Synthesis and Anticancer Evaluation of 4-Chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol Analogues: An Insight into Experimental and Theoretical Studies
Abstract
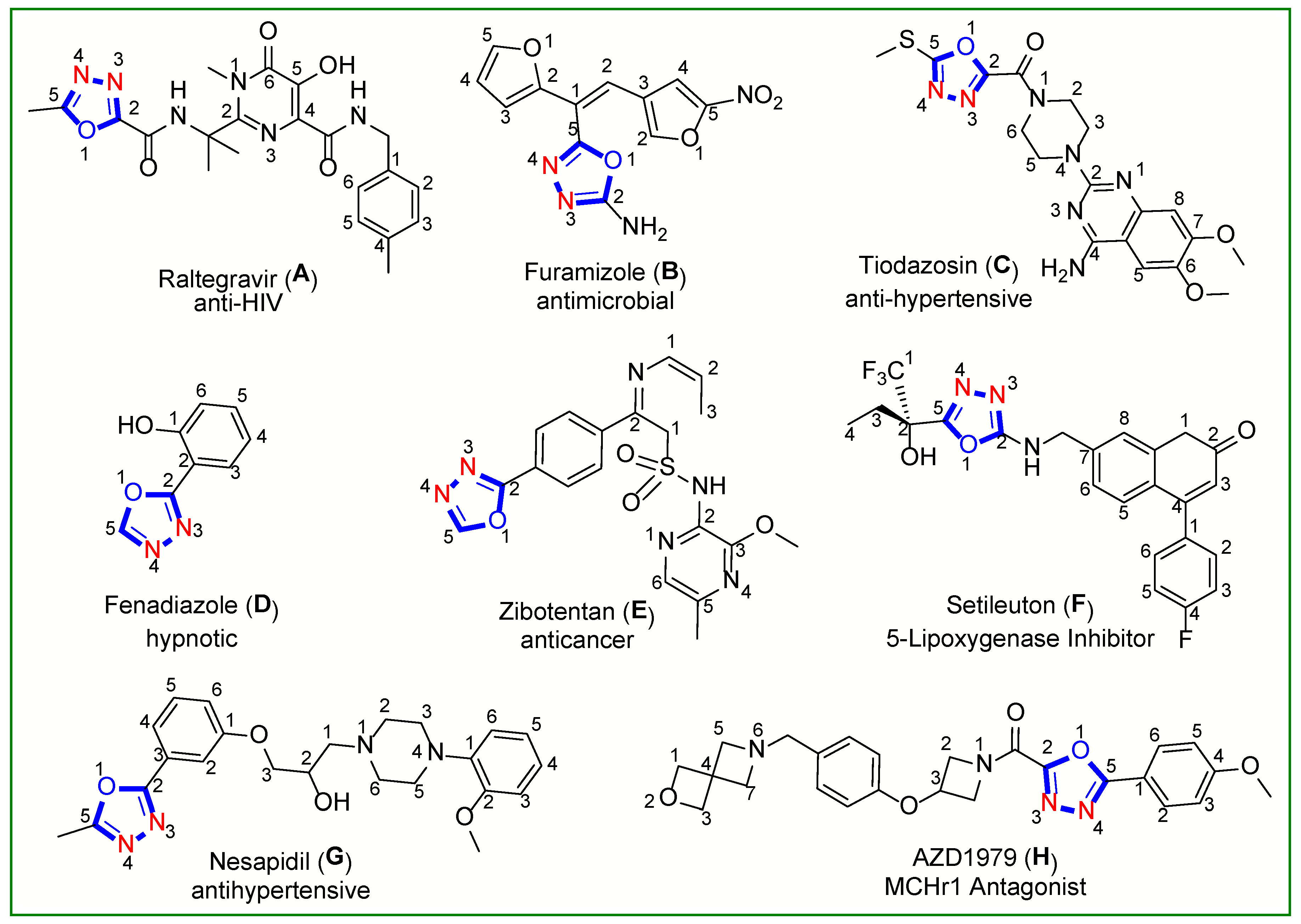
:1. Introduction
2. Results
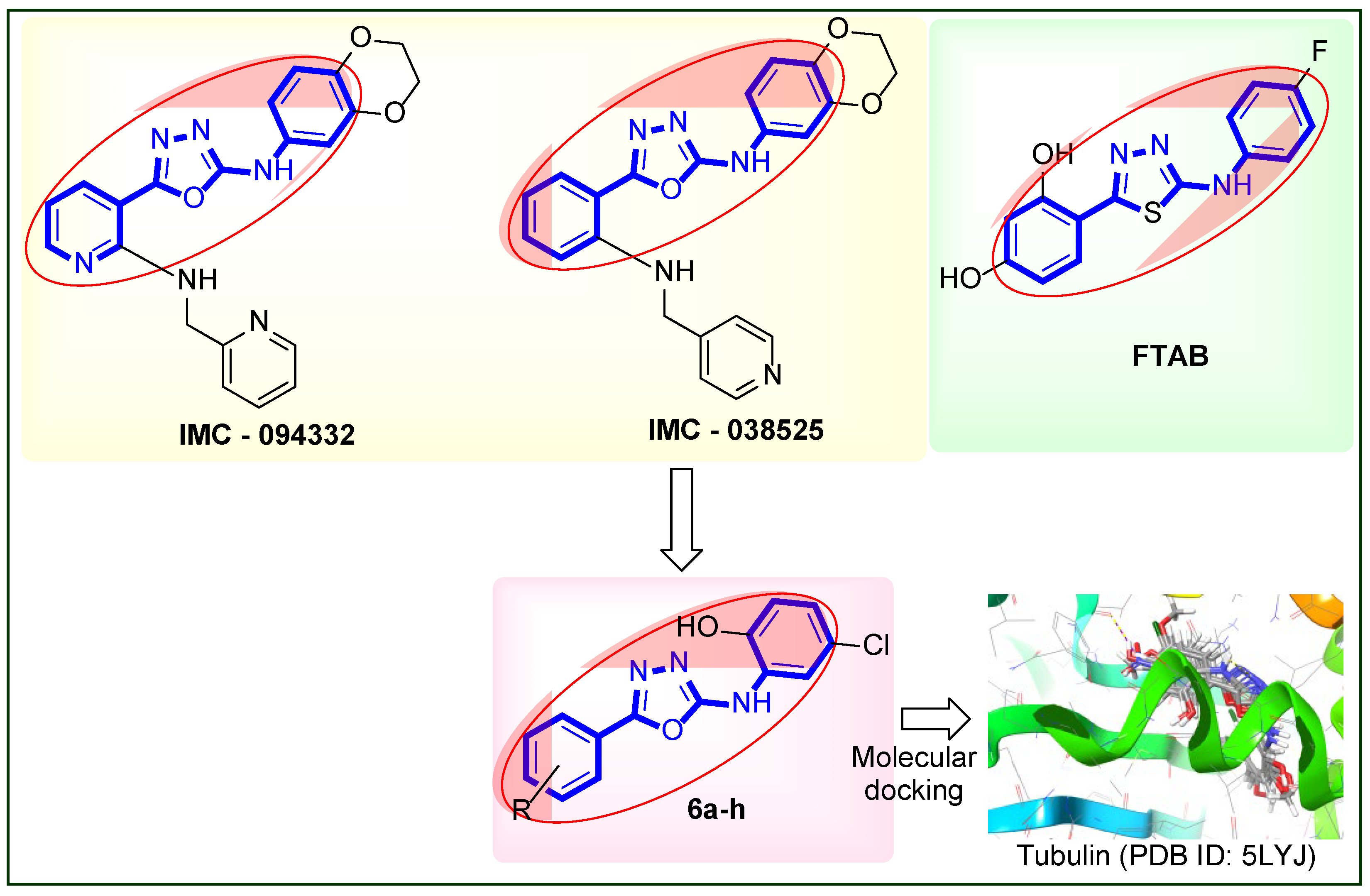
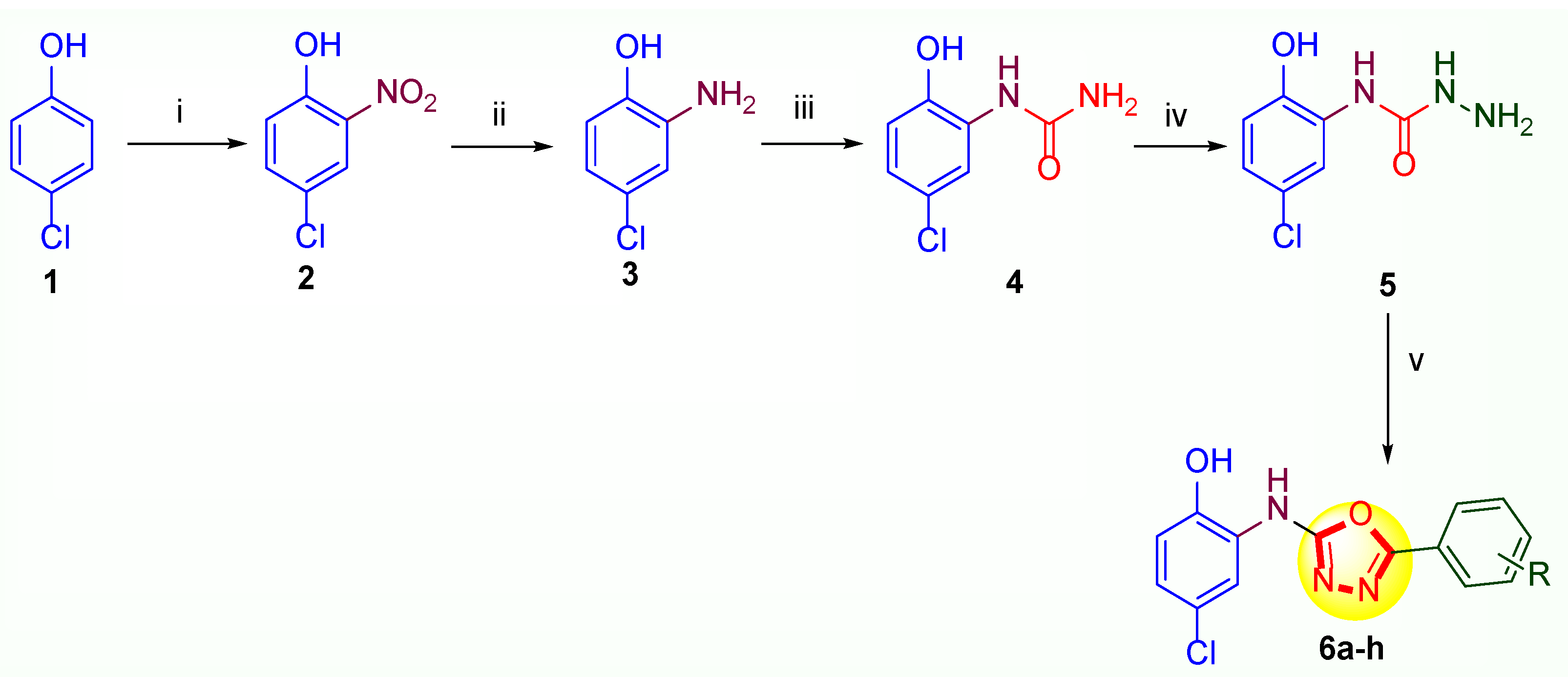
2.1. Chemistry
2.2. Anticancer Activity
2.3. Molecular Docking
2.4. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Method for the Synthesis of 4-Chloro-2-nitrophenol (2)
4.1.2. Method for the Synthesis of 2-Amino-4-chlorophenol (3)
4.1.3. Method for the Synthesis of 1-(5-Chloro-2-hydroxyphenyl)urea (4)
4.1.4. Method for the Synthesis of N-(5-Chloro-2-hydroxyphenyl)hydrazinecarboxamide (5)
4.1.5. General Method for the Synthesis of 4-Chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol Analogues (6a-h)
4.2. Anticancer Activity
4.3. Molecular Docking Studies
4.4. Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khalilullah, H.; Khan, S.; Ahsan, M.J.; Ahmed, B. 1,3,4-Oxadiazole: A Biologically Active Scaffold. Mini-Rev. Med. Chem. 2012, 12, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J. 1,3,4-Oxadiazole containing compounds as therapeutic targets for cancer therapy. Mini-Rev. Med. Chem. 2022, 22, 144–197. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef]
- Cocohoba, J.; Dong, B.J. Raltegravir: The First HIV Integrase Inhibitor. Clin. Ther. 2008, 30, 1747–1765. [Google Scholar] [CrossRef]
- Vardan, S.; Smulyan, H.; Mookherjee, S.; Eich, R. Effects of tiodazosin, a new antihypertensive, hemodynamics and clinical variables. Clin. Pharm. Ther. 1983, 34, 290–296. [Google Scholar] [CrossRef]
- Schlecker, R.; Thieme, P.C. The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl)phenoxypropanolahines. Tetrahedron 1988, 44, 3289–3294. [Google Scholar] [CrossRef]
- Ogata, M.; Atobe, H.; Kushida, H.; Yamamoto, K. In Vitro Sensitivity of Mycoplasmas Isolated From Various Animals and Sewage to Antibiotics and Nitrofurans. J. Antibiot. 1971, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Growcott, J.W. Zibotentan. Drugs Fut. 2009, 34, 624–633. [Google Scholar] [CrossRef]
- Ducharme, Y.; Blouin, M.; Brideau, C.; Chateauneuf, A.; Gareau, Y.; Grimm, E.L.; Juteau, H.; Laliberte, S.; MacKay, B.; Masse, F.; et al. The Discovery of Setileuton, a Potent and Selective 5-Lipoxygenase Inhibitor. ACS Med. Chem. Lett. 2010, 1, 170–174. [Google Scholar] [CrossRef]
