Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol
Abstract
:1. Introduction
2. Results and Discussions
2.1. Exploration of Various Characterization Results
2.2. Performance of CO2 Feed Gas to CH3OH Conversion Catalyzed by MoS2, MoS2/Nix, and MoS2/Cox
2.3. Intermediates and Mechanisms Properties Involved in CO2 Hydrogenation to Methanol
2.3.1. Determination of Intermediates through In Situ Infrared Spectroscopy
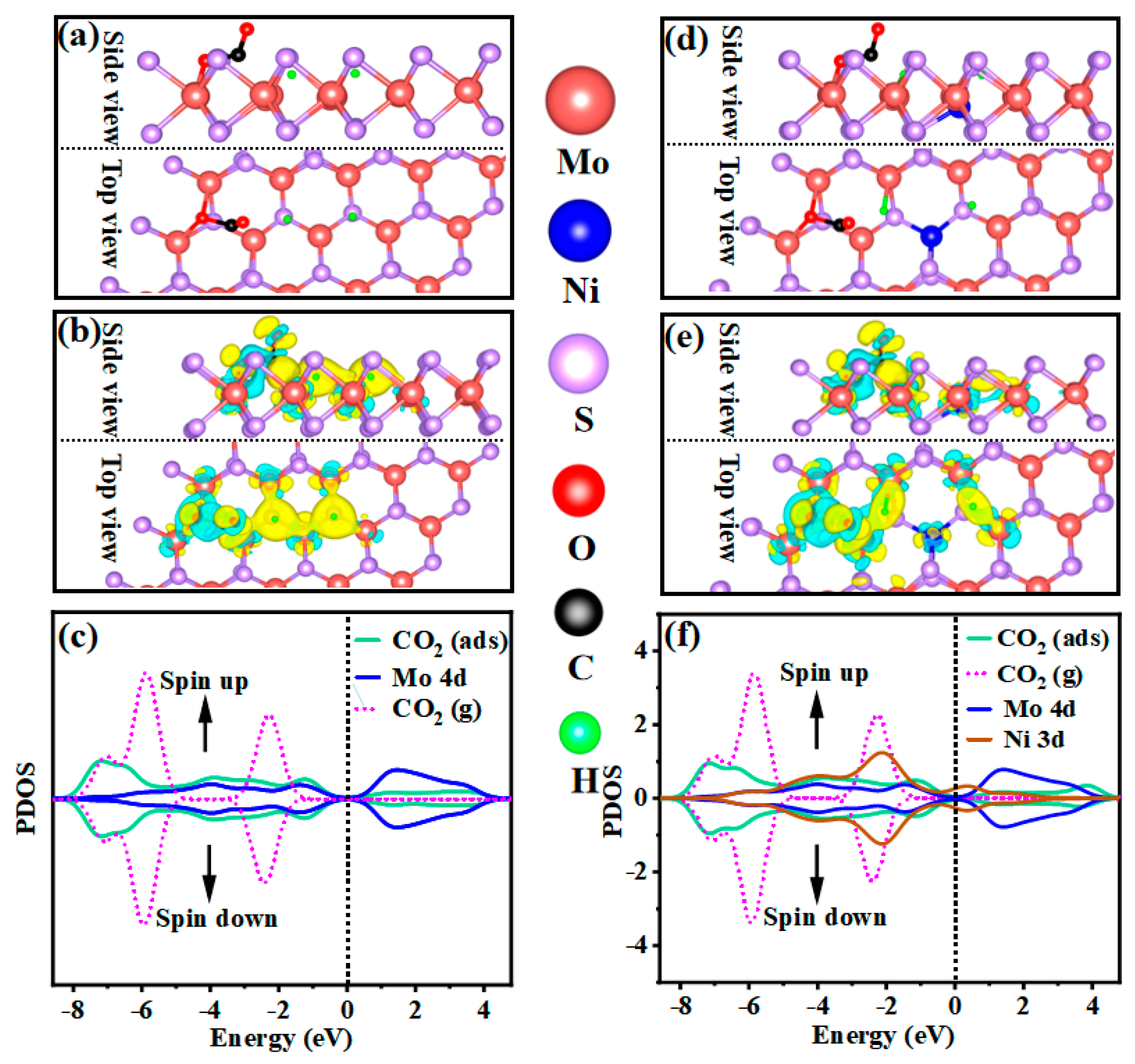
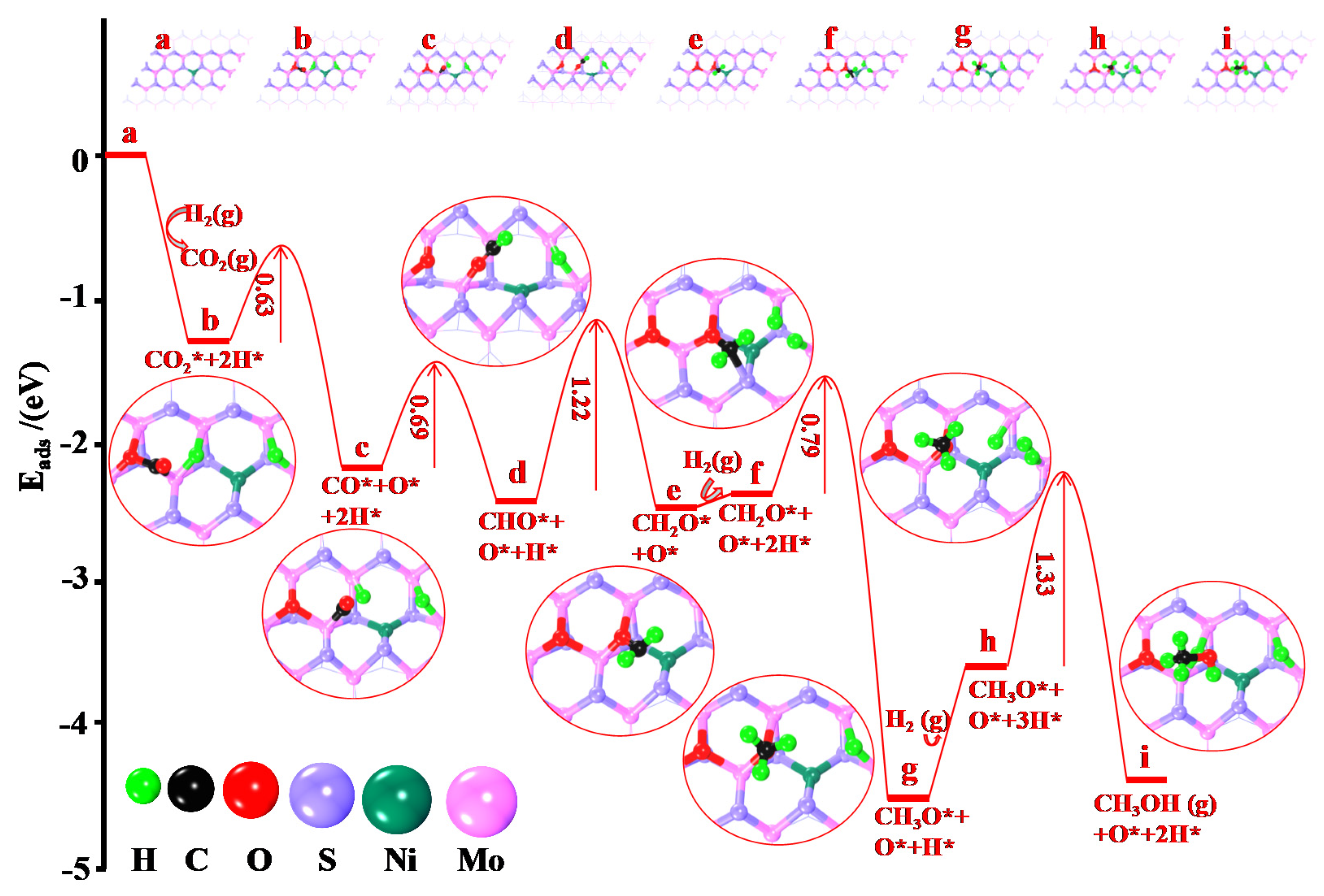
2.3.2. Determination of Mechanisms Properties through DFT Calculation
3. Material and Methods
3.1. Materials
3.2. Experimental Apparatus
3.3. Preparation of MoS2, MoS2/Ni, and MoS2/Co Materials
3.4. Characterization Methods
3.5. Catalytic Performance Test
3.6. Calculation Methods for Reactant Conversion as Well as Product Selectivity
3.7. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wei, J.; Yao, R.W.; Han, Y.; Ge, Q.J.; Sun, J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem. Soc. Rev. 2021, 50, 10764–10805. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [PubMed]
- Freeman, B.W.J.; Evans, C.D.; Musarika, S.; Morrison, R.; Newman, T.R.; Page, S.E.; Wiggs, G.F.S.; Bell, N.G.A.; Styles, D.; Wen, Y.; et al. Responsible agriculture must adapt to the wetland character of mid-latitude peatlands. Glob. Chang. Biol. 2022, 28, 3795–3811. [Google Scholar] [CrossRef]
- Asare Bediako, B.B.; Qian, Q.; Han, B. Synthesis of C2+ Chemicals from CO2 and H2 via C-C Bond Formation. Acc. Chem. Res. 2021, 54, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Ma, W.; Hu, J.; Zhou, L.; Wu, Y.; Geng, J.; Hu, X. Efficient hydrogenation of CO2 to formic acid in water without consumption of a base. Green Chem. 2022, 24, 6727–6732. [Google Scholar] [CrossRef]
