, X.J.; Liu, Z.B.; Wei, Y.C.; Zhang, J.; Zhao, N.N.; Yu, Z.; Yao, B.H. Boosting principles for the photocatalytic performance of Cr-doped Cu2O crystallites and mechanisms of photocatalytic oxidation for levofloxacin. Appl. Surf. Sci. 2021, 576, 151842. [Google Scholar] [CrossRef]
- Liu, Z.B.; Yu, X.J.; Gao, P.H.; Nie, J.K.; Yang, F.; Guo, B.Q.; Zhang, J. Preparation of BiOCl/Cu2O composite particles and its photocatalytic degradation of moxifloxacin. Opt. Mater. 2022, 128, 112432. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.; Ruan, Z.H.; Yuan, Y.; Lin, K.F. Extensive solar light utilizing by ternary C-dots/Cu2O/SrTiO3: Highly enhanced photocatalytic degradation of antibiotics and inactivation of E. coli. Chemosphere 2022, 290, 133340. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2021, 145, 111561. [Google Scholar] [CrossRef]
- Su, X.P.; Chen, W.; Han, Y.N.; Wang, D.C.; Yao, J.M. In-situ synthesis of Cu2O on cotton fibers with antibacterial properties and reusable photocatalytic degradation of dyes. Appl. Surf. Sci. 2021, 536, 147945. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.M.; Zhao, H.; Yao, F.B.; Cao, J.; Chen, Z.; Wang, D.B.; Yang, Q. Core-shell structured Cu2O@HKUST-1 heterojunction photocatalyst with robust stability for highly efficient tetracycline hydrochloride degradation under visible light. Chem. Eng. J. 2021, 426, 131255. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, T.X.; Zhao, W.S.; Cui, S.C.; Guo, R.; Li, D.; Kadasala, N.R.; Han, D.L.; Jiang, Y.H.; Liu, Y. Multifunctional magnetic Fe3O4/Cu2O-Ag nanocomposites with high sensitivity for SERS detection and efficient visible light-driven photocatalytic degradation of polycyclic aromatic hydrocarbons (PAHs). J. Colloid Interf. Sci. 2022, 628, 315–326. [Google Scholar] [CrossRef]
