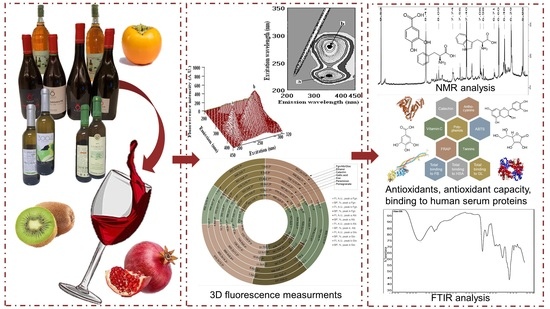
Characterization of Bioactivity of Selective Molecules in Fruit Wines by FTIR and NMR Spectroscopies, Fluorescence and Docking Calculations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bioactivity of Wine Samples
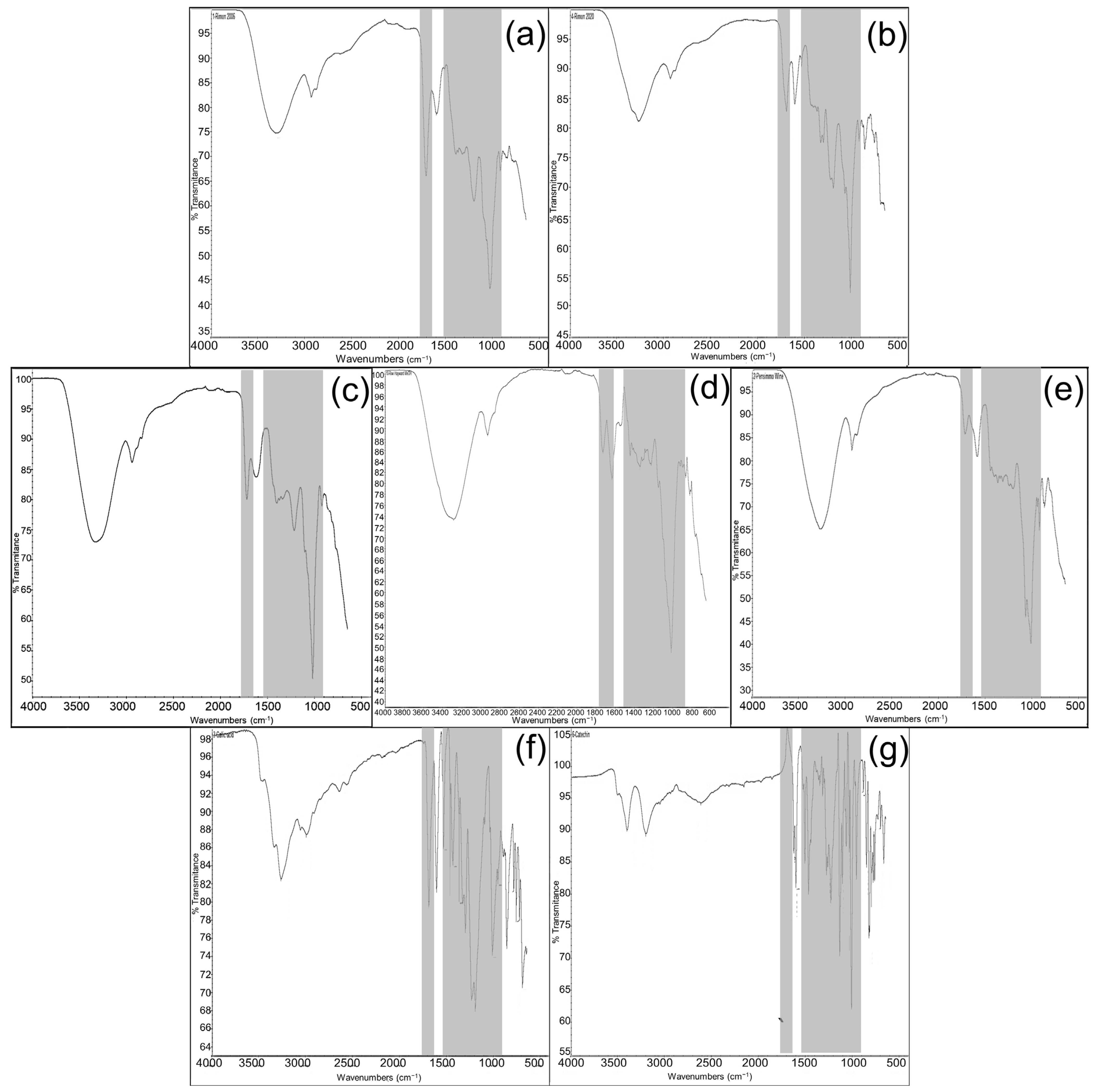
2.2. FTIR Spectroscopy
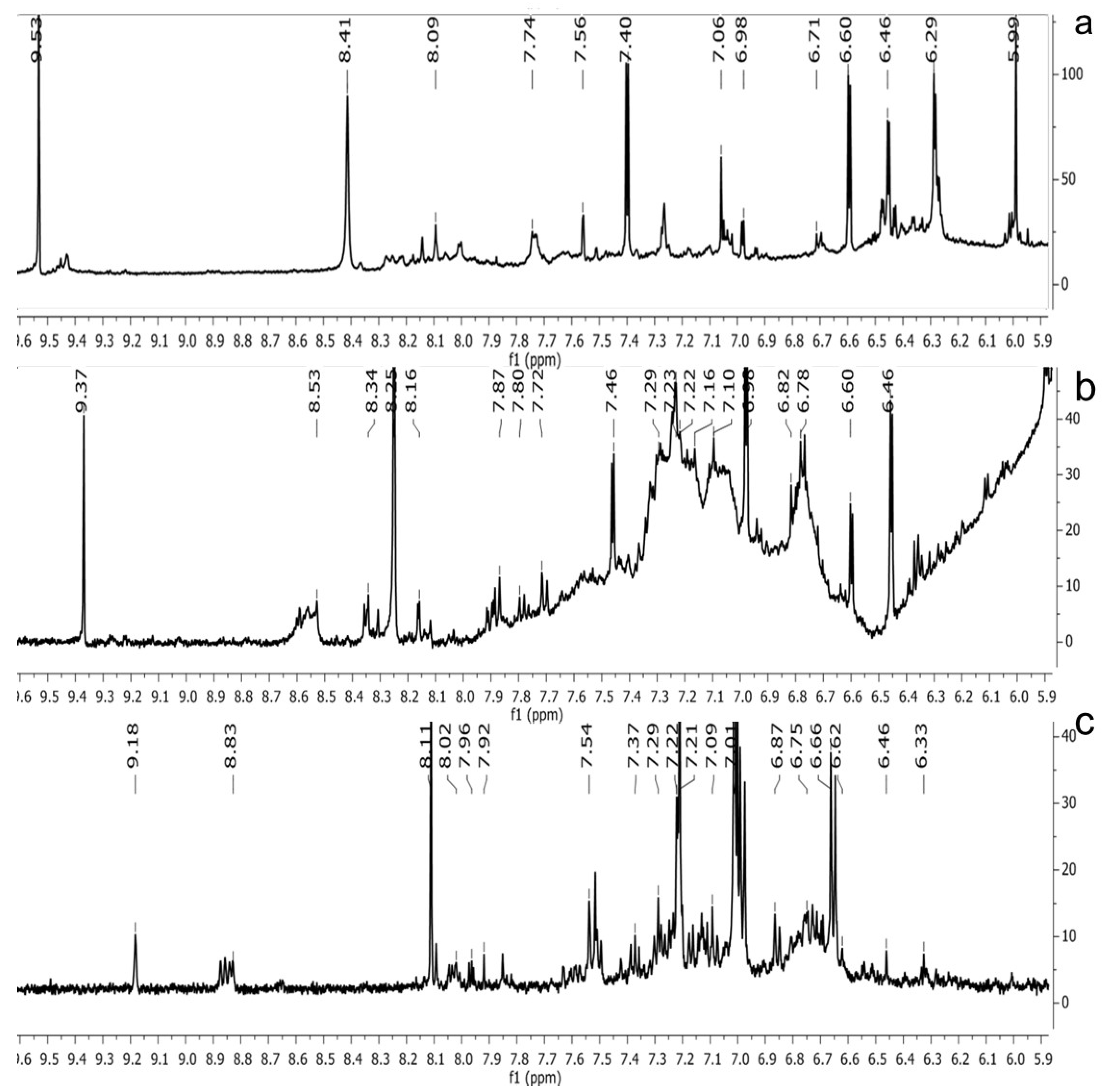
2.3. NMR Spectra
| No. | Tentative Compound | Structure | Persimmon | Kiwi | Pomegranate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +/− | δH(ppm), Multiplicity, J Value (Hz) | Lit. | +/− | δH(ppm), Multiplicity, J Value (Hz) | Lit. | +/− | δH(ppm), Multiplicity, J Value (Hz) | Lit. | |||
| 1 | Phenylalanine | 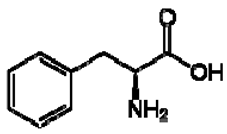 | + | 7.32, d, 7.4 | [52,53,54] | + | 7.40, m (2H) 7.35, m 7.30, d, 7.4 (2H) | [45,55] | + | nd | [12] |
| 2 | Kaempferol |  | + | 8.01, d, 8.0 6.95, d, 8.0 6.32, br d (small d) 6.10, br d (small d) | [55] | + | 8.01, d, 8.0 6.95, d, 8.0 6.32, br d (small d) 6.10, br d (small d) | [55] | − | - | - |
| 3 | Rutin | 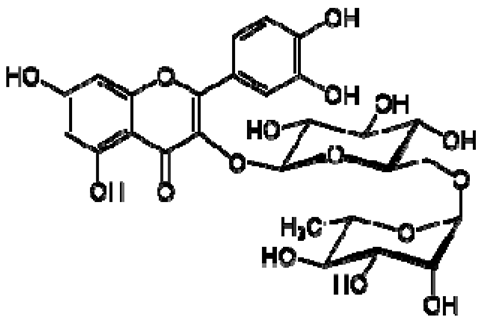 | − | - | - | + | 7.65, d, 2.0 7.60, dd, 6.82, d, 8.5 6.38, d, 6.19, d, 1.05, d, 7.0 4.51, br s (small d) 5.05, d, 8.0 | [45,55] | − | - | - |
| 4 | Tryptophan | 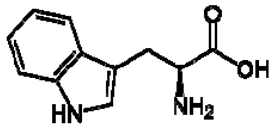 | + | 7.31 | [52,53,54] | + | 7.70, d, 8.0 7.54, d, 8.0 7.20, t, 7.0 | [45,55] | + | nd | [12] |
| 5 | Tyrosine |  | − | - | - | + | 3.94, m 7.15, d, 8.0 6.82, d, 8.0 | [45,55] | + | nd | [12] |
| 6 | Caffeic acid derivatives | 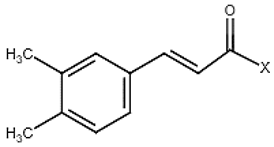 | − | - | - | + | 7.57, d, 13.0 7.28, br s (small d) 7.22, d, 8.0 6.95, d, 8.0 6.55, d, 13.0 | [45,55] | − | - | - |
| 7 | Protocatechuic acid | 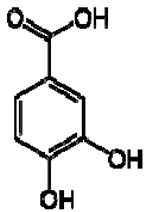 | + | 7.39, br s (small d) 7.35, br d (dd), 8.0 6.92, d, 8.0 | [43,55] | + | 7.39, br s (small d) 7.35, br d (dd), 8.0 6.92, d, 8.0 | [45] | + | 6.94 (d, J = 7.0), 7.23 (dd, J = 8.1, 2.0) | [51] |
| 8 | Catechol | 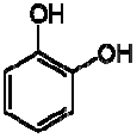 | − | - | - | + | 6.776.84, m 4.52, d, 7.20 2.94, dd, 15.7, 6.2 2.47, dd, 15.0, 8.0 | [45,55] | − | - | - |
| 9 | Syringic acid | 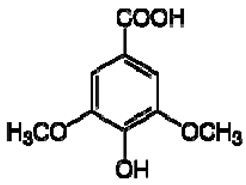 | − | - | - | + | 7.26, s, 2H 3.89, s | [45] | − | - | - |
| 10 | Afzelechin | 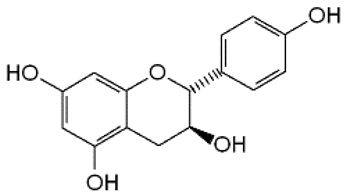 | − | - | - | + | 2.83, 2.80; 2.79, dd,15.6, 4.8 2.68, d 6.85, d, 8.0 (2H) 7.17, d, 8.0 (2H) | [55] | − | - | - |
| 11 | Kaempferol derivatives | 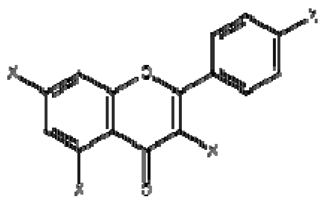 | + | 6.97, d, 2.7 6.46, d, 2.7 | [55] | − | - | - | − | - | - |
| 12 | Quercetin derivatives | 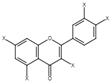 | + | 7.52, d, 3.5 6.66, d, 3.5 | [55] | + | 7.52, d, 3.5 6.66, d, 3.5 | [45] | − | - | - |
| 13 | Gallic acid | 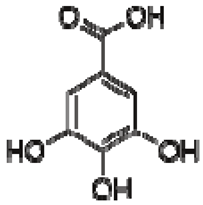 | + | 7.01 (s) | [43,54,55] | − | - | - | + | 7.04 (s) | [20,51] |
