N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
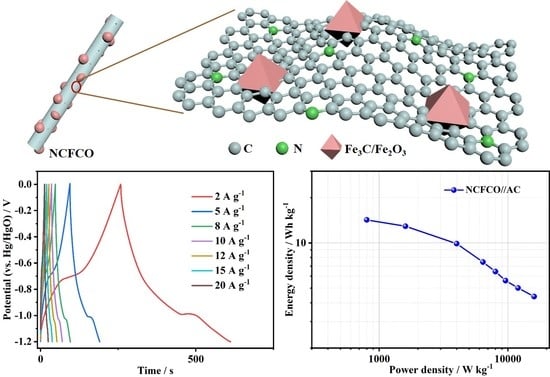
2.1. Characterizations
2.2. Electrochemical Measurements
3. Experimental
3.1. Chemicals and Regents
3.2. Preparations of the NCFC, NCFCO, and NCFO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, X.; Lou, D.; Wang, H.; Sun, X.; Li, J.; Liu, Y.N. Flexible supercapacitor based on organohydrogel electrolyte with long-term anti-freezing and anti-drying property. Adv. Funct. Mater. 2020, 30, 2007291. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Cui, Y.; Wang, X.; Yong, Z.; Liang, D.; Chi, Y.; Wang, Z. Sandwich-like MXene/alpha-Fe2O3-C-MoS2-PEDOT:PSS/MXene film electrodes with ultrahigh area capacitance for flexible supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 9172–9182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- He, Y.; Wei, Q.; An, N.; Meng, C.; Hu, Z. Organic small-molecule electrodes: Emerging organic composite materials in supercapacitors for efficient energy storage. Molecules 2022, 27, 7692. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.-S.; Vinodh, R.; Pollet, B.G.; Babu, R.S.; Ramkumar, V.; Kim, S.-C.; Krishnakumar, K.; Kim, H.-J. A high-performance asymmetric supercapacitor consists of binder free electrode materials of bimetallic hydrogen phosphate (MnCo(HPO4)) hexagonal tubes and graphene ink. Electrochim. Acta 2022, 426, 140763. [Google Scholar] [CrossRef]
- Li, B.; Yu, M.; Li, Z.; Yu, C.; Wang, H.; Li, Q. Constructing flexible all-solid-state supercapacitors from 3D nanosheets active bricks via 3D manufacturing technology: A perspective review. Adv. Funct. Mater. 2022, 32, 2201166. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Zhu, G.; Shi, J.; Lu, T.; Pan, L. Biomass-based N, P, and S self-doped porous carbon for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 12052–12060. [Google Scholar] [CrossRef]
- Qiu, H.; Cheng, H.; Meng, J.; Wu, G.; Chen, S. Magnetothermal microfluidic-assisted hierarchical microfibers for ultrahigh-energy-density supercapacitors. Angew. Chem. Int. Ed. 2020, 59, 7934–7943. [Google Scholar] [CrossRef]
- Roy, B.K.; Tahmid, I.; Rashid, T.U. Chitosan-based materials for supercapacitor applications: A review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded graphite-based materials for supercapacitors: A review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Yang, L.; Jia, J.; Cheng, S.; Chen, H.; Wu, Z.-S.; Passarello, D.; Liu, M. Targeted synthesis and reaction mechanism discussion of Mo2C based insertion-type electrodes for advanced pseudocapacitors. J. Mater. Chem. A 2020, 8, 7819–7827. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational design of electrode materials for advanced supercapacitors: From lab research to commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Li, Z.; Shi, Z.; Zhu, J.; Mei, H. Fe2O3/N doped rGO anode hybridized with NiCo LDH/Co(OH)2 cathode for battery-like supercapacitor. Chem. Eng. J. 2021, 403, 126325. [Google Scholar] [CrossRef]
- Chen, L.; Liang, B.; Lv, J.; Chen, M.; Hu, J.; Zeng, K.; Yang, G. Route to a porous carbon nanofiber membrane containing FexCy/Fe by facile in situ ion-exchange functionalization of the PAA carboxyl group: Exemplified by a supercapacitor. ACS Appl. Energy Mater. 2022, 5, 1580–1594. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.; Xiao, J.; Cen, W.; Yuan, S. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, S.; Zhou, W.; Jia, J.; Yang, L.; Yao, M.; Wang, M.; Zhou, J.; Wu, P.; Liu, M. Construction and performance characterization of α-Fe2O3/rGO composite for long-cycling-life supercapacitor anode. ACS Sustain Chem. Eng. 2017, 5, 5067–5074. [Google Scholar] [CrossRef]
- Jiang, S.-H.; Ding, J.; Wang, R.-H.; Chen, F.-Y.; Sun, J.; Deng, Y.-X.; Li, X.-L. Solvothermal-induced construction of ultra-tiny Fe2O3 nanoparticles/graphene hydrogels as binder-free high-capacitance anode for supercapacitors. Rare Met. 2021, 40, 3520–3530. [Google Scholar] [CrossRef]