- Altynbaeva, L.S.; Barsbay, M.; Aimanova, N.A.; Jakupova, Z.Y.; Nurpeisova, D.T.; Zdorovets, M.V.; Mashentseva, A.A. A Novel Cu2O/ZnO@PET Composite Membrane for the Photocatalytic Degradation of Carbendazim. Nanomaterials 2022, 12, 1724. [Google Scholar] [CrossRef]
- Yanagida, S.; Yajima, T.; Takei, T.; Kumada, N. Removal of hexavalent chromium from water by Z-scheme photocatalysis using TiO2 (rutile) nanorods loaded with Au core-Cu2O shell particles. J. Environ. Sci. 2022, 115, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Qiang, T.T.; Wang, S.T.; Ren, L.F.; Gao, X.D. Novel 3D Cu2O/N-CQD/ZIF-8 composite photocatalyst with Z-scheme heterojunction for the efficient photocatalytic reduction of Cr(VI). J. Environ. Chem. Eng. 2022, 10, 108784. [Google Scholar] [CrossRef]
- Ma, J.; Liang, C.J.; Yu, C.J.; Li, H.M.; Xu, H.; Hua, Y.J.; Wang, C.T. BiOBr microspheres anchored with Cu2O nanoparticles and rGO: A Z-scheme heterojunction photocatalyst for efficient reduction of Cr(VI) under visible light irradiation. Appl. Surf. Sci. 2023, 609, 155247. [Google Scholar] [CrossRef]
- An, X.Q.; Liu, H.J.; Qu, J.H.; Moniz, S.J.A.; Tang, J.W. Photocatalytic mineralisation of herbicide 2,4,5-trichlorophenoxyacetic acid: Enhanced performance by triple junction Cu-TiO2-Cu2O and the underlying reaction mechanism. New J. Chem. 2015, 39, 314–320. [Google Scholar] [CrossRef]