- Yarmohammadi, E.; Beyzaei, H.; Aryan, R.; Moradi, A. Ultrasound-assisted, low-solvent and acid/base-free synthesis of 5-substituted 1,3,4-oxadiazole-2-thiols as potent antimicrobial and antioxidant agents. Mol. Divers. 2021, 25, 2367–2378. [Google Scholar] [CrossRef]
- Johansson, A.; Löfberg, C.; Antonsson, M.; Von Unge, S.; Hayes, M.A.; Judkins, R.; Ploj, K.; Benthem, L.; Lindén, D.; Brodin, P. Discovery of (3-(4-(2-Oxa-6-Azaspiro[3.3]Heptan-6-Ylmethyl)Phenoxy)Azetidin-1-Yl)(5-(4-Methoxyphenyl)-1,3,4-Oxadiazol-2-Yl)Methanone (AZD1979), a melanin concentrating hormone receptor 1 (MCHr1) antagonist with favourable physicochemical properties. J. Med. Chem. 2016, 59, 2497–2511. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; Alhamzani, A.G.; Abou-Krisha, M.A.; Kanthimathi, G.; Raghu, M.S.; Kumar, K.Y.; Prashanth, M.K.; Jeon, B.H. Synthesis, molecular docking study and anticancer activity of novel 1,3,4-oxadiazole derivatives as potential tubulin inhibitors. Heliyon 2023, 9, e13460. [Google Scholar] [CrossRef]
- Nieddu, V.; Pinna, G.; Marchesi, I.; Sanna, L.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Synthesis and Antineoplastic Evaluation of Novel Unsymmetrical 1,3,4-Oxadiazoles. J. Med. Chem. 2016, 59, 10451–10469. [Google Scholar] [CrossRef]
- Verma, G.; Chashoo, G.; Ali, A.; Khan, M.F.; Akhtar, W.; Ali, I.; Akhtar, M.; Alam, M.M.; Shaquiquzzaman, M. Synthesis of pyrazole acrylic acid based oxadiazole and amide derivatives as antimalarial and anticancer agents. Bioorg. Chem. 2018, 77, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Karabanovich, G.; Zemanová, J.; Smutný, T.; Székely, R.; Šarkan, M.; Centárová, I.; Vocat, A.; Pávková, I.; Čonka, P.; Němeček, J.; et al. Development of 3,5-Dinitrobenzylsulfanyl-1,3,4-oxadiazoles and thiadiazoles as selective antitubercular agents active against replicating and nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 2362–2380. [Google Scholar] [CrossRef]
- Irfan, A.; Faisal, S.; Zahoor, A.F.; Noreen, R.; Al-Hussain, S.A.; Tuzun, B.; Javaid, R.; Elhenawy, A.A.; Zaki, M.E.A.; Ahmad, S.; et al. In Silico Development of Novel Benzofuran-1,3,4-Oxadiazoles as Lead Inhibitors of M. tuberculosis Polyketide Synthase 13. Pharmaceuticals 2023, 16, 829. [Google Scholar] [CrossRef]
