Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Co-Doped LuFeO3 Powders and Structure Analysis
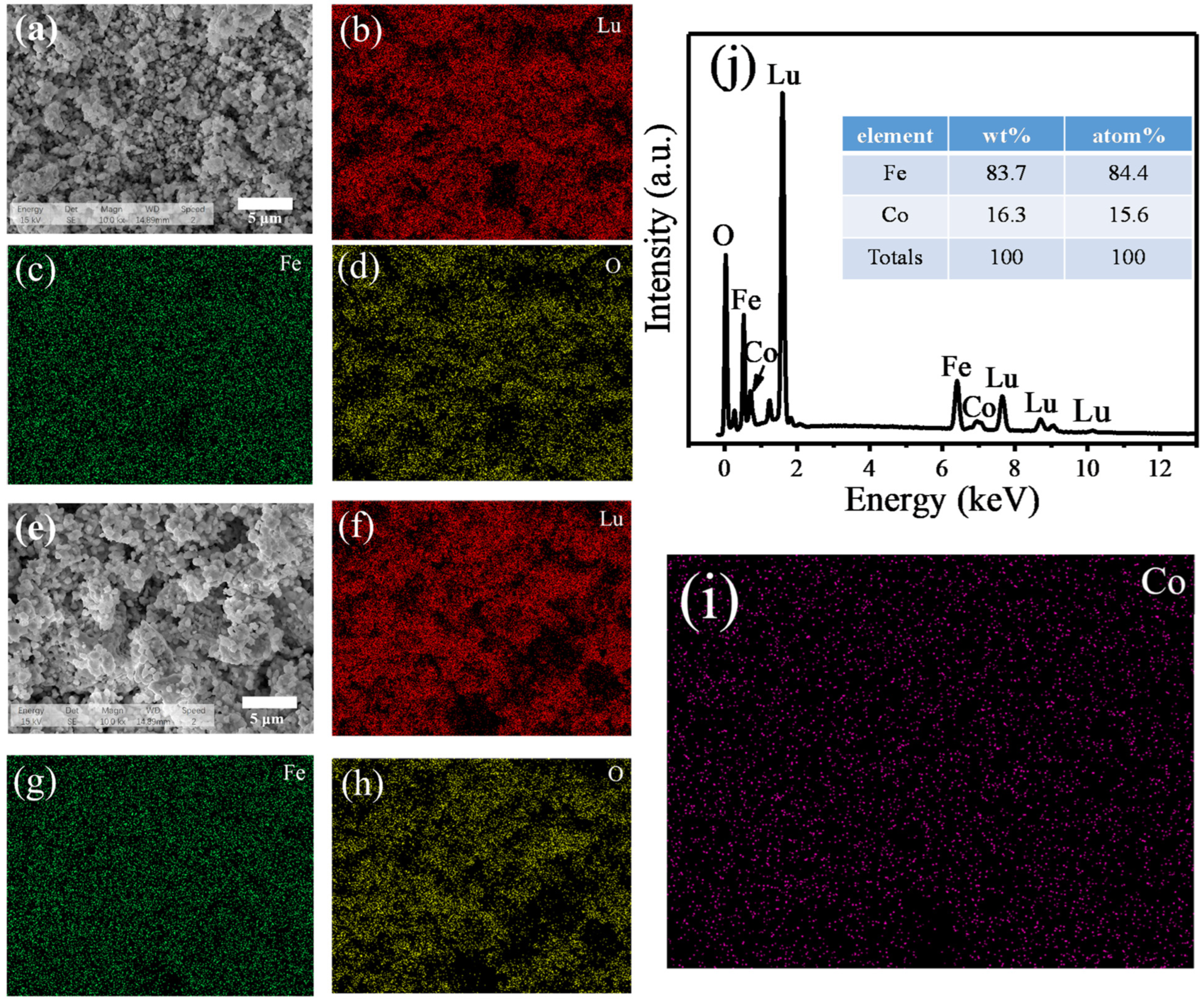
2.2. Morphology and Compositional Analysis
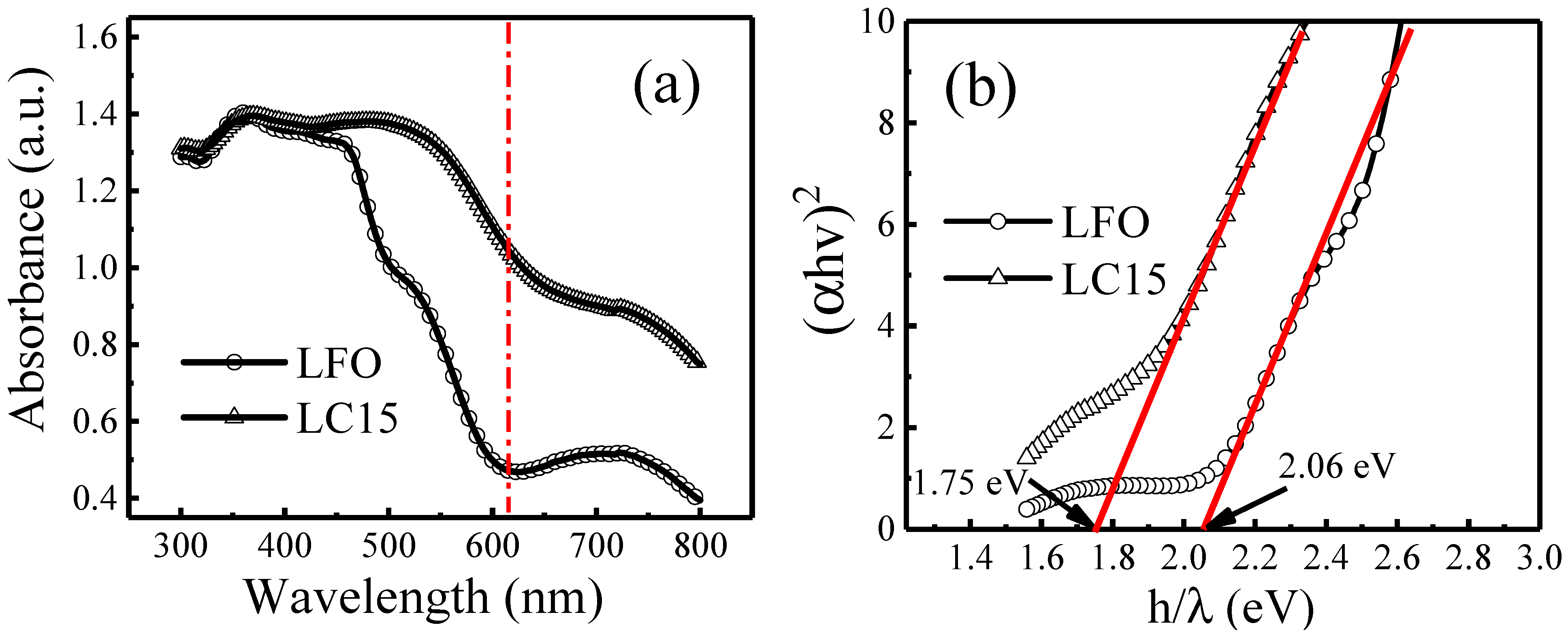
2.3. UV–Vis Absorbance Analysis
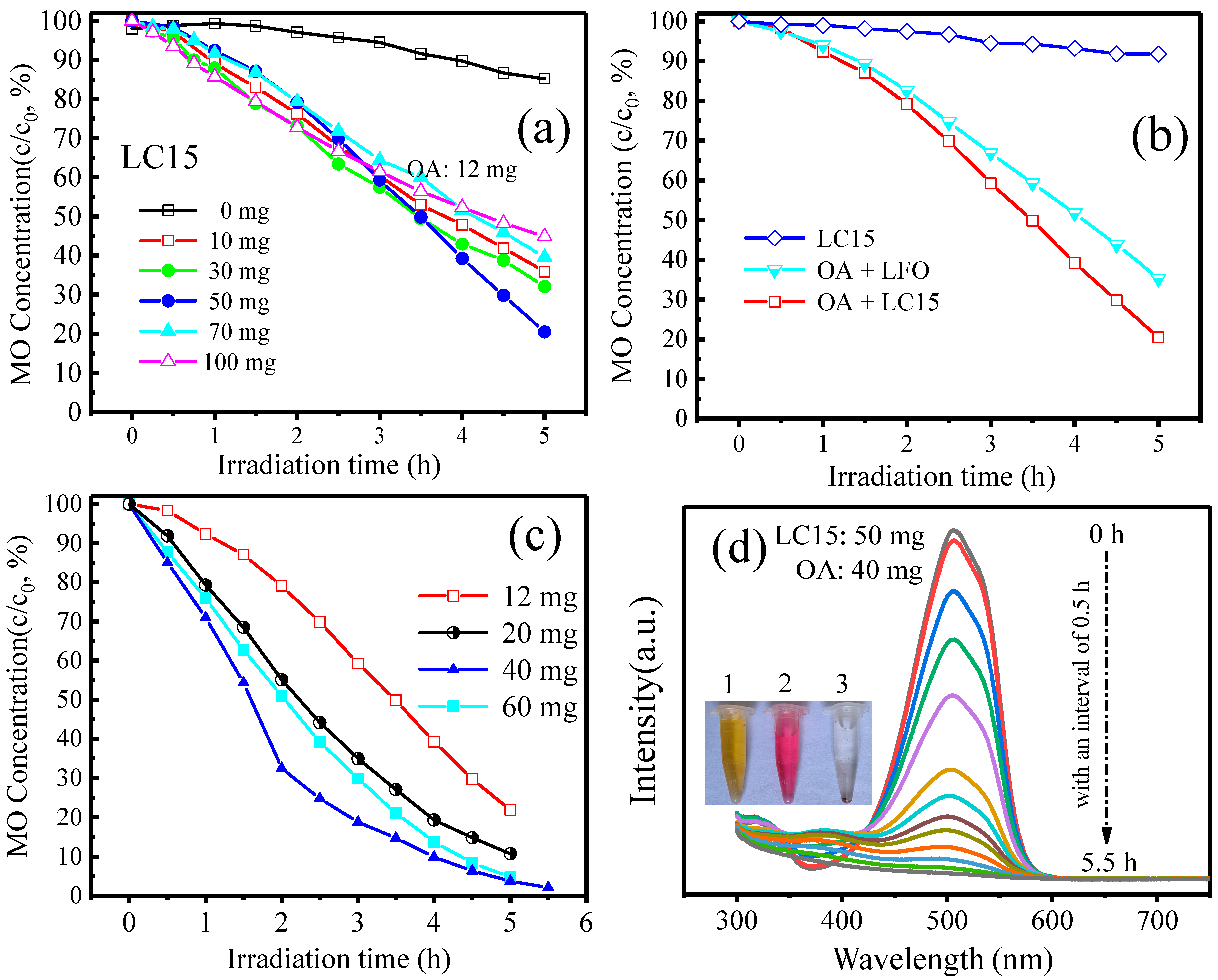
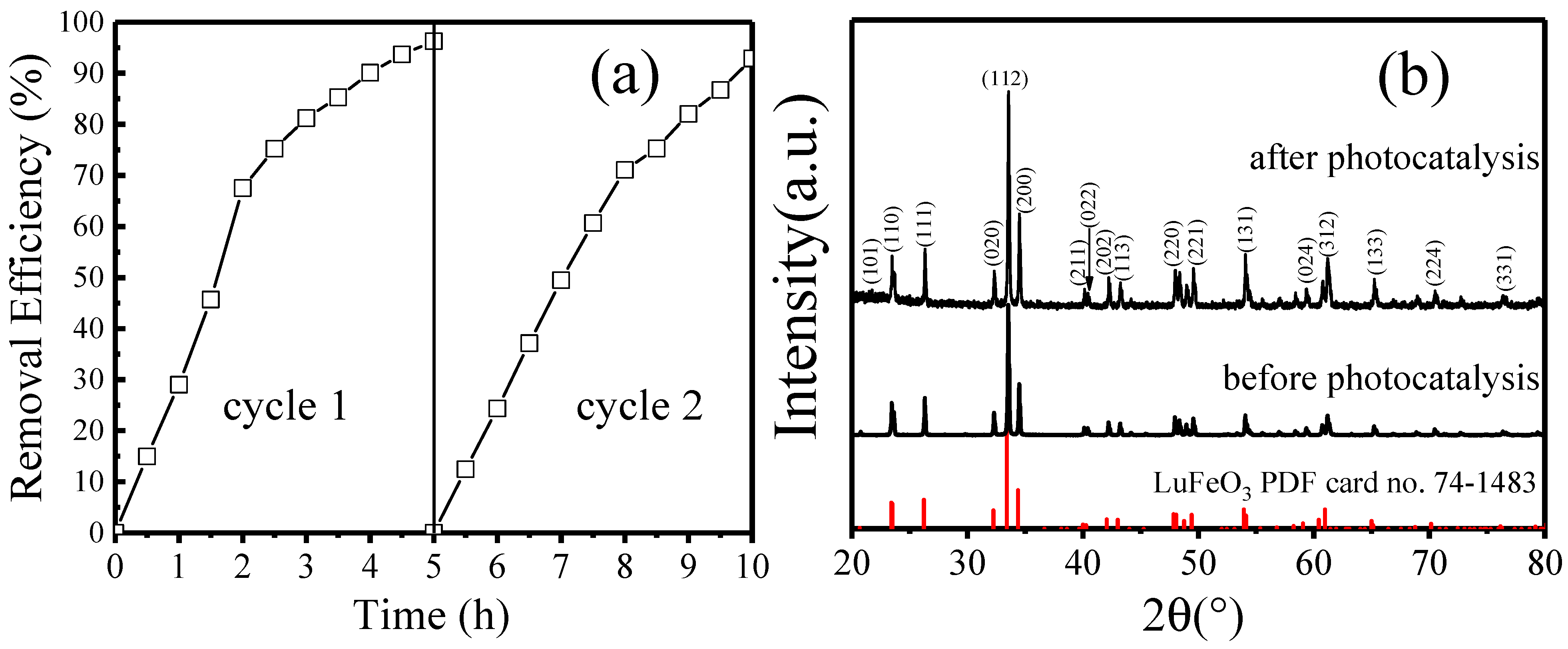
2.4. Photocatalytic Degradation of MO under Visible Light Irradiation
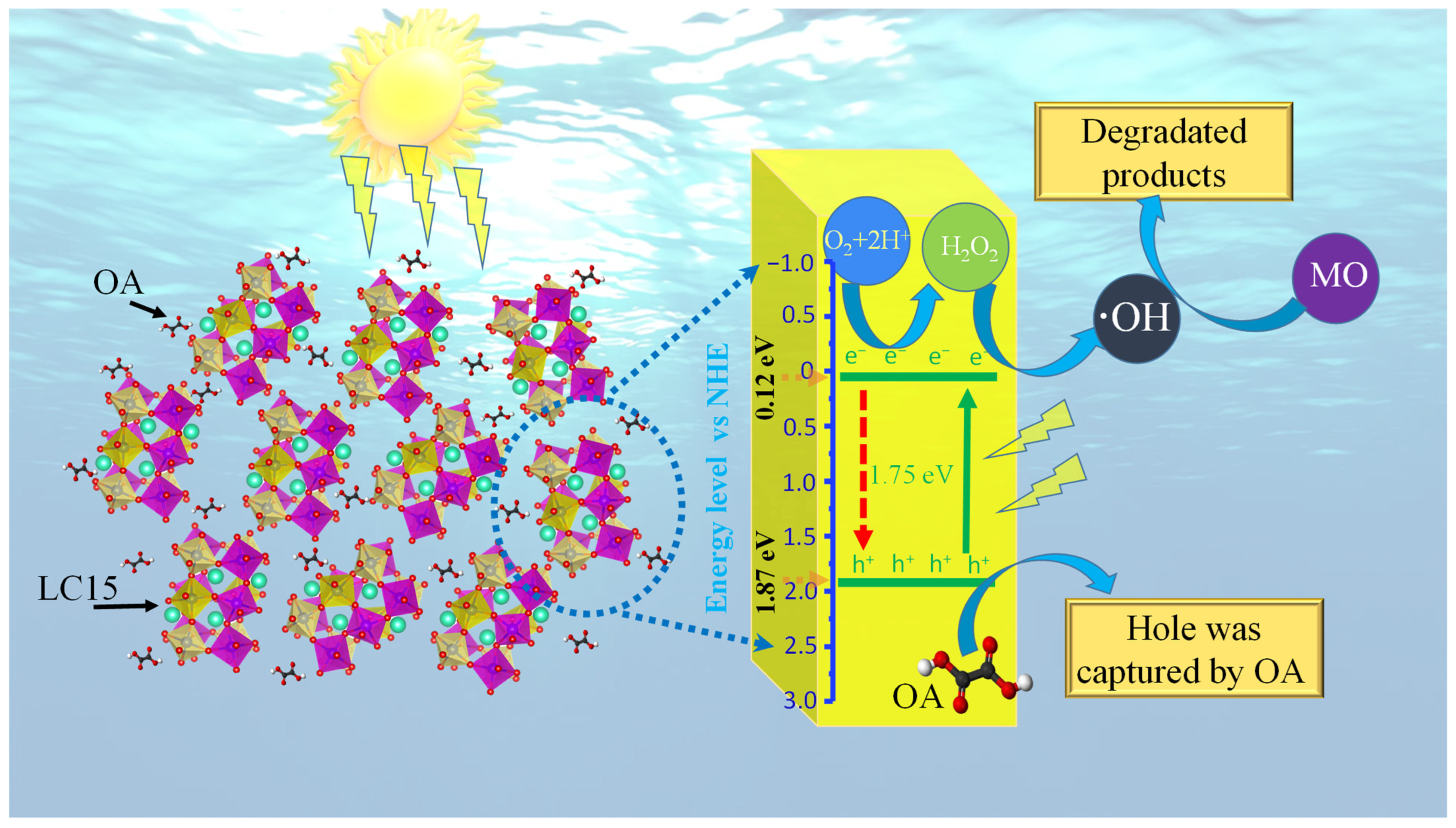
2.5. Possible Degradation Mechanism
3. Experimental Section
3.1. Chemicals
3.2. Preparation of B-Site Co-Doped LuFeO3 Powders
3.3. Degradation of MO under Visible Light Irradiation
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dong, S.; Xiang, H.; Dagotto, E. Magnetoelectricity in multiferroics: A theoretical perspective. Natl. Sci. Rev. 2019, 6, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Baltz, V.; Manchon, A.; Tsoi, M.; Moriyama, T.; Ono, T.; Tserkovnyak, Y. Antiferromagnetic spintronics. Rev. Mod. Phys. 2018, 90, 015005. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Yang, H.; Xian, T.; Zhang, C.R. A new photocatalyst of LuFeO3 for the dye degradation. Phys. Scr. 2015, 90, 085808. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Jiang, H.; Zhang, L.; Zhang, H.; Cao, Y. Catalytic removal of toluene over three-dimensionally ordered macroporous Eu1–xSrxFeO3. Chem. Eng. J. 2013, 214, 262–271. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Niu, X.; Du, W.; Du, W. Preparation, characterization and gas-sensing properties of rare earth mixed oxides. Sens. Actuators B-Chem. 2004, 99, 399–404. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, Y.; Taguchi, Y.; Arima, T.-H.; Tokura, Y. Electric-field-induced generation and reversal of ferromagnetic moment in ferrites. Nat. Phys. 2012, 8, 838–844. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Heterogeneous photo-Fenton degradation of organics using highly efficient Cu-doped LaFeO3 under visible light. J. Ind. Eng. Chem. 2018, 61, 53–64. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Zhang, Y.; Li, X.; Cui, X.; Yang, G. Single Selenium Atomic Vacancy Enabled Efficient Visible-Light-Response Photocatalytic NO Reduction to NH3 on Janus WSSe Monolayer. Molecules 2023, 28, 2959. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Li, J.; Dong, H.; Yang, S.; Gao, Y.; Liu, W. Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction. Molecules 2023, 28, 4602. [Google Scholar] [CrossRef] [PubMed]
- Nethercot, A.H. Prediction of Fermi Energies and Photoelectric Thresholds Based on Electronegativity Concepts. Phys. Rev. Lett. 1974, 33, 1088–1091. [Google Scholar] [CrossRef]
- White, R.L. Review of Recent Work on the Magnetic and Spectroscopic Properties of the Rare-Earth Orthoferrites. J. Appl. Phys. 1969, 40, 1061–1069. [Google Scholar] [CrossRef]
- Yamaguchi, T. Theory of spin reorientation in rare-earth orthochromites and orthoferrites. J. Phys. Chem. Solids 1974, 35, 479–500. [Google Scholar] [CrossRef]
- Zhu, W.; Pi, L.; Tan, S.; Zhang, Y. Anisotropy and extremely high coercivity in weak ferromagnetic LuFeO3. Appl. Phys. Lett. 2012, 100, 052407. [Google Scholar] [CrossRef]
- Sun, H.H.; Kim, U.-H.; Park, J.-H.; Park, S.-W.; Seo, D.-H.; Heller, A.; Mullins, C.B.; Yoon, C.S.; Sun, Y.-K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Ojha, G.P.; Kim, A.A.; Kim, H.J. Manganese-doped tungsten disulfide microcones as binder-free electrode for high performance asymmetric supercapacitor. J. Energy Storage 2022, 47, 103674. [Google Scholar] [CrossRef]