- Sharma, P.; Sebastian, J.; Ghosh, S.; Creaser, D.; Olsson, L. Recent advances in hydrogenation of CO2 into hydrocarbons via methanol intermediate over heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1665–1697. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Wei, Y.; Zhu, Z.; Du, R.; Liu, S. Comprehensive study on unregulated emissions of heavy-duty SI pure methanol engine with EGR. Fuel 2022, 320, 123974. [Google Scholar] [CrossRef]
- Chein, R.; Chen, W.; Chyuan Ong, H.; Loke Show, P.; Singh, Y. Analysis of methanol synthesis using CO2 hydrogenation and syngas produced from biogas-based reforming processes. Chem. Eng. J. 2021, 426, 130835. [Google Scholar] [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based nanocatalysts for CO2 hydrogenation to methanol. Energy Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Cored, J.; Lopes, C.W.; Liu, L.; Soriano, J.; Agostini, G.; Solsona, B.; Sánchez-Tovar, R.; Concepción, P. Cu-Ga3+-doped wurtzite ZnO interface as driving force for enhanced methanol production in co-precipitated Cu/ZnO/Ga2O3 catalysts. J. Catal. 2022, 407, 149–161. [Google Scholar] [CrossRef]
- Bahri, S.; Pathak, S.; Singhahluwalia, A.; Malav, P.; Upadhyayula, S. Meta-analysis approach for understanding the characteristics of CO2 reduction catalysts for renewable fuel production. J. Clean. Prod. 2022, 339, 130653. [Google Scholar] [CrossRef]
- Khobragade, R.; Roškarič, M.; Žerjav, G.; Košiček, M.; Zavašnik, J.; Van de Velde, N.; Jerman, I.; Tušar, N.N.; Pintar, A. Exploring the effect of morphology and surface properties of nanoshaped Pd/CeO2 catalysts on CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2021, 627, 118394. [Google Scholar] [CrossRef]