| 14 | Ellagic acid | 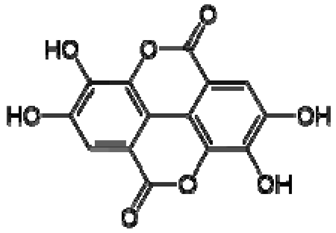 | − | - | - | − | - | - | + | 7.47 (s) | [51] |
| 15 | Punicalagin | 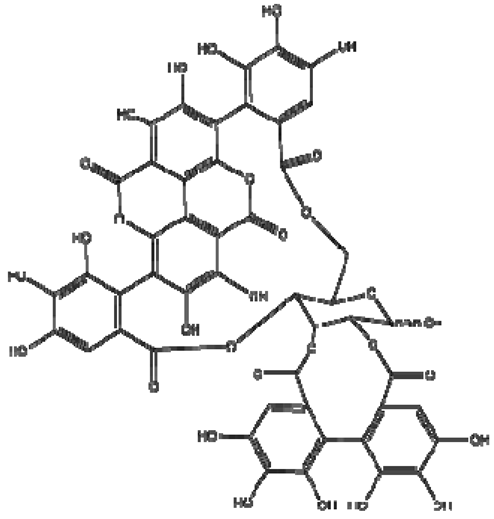 | − | - | - | − | - | - | + | (α): 7.21 (s), 7.01 (s), 6.88 (s) (β): 7.24 (s), 7.05 (s), 6.92 (s) 6.53, d 9.89 Hz | [51,56] |
| 16 | Pelargonidin-3,5-di-O-glucoside | 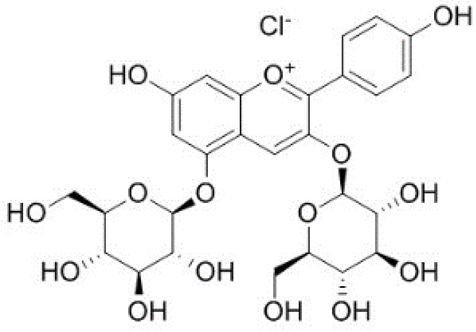 | − | - | - | − | - | - | + | 8.93 (s), 8.14 (d), 6.99 (s), 6.96 (s) | [51] |
| 17 | Delphinidin-3-O-glucoside | 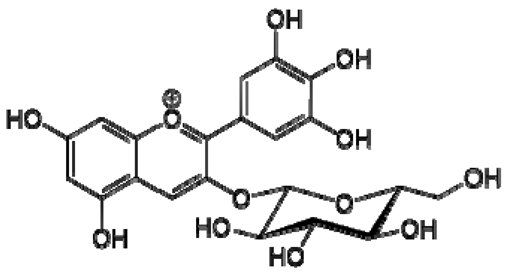 | − | - | - | − | - | - | + | 8.95 (s), 7.91 (s), 6.88 (d, J = 1.5), 6.71(d, J = 1.5) | [51] |
| 18 | Delphinidin-3,5-di-O-glucoside | 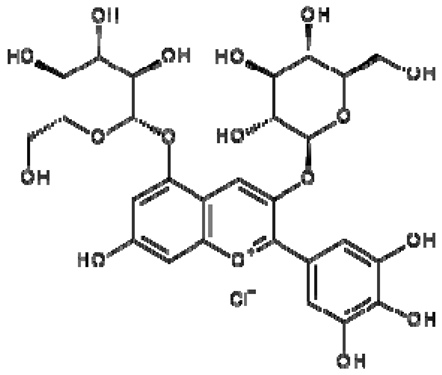 | − | - | - | − | - | - | + | 8.57 (s), 7.09 (s), 6.81(s), 6.62 (s) | [51] |
| 19 | Cyanidin-3,5-di-O-glucoside |  | − | - | - | − | - | - | + | 9.25 (s), 8.85 (d, J = 7.8), 8.10 (d, J = 2.0), 6.91(s) | [20,51] |
| 20 | Quercetin |  | − | - | - | − | - | - | + | 6.22 (s), 6.40 (s), 7.41 (d, J = 8.4) | [51] |
| 21 | Cyanidin-3-O-glucoside |  | − | - | - | − | - | - | + | nd | [20] |
| 22 | Pelargonidin-3-O-glucoside | 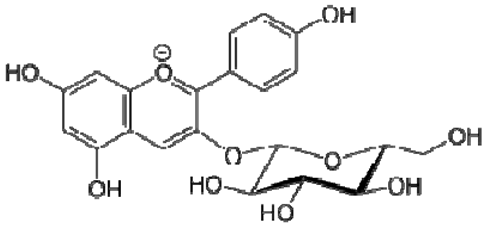 | − | - | - | − | - | - | + | nd | [20] |
| 23 | p-coumaric acid | 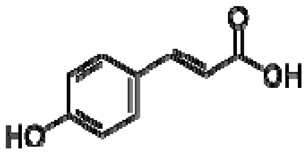 | − | - | - | − | - | - | + | nd | [20] |
| 24 | Chlorogenic acid | 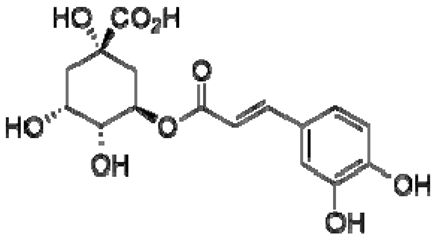 | − | - | - | − | - | - | + | nd | [20] |
| 25 | Caffeic acid | 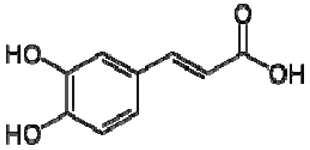 | + | 7.33 (d, J = 16.0 Hz), 7.13 (d, J = 1.9 Hz), 7.00 (dd, J = 8.0, 2.0 Hz), 6.86 (d, J = 8.0 Hz), 6.35 (d, J = 16.0 Hz) | [43] | − | - | - | + | nd | [20] |
| 26 | Niacin | 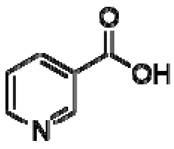 | − | - | - | + | 8.96, s 8.54, br s 8.25, br s | [45] | − | - | - |
| 27 | Hesperidin |  | − | - | - | + | 6.97, d, 2.7 6.46, d, 2. | [45] | − | - | - |
| 28 | Trigonelline | 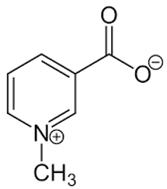 | + | 9.14 (s), 8.83 (m), 8.07 (m), 4.44 (br s) | [43,52,54] | − | - | - | + | 9.12, s; 8.07, t 6.90 Hz | [12,56] |
| 29 | Uridine |  | + | 7.93 (d, J = 7.9 Hz), 5.80 (m), 4.34 (m), 4.22 (m), 4.12 (m), 3.90 (m), 3.80 (m) | [43,52,54] | − | - | - | − | - | - |
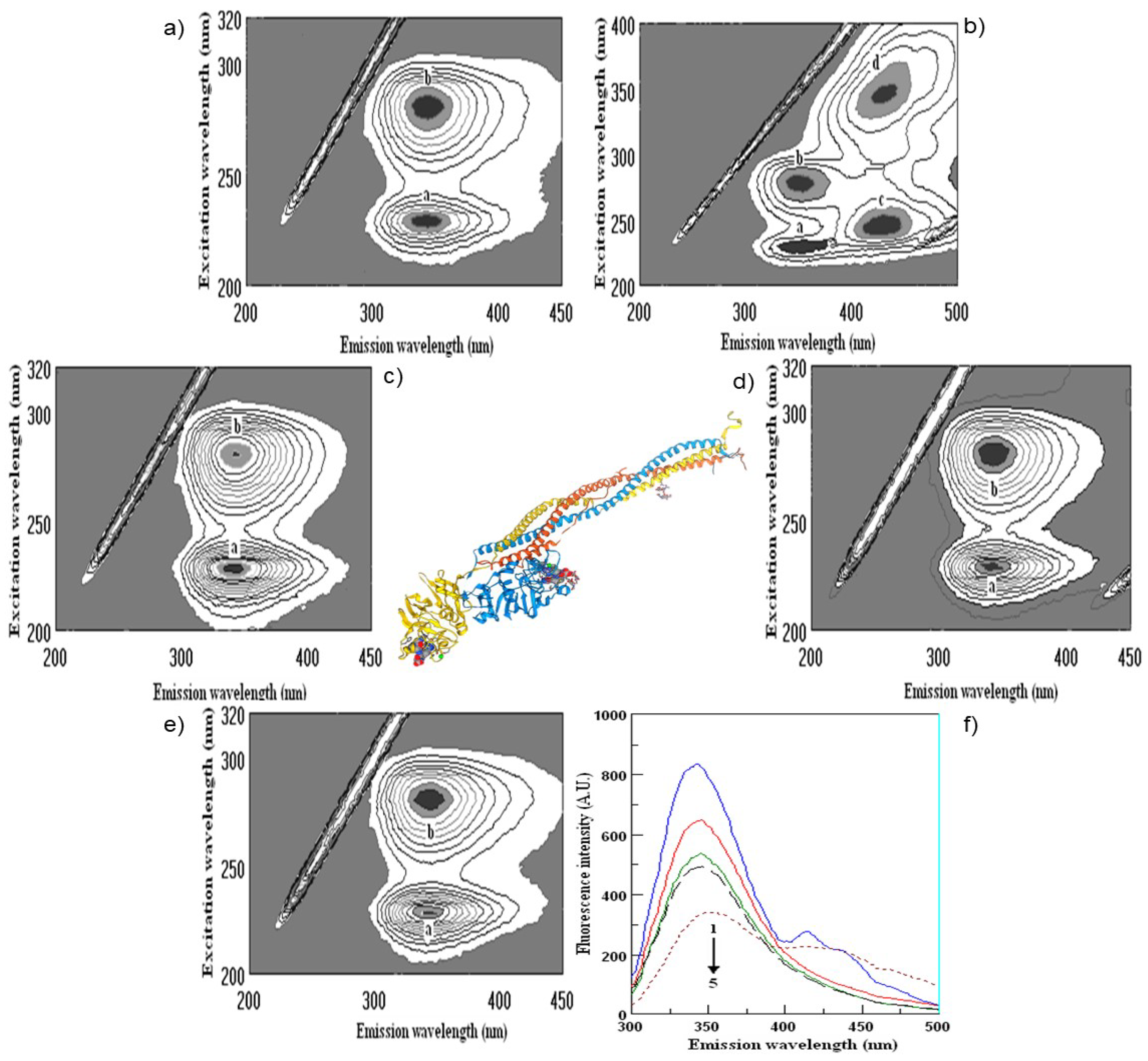
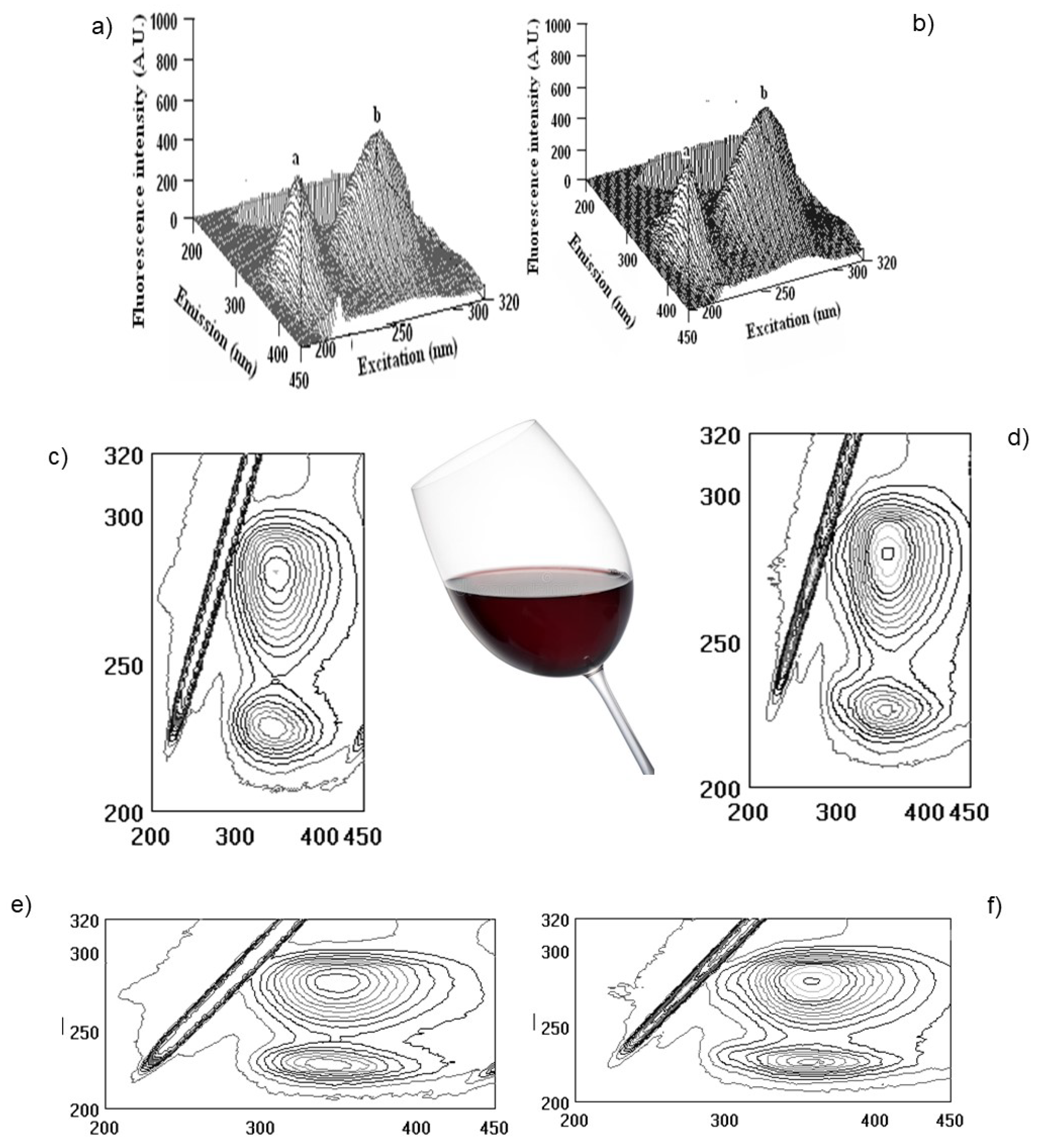
2.4. Fluorescence Measurements
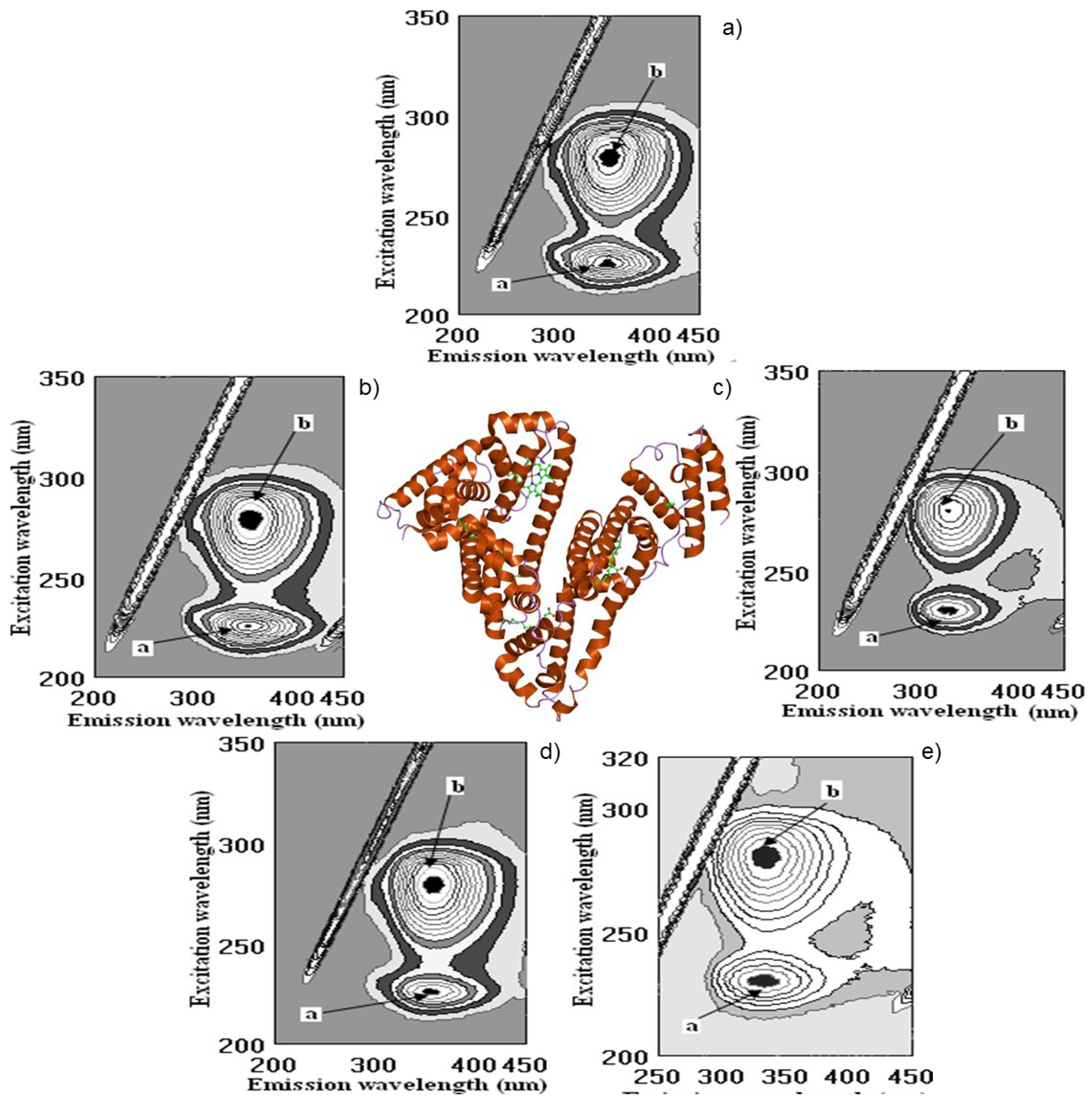
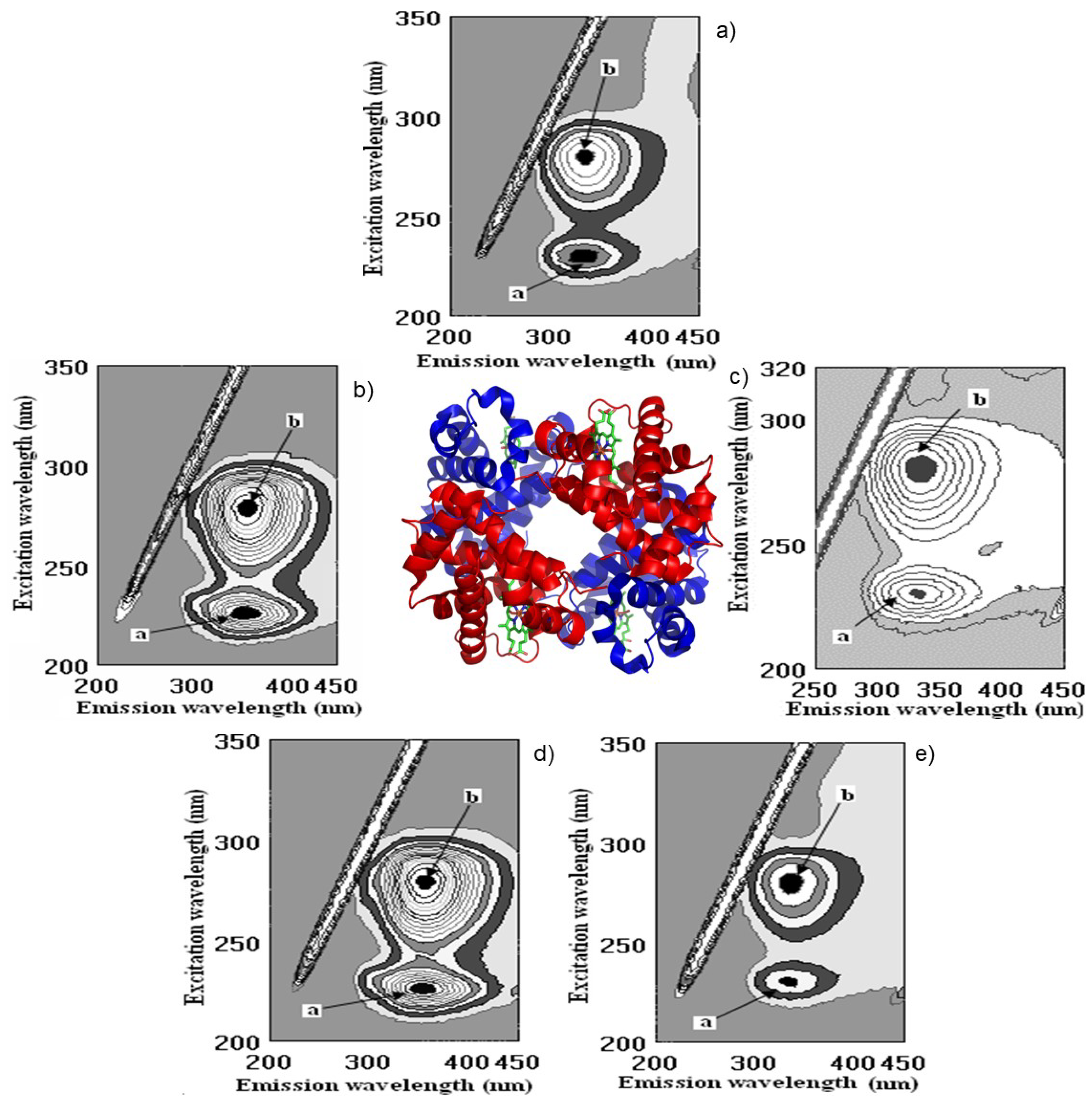
2.5. Molecular Docking Study
3. Materials and Methods
3.1. Materials
3.2. Wine Samples
3.3. Analyses of Bioactivity in Wine Samples
3.4. Fourier Transform Infrared Spectra of Polyphenols
3.5. 1H NMR Spectroscopy
3.6. Fluorometric Studies
3.7. Molecular Docking Study
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Gorinstein, S.; Caspi, A.; Libman, I.; Lerner, H.T.; Huang, D.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Katrich, E.; Feng, S.; et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: Studies in vitro and in humans. J. Agric. Food Chem. 2006, 54, 1887–1892. [Google Scholar] [CrossRef]
