Micro-Executor of Natural Products in Metabolic Diseases
Abstract
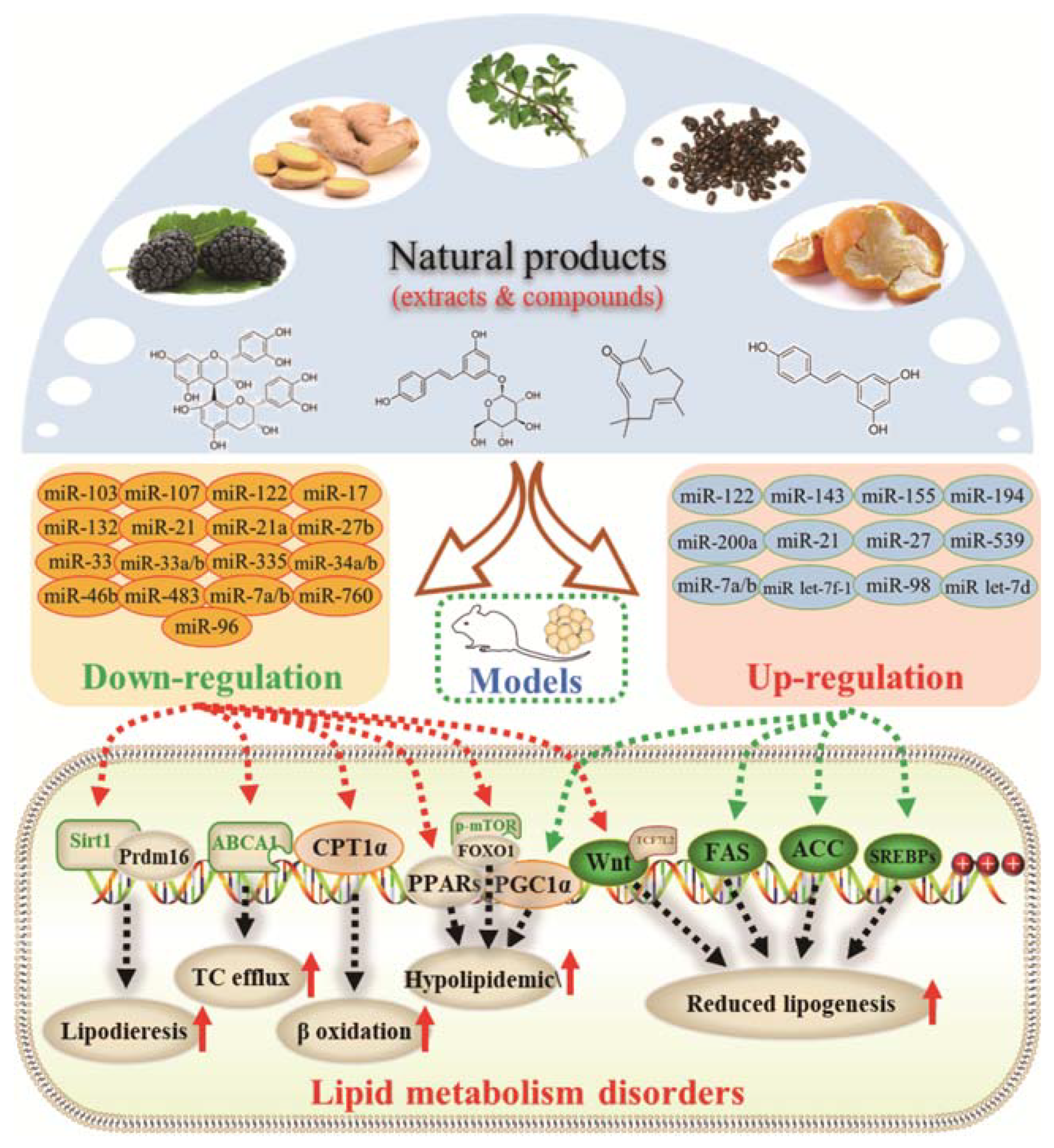
:1. Introduction
2. Research Methodology
3. Effects of Natural Products on Lipid Metabolism Disorders
3.1. Regulatory Effects on Fatty Acid Synthesis and Decomposition
3.2. Inhibitory Effects on Adipocyte Differentiation and Accumulation
4. Effects of Natural Products on Glucose Metabolism Disorders
4.1. Hypoglycemic Action
4.2. Restraining Effects on Oxidative Stress
5. Effects of Natural Products on Cardiovascular Diseases
5.1. Therapeutic Effects on Myocardial Cell Injury
| Natural Products (Extracts) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
| Açaí and red muscadine grape polyphenolics | miR-126↑ | 5–20 mg GAE/L for 30 min | Cell culture | HUVECs | VCAM-1 | ● Protected HUVEC against glucose-induced oxidative stress and inflammation; ● Inhibited gene expression of adhesion molecules and NF-κB activation. | [132] |
| Astragalus root dry extract | miR-1↓ | 20 mg/kg/d for 7 days | Intraperitoneal injection | CVB3-treated mice | Cx43 | ● Prevented the increase of immune cell infiltration and arrhythmia. | [90] |
| Crataegus persica extract | miR-126↑ | 300 mg/kg/d for 10 weeks | Gavage | Diabetic rats | / | ● Decreased elevated levels of renal oxidative stress, glomerular filtration rate, insulin sensitivity, and pathological score; ● Ameliorated myocardial ischemia-reperfusion-induced renal injury. | [79] |
| Panax notoginseng saponins | miR-29c↑ | 150 mg/kg/d for 20 days | Intraperitoneal injection | ISO-treated mice | Cols, Fbn1 | ● Alleviated ISO-induced myocardial injury and fibrotic alterations; ● Cardioprotective effects. | [18] |
| Portulaca oleracea extract | miR-146↑ miR-let-7↑ | 300 mg/kg/d for 35 days | Gavage | Lipopolysaccharide treated mice | / | ● Protected from LPS-induced neuroinflammation and memory decline through antioxidant and anti-inflammatory effects. | [115] |
| Resina draconis | miR-423↑ | 0.25, 0.5 and 1.0 mg/mL | Intramuscular injection | Ischemia-reperfusion tree shrew | ERK | ● Reduced the infarct size, enhanced the superoxide dismutase expression, and downregulated the malondialdehyde concentration; ● Suppressed the ischemia-reperfusion-induced apoptosis. | [118] |
| Salvianolate | miR-122↓ | 12, 24 and 48 mg/kg/d for 2 weeks | Intraperitoneal injection | Myocardial infarction rats | / | ● Induced the anti-apoptosis mechanism of cardiomyocytes. | [119] |
| Xiaoxianggou | miR-203↓ | 10, 20, and 40 g/kg, two times one week for 16 weeks | Gavage | Endogenous high Ang II ApoE −/− mice | Ets2 | ● Reduced the atherosclerotic plaque area and serum autoantibodies against oxLDL. | [133] |
| Natural Products (Compounds) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
Astragaloside IV | miR-135a↑ | 10 mg/kg/d for 9 days | Gavage | Isoproterenol-treated rats | TPRM7 | ● Inhibited cardiac fibrosis by targeting the miR-135a-TRPM7-TGF-β/Smads pathway. | [131] |
Berberine | miR-133a↓ | 1.0 g/kg/d for 8 weeks | Gavage | STZ-induced diabetic rats | / | ● Improved vascular dementia; ● Improved impairments of learning and memory. | [134] |
Celastrol | miR-21↓ | 1 mg/kg/d for 3 and 12 weeks | Intraperitoneal injection | Transverse aortic constriction mice | ERK1/2 | ● Attenuated TAC-induced cardiac hypertrophy; ● Reduced the increased collagen deposition and downregulated α-smooth muscle actin; ● Attenuated pathological myocardial fibrosis. | [129] |
Curcumin | miR-7a/b↑ | 50 mg/kg/d for 1 week | Gavage | Myocardial infarction mice | SP1 | ● Reduced the infarct size; ● Protected against hypoxia-induced cardiac myocytes apoptosis. | [122] |