- Sharma, K.; Maiti, K.; Kim, N.H.; Hui, D.; Lee, J.H. Green synthesis of glucose-reduced graphene oxide supported Ag-Cu2O nanocomposites for the enhanced visible-light photocatalytic activity. Compos. Part. B-Eng. 2018, 138, 35–44. [Google Scholar] [CrossRef]
- Lv, Q.Y.; Li, L.L.; Li, Y.F.; Mao, J.H.; Chen, T.; Shao, D.W.; Li, M.M.; Tan, R.S.; Zhao, J.Q.; Shi, S.H. A DFT Study of Electronic Structures and Photocatalytic Properties of Mn-Cu2O. Russ. J. Phys. Chem. A 2020, 94, 641–646. [Google Scholar]


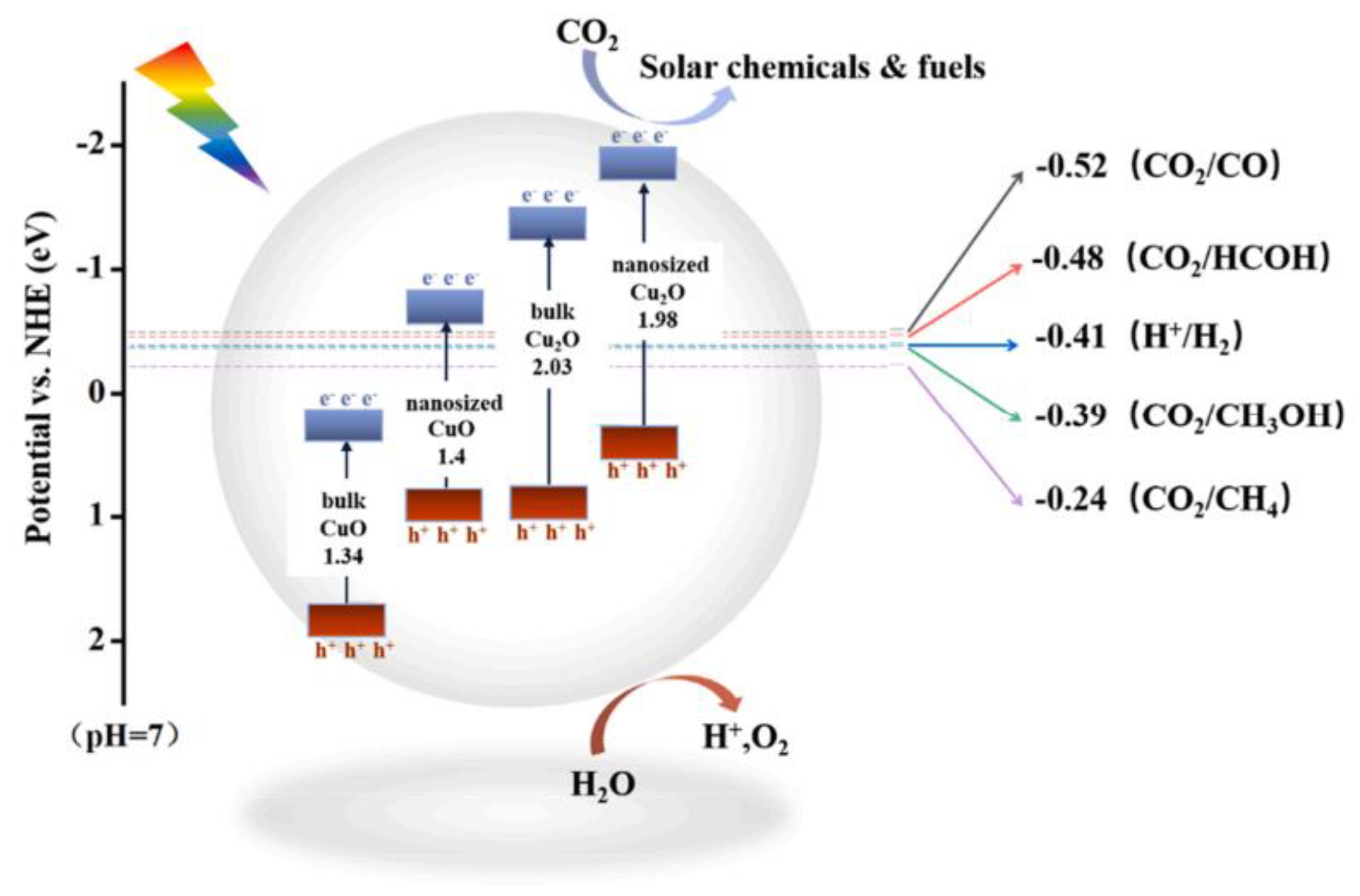

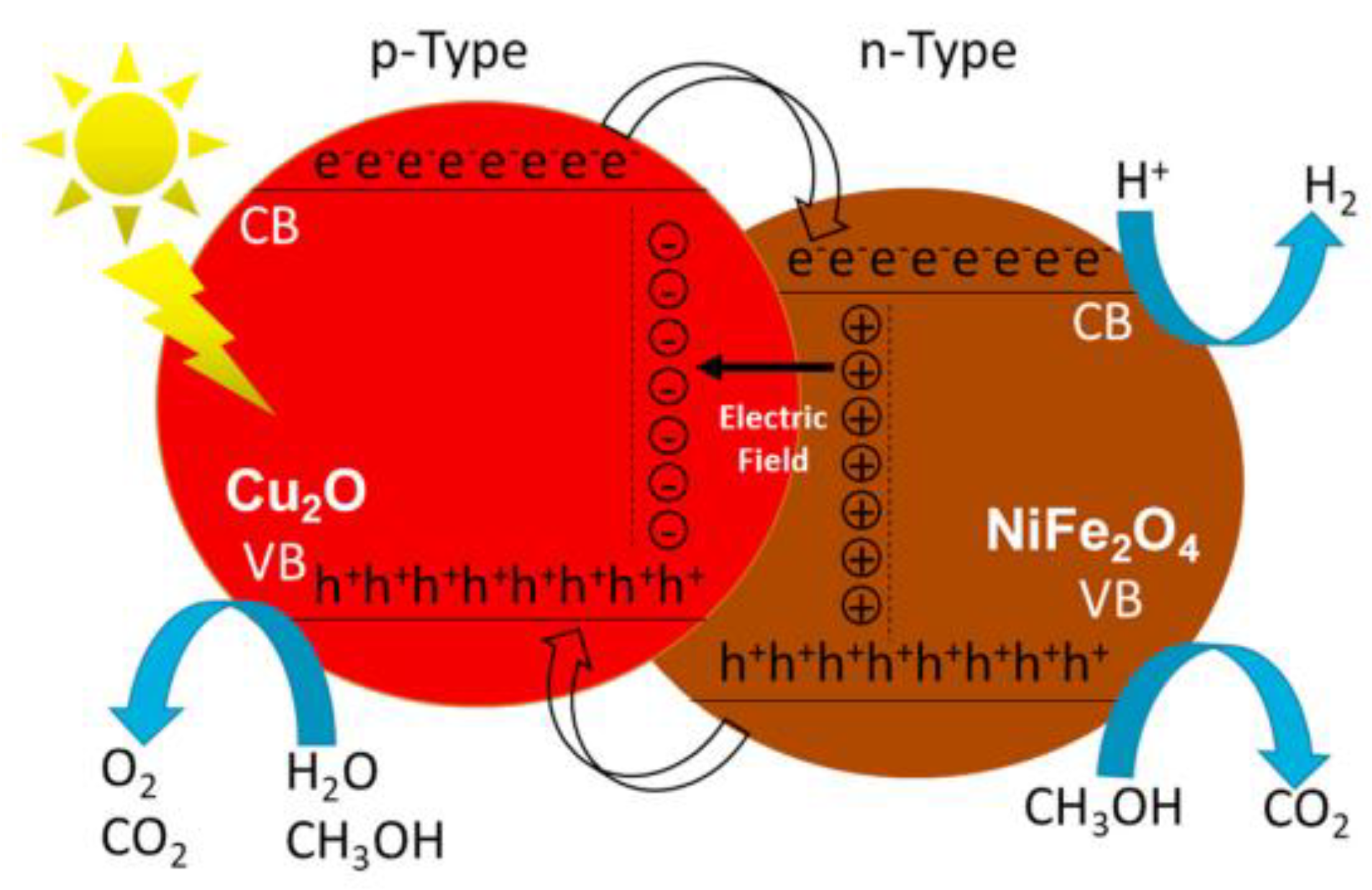
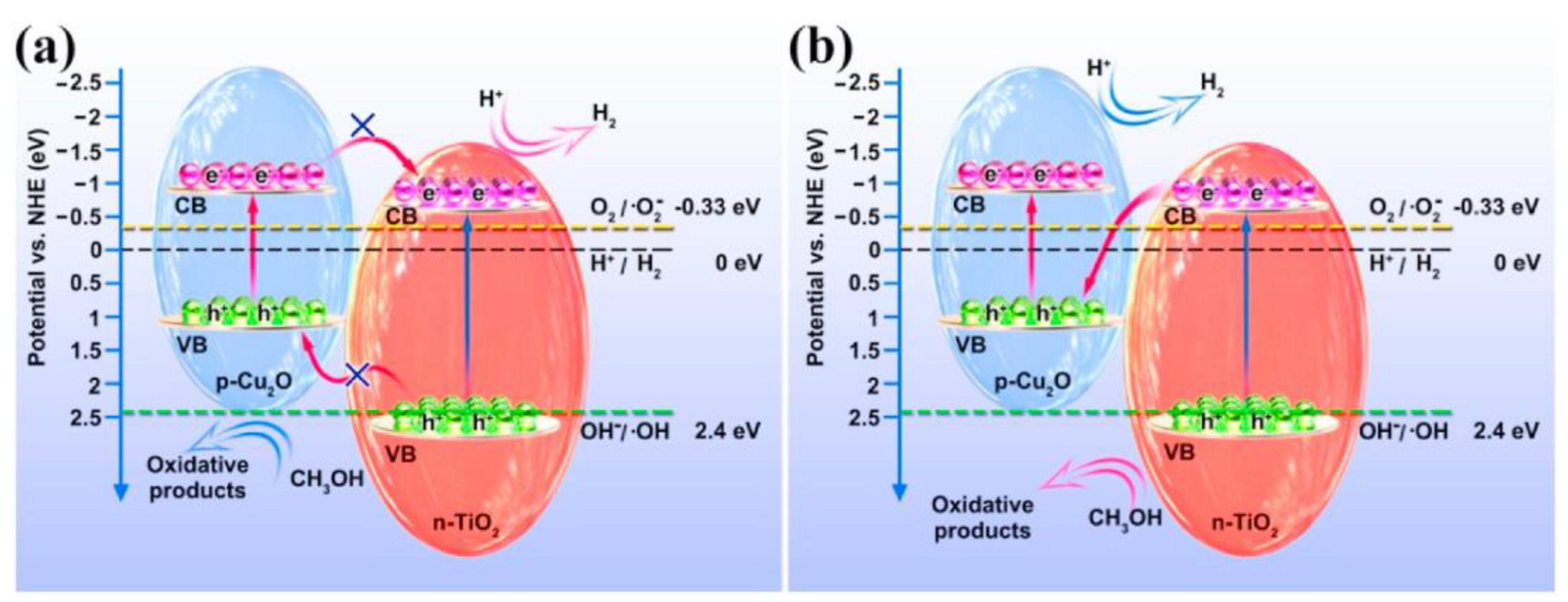
| Photocatalyst | Synthesis Method | Morphology and Structure | Size | Bandgap (Eg) | Light Resource | Product | Yield (μmol∙g−1 h−1) | Energy Conversion Efficiency/Selectivity | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Cu2O/Cu/CVO | Hydrothermal and wet chemical reduction methods | Cu2O nanoclusters and Cu NPs cover the surface of elliptic CVO NPs | ~100 nm | EgCVO: 2.34 eV; EgCu2O: 1.87 eV | 300 W Xe lamp (λ > 400 nm) | CO and CH4 | 6.97 and 1.62 | Selectivity: 51.3% for CO | [78] |
| 3D porous Cu2O | Electrodeposition and thermal oxidation. | 3D porous structure | 23–25 μm | 2.0 eV | 300 W Xe lamp (λ > 420 nm) | CO, CH4, and C2H4 | 26.8, 4.04, and 0.66 | — | [79] |
| Spherical Cu/Cu2O | Solution chemical method | Spherical structure | 1 μm | — | 300 W Xe lamp (λ > 420 nm) | CO, CH3OH, and H2 | 87.7, 10.2, and 5.4 | — | [80] |
| Cu2O-Pd | AA reduction and in situ methods | Cube | ~2 μm | 1.90 eV | 300 W Xe lamp (λ > 420 nm) | CO | 0.13 | — | [81] |