- Kiin-Kabari, D.B.; Igbo, Q.; Barber, L.I. Production and Evaluation of Table Wine Using Two Different Varieties of Pawpaw (Carica papaya). J. Food Sci. Eng. 2019, 9, 199–209. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [Green Version]
- Tandee, K.; Kittiwachana, S.; Mahatheeranont, S. Antioxidant activities and volatile compounds in longan (Dimocarpus longan Lour.) wine produced by incorporating longan seeds. Food Chem. 2021, 348, 128921. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wen, S.; Lihong, W.; Chuang, W.; Chaoyun, W. Molecular structures of nonvolatile components in the Haihong fruit wine and their free radical scavenging effect. Food Chem. 2021, 353, 129298. [Google Scholar] [CrossRef]
- Liu, M.; Yang, K.; Qi, Y.; Zhang, J.; Fan, M.; Wei, X. Fermentation temperature and the phenolic and aroma profile of persimmon wine. J. Inst. Brew. 2018, 124, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Park, Y.S.; Park, Y.K.; Ham, K.S.; Kang, S.G.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Phytochemical analysis of two main varieties of persimmon and kiwifruit and their antioxidative and quenching capacities. Eur. Food Res. Technol. 2020, 246, 1259–1268. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef]
- Nor, S.M.; Ding, P.; Abas, F.; Mediani, A. 1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening. Agriculture 2022, 12, 156. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Rosas-Bautista, A.; Rico-Arzate, E.; Cruz-Narvaez, Y.; Zepeda-Vallejo, L.G.; Lalaleo, L.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Study of nutritional quality of pomegranate (Punica granatum L.) juice using 1H NMR-based metabolomic approach: A comparison between conventionally and organically grown fruits. LWT 2020, 134, 110222. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chem. 2011, 124, 1760–1769. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, R.; Delfini, M. Monitoring of metabolic profiling and water status of Hayward kiwifruits by nuclear magnetic resonance. Talanta 2010, 82, 1826–1838. [Google Scholar] [CrossRef]
- Ferrari, E.; Foca, G.; Vignali, M.; Tassi, L.; Ulrici, A. Adulteration of the anthocyanin content of red wines: Perspectives for authentication by Fourier Transform-Near InfraRed and 1H NMR spectroscopies. Anal. Chim. Acta 2011, 701, 139–151. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Simeonov, V.; Namieśnik, J. Characterization of home-made and regional fruit wines by evaluation of correlation between selected chemical parameters. Microchem. J. 2018, 140, 66–73. [Google Scholar] [CrossRef]
- Palanisami, M.; Kaur, K.; Sahu, B.K.; Kataria, S.; Chandel, M.; Sharma, A.; Elumalai, S.; Ramaraj, R.; Shanmugam, V. Excellent enzymeless anti-oxidant sensor for fruit juice and wine using nano gold/metal selenide urchins decorated 2D-composite. Microchem. J. 2022, 183, 108078. [Google Scholar] [CrossRef]