- Arshad, Y.; Khan, S.; Hashmi, M.A.; Ayub, K. Transition metal doping: A new and effective approach for remarkably high nonlinear optical response in aluminum nitride nanocages. New J. Chem. 2018, 42, 6976–6989. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Recent development of transition metal doped carbon materials derived from biomass for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 32436–32454. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Synthesis of high-performance nickel hydroxide nanosheets/gadolinium doped-α-MnO2 composite nanorods as cathode and Fe3O4/GO nanospheres as anode for an all-solid-state asymmetric supercapacitor. J. Energy Chem. 2022, 64, 475–484. [Google Scholar] [CrossRef]
- Polat, O.; Caglar, M.; Coskun, F.M.; Coskun, M.; Caglar, Y.; Turut, A. An experimental investigation: The impact of cobalt doping on optical properties of YbFeO3−ẟ thin film. Mater. Res. Bull. 2019, 119, 110567. [Google Scholar] [CrossRef]
- Ishihara, T.; Furutani, H.; Honda, M.; Yamada, T.; Shibayama, T.; Akbay, T.; Sakai, N.; Yokokawa, H.; Takita, Y. Improved Oxide Ion Conductivity in La0.8Sr0.2Ga0.8Mg0.2O3 by Doping Co. Chem. Mater. 1999, 11, 2081–2088. [Google Scholar] [CrossRef]
- Feng, G.; Xue, Y.; Shen, H.; Feng, S.; Li, L.; Zhou, J.; Yang, H.; Xu, D. Sol–gel synthesis, solid sintering, and thermal stability of single-phase YCoO3. Phys. Status Solidi A 2012, 209, 1219–1224. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, J.; Han, Z.; Zhao, M.; Wu, Z.; Zhang, Y.; Qin, H. Electrical and CO-sensing properties of NdFe1-xCoxO3 perovskite system. J. Rare Earth 2010, 28, 591–595. [Google Scholar] [CrossRef]
- Somvanshi, A.; Husain, S.; Khan, W. Investigation of structure and physical properties of cobalt doped nano-crystalline neodymium orthoferrite. J. Alloys Compd. 2019, 778, 439–451. [Google Scholar] [CrossRef]