| Uio-66-NH2/Cu2O/Cu | Hydrothermal method | Octahedron UiO-66-NH2 and Cu attached to the surface of polyhedron Cu2O | 1.5 μm | 2.79 eV | 300 W Xe lamp | CO | 4.54 | — | [82] |
| Cu2O-111-Cu0 | One-pot method | Octahedral structure | side length of ~1 μm | 1.98 eV | 300 W Xe lamp | CH4 | 78.4 | 97% | [83] |
| Ag4/Cu2O@rGO | Water bath combining with gas-bubbling-assisted membrane reduction | Ultrathin rGO nanosheet and Ag NPs supported on Cu2O octahedral nanocrystals | Cu2O: 300 nm; Ag: 10.7 nm; rGO: 1.0 nm (thickness) | EgAg/Cu2O: 1.92 eV; EgCu2O: 2.0 eV | 300 W Xe lamp (λ > 380 nm) | CH4 | 82.6 | AQE: 1.26%. Selectivity: 95.4% | [84] |
| 1D Cu2O@Cu NRs | In situ reduction method | One-dimensional nanorod arrays | <100 nm | 2.03 eV | 350 W Xe lamp (λ > 420 nm) | CH4 and C2H4 | — | AQE: 2.4% | [85] |
| RT-Cu0.75 | Low temperature thermochemical reduction and photo-deposition | — | — | 2.72 eV | 100 W solar simulator with an AM 1.5 filter | CH4 | 77 nmol·g−1 h−1 | AQE: 0.012% | [86] |
| U-Cu2O-LTH@PCN-X | In situ reduction | Ultrafine nanoclusters | <3 nm | EgPCN: 2.62 eV; EgU-Cu2O-LTH: 2.07 eV | 300 W Xe lamp (λ > 400 nm) | CH3OH | 51.22 | AQE: 1.01% | [87] |
| Fe3O4@N-C/Cu2O | AA reduction and aerobic oxidation | Rod-shaped core–shell nanostructure | 5 nm (thickness of NC shell layer) | — | 5 W Xe HID lamp | CH3OH | 146.7 | — | [88] |
| Dodeca-Cu2O/rGO | Solution-chemistry | Rhombic dodecahedra | 400–700 nm | 2.16 eV | 300 W Xe lamp (λ > 420 nm) | CH3OH | 17.765 | — | [89] |
| Carbon layer@CQDs/Cu2O | Hydrothermal method | Nearly spherical structure | ~2 µm diameter | 2.09 eV | 300 W Xe lamp | CH3OH | 99.6 | — | [90] |