- Gholinejad, M.; Khosravi, F.; Afrasi, M.; Sansano, J.M.; Nájera, C. Applications of bimetallic PdCu catalysts. Catal. Sci. Technol. 2021, 11, 2272–2652. [Google Scholar] [CrossRef]
- Xu, D.; Hong, X.; Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 2021, 393, 207–214. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Kong, L.; Wang, J.; Lv, P.; Hao, J.; Gao, X.; Yu, G. The homojunction formed by h-In2O3(110) and c-In2O3(440) promotes carbon dioxide hydrogenation to methanol on graphene oxide modified In2O3. J. Colloid Interface Sci. 2022, 623, 1048–1062. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2022, 7, 807–817. [Google Scholar] [CrossRef]
- Primo, A.; He, J.; Jurca, B.; Cojocaru, B.; Bucur, C.; Parvulescu, V.I.; Garcia, H. CO2 methanation catalyzed by oriented MoS2 nanoplatelets supported on few layers graphene. Appl. Catal. B Environ. 2019, 245, 351–359. [Google Scholar] [CrossRef]
- Prašnikar, A.; Likozar, B. Sulphur poisoning, water vapour and nitrogen dilution effects on copper-based catalyst dynamics, stability and deactivation during CO2 reduction reactions to methanol. React. Chem. Eng. 2022, 7, 1073–1082. [Google Scholar] [CrossRef]
- Liu, P.; Choi, Y.; Yang, Y.; White, M.G. Methanol Synthesis from H2 and CO2 on a Mo6S8 Cluster: A Density Functional Study. J. Phys. Chem. A 2010, 114, 3888–3895. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Dai, Y.; Pu, Z.; Lao, Z.; Chen, Y.; Wang, M.; Zheng, X.; Zhu, J.; Zhang, W.; et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 2018, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cheng, Y.; Li, S.; Yang, Z.; Wu, R. CO2 thermoreduction to methanol on the MoS2 supported single Co atom catalyst: A DFT study. Appl. Surf. Sci. 2020, 528, 147047. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics liquid-metal-amorphous-semiconductor simulation of the transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Wang, Q.; Xiao, C.; Zhang, C.; Gao, T. Interstitial diffusion of a helium atom in bulk Li4SiO4 crystal from first-principles calculations. Ceram. Int. 2020, 46, 8192–8199. [Google Scholar] [CrossRef]
- Djamil, J.; Segler, S.A.; Dabrowski, A.; Bensch, W.; Lotnyk, A.; Schürmann, U.; Kienle, L.; Hansen, S.; Beweries, T. The influence of carbon content on the structure and properties of MoSxCy photocatalysts for light-driven hydrogen generation. Dalton Trans. 2013, 42, 1287–1292. [Google Scholar] [CrossRef]
- Berhault, G.; Perez De La Rosa, M.; Mehta, A.; Yácaman, M.J.; Chianelli, R.R. The single-layered morphology of supported MoS2-based catalysts-the role of the cobalt promoter and its effects in the hydrodesulfurization of dibenzothiophene. Appl. Catal. A Gen. 2008, 345, 80–88. [Google Scholar] [CrossRef]
- Huang, Z.; Su, M.; Yang, Q.; Li, Z.; Chen, S.; Li, Y.; Zhou, X.; Li, F.; Song, Y. A general patterning approach by manipulating the evolution of two-dimensional liquid foams. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, D.; Liu, H.; Umar, A.; Wu, X. High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide. Chin. Chem. Lett. 2019, 30, 1105–1110. [Google Scholar] [CrossRef]