- Radunić, M.; Jukić Špika, M.; Goreta Ban, S.; Gadže, J.; Díaz-Pérez, J.C.; Maclean, D. Physical and chemical properties of pomegranate fruit accessions from Croatia. Food Chem. 2015, 177, 53–60. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Cao, Y.; Qi, Y.; Yang, K.; Wei, X.; Xu, Y.; Fan, M. Effect of glutathione-enriched inactive dry yeast on color, phenolic compounds, and antioxidant activity of kiwi wine. J. Food Process. Preserv. 2019, 44, e14347. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-álvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–35. [Google Scholar] [PubMed]
- Borazan, A.A.; Bozan, B. The influence of pectolytic enzyme addition and prefermentative mash heating during the winemaking process on the phenolic composition of Okuzgozu red wine. Food Chem. 2013, 138, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mousavinejad, G.; Emam-Djomeh, Z.; Rezaei, K.; Khodaparast, M.H.H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009, 115, 1274–1278. [Google Scholar] [CrossRef]
- Anli, R.E.; Vural, N. Antioxidant phenolic substances of Turkish red wines from different wine regions. Molecules 2009, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef]
- Ferrara, G.; Cavoski, I.; Pacifico, A.; Tedone, L.; Mondelli, D. Morpho-pomological and chemical characterization of pomegranate (Punica granatum L.) genotypes in Apulia region, Southeastern Italy. Sci. Hortic. 2011, 130, 599–606. [Google Scholar] [CrossRef]
- Asadi-Gharneh, H.A.; Mohammadzamani, M.; Karimi, S. Evaluation of Physico-Chemical Properties and Bioactive Compounds of Some Iranian Pomegranate Cultivars. Int. J. Fruit Sci. 2017, 17, 175–187. [Google Scholar] [CrossRef]
- Solovyev, P.A.; Fauhl-Hassek, C.; Riedl, J.; Esslinger, S.; Bontempo, L.; Camin, F. NMR spectroscopy in wine authentication: An official control perspective. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2040–2062. [Google Scholar] [CrossRef]
- Amargianitaki, M.; Spyros, A. NMR-based metabolomics in wine quality control and authentication. Chem. Biol. Technol. Agric. 2017, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Scano, P. Characterization of the medium infrared spectra of polyphenols of red and white wines by integrating FT IR and UV–Vis spectral data. LWT 2021, 147, 111604. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, S.; Wen, L.; Jiang, Y.; Zhao, M.; Chen, F.; Prasad, K.N.; Duan, X.; Yang, B. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013, 136, 563–568. [Google Scholar] [CrossRef]
- Silva, S.D.; Feliciano, R.P.; Boas, L.V.; Bronze, M.R. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; El Orche, A.; Naiker, M. Prediction of anthocyanin content and variety in plum extracts using ATR-FTIR spectroscopy and chemometrics. Vib. Spectrosc. 2022, 121, 103406. [Google Scholar] [CrossRef]