| Ti3C2 QDs/Cu2O NWs/Cu | Self-assembly strategy | QDs incorporated onto NWs | ~500 nm (diameter of NWs) | 2.02 eV | AM 1.5, 300 W Xe lamp | CH3OH | 78.50 | — | [91] |
| Cu@Cu2O | Thermal treatment | Core–shell nanoparticles | ~70 nm diameter | — | Xe lamp (420–780 nm) | HCOOH | 67.35 | AQE: 0.12% at 560 nm | [92] |
| NH2-C@Cu2O | Low temperature annealing | Octahedral structure | — | 1.79 eV | 300 W Xe lamp (λ > 420 nm) | HCOOH | 138.65 | Selectivity: 92% | [93] |
| Photocatalyst | Synthesis Method | Morphology and Structure | Size | Bandgap (Eg) | Light Resource | Yield (μmol∙g−1 h−1) | Energy Conversion Efficiency/Selectivity | Refs. |
|---|---|---|---|---|---|---|---|---|
| Cu2O/TiO2 | Ball-milling | Irregular shapes | Anatase: 16.2 nm Rutile: 30.5 nm | 3.08 eV | High-pressure Hg lamp (125 W) | 200 | AQE: 1.51% Light-to-chemical energy efficiency: 0.6% | [99] |
| In(OH)3-In2S3-Cu2O | Hydrothermal, wet chemical and electrospinning process | Nanofiber | 100–200 nm of diameter | EgIn(OH)3: 5.15 eV EgIn2S3: 1.98 eV EgCu2O: 2.17 eV | 5 W blue light LED (λmax = 420 nm) | 1786.5 | — | [100] |
| Au@Cu2O | Sequential ion-exchange reaction | Core–shell architectures | 54.4 ± 4.8 nm | EgCu2O: 2.40 eV | Xenon lamp and AM 1.5G filter | 55.5 | AQE: 0.29% at 420 nm | [101] |
| Na2Ti6O13/CuO/Cu2O | Solid-state and impregnation method | Belt morphology | 1 μm | 3.61 eV | UV/vis lamp (254 nm, 4400 μW/cm2) | 33 | — | [102] |
| C@Cu2O/CuO | Calcination | Chrysanthemum-like crystalline | — | 2.0 eV | 350 W Xe lamp (40 mW/cm2) | 26,700 | External quantum efficiency (EQE): 52.4% | [103] |