- Khan, M.H.; Hameed, S.; Akhtar, T.; Al-Masoudi, W.A.; Jones, P.G.; Pannecouque, C. Synthesis, crystal structure, and molecular docking study of new thiazdiazole and thiazole analogues as potential anti-HIV agents. Med. Chem. Res. 2016, 25, 2399–2409. [Google Scholar] [CrossRef]
- Khalilullah, H.; Shamshir, K.M.; Shivli, N.; Bahar, A. Synthesis, characterization and antimicrobial activity of benzodioxane ring containing 1,3,4-oxadiazole derivatives. Arab. J. Chem. 2016, 2, 1029–1035. [Google Scholar] [CrossRef]
- Rana, S.M.; Islam, M.; Saeed, H.; Rafique, H.; Majid, M.; Aqeel, M.T.; Imtiaz, F.; Ashraf, Z. Synthesis, Computational Studies, Antioxidant and Anti-Inflammatory Bio-Evaluation of 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2023, 16, 1045. [Google Scholar] [CrossRef]
- Tabatabai, S.A.; Lashkari, S.B.; Zarrindast, M.R.; Golibeikian, M.; Shafiee, A. Design, Synthesis and Anticonvulsant Activity of 2-(2-Phenoxy)phenyl-1,3,4-oxadiazole Derivatives. Iranian J. Pharm. Sci. 2013, 12, 105–111. [Google Scholar]
- Vaidya, A.; Jain, S.; Jain, P.; Jain, P.; Tiwari, N.; Jain, R.; Jain, R.; Jain, A.K.; Agrawal, R.K. Synthesis and Biological Activities of Oxadiazole Derivatives: A Review. Mini-Rev. Med. Chem. 2016, 16, 825–845. [Google Scholar] [CrossRef]
- Salahuddin; Mazumdar, A.; ShaharYar, M.; Mazumdar, R.; Chakraborthy, G.S.; Ahsan, M.J.; Rahman, M. Updates on synthesis and biological activities of 1,3,4-oxadiazole: A review. Synth. Comm. 2017, 47, 1805–1847. [Google Scholar] [CrossRef]
- Verma, G.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Akhter, M.; Shquiquzzaman, M. A Review Exploring Therapeutic Worth of 1,3,4-Oxadiazole Tailored Compounds. Mini-Rev. Med. Chem. 2019, 6, 477–509. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, R.M.; Kadry, H.H.; Taher, A.T.; Abou-Seri, S.M. Comparative Study of the Synthetic Approaches and Biological Activities of the Bioisosteres of 1,3,4-Oxadiazoles and 1,3,4-Thiadiazoles over the Past Decade. Molecules 2022, 27, 2709. [Google Scholar] [CrossRef]
- Sung, H.; Ferley, J.; Siegel, R.L.; Laversanne, M.; Soerjomartaram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Cancer Reports, 2020; WHO: Geneva, Switzerland, 2020; ISBN 978-92-4-000129-9. [Google Scholar]