Dioscin | miR-140↓ | Rats treated with 60, 30, and 15 mg/kg/d for 7 days; mice treated with 80, 40, and 20 mg/kg/d for 7 days | Gavage | Doxorubicin-treated rats and mice | Sirt2, Nrf2 | ● Improved histopathological and electrocardiogram changes; ● Inhibited myocardial oxidative insult; ● Alleviated doxorubicin-induced cardiotoxicity. | [116] |
Emodin | miR-126↑ | 40 mg/kg/d for 7 weeks | Gavage | Balloon-injured carotid artery rats | Wnt4, Dvl-1, β-catenin | ● Prevented intimal thickening via Wnt4/Dvl-1/β-catenin signaling pathway. | [135] |
Ginsenoside Rb1 | miR-208↓ | 40 μM for 24 h | Cell culture | Neonatal rat cardiomyocytes | NLK | ● Ameliorated cardiomyocytes apoptosis; ● Protected cardiomyocytes injuries. | [124] |
Ginsenoside Rg1 | miR-23a↓ | 150 nM for 24 h | Cell culture | HUVECs | MET | ● Increased MET protein expression in a time-dependent manner; ● Induced angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression. | [136] |
Gypenoside A | miR-143↓ | 100 mg/kg 1 h before I/R administration | Gavage | Myocardial I/R injured rats | / | ● Cardio-protective effect; ● Attenuated I/R-induced injures; ● Activated AMPK signaling. | [114] |
Luteolin-7-diglucuronide | miR-29c, miR-30c, miR-133b: ↑; miR-21↓ | 40 mg/kg/d for 11 days | Intraperitoneal injection | Isoproterenol-treated mice | Cols, elastin and Fbn1; CTGF | ● Attenuated ISO-induced myocardial fibrosis; ● Suppresses ISO-induced oxidative stress and upregulation of NADPH oxidase; ● Reduced myocardial fibrotic lesions. | [130] |
Puerarin | miR-22↓ | 100 μM for 20 days | Cell culture | The mES cell line D3 and its transgenic cell line αPIG (clone 44) | Cav3 | ● Improved the myofibrillar alignment and sarcomere development; ● Promoted the development of t-tubules; ● Upregulated the t-tubules biogenesis-related genes. | [123] |
Resveratrol | miR-34a↓ | 20, 50 or 100 mΜ for 48 h | Cell culture | Rat heart-derived H9c2 cells | / | ● Enhanced cell viability; ● Reduced cell apoptosis; ● Protective effect on cardiomyocytes. | [113] |
| miR-328↓ | 100 mg/kg/d for 8 weeks | Gavage | Cold-treated mice | / | ● Inhibited alteration of cardiac structure; ● Improved ultrastructure of myocardium; ● Improved cardiac function; ● Suppressed cold-induced hypertension; ● Suppressed apoptosis of myocardium. | [120] | |
| miR-29b↓ | 0.1 mg/mL for 2 months | Drinking | Fbn1C1039G/+ Marfan mice | Bcl-2 | ● Promoted elastin integrity and smooth muscle cell survival; ● Inhibited aortic root dilatation. | [137] | |
Sodium Tanshinone IIA Sulfonate | miR-133a↓ | 10 mg/kg/d for 3 weeks | Gavage | PAD mice | / | ● Improved perfusion recovery, increased capillary densities, decreased ROS level in the ischemic hindlimb in diabetic mice; ● Improved angiogenesis via inhibiting miR-133a expression and increasing GCH-1 protein levels. | [89] |
Tanshinone IIA | miR-375↓ | 10 mg/kg for 20 weeks | Intraperitoneal injection | HFD-fed ApoE-/- mice | KLF4 | ● Attenuated atherosclerosis. | [138] |
| miR-1↓ | 20 mg/kg/d for 3 months | Gavage | Myocardial infarction rats | Cx43 | ● Improved the hemodynamic parameters; ● Regulated P38 MAPK pathway; ● Relieved ischemia-induced injury. | [109]. | |
| miR-1↓ | 10 mg/kg/d for 3 months | Gavage | Myocardial infarction rats | KCNJ2, SRF | ● Raised survival rates; ● Ameliorated dysfunction of IK1; ● Suppressed ischemic arrhythmias and cardiac mortality. | [111] | |
| miR-133↑ | 10 µM for 30 min | Cell culture | Neonatal rat cardiomyocytes | ERK1/2 | ● Increased cell viability; ● Protected cell against apoptosis; ● Activated MAPK ERK1/2 signaling. | [110] | |
Theaflavin | miR-24↑ | 5, 10 mg/kg/d for 12 weeks | Gavage | HFD-fed ApoE−/− mice | Nrf2, HO-1 | ● Promoted the activities of antioxidant enzymes (SOD, CAT, and GSH-Px); ● Inhibited the formation of atherosclerotic plaque and the process of histological alterations in the aorta. | [125] |