- Malinda, K.; Sutanto, H.; Darmawan, A. Characterization and antioxidant activity of gallic acid derivative. AIP Conf. Proc. 2017, 1904, 020030. [Google Scholar] [CrossRef]
- Zhou, R.Q.; Li, X.L.; He, Y.; Jin, J.J. Determination of catechins and caffeine content in tea (Camellia sinensis L.) leaves at different positions by fourier-transform infrared spectroscopy. Trans. ASABE 2018, 61, 1221–1230. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Grijalva-Verdugo, C.; Hernández-Martínez, M.; Meza-Márquez, O.G.; Gallardo-Velázquez, T.; Osorio-Revilla, G. FT-MIR spectroscopy and multivariate analysis for determination of bioactive compounds and antioxidant capacity in Cabernet Sauvignon wines. CyTA-J. Food 2018, 16, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoǧlu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids-La(III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef]
- Li, S.; Wilkinson, K.L.; Mierczynska-Vasilev, A.; Bindon, K.A.; Maria, R.; Perestrelo, S.; Câmara, J.S. Applying Nanoparticle Tracking Analysis to Characterize the Polydispersity of Aggregates Resulting from Tannin–Polysaccharide Interactions in Wine-Like Media. Molecules 2019, 24, 2100. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Wu, S.; Yu, W.J.; Xu, X.; Huang, M.; Tang, Y.; Yang, Z. Wine Authentication Using Integration Assay of MIR, NIR, E-tongue, HS-SPME-GC-MS, and Multivariate Analyses: A Case Study for a Typical Cabernet Sauvignon Wine. J. AOAC Int. 2019, 102, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Camiña, J.M.; Callejón, R.M.; Azcarate, S.M. Spectralprint techniques for wine and vinegar characterization, authentication and quality control: Advances and projections. TrAC Trends Anal. Chem. 2021, 134, 116121. [Google Scholar] [CrossRef]
- Maulidiani, M.; Mediani, A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. 1H NMR and antioxidant profiles of polar and non-polar extracts of persimmon (Diospyros kaki L.)—Metabolomics study based on cultivars and origins. Talanta 2018, 184, 277–286. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic characterization of Palatinate German white wines according to sensory attributes, varieties, and vintages using NMR spectroscopy and multivariate data analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S.; Namieśnik, J.; et al. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Wu, Q.; Zhao, Y.; Jiang, Y.; John, A.; Wen, L.; Li, T.; Jian, Q.; Yang, B. Analyses of quality and metabolites levels of okra during postharvest senescence by 1H-high resolution NMR. Postharvest Biol. Technol. 2017, 132, 171–178. [Google Scholar] [CrossRef]
- Murray, N.J.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. Eur. J. Biochem. 1994, 219, 923–935. [Google Scholar] [CrossRef]