- Nforna, E.A.; Tsobnang, P.K.; Fomekong, R.L.; Tedjieukeng, H.M.K.; Lambi, J.N.; Ghogomu, J.N. Effect of B-site Co substitution on the structure and magnetic properties of nanocrystalline neodymium orthoferrite synthesized by auto-combustion. R. Soc. Open Sci. 2021, 8, 201883. [Google Scholar] [CrossRef]
- Somvanshi, A.; Husain, S.; Manzoor, S.; Zarrin, N.; Ahmad, N.; Want, B.; Khan, W. Tuning of magnetic properties and multiferroic nature: Case study of cobalt-doped NdFeO3. Appl. Phys. A 2021, 127, 174. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, T.L.; Mittova, I.Y.; Mittova, V.O.; Nguyen, T.L.T.; Nguyen, H.V.; Bui, V.X. Co-Doped NdFeO3 Nanoparticles: Synthesis, Optical, and Magnetic Properties Study. Nanomaterials 2021, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.; Coskun, M.; Coskun, F.M.; Zlamal, J.; Kurt, B.Z.; Durmus, Z.; Caglar, M.; Turut, A. Co doped YbFeO3: Exploring the electrical properties via tuning the doping level. Ionics 2019, 25, 4013–4029. [Google Scholar] [CrossRef]
- Qahtan, A.A.A.; Husain, S.; Somvanshi, A.; Fatema, M.; Khan, W. Investigation of alteration in physical properties of dysprosium orthochromite instigated through cobalt doping. J. Alloys Compd. 2020, 843, 155637. [Google Scholar] [CrossRef]
- Haque, A.; Bhattacharya, S.; Das, R.; Hossain, A.; Gayen, A.; Kundu, A.K.; Vasundhara, M.; Seikh, M.M. Effects of Bi doping on structural and magnetic properties of cobalt ferrite perovskite oxide LaCo0.5Fe0.5O3. Ceram. Int. 2022, 48, 16348–16356. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lan, Y.-S.; Zeng, Z.-Y.; Chen, X.-R.; Chen, Q.-F. Magnetic structures and optical properties of rare-earth orthoferrites RFeO3 (R=Ho, Er, Tm and Lu). Solid State Commun. 