5.2. Protective Effects on Vascular Endothelial Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Cawley, J.; Wen, K. Policies to Prevent Obesity and Promote Healthier Diets: A Critical Selective Review. Clin. Chem. 2018, 64, 163–172. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Velliquette, R.A.; Grann, K.; Burns, C.R.; Scholten, J.; Tian, F.; Zhang, Q.; Gui, M. A novel botanical formula prevents diabetes by improving insulin resistance. BMC Complement. Altern. Med. 2017, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Gupta, N.; Guo, Y.; Beckman, J.A.; Bangalore, S.; Berger, J.S. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart 2018, 104, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Horvath, T.L. Limitations in anti-obesity drug development: The critical role of hunger-promoting neurons. Nat. Rev. Drug Discov. 2012, 11, 675–691. [Google Scholar] [CrossRef]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Shukla, S.K.; Gupta, S.; Ojha, S.K.; Sharma, S.B. Cardiovascular friendly natural products: A promising approach in the management of CVD. Nat. Prod. Res. 2010, 24, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A MicroRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A.; Blade, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345. [Google Scholar] [CrossRef]
- Zhang, L.; He, S.; Yang, F.; Yu, H.; Xie, W.; Dai, Q.; Zhang, D.; Liu, X.; Zhou, S.; Zhang, K. Hyperoside ameliorates glomerulosclerosis in diabetic nephropathy by downregulating miR-21. Can. J. Physiol. Pharmacol. 2016, 94, 1249–1256. [Google Scholar] [CrossRef]
- Liu, L.; Ning, B.; Cui, J.; Zhang, T.; Chen, Y. miR-29c is implicated in the cardioprotective activity of Panax notoginseng saponins against isoproterenol-induced myocardial fibrogenesis. J. Ethnopharmacol. 2017, 198, 1–4. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol. Nutr. Food Res. 2017, 62, 1700505. [Google Scholar] [CrossRef]
- Pang, D.; You, L.; Zhou, L.; Li, T.; Zheng, B.; Liu, R.H. Averrhoa carambola free phenolic extract ameliorates nonalcoholic hepatic steatosis by modulating mircoRNA-34a, mircoRNA-33 and AMPK pathways in leptin receptor-deficient db/db mice. Food Funct. 2017, 8, 4496–4507. [Google Scholar] [CrossRef]
- Liu, S.; Chang, X.; Yu, J.; Xu, W. Cerasus humilis Cherry Polyphenol Reduces High-Fat Diet-Induced Obesity in C57BL/6 Mice by Mitigating Fat Deposition, Inflammation, and Oxidation. J. Agric. Food Chem. 2020, 68, 4424–4436. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Liu, H.; Qi, X.; Dong, L.; Zhang, R.; Zhang, J. Citrus peel flavonoids improve lipid metabolism by inhibiting miR-33 and miR-122 expression in HepG2 cells. Biosci. Biotechnol. Biochem. 2019, 83, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger Extract Ameliorates Obesity and Inflammation via Regulating MicroRNA-21/132 Expression and AMPK Activation in White Adipose Tissue. Nutrients 2018, 10, 1567. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Chronic supplementation of proanthocyanidins reduces postprandial lipemia and liver miR-33a and miR-122 levels in a dose-dependent manner in healthy rats. J. Nutr. Biochem. 2014, 25, 151–156. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012, 56, 1636–1646. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Arola-Arnal, A.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Blade, C. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS ONE 2013, 8, e69817. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, M.; Xu, L.; Fang, Z.; Wu, W.; Zhang, Q.; Chung, P.; Lin, Y.; Wang, S.; Zhang, Y. miR-96 and autophagy are involved in the beneficial effect of grape seed proanthocyanidins against high-fat-diet-induced dyslipidemia in mice. Phytother. Res. 2019, 33, 1222–1232. [Google Scholar] [CrossRef]