- Shi, T.-Z.; Feng, Y.-L.; Peng, T.; Yuan, B.-G. Sea urchin-shaped Fe2O3 coupled with 2D MXene nanosheets as negative electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 2021, 381, 138245. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Chen, Z.; Jiang, Z.; Li, H.; Cao, H.; Liu, Y.; Zhu, Y.; Fang, Z.; Yu, X. Rational design of novel ultra-small amorphous Fe2O3 nanodots/graphene heterostructures for all-solid-state asymmetric supercapacitors. Nano Res. 2020, 14, 953–960. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Shang, X.; Xu, K.; Yang, J.; Huang, M.; Liu, J. Structure-tunable Mn3O4-Fe3O4@C hybrids for high-performance supercapacitor. J. Colloid. Interf. Sci. 2021, 581, 66–75. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-step preparation of ultrasmall iron oxide-embedded carbon nanotubes on carbon cloth with excellent superhydrophilicity and enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2021, 13, 45670–45678. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, M.; Xu, X.; Lu, T.; Sun, C.Q.; Pan, L. Phosphorus-doped 3D carbon nanofiber aerogels derived from bacterial-cellulose for highly-efficient capacitive deionization. Carbon 2018, 130, 377–383. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, J.; Li, J.; Cong, Z.; Wei, L.; Liu, M.; Zhai, S.; Wang, K.; An, Q. Lignin-derived electrode materials for supercapacitor applications: Progress and perspectives. J. Mater. Chem. A 2023, 11, 1061–1082. [Google Scholar] [CrossRef]
- Cai, D.; Du, J.; Zhu, C.; Cao, Q.; Huang, L.; Wu, J.; Zhou, D.; Xia, Q.; Chen, T.; Guan, C.; et al. Iron oxide nanoneedles anchored on N-doped carbon nanoarrays as an electrode for high-performance hybrid supercapacitor. ACS Appl. Energ. Mater. 2020, 3, 12162–12171. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, S.; Zhang, J.; Ning, F.; Ma, C.; Qiu, Z. Facile fabrication of oxygen vacancy-rich α-Fe2O3 microspheres on carbon cloth as negative electrode for supercapacitors. Electrochim. Acta 2020, 338, 135820. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Yan, J.; Guan, C.; Wang, J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ. Mater. 2020, 4, 502–521. [Google Scholar] [CrossRef]
- Tong, Z.; Huang, L.; Lei, W.; Zhang, H.; Zhang, S. Carbon-containing electrospun nanofibers for lithium-sulfur battery: Current status and future directions. J. Energy Chem. 2021, 54, 254–273. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Xu, X.; Chen, L.; Lu, T.; Hill, J.P.; Pan, L.; Yamauchi, Y. Embedding metal-organic frameworks for the design of flexible hybrid supercapacitors by electrospinning: Synthesis of highly graphitized carbon nanofibers containing metal oxide nanoparticles. Small Struct. 2022, 3, 2200015. [Google Scholar] [CrossRef]
- Wang, H.; Yao, L.; Zuo, H.; Ruan, F.; Wang, H. Fabrication of porous carbon nanofibers from polymer blends using template method for electrode-active materials in supercapacitor. Molecules 2023, 28, 2228. [Google Scholar] [CrossRef]
- Zhang, X.; Han, R.; Liu, Y.; Li, H.; Shi, W.; Yan, X.; Zhao, X.; Li, Y.; Liu, B. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: A review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar] [CrossRef]
- Dong, K.; Liang, J.; Wang, Y.; Xu, Z.; Liu, Q.; Luo, Y.; Li, T.; Li, L.; Shi, X.; Asiri, A.M.; et al. Honeycomb carbon nanofibers: A superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. 2021, 60, 10583–10587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, X.; Bai, Y.; Yang, H.; Dong, R.; Wang, X.; Xu, H.; Wang, Q.; Li, H.; Gao, H.; et al. Probing the energy storage mechanism of quasi-metallic Na in hard carbon for sodium-ion batteries. Adv. Energy Mater. 2021, 11, 2003854. [Google Scholar] [CrossRef]