| NiCo-LDH/Cu2O | Electrostatic self-assembly | 3D flower cluster | — | EgNiCo-LDH: 1.78 eV EgCu2O: 1.89 eV | 5 W LED (λ ≥ 420 nm) | 3666 | — | [104] |
| Cu2O/TiO2 | DES-assisted synthesis | Cu2O nanoclusters on TiO2 surfaces | 1.5 nm of Cu2O nanoclusters and 25.8 nm of TiO2 particles | EgTiO2: 3.12 eV EgCu2O: 2.13 eV | 300 W Xe lamp | 24,210 | — | [105] |
| Cu@TiO2-Cu2O | Hydrothermal and NaBH4 treatment | Urchin-like hierarchical spheres | — | EgTiO2: 3.18 eV EgCu2O: 2.05 eV | 300 W Xenon lamp | 12,000.6 | AQE: 8.26% | [106] |
| Cu/Cu2O | Microwave-assisted heating | Hollow spherical morphology | 430 ± 1.2 nm in diameter | 2.0 eV | LED light (20 W) | 141 | — | [107] |
| Cu2O/SiO2/CdIF | Reactive deposition | Core–shell structure | — | EgCdIF: 5.09 eV EgCu2O: 2.22 eV | 300 W xenon lamp (340–780 nm) | 2879.09 | AQE: 0.040% at 420 nm | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Zuo, C.; Liu, M.; Tai, X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules 2023, 28, 5576. https://doi.org/10.3390/molecules28145576
Su Q, Zuo C, Liu M, Tai X. A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules. 2023; 28(14):5576. https://doi.org/10.3390/molecules28145576
Chicago/Turabian StyleSu, Qian, Cheng Zuo, Meifang Liu, and Xishi Tai. 2023. "A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications" Molecules 28, no. 14: 5576. https://doi.org/10.3390/molecules28145576
APA StyleSu, Q., Zuo, C., Liu, M., & Tai, X. (2023). A Review on Cu2O-Based Composites in Photocatalysis: Synthesis, Modification, and Applications. Molecules, 28(14), 5576. https://doi.org/10.3390/molecules28145576