- Cao, H.; Wang, H.; Huang, Y.; Sun, Y.; Shi, S.; Tang, M. Quantification of gold (III) in solution and with a test stripe via the quenching of the fluorescence of molybdenum disulfide quantum dots. Microchim. Acta 2017, 184, 91–100. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Yang, L.; Jiang, Y.; Zhang, Y.; Hong, W.; Tian, Y.; Zhao, H.; Hu, J.; Zhou, L.; et al. Nickel chelate derived NiS2 decorated with bifunctional carbon: An efficient strategy to promote sodium storage performance. Adv. Funct. Mater. 2018, 28, 1803690. [Google Scholar] [CrossRef]
- Lin, Q.; Dong, X.; Wang, Y.; Zheng, N.; Zhao, Y.; Xu, W.; Ding, T. Molybdenum disulfide with enlarged interlayer spacing decorated on reduced graphene oxide for efficient electrocatalytic hydrogen evolution. J. Mater. Sci. 2020, 55, 6637–6647. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Song, A.; Wang, L.; Wang, Y.; Dong, H.; Shao, G. A novel structure of Ni-(MoS2/GO) composite coatings deposited on Ni foam under supergravity field as efficient hydrogen evolution reaction catalysts in alkaline solution. Electrochim. Acta 2017, 249, 52–63. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Wang, X.; Li, S.; Huang, W.; Dong, L.; Liu, C.; Li, Y.; Lan, Y. Molybdenum Disulfide/Nitrogen-Doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Zhang, H.; Zhang, Y.; Liu, L.; Fang, L.; Yang, X.; Gu, X.; Wang, Y. Unique three-dimensional Mo2C@MoS2 heterojunction nanostructure with S vacancies as outstanding all-pH range electrocatalyst for hydrogen evolution. J. Catal. 2019, 371, 20–26. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Goodman, K.R.; Head, A.R.; Tong, X.; Stacchiola, D.J.; White, M.G. Reactivity of a zirconia-copper inverse catalyst for CO2 hydrogenation. J. Phys. Chem. C 2020, 124, 22158–22172. [Google Scholar] [CrossRef]