- Gao, W.; Li, Y.; Zhao, J.; Zhang, Z.; Tang, W.; Wang, J.; Wu, Z.; Li, Z. Design and preparation of graphene/Fe2O3 nanocomposite as negative material for supercapacitor. Chem. Res. Chin. Univ. 2022, 38, 1097–1104. [Google Scholar] [CrossRef]
- Su, X.; Ye, C.; Li, X.; Guo, M.; Cao, R.; Ni, K.; Zhu, Y. Heterogeneous stacking carbon films for optimized supercapacitor performance. Energy Storage Mater. 2022, 50, 365–372. [Google Scholar] [CrossRef]
- Hou, X.; Ren, P.; Dai, Z.; Chen, H.; Tang, W.; Chen, Z.; Ren, F.; Jin, Y. Ultrahigh voltage window, preeminent energy density aqueous supercapacitor derived from honeycomb-like porous carbon decorated with carbon dots. Electrochim. Acta 2022, 425, 140336. [Google Scholar] [CrossRef]
- Goswami, R.N.; Mourya, P.; Behera, B.; Khatri, O.P.; Ray, A. Graphene-polyaniline nanocomposite based coatings: Role of convertible forms of polyaniline to mitigate steel corrosion. Appl. Surf. Sci. 2022, 599, 153939. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Wang, P.; Liang, C.; Yu, K. Preparation and mechanism of biomass-derived graphene-like Li+/Na+ battery anodes controlled by N/O functional groups and interlayer spacing. J. Alloys Compd. 2022, 918, 165785. [Google Scholar] [CrossRef]
- Wee, J.-H.; Kim, Y.A.; Yang, C.-M. Sequential doping of nitrogen and oxygen in single-walled carbon nanohorns for use as supercapacitor electrodes. Micropor. Mesopor. Mater. 2021, 310, 110595. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Liu, S.; Che, X.; He, S.; Chen, G.; Qiu, J. Nitrogen-doped porous carbon electrode for aqueous iodide redox supercapacitor. Chem. Eng. J. 2023, 451, 138501. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Wang, S. Self-assembly of biomass derivatives into multiple heteroatom-doped 3D-interconnected porous carbon for advanced supercapacitors. Carbon 2022, 199, 258–267. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, G.; Ma, D.; Chen, L.; Zhu, G.; Zhu, J.; Shen, X. Phosphate functionalized CoS nanoparticles coupled with Fe2O3 nanocrystals decorated on N, S co-doped porous carbon spheres for advanced hybrid supercapacitors. Inorg. Chem. Front. 2023, 10, 406–416. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Jiang, H.; Ding, Y.; He, G.; Guo, J.; Chu, Z.; Yu, G. Pulverizing Fe2O3 nanoparticles for developing Fe3C/N-codoped carbon nanoboxes with multiple polysulfide anchoring and converting activity in Li-S batteries. Adv. Funct. Mater. 2021, 31, 2011249. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, D.; Wang, T. N-doped carbon nanosheet supported Fe2O3/Fe3C nanoparticles as efficient electrode materials for oxygen reduction reaction and supercapacitor application. Inorg. Chem. Commun. 2020, 117, 107952. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.; Zhong, Y.; Zheng, J.; Deng, K.; Lv, X.; Li, H.; Tian, W.; Ji, J. N, S co-doped branched carbon nanotubes with hierarchical porous structure and electron/ion transfer pathways for supercapacitors and lithium-ion batteries. Carbon 2022, 198, 91–100. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Cui, C.; Qian, W.; Jin, Y. The progress and comprehensive analysis of supercapacitors for alternating current line filtering: A review. Batter. Supercaps 2023, 6, 202200566. [Google Scholar] [CrossRef]
- Dai, S.; Bai, Y.; Shen, W.; Zhang, S.; Hu, H.; Fu, J.; Wang, X.; Hu, C.; Liu, M. Core-shell structured Fe2O3@Fe3C@C nanochains and Ni-Co carbonate hydroxide hybridized microspheres for high-performance battery-type supercapacitor. J. Power Source 2021, 482, 228915. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Guo, X.; Wang, Z.; Mao, F.; Su, L.; Wu, H.; Wang, K.; Zhang, Q. An asymmetric supercapacitor with an interpenetrating crystalline Fe-MOF as the positive electrode and its congenetic derivative as the negative electrode. Inorg. Chem. Front. 2021, 8, 4878–4886. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, R.; Xu, W.; Ding, J.; Wang, Y.; Yan, X.; Shi, W.; Wang, M. Soluble salt assisted synthesis of hierarchical porous carbon-encapsulated Fe3C based on MOFs gel for all-solid-state hybrid supercapacitor. Chem. Eng. J. 2021, 419, 129576. [Google Scholar] [CrossRef]