- Tuma, M.C.; Malikzay, A.; Ouyang, X.; Surguladze, D.; Fleming, J.; Mitelman, S.; Camara, M.; Finnerty, B.; Doody, J.; Chekler, E.L.P.; et al. Antitumor activity of IMC-038525, a novel oral tubulin polymerization inhibitor. Trans. Oncol. 2010, 3, 318–325. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, X.; Piatnitski, E.L.; Kiselyov, A.S.; He, H.Y.; Mao, Y.; Pattaropong, V.; Yu, Y.; Kim, K.H.; Kincaid, J.; et al. Synthesis and structure-activity relationships of 1,2,4-triazoles as a novel class of potent tubulin polymerization inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5154–5159. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, M.; Matysiak, J.; Szeliga, M.; Pozarowski, P.; Niewiadomy, A.; Albrecht, J.; Rzeski, W. 2-Amino-1,3,4-thiadiazole derivative (FABT) inhibits extracellular signal-regulated kinase pathway and induces cell cycle arrest in human non-small lung carcinoma cells. Bioorg. Med. Chem. Lett. 2012, 22, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Binarová, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef]
- Dawbaa, S.; Nuha, D.; Evren, A.E.; Cankilic, M.Y.; Yurttas, L.; Turan, G. New oxadiazole/triazole derivatives with antimicrobial and antioxidant properties. J. Mol. Struct. 2023, 1282, 135213. [Google Scholar] [CrossRef]
- Salahuddin; Mazumder, A.; Shaharyar, M. Synthesis, antibacterial and anticancer evaluation of 5-substituted (1,3,4-oxadiazol-2-yl)quinoline. Med. Chem. Res. 2015, 24, 2514–2528. [Google Scholar] [CrossRef]
- Ahsan, M.J. Rationale design, synthesis and anticancer activity of 2,5-disubstituted-1,3,4-oxadiazole analogues. ChemistrySelect 2016, 1, 4713–4720. [Google Scholar] [CrossRef]
- Agarwal, M.; Singh, V.; Sharma, S.K.; Sharma, P.; Ansari, M.Y.; Jadav, S.S.; Yasmin, S.; Sreenivasulu, R.; Hassan, M.Z.; Saini, V.; et al. Design and synthesis of new 2,5-disubstituted-1,3,4- oxadiazole analogues as anticancer agents. Med. Chem. Res. 2016, 25, 2289–2303. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Chabukswar, A.R.; Shinde, B. Microwave assisted one pot synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazoles as antifungal agents. Bioorg. Med. Chem. Lett. 2011, 21, 444–448. [Google Scholar] [CrossRef]
- Development Therapeutic Program NCI/NIH (2014). Available online: http://dtp.nci.nih.gov (accessed on 20 January 2022).