- Liu, T.; Xu, D.; Wu, D.; Liu, G.; Hong, X. Spinel ZnFe2O4 regulates copper sites for CO2 hydrogenation to methanol. ACS Sustain. Chem. Eng. 2021, 9, 4033–4041. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Fluorine-modified Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Catal. Commun. 2014, 50, 78–82. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Sun, M.; Adjaye, J.; Nelson, A.E. Theoretical investigations of the structures and properties of molybdenum-based sulfide catalysts. Appl. Catal. A Gen. 2004, 263, 131–143. [Google Scholar] [CrossRef]
- Yoosuk, B.; Kim, J.H.; Song, C.; Ngamcharussrivichai, C.; Prasassarakich, P. Highly active MoS2, CoMoS2 and NiMoS2 unsupported catalysts prepared by hydrothermal synthesis for hydrodesulfurization of 4,6-dimethyldibenzothiophene. Catal. Today 2008, 130, 14–23. [Google Scholar] [CrossRef]
- Park, K.; Kim, T. Interactive mechanism of plasma-assisted CO2 capture for calcium looping cycle via in-situ DRIFTS. J. CO2 Util. 2022, 56, 101800. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Z.; Song, L.; Fu, Q. An investigation of Zr/Ce ratio influencing the catalytic performance of CuO/Ce1-Zr O2 catalyst for CO2 hydrogenation to CH3OH. J. Energy Chem. 2020, 47, 18–28. [Google Scholar] [CrossRef]
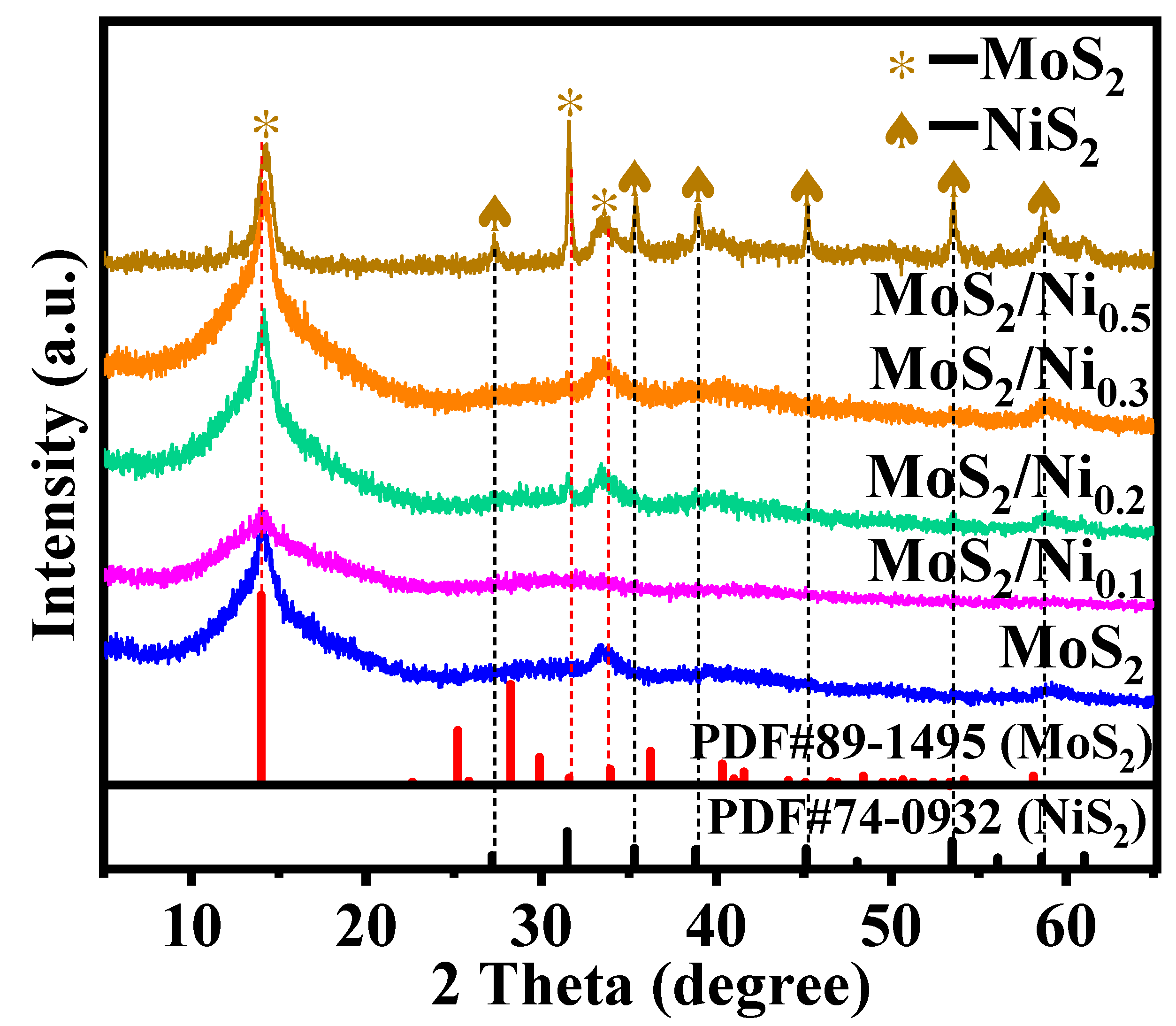
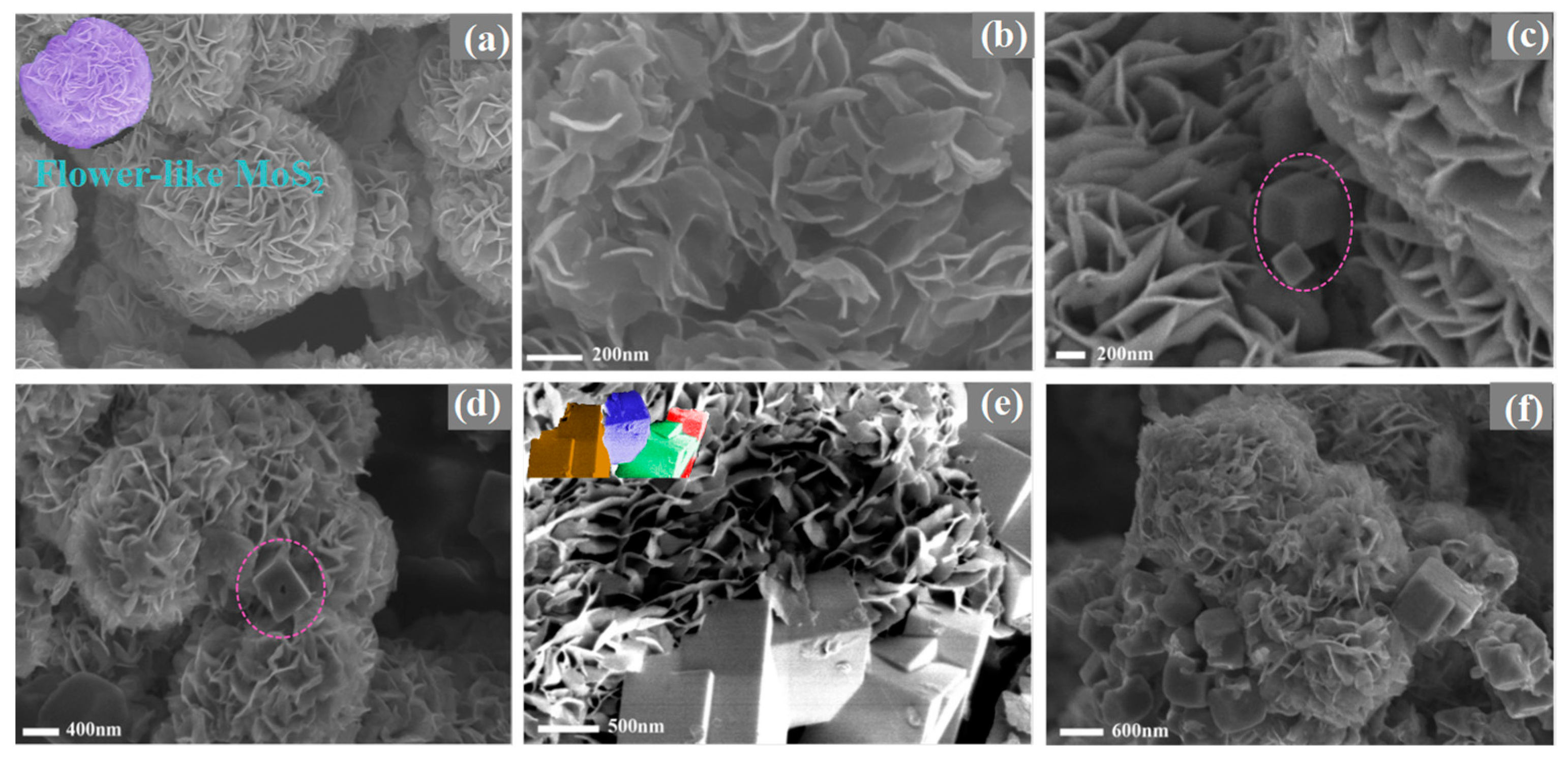

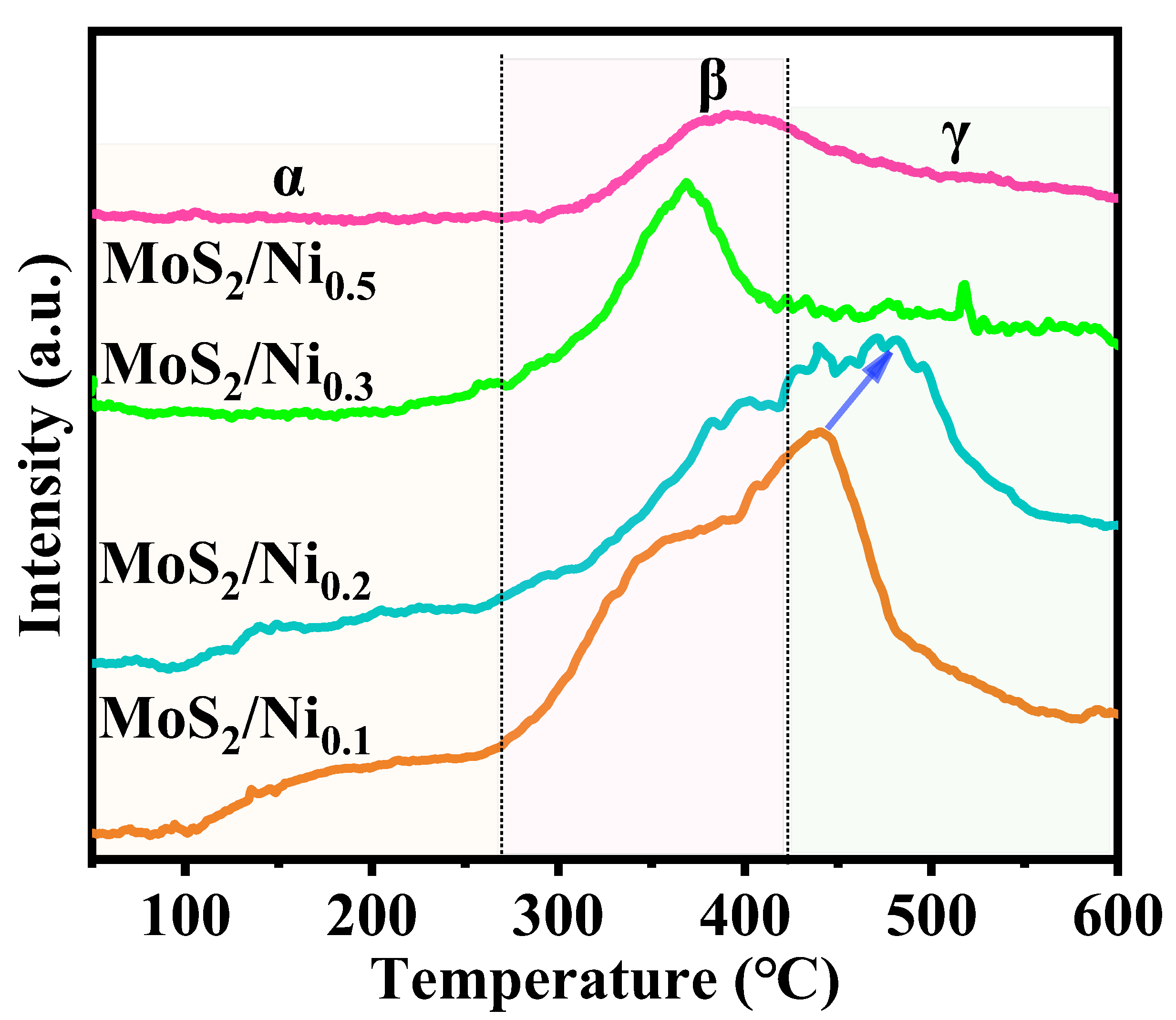
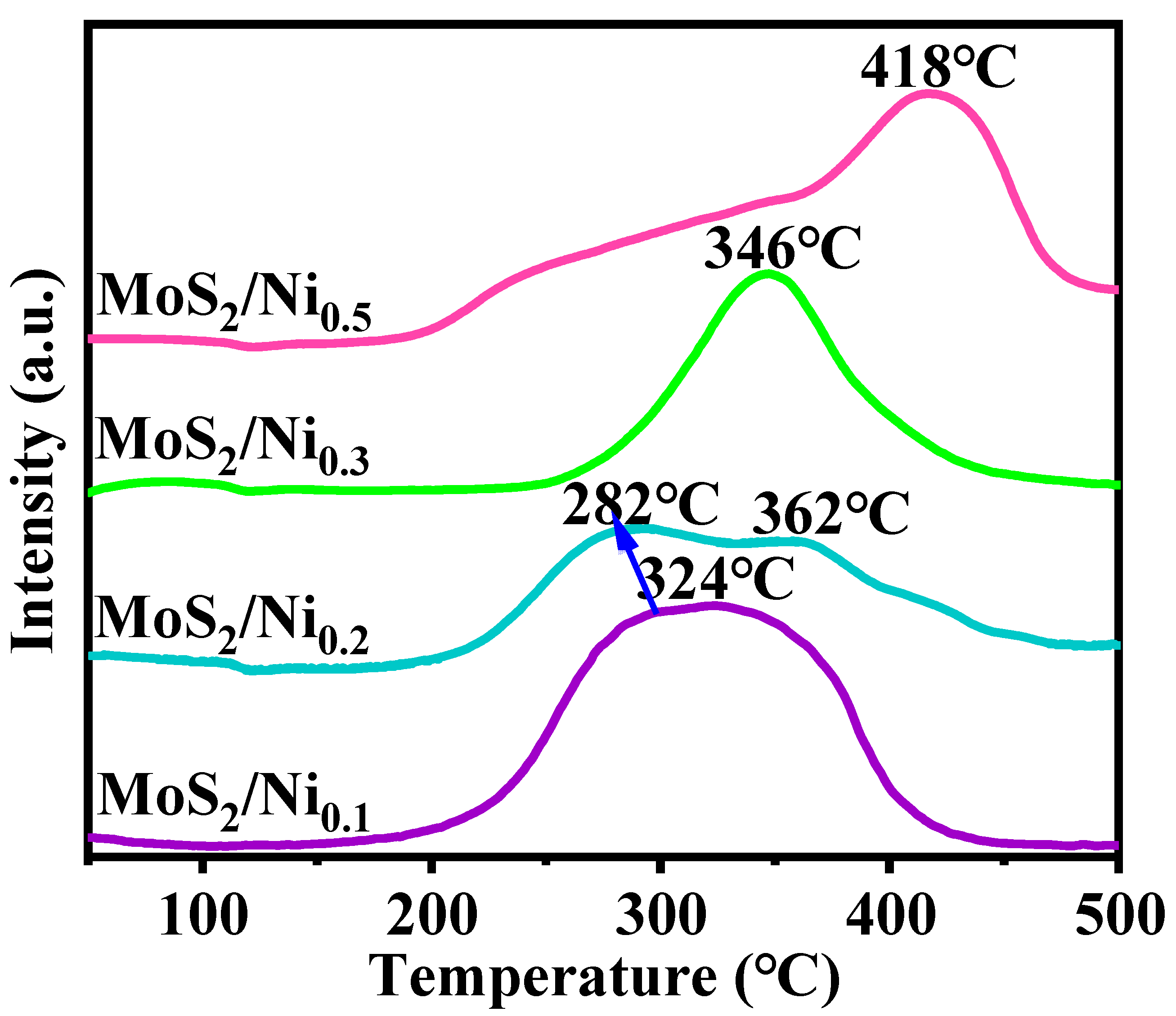
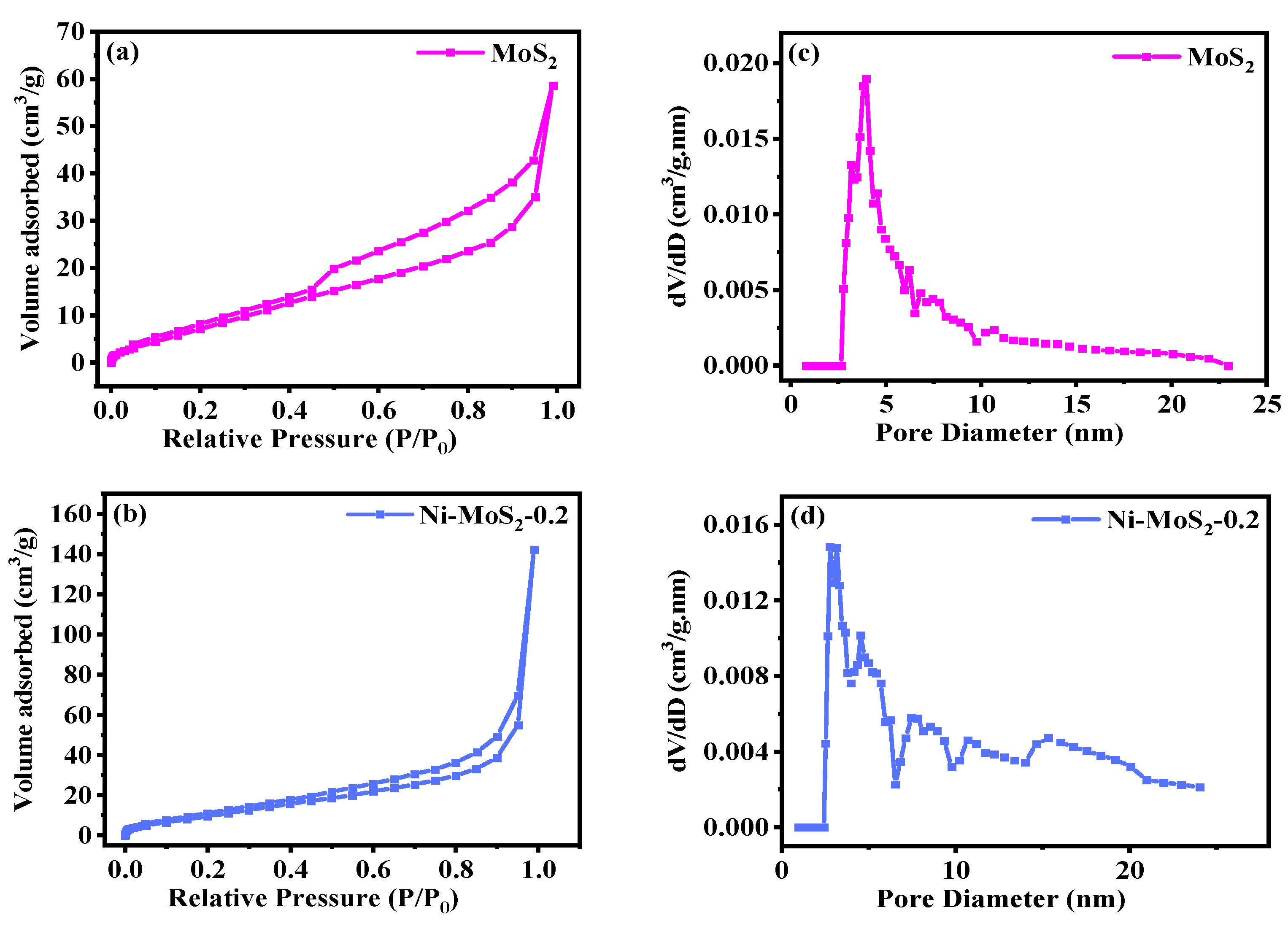



| Sample | BET Specific Surface Area (m2·g−1) | Surface Pore Diameter (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| MoS2 | 41.70 | 20.65 | 0.214 |
| MoS2/Ni0.2 | 40.73 | 10.13 | 0.090 |
| Chemical Reagent Name | Chemical Formula | Specification | Manufacturer |