- Košir, I.J.; Kidrič, J. Use of modern nuclear magnetic resonance spectroscopy in wine analysis: Determination of minor compounds. Anal. Chim. Acta 2002, 458, 77–84. [Google Scholar] [CrossRef]
- Lucarini, M.; Sciubba, F.; Capitani, D.; Di Cocco, M.E.; D’Evoli, L.; Durazzo, A.; Delfini, M.; Lombardi Boccia, G. Role of catechin on collagen type I stability upon oxidation: A NMR approach. Nat. Prod. Res. 2019, 34, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kraszni, M.; Marosi, A.; Larive, C.K. NMR assignments and the acid-base characterization of the pomegranate ellagitannin punicalagin in the acidic pH-range. Anal. Bioanal. Chem. 2013, 405, 5807–5816. [Google Scholar] [CrossRef]
- Hasanpour, M.; Saberi, S.; Iranshahi, M. Metabolic Profiling and Untargeted 1H-NMR-Based Metabolomics Study of Different Iranian Pomegranate (Punica granatum) Ecotypes. Planta Med. 2020, 86, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.D.d.C.; Fonseca, F.A.; Dutra, L.M.; Santos, M.d.F.C.; Menezes, L.R.A.; Campos, F.R.; Nagata, N.; Ayub, R.; Barison, A. 1H HR-MAS NMR-based metabolomics study of different persimmon cultivars (Diospyros kaki) during fruit development. Food Chem. 2018, 239, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Muramatsu, T.; Furihata, K.; Wei, F.; Koda, M.; Miyakawa, T.; Tanokura, M. NMR-based metabolic profiling and comparison of Japanese persimmon cultivars. Sci. Rep. 2019, 9, 15011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.; Furihata, K.; Koda, M.; Wei, F.; Miyakawa, T.; Tanokura, M. NMR-based analysis of the chemical composition of Japanese persimmon aqueous extracts. Magn. Reson. Chem. 2016, 54, 213–221. [Google Scholar] [CrossRef]
- Kim, Y.M.; Abas, F.; Park, Y.S.; Park, Y.-K.; Ham, K.S.; Kang, S.G.; Lubinska-Szczygeł, M.; Ezra, A.; Gorinstein, S. Bioactivities of Phenolic Compounds from Kiwifruit and Persimmon. Molecules 2021, 26, 4405. [Google Scholar] [CrossRef]
- Tang, F.; Hatzakis, E. NMR-Based Analysis of Pomegranate Juice Using Untargeted Metabolomics Coupled with Nested and Quantitative Approaches. Anal. Chem. 2020, 92, 11177–11185. [Google Scholar] [CrossRef]
- Viskić, M.; Bandić, L.M.; Korenika, A.M.J.; Jeromel, A. NMR in the Service of Wine Differentiation. Foods 2021, 10, 120. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H NMR-based metabonomics for the classification of Greek wines according to variety, region, and vintage. Comparison with HPLC data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef]
- Pinto, J.; Oliveira, A.S.; Azevedo, J.; De Freitas, V.; Lopes, P.; Roseira, I.; Cabral, M.; Guedes de Pinho, P. Assessment of oxidation compounds in oaked Chardonnay wines: A GC-MS and 1H NMR metabolomics approach. Food Chem. 2018, 257, 120–127. [Google Scholar] [CrossRef]