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a highflux anticancer drug screening using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Collins, A.H. Microbiological Methods, 2nd ed.; Butterworth: London, UK, 1976. [Google Scholar]
- Cruickshank, R.; Duguid, J.P.; Marmion, B.P.; Swain, R.H.A. Medicinal Microbiology, 12th ed.; Churchill Livingstone: London, UK, 1975; Volume 2, p. 196. [Google Scholar]
- Ahsan, M.J.; Ali, A.; Ali, A.; Afzal, O.; Salahuddin; Yusuf, M.; Altamimi, A.S.A.; Sharma, O.; Alossaimi, M.A.; Bakht, M.A. Synthesis of New 4’-(Substituted phenyl)spiro[indoline-3,3’-[1,2,4]triazolidine]-2,5’-diones as Antimicrobial, Antitubercular, and Antifungal Agents: An Insight into the ADME and Toxicity Prediction as well as in-silico Molecular Docking Studies. J. Mol. Struct. 2023, 1290, 135846. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogels’ Textbook of Practical Organic Chemistry, 5th ed.; Longman Group UK Limited: London, UK, 1989. [Google Scholar]
- Ali, A.; Ali, A.; Tahir, A.; Bakht, M.A.; Salahuddin; Ahsan, M.J. Molecular Engineering of Curcumin, an Active Constituent of Curcuma longa L. (Turmeric) of the Family Zingiberaceae with Improved Antiproliferative Activity. Plants 2021, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- X-Ray Crystallographic Structure of Tubulin-Combrestatin A4 Complex. Available online: https://www.rcsb.org/structure/5lyj (accessed on 12 January 2023).
- Gaspari, R.; Prota, A.E.; Bargsten, K.; Cavalli, A.; Steinmetz, M.O. Structural Basis of cis- and trans-Combretastatin Binding to Tubulin. Chem 2017, 2, 102–113. [Google Scholar] [CrossRef]
- X-Ray Crystal of Cytochrome Bacterial DNA Gyrase (PDB ID: 6KZV). Available online: https://www.rcsb.org/structure/6KZV (accessed on 16 March 2023).
- Ushiyama, F.; Amada, H.; Takeuchi, T.; Tanaka-Yamamoto, N.; Kanazawa, H.; Nakano, K.; Mima, M.; Masuko, A.; Takata, I.; Hitaka, K.; et al. Lead Identification of 8-(Methylamino)-2-oxo-1,2-dihydroquinoline Derivatives as DNA Gyrase Inhibitors: Hit-to-Lead Generation Involving Thermodynamic Evaluation. ACS Omega 2020, 5, 10145–10159. [Google Scholar] [CrossRef] [PubMed]



| Panel | Cell Lines | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia | CCRF-CEM | 95.31 | 90.61 | 97.41 | 98.1 | 99.52 | 98.76 | 83.84 | 95.14 |
| HL-60(TB) | 91.94 | 96.58 | 103.03 | 98.47 | 103.4 | 99.51 | 119.06 | 111.48 | |
| K-562 | 93.01 | 91.61 | 96.74 | 94.05 | 97.96 | 92.24 | 100.44 | 95.61 | |
| MOLT-4 | 93.23 | 94.59 | 100.15 | 95.64 | 96.06 | 96.19 | 91.33 | 103.1 | |
| RPMI-8226 | 92.5 | 97.08 | 91.83 | 92.27 | 92.84 | 93.76 | 92.45 | 102.57 | |
| SR | 95.73 | 90.53 | 93.7 | 98.04 | 93.42 | 102.11 | 101.26 | 98.87 | |
| Non-small cell lung cancer | A549/ATCC | 94.38 | 87.15 | 92.88 | 93.37 | 94.35 | 89.37 | 93.03 | 65.15 |
| EKVX | 102.36 | 94 | 91.25 | 99.04 | 105.1 | 96.06 | 91.13 | 82 | |
| HOP-62 | 105.99 | 102.74 | 97.05 | 101.95 | 105.83 | 104.42 | 94.4 | 59.72 | |
| HOP-92 | 120.92 | 119.71 | 100.76 | 117.37 | 117.06 | 119.62 | 119.37 | 115.91 | |