|---|---|---|---|
| Cobalt nitrate hexahydrate | Co(NO3)2·6H2O | Analytical pure AR | Sinopharm group chemical reagent Co., Ltd. (Shanghai, China) |
| Nickel nitrate hexahydrate | Ni(NO3)2·6H2O | Analytical pure AR | Sinopharm group chemical reagent Co., Ltd. |
| Ammonium molybdate tetrahydrate | (NH4)6Mo7O24·4H2O | Analytical pure AR | Sinopharm group chemical reagent Co., Ltd. |
| Thiourea | CH4N2S | Analytical pure AR | Tianjin damao chemical reagent factory (Tianjin, China) |
| Quartz sand | SiO2 | Analytical pure AR | Shanghai mclean biochemical technology Co., Ltd. (Shanghai, China) |
| Raw gas | H2/CO2/N2 | 72/24/4 (molar ratio) | Ningxia guangli comprehensive trading Co., Ltd. (Yinchuan, China) |
| Reducing gas | H2/N2 | H2/N2 = 30/70 | Beijing yanan weiye gas Co., Ltd. (Beijing, China) |
| High purity nitrogen | N2 | 99.999% | Ningxia guangli comprehensive Trading Co., Ltd. |
| High purity helium | He | 99.999% | Ningxia guangli comprehensive trading Co., Ltd. |
| Air | Air | 100% | air generator (Yinchuan, China) |
| Equipment Name | Instrument Model | Manufacturer |
|---|---|---|
| Carbon dioxide catalytic conversion evaluation device | Φ240 × Φ22 × Φ510 | Beijing Kunlun yongtai Technology Co., Ltd. (Beijing, China) |
| Gas chromatograph | Agilent GC8890 | Agilent Technologies (China) Co., Ltd. (Beijing, China) |
| Electric heating constant temperature blast drying oven | GZX-9240MBE | Shanghai boxun industrial Co., Ltd. medical equipment factory (Shanghai, China) |
| Tube furnace | TL1200 | Nanjing boyuntong instrument technology Co., Ltd. (Nanjing, China) |
| Desktop high-speed centrifuge | H/T16MM | Hunan hercy instrument equipment Co., Ltd. (Changsha, China) |