- Lu, R.H.; Qin, C.B.; Yang, F.; Zhang, W.Y.; Zhang, Y.R.; Yang, G.K.; Yang, L.P.; Meng, X.L.; Yan, X.; Nie, G.X. Grape seed proanthocyanidin extract ameliorates hepatic lipid accumulation and inflammation in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 1665–1677. [Google Scholar] [CrossRef]
- Torres, L.F.; Cogliati, B.; Otton, R. Green Tea Prevents NAFLD by Modulation of miR-34a and miR-194 Expression in a High-Fat Diet Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 4168380. [Google Scholar] [CrossRef]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Lima, N.D.S.; Numata, E.P.; Mesquita, L.M.S.; Dias, P.H.; Vilegas, W.; Gambero, A.; Ribeiro, M.L. Modulatory Effects of Guarana (Paullinia cupana) on Adipogenesis. Nutrients 2017, 9, 635. [Google Scholar] [CrossRef]
- Su, D.; Zhang, R.; Hou, F.; Chi, J.; Huang, F.; Yan, S.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, M. Lychee pulp phenolics ameliorate hepatic lipid accumulation by reducing miR-33 and miR-122 expression in mice fed a high-fat diet. Food Funct. 2017, 8, 808–815. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.S.; Chang, E.; Lee, Y.; Lee, J.; Kim, J.; Kim, C.T.; Kim, I.H.; Kim, Y. Mulberry Fruit Extract Promotes Serum HDL-Cholesterol Levels and Suppresses Hepatic microRNA-33 Expression in Rats Fed High Cholesterol/Cholic Acid Diet. Nutrients 2020, 12, 1499. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Yu, M.H.; Wu, Y.L.; Hong, C.C.; Chen, C.S.; Chan, K.C.; Wang, C.J. Mulberry Leaf (Morus alba L.) Extracts and Its Chlorogenic Acid Isomer Component Improve Glucolipotoxicity-Induced Hepatic Lipid Accumulation via Downregulating miR-34a and Decreased Inflammation. Nutrients 2022, 14, 4808. [Google Scholar] [CrossRef]
- Monraz-Méndez, C.A.; Escutia-Gutiérrez, R.; Rodriguez-Sanabria, J.S.; Galicia-Moreno, M.; Monroy-Ramírez, H.C.; Sánchez-Orozco, L.; García-Bañuelos, J.; de la Rosa-Bibiano, R.; Santos, A.; Armendáriz-Borunda, J.; et al. Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients 2022, 14, 4225. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca Oleracea Extract on Acute Alcoholic Liver Injury of Rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, M.S.; Kang, S.A.; Kim, C.T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330. [Google Scholar] [CrossRef]
- Stefanon, B.; Pomari, E.; Colitti, M. Effects of Rosmarinus officinalis extract on human primary omental preadipocytes and adipocytes. Exp. Biol. Med. 2015, 240, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zou, B.; Nie, R.; Zhang, Y.; Li, C.M. A-type ECG and EGCG dimers disturb the structure of 3T3-L1 cell membrane and strongly inhibit its differentiation by targeting peroxisome proliferator-activated receptor gamma with miR-27 involved mechanism. J. Nutr. Biochem. 2015, 26, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor gamma with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Su, T.C.; Yang, M.J.; Chen, W.T.; Siao, A.C.; Huang, L.R.; Lin, Y.Y.; Kuo, Y.C.; Chung, J.F.; Cheng, C.F.; et al. Green tea epigallocatechin gallate suppresses 3T3-L1 cell growth via microRNA-143/MAPK7 pathways. Exp. Biol. Med. 2022, 247, 1670–1679. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674. [Google Scholar] [CrossRef]
- Rohm, B.; Holik, A.K.; Kretschy, N.; Somoza, M.M.; Ley, J.P.; Widder, S.; Krammer, G.E.; Marko, D.; Somoza, V. Nonivamide enhances miRNA let-7d expression and decreases adipogenesis PPARgamma expression in 3T3-L1 cells. J. Cell. Biochem. 2015, 116, 1153–1163. [Google Scholar] [CrossRef]
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1beta axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2017, 31, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Ge, Z.; Zhu, W.; Xu, Z.; Li, C. Persimmon tannin represses 3T3-L1 preadipocyte differentiation via up-regulating expression of miR-27 and down-regulating expression of peroxisome proliferator-activated receptor-gamma in the early phase of adipogenesis. Eur. J. Nutr. 2015, 54, 1333–1343. [Google Scholar] [CrossRef]