| NCI-H226 | 105.72 | 107.83 | 99.08 | 97.66 | 109.28 | 99.25 | 93.86 | 57.41 | |
| NCI-H23 | 101.36 | 97.21 | 89.12 | 100.38 | 96.67 | 95.13 | 93.66 | 79.18 | |
| NCI-H322M | 99.79 | 98.15 | 97.07 | 101.97 | 101.53 | 98.01 | 102.39 | 66.42 | |
| NCI-H460 | 104.48 | 101.02 | 104.92 | 105.25 | 80.45 | 99.18 | 102.16 | 44.39 | |
| NCI-H522 | 76.68 | 86.11 | 87.33 | 85.94 | 82.27 | 79.68 | 88.6 | 82.71 | |
| Colon cancer | HCC-2998 | 99 | 104.62 | 105.4 | 112.79 | 106.12 | 95.16 | 107.17 | 86 |
| HCT-116 | 104.58 | 103.43 | 99.6 | 99.17 | 89.72 | 79.85 | 93.39 | 64.41 | |
| HCT-15 | 95.67 | 95.25 | 93.24 | 95.62 | 91.28 | 87.97 | 95.78 | 93.96 | |
| HT29 | 99.63 | 92.61 | 98.93 | 101.07 | 98.03 | 88.79 | 102.91 | 89.66 | |
| KM12 | 99.8 | 99.76 | 99.97 | 100.21 | 98.14 | 99.38 | 100.05 | 99.39 | |
| SW-620 | 103.18 | 95.56 | 101.61 | 106.56 | 96.99 | 98.15 | 97.46 | 87.4 | |
| CNS cancer | SF-268 | 101.69 | 103.23 | 98.7 | 99.96 | 102.59 | 95.4 | 96.32 | 70.04 |
| SF-295 | 94.74 | 94.54 | 93.94 | 95.86 | 97.97 | 90.94 | 96.1 | 54.74 | |
| SF-539 | 96.75 | 102.67 | 94.36 | 97.09 | 97.61 | 99.35 | 96.03 | 56.21 | |
| SNB-19 | 96.86 | 95.17 | 93.49 | 95.69 | 101.78 | 96.14 | 101.73 | 34.88 | |
| SNB-75 | 106 | 104.3 | 88.1 | 110.01 | 102.53 | 85.25 | 95.7 | 45.32 | |
| U251 | 108.42 | 101.95 | 106.8 | 101.81 | 110.38 | 98.86 | 101.29 | 73.96 | |
| Melanoma | LOX IMVI | 105.56 | 96.63 | 92.71 | 105.81 | 95.88 | 99.59 | 93.23 | 93.26 |
| MALME-3M | 99.56 | 94.69 | 95.31 | 98.67 | 99.03 | 96.85 | 91.74 | 87.03 | |
| M14 | 101.31 | 101.56 | 104.02 | 108.72 | 96.97 | 102.72 | 101.42 | 102.06 | |
| MDA-MB-435 | 98.9 | 92.79 | 103.33 | 103.96 | 95.59 | 97.75 | 108.74 | 89.29 | |
| SK-MEL-28 | 103.13 | 107.19 | 101.02 | 105.24 | 109.05 | 101.02 | 118.11 | 99.89 | |
| SK-MEL-5 | 98.18 | 99.34 | 97.51 | 97.81 | 100.18 | 96.24 | 100.75 | 94.83 | |
| UACC-257 | 97.67 | 87.48 | 95.1 | 102.94 | 102.24 | 95.07 | 89.74 | 89.39 | |
| UACC-62 | 86 | 86.98 | 87.06 | 86.4 | 89.93 | 78.75 | 83.7 | 95.39 | |
| Ovarian cancer | IGROV1 | 106.78 | 110.01 | 98.18 | 103.66 | 112.29 | 99.07 | 111.38 | 95.15 |
| OVCAR-3 | 108.43 | 106.72 | 105.56 | 108.82 | 109.51 | 107.84 | 113.11 | 93.72 | |
| OVCAR-4 | 106.23 | 92.66 | 105.72 | 112.65 | 107.87 | 98.46 | 97.77 | 59.97 | |
| OVCAR-5 | 96.14 | 100.93 | 95.05 | 96.83 | 103.38 | 95.35 | 97.86 | 99.22 | |
| NCI/ADR-RES | 100.27 | 94.74 | 100.36 | 103.69 | 99.8 | 98.12 | 97.93 | 51.72 | |
| SK-OV-3 | 117.64 | 102.62 | 99.89 | 112.57 | 115.3 | 105.56 | 104.71 | 81.81 | |
| Renal cancer | 786–0 | 94.95 | 98.08 | 100.57 | 100.28 | 108.08 | 92.93 | 111.45 | 79.58 |
| A498 | 113.51 | 103.52 | 117.23 | 121.53 | 127.23 | 104.1 | 130.65 | 101.82 | |
| ACHN | 106.84 | 107.22 | 103.61 | 105.68 | 101.8 | 106.06 | 103.15 | 72.01 | |
| CAKI-1 | 103.12 | 95.64 | 87.11 | 94.99 | 98.39 | 88.89 | 92.01 | 81.17 | |
| RXF 393 | 112.18 | 107.36 | 108.62 | 112.07 | 117.5 | 94.92 | 106.85 | 90.15 | |
| SN 12C | 92.59 | 96.79 | 91.49 | 101.31 | 101.82 | 97.73 | 95.3 | 76.76 | |