| Magnetic stirrer | DF-101S | Shandong juancheng hualu electric heating instrument Co., Ltd. (Heze, China) |
| Powder tablet press | 35 mm | Hefei kejing material technology Co., Ltd. (Hefei, China) |
| Electronic balance | PL602 | Mettler toledo Instruments Ltd. (Zurich, Switzerland) |
| Hydrogen generator | SPH-300 | Beijing China HP analytical technology research institute (Beijing, China) |
| Air generator | SPB-3 | Beijing China HP analytical technology research institute |
| Column (TCD) | HP-PLOT Q | Agilent Technologies (China) Co., Ltd. (Beijing, China) |
| Column (FID) | MolSieve 5A | Agilent Technologies (China) Co., Ltd. |
| Component i | CO2 | CO | CH3OH | CH4-TCD | CH4-FID |
|---|---|---|---|---|---|
| Correction factor fi | 1.06 | 1.24 | 2.13 | 1.66 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Qi, L.; Gao, Z.; Guo, T.; Zhai, D.; He, Y.; Ma, J.; Guo, Q. Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol. Molecules 2023, 28, 5796. https://doi.org/10.3390/molecules28155796
Yuan Y, Qi L, Gao Z, Guo T, Zhai D, He Y, Ma J, Guo Q. Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol. Molecules. 2023; 28(15):5796. https://doi.org/10.3390/molecules28155796
Chicago/Turabian StyleYuan, Yongning, Liyue Qi, Zhuxian Gao, Tuo Guo, Dongdong Zhai, Yurong He, Jingjing Ma, and Qingjie Guo. 2023. "Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol" Molecules 28, no. 15: 5796. https://doi.org/10.3390/molecules28155796
APA StyleYuan, Y., Qi, L., Gao, Z., Guo, T., Zhai, D., He, Y., Ma, J., & Guo, Q. (2023). Performance Exploration of Ni-Doped MoS2 in CO2 Hydrogenation to Methanol. Molecules, 28(15), 5796. https://doi.org/10.3390/molecules28155796