- Gai, Y.; Li, Y.; Xu, Z.; Chen, J. Pseudoprotodioscin inhibits SREBPs and microRNA 33a/b levels and reduces the gene expression regarding the synthesis of cholesterol and triglycerides. Fitoterapia 2019, 139, 104393. [Google Scholar] [CrossRef] [PubMed]
- Gracia, A.; Fernandez-Quintela, A.; Miranda, J.; Eseberri, I.; Gonzalez, M.; Portillo, M.P. Are miRNA-103, miRNA-107 and miRNA-122 Involved in the Prevention of Liver Steatosis Induced by Resveratrol? Nutrients 2017, 9, 360. [Google Scholar] [CrossRef]
- Gracia, A.; Miranda, J.; Fernandez-Quintela, A.; Eseberri, I.; Garcia-Lacarte, M.; Milagro, F.I.; Martinez, J.A.; Aguirre, L.; Portillo, M.P. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016, 7, 1680–1688. [Google Scholar] [CrossRef]
- Eseberri, I.; Lasa, A.; Miranda, J.; Gracia, A.; Portillo, M.P. Potential miRNA involvement in the anti-adipogenic effect of resveratrol and its metabolites. PLoS ONE 2017, 12, e0184875. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984–36995. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Naar, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Moore, K.J.; Rayner, K.J.; Suarez, Y.; Fernandez-Hernando, C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu. Rev. Nutr. 2011, 31, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.P.; Lv, Q.T.; Dongol, S.; Wang, C.; Jiang, J. Single nucleotide polymorphism of SREBF-1 gene associated with an increased risk of endometrial cancer in Chinese women. PLoS ONE 2014, 9, e90491. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Nagai, Y.; Yonemitsu, S.; Erion, D.M.; Iwasaki, T.; Stark, R.; Weismann, D.; Dong, J.; Zhang, D.; Jurczak, M.J.; Loffler, M.G.; et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009, 9, 252–264. [Google Scholar] [CrossRef]
- Bennett, M.K.; Lopez, J.M.; Sanchez, H.B.; Osborne, T.F. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J. Biol. Chem. 1995, 270, 25578–25583. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Brocklehurst, K.J.; Marley, A.E.; Carey, F.; Carling, D.; Beri, R.K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994, 353, 33–36. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Saha, A.K.; Schwarsin, A.J.; Roduit, R.; Masse, F.; Kaushik, V.; Tornheim, K.; Prentki, M.; Ruderman, N.B. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J. Biol. Chem. 2000, 275, 24279–24283. [Google Scholar] [CrossRef]
- Bengestrate, L.; Virtue, S.; Campbell, M.; Vidal-Puig, A.; Hadaschik, D.; Hahn, P.; Bielke, W. Genome-wide profiling of microRNAs in adipose mesenchymal stem cell differentiation and mouse models of obesity. PLoS ONE 2011, 6, e21305. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Nickels, J.D.; Chatterjee, S.; Stanley, C.B.; Qian, S.; Cheng, X.; Myles, D.A.A.; Standaert, R.F.; Elkins, J.G.; Katsaras, J. The in vivo structure of biological membranes and evidence for lipid domains. PLoS Biol. 2017, 15, e2002214. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Price, N.L.; Fernandez-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Dogan, A.; Demirci, S.; Apdik, H.; Bayrak, O.F.; Gulluoglu, S.; Tuysuz, E.C.; Gusev, O.; Rizvanov, A.A.; Nikerel, E.; Sahin, F. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/beta-catenin pathway. Metabolism 2017, 69, 130–142. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, D.F.; Li, J.; Musuka, G.; Awad, S.F. Spatial epidemiology of diabetes: Methods and insights. World J. Diabetes 2021, 12, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [PubMed]
- Kim, A.; Lee, W.; Yun, J.M. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr. Res. Pract. 2017, 11, 430–434. [Google Scholar] [CrossRef]