| UO-31 | 92.04 | 93.53 | 85.65 | 92.2 | 104.92 | 84.53 | 81.99 | 82.82 | |
| Prostate cancer | PC-3 | 94.36 | 85.87 | 89.66 | 90.97 | 92.45 | 88.87 | 82.21 | 89.9 |
| DU-145 | 104.69 | 102.62 | 106.63 | 106.77 | 101.93 | 104.47 | 109.31 | 87.7 | |
| Breast cancer | MCF7 | 88.75 | 83.92 | 65.54 | 82.19 | 75.21 | 85.96 | 84.23 | 81.18 |
| MDA-MB-231 | 107.86 | 97.25 | 95.31 | 93.6 | 104.79 | 99.02 | 90.49 | 57.42 | |
| HS 578T | 111.42 | 100.53 | 100.25 | 105.9 | 109.39 | 103.85 | 93.65 | 52.78 | |
| BT-549 | 115.53 | 112.05 | 108.47 | 111.75 | 101.27 | 104.77 | 111.86 | 98 | |
| T-47D | 106.36 | 102.08 | 95.87 | 111.22 | 102.18 | 97.96 | 83.03 | 81.98 | |
| MDA-MB-468 | 101.42 | 90.64 | 73.99 | 87.2 | 91.04 | 100.03 | 108.19 | 82.26 | |
| Mean Growth Percent | 100.91 | 98.2 | 96.97 | 101.09 | 100.79 | 96.45 | 98.85 | 81.49 | |
| Compounds | Cell Line (Percent Growth Inhibition; % GI) | |||
|---|---|---|---|---|
| 69.99 to 50.00 | 49.99 to 40.00 | 39.99 to 20.00 | 19.99 to 10.00 | |
| 6a | − | − | NCI-H522 (23.32) | CCRF-CEM (16.16) UACC-62 (14) MCF7 (11.25) |
| 6b | − | − | − | MCF7 (16.08) PC-3 (14.13) NCI-H522 (13.89) UACC-62 (13.02) A549 (12.85) UACC-257 (12.52) |
| 6c | − | − | MCF7 (34.46) | UO-31 (14.35) UACC-62 (12.94) CAKI-1 (12.89) NCI-H522 (12.67) NCI-H23 (10.88) PC-3 (10.34) |
| 6d | − | − | MDA-MB-468 (26.01) MCF7 (24.79) | MCF7 (17.81) NCI-H522 (14.06) UACC-62 (13.06) MDA-MB-468 (12.08) |
| 6e | − | − | − | NCI-H460 (19.55) NCI-H522 (17.73) HCT-116 (10.28) UACC-62 (10.07) |
| 6f | − | − | UACC-62 (21.25) NCI-H522 (20.32) HCT-116 (20.15) | UO-31 (15.47) SNB-75 (14.75) MCF7 (14.04) HCT-15 (12.03) HT29 (11.21) PC-3 (11.13) CAKI-1 (11.11) A549 (10.63) |
| 6g | − | − | − | UO-31 (18.01) PC-3 (17.79) T-47D (16.97) UACC-62 (16.3) MCF7 (15.77) NCI-H522 (11.4) UACC-257 (10.26) |
| 6h | SNB-19 (65.12) NCI-H460 (55.61) SNB-75 (54.68) | NCI/ADR-RES (48.28) HS 578T (47.22) SF-295 (45.26) SF-539 (43.79) NCI-H226 (42.59) MDA-MB-231 (42.58) HOP-62 (40.28) OVCAR-4 (40.03) | A459 (34.85) HCT-116 (35.59) NCI-H322M (33.58) SF-268 (29.96) ACNH (27.99) U251 (26.04) SN 12C (23.24) NCI-H23 (20.82) 786-O (20.42) | CAKI-1 (18.83) MCF7 (18.82) SK-OV-3 (18.19) T-47D (18.02) MDA-MB-468 (17.74) NCI-H522 (17.29) UO-31 (17.18) HCC-2998 (14.0) MALME-3M (12.97) DU-145 (12.3) MDA-MB-435 (10.71) UACC-257 (10.61) HT29 (10.34) PC-3 (10.1) |
| Panels | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h | Imatinib |
|---|---|---|---|---|---|---|---|---|---|
| Leukemia | 6.38 | 6.5 | 2.86 | 3.91 | 2.8 | 2.91 | 1.94 | −1.13 | 9 |
| Non-Small Cancer Cell | −1.29 | 0.68 | 4.50 | −0.33 | 0.83 | 2.14 | 2.38 | 27.46 | 15.68 |
| Colon Cancer | −0.31 | 1.46 | 0.21 | −2.57 | 3.29 | 8.45 | 0.54 | 13.19 | 5.34 |
| CNS Cancer | −1.29 | 0.68 | 4.50 | −0.33 | 0.83 | 2.14 | 2.38 | 27.46 | 5.8 |
| Melanoma | 1.21 | 4.17 | 2.99 | −1.19 | 1.39 | 4.00 | 1.57 | 6.11 | −0.87 |
| Ovarian Cancer | −5.92 | −1.28 | −0.79 | −6.37 | −8.03 | −0.73 | −3.79 | 19.74 | −7.16 |
| Renal Cancer | −2.18 | −0.31 | 0.82 | −4.01 | −8.53 | 4.41 | −3.06 | 16.53 | 3.25 |