2019, 288, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, W.; Zhang, J.; Huang, J.; Dong, M.; Yuan, H.; Xu, T.; Shi, L.; Dai, Y.; Liu, Q.; et al. Effects of mechanochemical activation on the structural and electrical properties of orthorhombic LuFeO3 ceramics. J. Am. Ceram. Soc. 2021, 104, 3019–3029. [Google Scholar] [CrossRef]
- Hill, R.J.; Howard, C.J. Quantitative phase analysis from neutron powder diffraction data using the Rietveld method. J. Appl. Crystallogr. 1987, 20, 467–474. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: London, UK, 1995; p. 308. [Google Scholar]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Baloch, A.A.B.; Alqahtani, S.M.; Mumtaz, F.; Muqaibel, A.H.; Rashkeev, S.N.; Alharbi, F.H. Extending Shannon’s ionic radii database using machine learning. Phys. Rev. Mater. 2021, 5, 043804. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Rao, C.N.R.; Ferraro, J.R. Infrared and Electronic Spectra of Rare Earth Perovskites: Ortho-Chromites, -Manganites and -Ferrites. Appl. Spectrosc. 1970, 24, 436–445. [Google Scholar] [CrossRef]
- Kaczmarek, W.; Paja̧k, Z.; Połomska, M. Differential thermal analysis of phase transitions in (Bi1−xLax)FeO3 solid solution. Solid State Commun. 1975, 17, 807–810. [Google Scholar] [CrossRef]
- Govindan, K.; Chandran, H.T.; Raja, M.; Maheswari, S.U.; Rangarajan, M. Electron scavenger-assisted photocatalytic degradation of amido black 10B dye with Mn3O4 nanotubes: A response surface methodology study with central composite design. J. Photochem. Photobiol. A Chem. 2017, 341, 146–156. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Zhu, L.P.; Deng, H.M.; Sun, L.; Yang, J.; Yang, P.X.; Chu, J.H. Optical properties of multiferroic LuFeO3 ceramics. Ceram. Int. 2014, 40 Pt A, 1171–1175. [Google Scholar] [CrossRef]