- Anjos, O.; Pedro, S.I.; Caramelo, D.; Semedo, A.; Antunes, C.A.L.; Canas, S.; Caldeira, I. Characterization of a Spirit Beverage Produced with Strawberry Tree (Arbutus unedo L.) Fruit and Aged with Oak Wood at Laboratorial Scale. Appl. Sci. 2021, 11, 5065. [Google Scholar] [CrossRef]
- Thanasi, V.; Catarino, S.; Ricardo-Da-Silva, J. Fourier transform infrared spectroscopy in monitoring the wine production. Ciência Técnica Vitivinícola 2022, 37, 79–99. [Google Scholar] [CrossRef]
- Basalekou, M.; Pappas, C.; Tarantilis, P.A.; Kallithraka, S. Wine Authenticity and Traceability with the Use of FT-IR. Beverages 2020, 6, 30. [Google Scholar] [CrossRef]
- Ranaweera, R.K.R.; Capone, D.L.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. A Review of Wine Authentication Using Spectroscopic Approaches in Combination with Chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T.; Apetrei, C.; Artem, V. Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination. Molecules 2019, 24, 4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Barnes, K.W.; Eisele, T.; Giusti, M.M.; Haché, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M.; Waterhouse, A.L. A Direct HPLC Separation of Wine Phenolics. Am. J. Enol. Vitic. 1994, 45, 1–5. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Macarena, L.S.; María, J.A.C.; Antonio, M.D. Olive Fruit Growth and Ripening as Seen by Vibrational Spectroscopy. J. Agric. Food Chem. 2009, 58, 82–87. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, F.R.; Delfini, M. Metabolic profiling and outer pericarp water state in zespri, CI.GI, and hayward kiwifruits. J. Agric. Food Chem. 2013, 61, 1727–1740. [Google Scholar] [CrossRef]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Kim, Y.M.; Deutsch, J.; Katrich, E.; Gorinstein, S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules 2021, 26, 6686. [Google Scholar] [CrossRef] [PubMed]
- Zaib, M.; Malik, M.N.H.; Shabbir, R.; Mushtaq, M.N.; Younis, W.; Jahan, S.; Ahmed, I.; Kharl, H.A.A. Imine Derivatives of Benzoxazole Attenuate High-Fat Diet-Induced Hyperlipidemia by Modulation of Lipid-Regulating Genes. ACS Omega 2023, 8, 15306–15317. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, E.; Venianakis, T.; Primikyri, A.; Papamokos, G.; Gerothanassis, I.P. Molecular Basis for the Selectivity of DHA and EPA in Sudlow’s Drug Binding Sites in Human Serum Albumin with the Combined Use of NMR and Docking Calculations. Molecules 2023, 28, 3724. [Google Scholar] [CrossRef] [PubMed]


| Indices | Pomegranate | Persimmon | Kiwifruit | Ethanol |
|---|---|---|---|---|
| Polyphenols, mg GAE | 1707.3 ± 11.4 a | 917.9 ± 8.4 b | 1325.8 ± 13.9 ab | |
| Anthocyanins, mgCGE | 88.2 ± 4.1 a | 79.2 ± 3.6 ab | 66.7 ± 2.2 b | |
| Tannins, mg | 182.7 ± 3.3 b | 337.8 ± 8.2 a | 157.1.6 ± 4.1 c | |
| Catechin, mg | 17.4 ± 1.3 a | 5.9 ± 0.4 b | 7.9 ± 0.8 ab | |
| Vitamin C, mg AA | 16.1 ± 1.4 b | 9.9 ± 0.8 b | 64.6 ± 5.3 a | |