| Prostate Cancer | 0.48 | 5.76 | 1.86 | 1.13 | 2.81 | 3.33 | 4.24 | 11.2 | 12.5 |
| Breast Cancer | −5.22 | 2.26 | 10.09 | 1.36 | 2.69 | 1.40 | 4.76 | 24.39 | 12.15 |
| S. No. | Compound | PDB ID: 5LYJ | ||
|---|---|---|---|---|
| Docking Score | Emodel Score | Interaction | ||
| 1 | 6a | −8.233 | −68.717 | H-bond (Asn258, 2.52 Å); halogen bond (Ala317, 3.17 Å) |
| 2 | 6b | −8.247 | −71.236 | H-bond (Asn258, 2.52 Å); halogen bond (Asn349, 3.43 Å) |
| 3 | 6c | −8.356 | −74.181 | H-bond (Asn258, 2.58 Å) |
| 4 | 6d | −8.389 | −73.546 | Halogen bond (Ala317, 3.35 Å) |
| 5 | 6e | −7.617 | −63.671 | − |
| 6 | 6f | −8.073 | −71.277 | Halogen bond (Ala317, 3.13 Å) |
| 7 | 6g | −8.266 | −74.933 | H-bond (Asn349, 2.39 Å); halogen bond (Ala317, 3.12 Å) |
| 8 | 6h | −8.030 | −71.237 | − |
| 9 | IMC-094322 | −7.669 | −42.387 | − |
| 10 | IMC-038525 | −7.941 | −52.924 | π-Cationic (Lys352, 6.46 Å) |
| S. No. | Compound | Zone of Inhibition in mm at 200 µg/mL | Minimum Inhibitory Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | S. aureus | B. subtilis | E. coli | P. aeruginosa | ||
| 1 | 6a | 12.0 ± 0.36 | 11.0 ± 0.25 | 13.0 ± 0.21 | 12.0 ± 0.26 | 16 | 32 | 16 | 16 |
| 2 | 6b | 11.0 ± 0.65 | 9.9 ± 0.30 | 11.0 ± 0.36 | 9.8 ± 0.25 | 32 | 64 | 32 | 64 |
| 3 | 6c | 17.0 ± 0.40 | 17.0 ± 0.21 | 17.0 ± 0.15 | 17.0 ± 0.21 | 8 | 8 | 8 | 8 |
| 4 | 6d | 5.5 ± 0.50 | 4.8 ± 0.75 | 5.6 ± 0.32 | 5.1 ± 0.15 | 256 | 512 | 256 | 256 |
| 5 | 6e | 16.0 ± 0.49 | 14.0 ± 0.45 | 16.0 ± 0.26 | 16.0 ± 0.40 | 8 | 32 | 8 | 8 |
| 6 | 6f | 5.2 ± 0.25 | 5.0 ± 0.10 | 5.4 ± 0.21 | 5.8 ± 0.10 | 256 | 256 | 256 | 256 |
| 7 | 6g | 5.6 ± 0.35 | 5.0 ± 0.23 | 5.8 ± 0.30 | 5.6 ± 0.21 | 256 | 256 | 256 | 256 |
| 8 | 6h | 6.7 ± 0.20 | 5.7 ± 0.29 | 6.0 ± 0.21 | 6.0 ± 0.23 | 128 | 256 | 128 | 128 |
| 9 | Ciprofloxacin | 18.0 ± 0.65 | 17.0 ± 0.26 | 18.0 ± 0.32 | 18.0 ± 0.31 | 4 | 4 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, O.; Ali, A.; Ali, A.; Altamimi, A.S.A.; Alossaimi, M.A.; Bakht, M.A.; Salahuddin; Alamri, M.A.; Ahsan, M.F.; Ahsan, M.J. Synthesis and Anticancer Evaluation of 4-Chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol Analogues: An Insight into Experimental and Theoretical Studies. Molecules 2023, 28, 6086. https://doi.org/10.3390/molecules28166086
Afzal O, Ali A, Ali A, Altamimi ASA, Alossaimi MA, Bakht MA, Salahuddin, Alamri MA, Ahsan MF, Ahsan MJ. Synthesis and Anticancer Evaluation of 4-Chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol Analogues: An Insight into Experimental and Theoretical Studies. Molecules. 2023; 28(16):6086. https://doi.org/10.3390/molecules28166086
Chicago/Turabian StyleAfzal, Obaid, Amena Ali, Abuzer Ali, Abdulmalik Saleh Alfawaz Altamimi, Manal A. Alossaimi, Md Afroz Bakht, Salahuddin, Mubarak A. Alamri, Md. Faiyaz Ahsan, and Mohamed Jawed Ahsan. 2023. "Synthesis and Anticancer Evaluation of 4-Chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol Analogues: An Insight into Experimental and Theoretical Studies" Molecules 28, no. 16: 6086. https://doi.org/10.3390/molecules28166086