- Adams, D.J.; Amadon, B. Study of the volume and spin collapse in orthoferrite LuFeO3 using LDA+U. Phys. Rev. B 2009, 79, 115114. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Yang, H.; Xian, T.; Ma, J.Y.; Zhang, H.M.; Feng, W.J.; Wei, Z.Q.; Jiang, J.L. Morphology-controlled synthesis of orthorhombic LuFeO3 particles via a hydrothermal route. J. Alloys Compd. 2014, 617, 855–862. [Google Scholar] [CrossRef]
- Polat, O.; Durmus, Z.; Coskun, F.M.; Coskun, M.; Turut, A. Engineering the band gap of LaCrO3 doping with transition metals (Co, Pd, and Ir). J. Mater. Sci. 2018, 53, 3544–3556. [Google Scholar] [CrossRef]
- Polat, O.; Coskun, M.; Coskun, F.M.; Durmus, Z.; Caglar, M.; Turut, A. Os doped YMnO3 multiferroic: A study investigating the electrical properties through tuning the doping level. J. Alloys Compd. 2018, 752, 274–288. [Google Scholar] [CrossRef]
- Polat, O.; Coskun, M.; Coskun, F.M.; Zengin Kurt, B.; Durmus, Z.; Caglar, Y.; Caglar, M.; Turut, A. Electrical characterization of Ir doped rare-earth orthoferrite YbFeO3. J. Alloys Compd. 2019, 787, 1212–1224. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Matin, M.A.; Hossain, M.N.; Mozahid, F.A.; Hakim, M.A.; Islam, M.F. Bandgap engineering of cobalt-doped bismuth ferrite nanoparticles for photovoltaic applications. Bull. Mater. Sci. 2019, 42, 190. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Chapter Two—Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar]
- Kuriechen, S.K.; Murugesan, S.; Raj, S.P.; Maruthamuthu, P. Visible light assisted photocatalytic mineralization of Reactive Red 180 using colloidal TiO2 and oxone. Chem. Eng. J. 2011, 174, 530–538. [Google Scholar] [CrossRef]