- Du, G.; Xiao, M.; Zhang, X.; Wen, M.; Pang, C.; Jiang, S.; Sang, S.; Xie, Y. Alpinia oxyphylla Miq. extract changes miRNA expression profiles in db-/db- mouse kidney. Biol. Res. 2017, 50, 9. [Google Scholar] [CrossRef]
- Wang, C.; Wang, K.; Li, P. Blueberry anthocyanins extract attenuated diabetic retinopathy by inhibiting endoplasmic reticulum stress via the miR-182/OGG1 axis. J. Pharmacol. Sci. 2022, 150, 31–40. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, H.; Yang, W.; Amat, R.; Peng, J.; Li, Y.; Deng, K.; Mao, X.; Jiao, Y. The Alcohol Extract of Coreopsis tinctoria Nutt Ameliorates Diabetes and Diabetic Nephropathy in db/db Mice through miR-192/miR-200b and PTEN/AKT and ZEB2/ECM Pathways. Biomed. Res. Int. 2019, 2019, 5280514. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Salehi, I.; Ranjbar, K.; Khosravi, M.; Zarrinkalam, E. Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2022, 153, 113411. [Google Scholar] [CrossRef]
- Yang, Y.M.; Seo, S.Y.; Kim, T.H.; Kim, S.G. Decrease of microRNA-122 causes hepatic insulin resistance by inducing protein tyrosine phosphatase 1B, which is reversed by licorice flavonoid. Hepatology 2012, 56, 2209–2220. [Google Scholar] [CrossRef]
- Balbaa, M.; Abdulmalek, S.A.; Khalil, S. Oxidative stress and expression of insulin signaling proteins in the brain of diabetic rats: Role of Nigella sativa oil and antidiabetic drugs. PLoS ONE 2017, 12, e0172429. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Zhang, D.; Han, W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 2022, 13, 8240–8254. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Q.; Gu, T.T.; Wang, W.; Song, L.; Chen, T.Y.; Xue, Q.C.; Zhou, F.; Li, J.M.; Kong, L.D. Curcumin protects against fructose-induced podocyte insulin signaling impairment through upregulation of miR-206. Mol. Nutr. Food Res. 2015, 59, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Tao, X.F.; Yin, L.H.; Xu, L.; Xu, Y.W.; Qi, Y.; Han, X.; Song, S.S.; Zhao, Y.Y.; Lin, Y.; et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. Br. J. Pharmacol. 2017, 174, 2512–2527. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Pang, X.; Yang, J.; Yu, H.; Zhang, Y.; Zhou, H.; Zhao, J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol. Lett. 2017, 277, 115–122. [Google Scholar] [CrossRef]
- Li, Y. Gypenoside A attenuates dysfunction of pancreatic β cells by activating PDX1 signal transduction via the inhibition of miR-150-3p both in vivo and in vitro. J. Biochem. Mol. Toxicol. 2022, 36, e23004. [Google Scholar] [CrossRef]
- Bae, S.; Lee, E.J.; Lee, J.H.; Park, I.C.; Lee, S.J.; Hahn, H.J.; Ahn, K.J.; An, S.; An, I.S.; Cha, H.J. Oridonin protects HaCaT keratinocytes against hydrogen peroxide-induced oxidative stress by altering microRNA expression. Int. J. Mol. Med. 2014, 33, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, W.; Peng, B.; Yuan, M.; Wang, N.; Wang, J.; Lu, W.; Wang, T. Sodium Tanshinone IIA sulfonate improves post-ischemic angiogenesis in hyperglycemia. Biochem. Biophys. Res. Commun. 2019, 520, 580–585. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhu, X.; Li, S.; Shi, X.; Li, Z.; Ai, M.; Sun, J.; Hou, B.; Cai, W.; et al. Protective Effects and Mechanisms of Vaccarin on Vascular Endothelial Dysfunction in Diabetic Angiopathy. Int. J. Mol. Sci. 2019, 20, 4587. [Google Scholar] [CrossRef]