- Guo, R.; Dang, L.; Liu, Z.; Lei, Z. Incorporation of electroactive NiCo2S4 and Fe2O3 into graphene aerogel for high-energy asymmetric supercapacitor. Colloids Surf. A 2020, 602, 125110. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, B.; Wang, Z.; Peter, F. Super long-life all solid-state asymmetric supercapacitor based on NiO nanosheets and α-Fe2O3 nanorods. Chem. Eng. J. 2016, 306, 193–203. [Google Scholar] [CrossRef]
- Lu, P.A.; Manikandan, M.R.; Yang, P.F.; He, Y.L.; Yang, F.; Dang, S.T.; Shi, Y.C.; Lou, W.B.; Liu, R.; Wu, S.J.; et al. Synthesis, analysis and characterization of alpha-Fe2O3 nanoparticles and their applications in supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 826. [Google Scholar] [CrossRef]
- Li, L.; Jia, C.; Shao, Z.; Wang, J.; Wang, F.; Wang, W.; Wang, H.; Zu, D.; Wu, H. Fe3O4/nitrogen-doped carbon electrodes from tailored thermal expansion toward flexible solid-state asymmetric supercapacitors. Adv. Mater. Interfaces 2019, 6, 1901250. [Google Scholar] [CrossRef]
- Peng, Z.; Huang, J.; Wang, Y.; Yuan, K.; Tan, L.; Chen, Y. Construction of a hierarchical carbon coated Fe3O4 nanorod anode for 2.6 V aqueous asymmetric supercapacitors with ultrahigh energy density. J. Mater. Chem. A 2019, 7, 27313–27322. [Google Scholar] [CrossRef]
- Su, S.; Lai, L.; Li, R.; Lin, Y.; Dai, H.; Zhu, X. Annealing-assisted dip-coating synthesis of ultrafine Fe3O4 nanoparticles/graphene on carbon cloth for flexible quasi-solid-state symmetric supercapacitors. ACS Appl. Energy Mater. 2020, 3, 9379–9389. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Yang, D.; Qin, L.; Zhang, S.; Xu, T.; Zhao, T.; Yu, Z.-Z. Ultraflexible reedlike carbon nanofiber membranes decorated with Ni-Co-S nanosheets and Fe2O3-C core-shell nanoneedle arrays as electrodes of flexible quasi-solid-state asymmetric supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1505–1516. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Bin, X.; Xia, C.; Que, W. 3D porous compact 1D/2D Fe2O3/MXene composite aerogel film electrodes for all-solid-state supercapacitors. Small 2022, 18, 2204917. [Google Scholar] [CrossRef]
- Kumar, A.; Das, D.; Sarkar, D.; Patil, S.; Shukla, A. Supercapacitors with prussian blue derived carbon encapsulated Fe/Fe3C nanocomposites. J. Electrochem. Soc. 2020, 167, 060529. [Google Scholar] [CrossRef]
- Khalafallah, D.; Miao, J.; Zhi, M.; Hong, Z. Confining self-standing CoSe2 nanostructures and Fe3C wrapped N-doped carbon frameworks with enhanced energy storage performances. Appl. Surf. Sci. 2021, 564, 150449. [Google Scholar] [CrossRef]







| C/% | N/% | O/% | Fe/% | |
|---|---|---|---|---|
| NCFC | 79.7 | 7.6 | 10.8 | 1.9 |
| NCFCO | 73.1 | 8.5 | 15.2 | 3.2 |
| NCFO | 27.8 | 2.5 | 46.2 | 23.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xie, F.; Wu, H.; Zhu, Y.; Zhang, P.; Li, Y.; Li, H.; Zhao, L.; Zhu, G. N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors. Molecules 2023, 28, 5751. https://doi.org/10.3390/molecules28155751
Li L, Xie F, Wu H, Zhu Y, Zhang P, Li Y, Li H, Zhao L, Zhu G. N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors. Molecules. 2023; 28(15):5751. https://doi.org/10.3390/molecules28155751
Chicago/Turabian StyleLi, Li, Fengting Xie, Heyu Wu, Yuanyuan Zhu, Pinghua Zhang, Yanjiang Li, Hengzheng Li, Litao Zhao, and Guang Zhu. 2023. "N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors" Molecules 28, no. 15: 5751. https://doi.org/10.3390/molecules28155751
APA StyleLi, L., Xie, F., Wu, H., Zhu, Y., Zhang, P., Li, Y., Li, H., Zhao, L., & Zhu, G. (2023). N-Doped Porous Carbon-Nanofiber-Supported Fe3C/Fe2O3 Nanoparticles as Anode for High-Performance Supercapacitors. Molecules, 28(15), 5751. https://doi.org/10.3390/molecules28155751