- Polat, O.; Coskun, F.M.; Yildirim, Y.; Sobola, D.; Ercelik, M.; Arikan, M.; Coskun, M.; Sen, C.; Durmus, Z.; Caglar, Y.; et al. The structural studies and optical characteristics of phase-segregated Ir-doped LuFeO3−δ films. Appl. Phys. A 2023, 129, 198. [Google Scholar] [CrossRef]
- Tomkiewicz, M. Scaling properties in photocatalysis. Catal. Today 2000, 58, 115–123. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Crittenden, J.; Minakata, D.; Chen, Y.; Wang, P. Effects of inorganic electron donors in photocatalytic hydrogen production over Ru/(CuAg)0.15In0.3Zn1.4S2 under visible light irradiation. J. Renew. Sustain. Energy 2014, 6, 033131. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Springer: New York, NY, USA, 1980; p. 183. [Google Scholar]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Cu2O/BiVO4 heterostructures: Synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and absolute hardness of Lewis acids and bases. J. Am. Chem. Soc. 1985, 107, 6801–6806. [Google Scholar] [CrossRef]
- Andersen, T.; Haugen, H.K.; Hotop, H. Binding Energies in Atomic Negative Ions: III. J. Phys. Chem. Ref. Data 1999, 28, 1511–1533. [Google Scholar] [CrossRef]
- Ning, C.; Lu, Y. Electron Affinities of Atoms and Structures of Atomic Negative Ions. J. Phys. Chem. Ref. Data 2022, 51, 021502. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Liu, J.; Cheng, K.; Sun, W.; Lin, J. Enhanced Visible Light Photocatalysis of Bi2O3 upon Fluorination. J. Phys. Chem. C 2013, 117, 20029–20036. [Google Scholar] [CrossRef]
- Tachikawa, T.; Fujitsuka, M.; Majima, T. Mechanistic Insight into the TiO2 Photocatalytic Reactions: Design of New Photocatalysts. J. Phys. Chem. C 2007, 111, 5259–5275. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Cheng, K.; Lin, J. Crystalline metallic Au nanoparticle-loaded α-Bi2O3 microrods for improved photocatalysis. Phys. Chem. Chem. Phys. 2012, 14, 12114–12121. [Google Scholar] [CrossRef]