- Azimova, K.; Rude, J.; Mallawaarachchi, I.; Dwivedi, A.; Sarosiek, J.; Mukherjee, D. Glucose levels and depression in Hispanic patients admitted to the cardiovascular intensive care unit: A cross-sectional study. Angiology 2015, 66, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, J.; Koricanac, G.; Culafic, T.; Romic, S.; Stojiljkovic, M.; Kostic, M.; Pantelic, M.; Tepavcevic, S. Low intensity exercise prevents disturbances in rat cardiac insulin signaling and endothelial nitric oxide synthase induced by high fructose diet. Mol. Cell. Endocrinol. 2016, 420, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor 1993, 3, 1–15. [Google Scholar] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxid. Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, L.; Li, H.; Sun, H.; Liu, J.; Wu, S.; Zhou, B. Progranulin causes adipose insulin resistance via increased autophagy resulting from activated oxidative stress and endoplasmic reticulum stress. Lipids Health Dis. 2017, 16, 25. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y. SIRT1: Role in cardiovascular biology. Clin. Chim. Acta 2015, 440, 8–15. [Google Scholar] [CrossRef]
- Jamal, J.; Mustafa, M.R.; Wong, P.F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Jeong, H.G. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic. Biol. Med. 2014, 74, 21–34. [Google Scholar]
- Yin, L.H.; Xu, Y.W.; Qi, Y.; Han, X.; Xu, L.N.; Peng, J.Y.; Sun, C.K. A green and efficient protocol for industrial-scale preparation of dioscin from Dioscorea nipponica Makino by two-step macroporous resin column chromatography. Chem. Eng. J. 2010, 165, 281–289. [Google Scholar] [CrossRef]
- Chen, W.; Gao, R.; Liu, L.; Zhu, M.; Wang, W.; Wang, Y.; Wu, Z.; Li, H.; Zheng, Z.; Jiang, L.; et al. Outline of the report on cardiovascular diseases in China, 2014. Eur. Heart J. Suppl. 2016, 18, F2–F11. [Google Scholar]
- Le Bouc, Y.; Brioude, F. Is there a relationship between the growth hormone dose and tumoral or cardiovascular complications? Bull. Acad. Natl. Med. 2012, 196, 127–135. [Google Scholar] [PubMed]
- Sadowska, J.; Bruszkowska, M. Comparing the effects of sucrose and high-fructose corn syrup on lipid metabolism and the risk of cardiovascular disease in male rats. Acta Sci. Pol. Technol. Aliment. 2017, 16, 231–240. [Google Scholar] [PubMed]
- Traverse, J.H.; Henry, T.D. Myocardial Injury as a New Target for Cell Therapy in Patients with Chronic Heart Failure When Something Bad Is Actually Good? Circ. Res. 2017, 120, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jing, X.; Dong, A.; Bai, B.; Wang, H. Overexpression of TIMP3 Protects Against Cardiac Ischemia/Reperfusion Injury by Inhibiting Myocardial Apoptosis through ROS/Mapks Pathway. Cell. Physiol. Biochem. 2017, 44, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, L.; Yang, K.; Fu, Y.; Liu, Y.; Chi, J.; Zhang, X.; Hong, S.; Ma, X.; Yin, X. H3 Relaxin Protects Against Myocardial Injury in Experimental Diabetic Cardiomyopathy by Inhibiting Myocardial Apoptosis, Fibrosis and Inflammation. Cell. Physiol. Biochem. 2017, 43, 1311–1324. [Google Scholar] [CrossRef]
- Li, F.; Zong, J.; Zhang, H.; Zhang, P.; Xu, L.; Liang, K.; Yang, L.; Yong, H.; Qian, W. Orientin Reduces Myocardial Infarction Size via eNOS/NO Signaling and thus Mitigates Adverse Cardiac Remodeling. Front. Pharmacol. 2017, 8, 926. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Chu, W.F.; Wang, B.; Zhang, J.L.; Zhao, M.; Li, X.L.; Li, B.X.; Lu, Y.J.; Yang, B.F.; et al. Tanshinone IIA Inhibits miR-1 Expression through p38 MAPK Signal Pathway in Post-infarction Rat Cardiomyocytes. Cell. Physiol. Biochem. 2010, 26, 991–998. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Li, Y.; Xu, C.; Li, X.; Zhu, D.; Zhang, Y.; Xing, S.; Wang, H.; Zhang, Z.; et al. Tanshinone IIA improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell. Physiol. Biochem. 2012, 30, 843–852. [Google Scholar] [CrossRef]
- Shan, H.; Li, X.; Pan, Z.; Zhang, L.; Cai, B.; Zhang, Y.; Xu, C.; Chu, W.; Qiao, G.; Li, B.; et al. Tanshinone IIA protects against sudden cardiac death induced by lethal arrhythmias via repression of microRNA-1. Br. J. Pharmacol. 2009, 158, 1227–1235. [Google Scholar] [CrossRef]