| ABTS, mM TE/L | 20.2 ± 1.9 a | 11.4 ± 0.9 c | 16.5 ± 1.7 b | |
| Total binding to Alb, % | 51.1 ± 3.9 a | 30.7 ± 2.9 b | 37.7 ± 3.8 ab | 2.6 ± 0.3 c |
| Total binding to Glo, % | 68.8 ± 5.8 a | 22.7 ± 2.3 c | 36.5 ± 4.6 b | 3.1 ± 0.3 c |
| Total binding to Fgn, % | 80.5 ± 4.3 a | 62.2 ± 6.7 ab | 43.6 ± 3.2 b | 2.9 ± 0.3 c |
| FRAP, mMTE/L | 7.4 ± 0.9 a | 3.4 ± 0.1 ab | 5.9 ± 0.8 b | |
| Gallic acid, mg | 108.8 ± 5.9 a | 48.1 ± 3.1 ab | 64.7 ± 4.2 b |
| Samples | Correlation | Q-Check Regions, cm−1 |
|---|---|---|
| Pomegr–Kiwi | 0.5559 | 3627.5–3285.5 |
| 0.5426 | 1697.5–1591.0 | |
| 0.5940 | 1204.5–1113.3 | |
| 0.8055 | 1094.5–1053.5 | |
| 0.5629 | 981–905.5 | |
| Pomegr–Persimm. | 0.5795 | 3085.5–2906.0 |
| 0.6523 | 1739.5–1568.5 | |
| 0.8645 | 1314.0–1221.5 | |
| 0.8762 | 1125.5–1037.5 | |
| 0.8068 | 1011.5–906.5 | |
| Kiwi–Persimm. | 0.5170 | 3568.5–3138.0 |
| 0.6663 | 1823.0–1665.0 | |
| 0.4315 | 1283.5–1139.0 |
| Ligand | Binding Affinity (Kcal/mol) | Amino Acids Involved in the Interaction (H-Bond) | Other Interactions |
|---|---|---|---|
| Catechin | −7.2 | ARG209 and ASP324 | Lys 212 (Pi-alkyl), Val 216 (Pi–sigma), Phe 228, Ser 232, Val 235, Val 325, Leu 331, Lys 351, Tyr 353, Glu 354 (van der Waals) |
| Gallic acid | −7.8 | Lys 199, His 288 and Glu 292 | Lys 195 and Ala 291 (Pi-alkyl), Tyr 50 (Pi-Pi T-shaped) |
| Vit C | −5.8 | Asp 108, Tyr 148, Arg 197 and Val 462 | Leu 103, Asn 109, Asp 107, Lys 466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Lubinska-Szczygeł, M.; Park, Y.-S.; Deutsch, J.; Ezra, A.; Luksrikul, P.; Beema Shafreen, R.M.; Gorinstein, S. Characterization of Bioactivity of Selective Molecules in Fruit Wines by FTIR and NMR Spectroscopies, Fluorescence and Docking Calculations. Molecules 2023, 28, 6036. https://doi.org/10.3390/molecules28166036
Kim Y-M, Lubinska-Szczygeł M, Park Y-S, Deutsch J, Ezra A, Luksrikul P, Beema Shafreen RM, Gorinstein S. Characterization of Bioactivity of Selective Molecules in Fruit Wines by FTIR and NMR Spectroscopies, Fluorescence and Docking Calculations. Molecules. 2023; 28(16):6036. https://doi.org/10.3390/molecules28166036
Chicago/Turabian StyleKim, Young-Mo, Martyna Lubinska-Szczygeł, Yong-Seo Park, Joseph Deutsch, Aviva Ezra, Patraporn Luksrikul, Raja Mohamed Beema Shafreen, and Shela Gorinstein. 2023. "Characterization of Bioactivity of Selective Molecules in Fruit Wines by FTIR and NMR Spectroscopies, Fluorescence and Docking Calculations" Molecules 28, no. 16: 6036. https://doi.org/10.3390/molecules28166036
APA StyleKim, Y. -M., Lubinska-Szczygeł, M., Park, Y. -S., Deutsch, J., Ezra, A., Luksrikul, P., Beema Shafreen, R. M., & Gorinstein, S. (2023). Characterization of Bioactivity of Selective Molecules in Fruit Wines by FTIR and NMR Spectroscopies, Fluorescence and Docking Calculations. Molecules, 28(16), 6036. https://doi.org/10.3390/molecules28166036