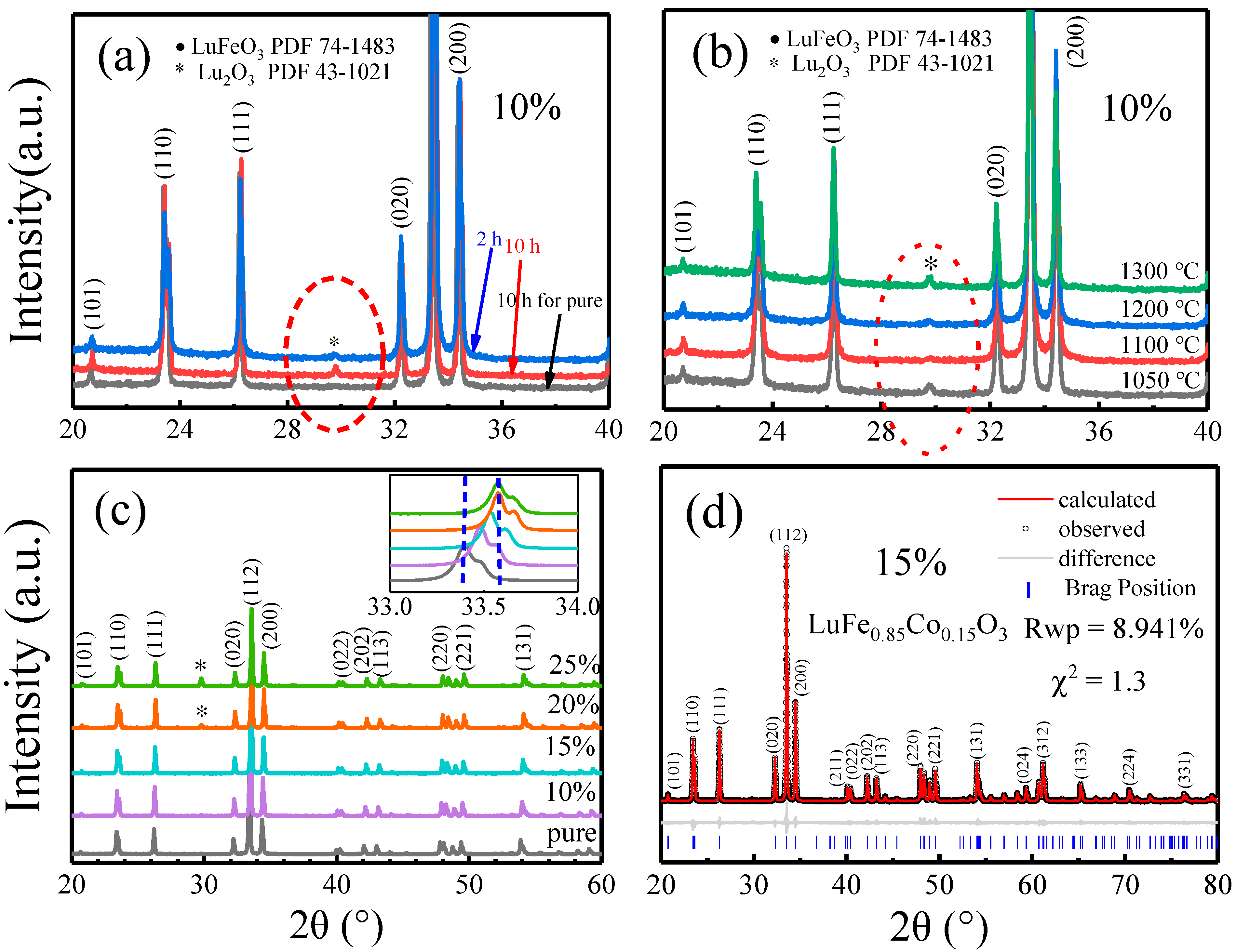

| Sample Space Group Rwp χ2 | Lattice Parameters (Å) | Atoms | Positions | Volume (Å3) | Angle Fe-O-Fe | |||
|---|---|---|---|---|---|---|---|---|
| Wyckoff | x | y | z | |||||
| LuFe0.85Co0.15O3 Pbnm 8.94 1.3 | a = 5.1976 ± 1 | Lu | 4c | 0.9800 | 0.0714 | 0.25 | 216.5313 ± 24 | |
| b = 5.5373 ± 1 | Fe(Co) | 4b | 0 | 0.5 | 0 | |||
| c = 7.5235 ± 1 | O1 | 4c | 0.1190 | 0.4539 | 0.25 | 140.601 | ||
| O2 | 8d | 0.6893 | 0.3071 | 0.0621 | 142.406 | |||
| LuFeO3 [33] Pbnm 7.74 1.46 | a = 5.2154 ± 1 | Lu | 4c | 0.9792 | 0.0715 | 0.25 | 219.0780 ± 35 | |
| b = 5.5537 ± 1 | Fe | 4b | 0 | 0.5 | 0 | |||
| c = 7.5637 ± 1 | O1 | 4c | 0.1179 | 0.4579 | 0.25 | 141.632 | ||
| O2 | 8d | 0.6890 | 0.3050 | 0.0638 | 142.121 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shi, C.; Li, P.; Wang, W.; Xiao, W.; Sun, T.; Zhang, J. Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance. Molecules 2023, 28, 5730. https://doi.org/10.3390/molecules28155730
Wang Z, Shi C, Li P, Wang W, Xiao W, Sun T, Zhang J. Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance. Molecules. 2023; 28(15):5730. https://doi.org/10.3390/molecules28155730
Chicago/Turabian StyleWang, Zhi, Changmin Shi, Pengfei Li, Wenzhu Wang, Wenzhen Xiao, Ting Sun, and Jing Zhang. 2023. "Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance" Molecules 28, no. 15: 5730. https://doi.org/10.3390/molecules28155730
APA StyleWang, Z., Shi, C., Li, P., Wang, W., Xiao, W., Sun, T., & Zhang, J. (2023). Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance. Molecules, 28(15), 5730. https://doi.org/10.3390/molecules28155730