- Miano, J.M.; Ramanan, N.; Georger, M.A.; de Mesy Bentley, K.L.; Emerson, R.L.; Balza, R.O., Jr.; Xiao, Q.; Weiler, H.; Ginty, D.D.; Misra, R.P. Restricted inactivation of serum response factor to the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 17132–17137. [Google Scholar] [CrossRef]
- Yang, B.; Ma, S.; Wang, Y.B.; Xu, B.; Zhao, H.; He, Y.Y.; Li, C.W.; Zhang, J.; Cao, Y.K.; Feng, Q.Z. Resveratrol exerts protective effects on anoxia/reoxygenation injury in cardiomyocytes via miR-34a/Sirt1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2734–2741. [Google Scholar]
- Chang, L.; Shi, R.; Wang, X.; Bao, Y. Gypenoside A protects ischemia/reperfusion injuries by suppressing miR-143-3p level via the activation of AMPK/Foxo1 pathway. Biofactors 2020, 46, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Youssef, A.M.; Magharbeh, M.K.; Al-Dalaen, S.M.; Al-Jawabri, N.A.; Al-Nawaiseh, T.N.; Al-Jwanieh, A.; Al-Ani, F.S. Protective Effect of Portulaca oleracea Extract Against Lipopolysaccharide-Induced Neuroinflammation, Memory Decline, and Oxidative Stress in Mice: Potential Role of miR-146a and miR-let 7. J. Med. Food 2022, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef]
- Qin, F.; Siwik, D.A.; Pimentel, D.R.; Morgan, R.J.; Biolo, A.; Tu, V.H.; Kang, Y.J.; Cohen, R.A.; Colucci, W.S. Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1453–H1463. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.R.; Zhang, T.; Mu, N.H.; Ruan, L.B.; Duan, J.L.; Zhang, R.P.; Miao, Y.B. Resina draconis inhibits the endoplasmic-reticulum-induced apoptosis of myocardial cells via regulating miR-423-3p/ERK signaling pathway in a tree shrew myocardial ischemia- reperfusion model. J. Biosci. 2019, 44, 53. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, X. Salvianolate Blocks Apoptosis During Myocardial Infarction by Down Regulating miR-122-5p. Curr. Neurovasc. Res. 2017, 14, 323–329. [Google Scholar] [CrossRef]
- Yin, K.; Zhao, L.; Feng, D.; Ma, W.; Liu, Y.; Wang, Y.; Liang, J.; Yang, F.; Bi, C.; Chen, H.; et al. Resveratrol Attenuated Low Ambient Temperature-Induced Myocardial Hypertrophy via Inhibiting Cardiomyocyte Apoptosis. Cell. Physiol. Biochem. 2015, 35, 2451–2462. [Google Scholar] [CrossRef]
- Lee, B.S.; Oh, J.; Kang, S.K.; Park, S.; Lee, S.H.; Choi, D.; Chung, J.H.; Chung, Y.W.; Kang, S.M. Insulin Protects Cardiac Myocytes from Doxorubicin Toxicity by Sp1-Mediated Transactivation of Survivin. PLoS ONE 2015, 10, e0135438. [Google Scholar] [CrossRef]
- Geng, H.H.; Li, R.; Su, Y.M.; Xiao, J.; Pan, M.; Cai, X.X.; Ji, X.P. Curcumin protects cardiac myocyte against hypoxia-induced apoptosis through upregulating miR-7a/b expression. Biomed. Pharmacother. 2016, 81, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.; Tang, M.; Hu, X.; Luo, H.; Hescheler, J.; Xi, J. Puerarin facilitates T-tubule development of murine embryonic stem cell-derived cardiomyocytes. Cell. Physiol. Biochem. 2014, 34, 383–392. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Wu, H.; Liu, Y.; Zheng, S.; Zhang, C.; Yang, C. Impact of miR-208 and its Target Gene Nemo-Like Kinase on the Protective Effect of Ginsenoside Rb1 in Hypoxia/Ischemia Injuried Cardiomyocytes. Cell. Physiol. Biochem. 2016, 39, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Deng, Z.; Zou, Y.; Liu, C.; Fu, H.; Gu, Y.; Chang, H. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating microRNA-24-mediated Nrf2/HO-1 signal. Phytother. Res. 2021, 35, 3418–3427. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xuan, L.; Liu, Y.; Shao, L.; Ge, H.; Gu, J.; Wei, C.; Zhao, M. Astragalus Root dry extract restores connexin43 expression by targeting miR-1 in viral myocarditis. Phytomedicine 2018, 46, 32–38. [Google Scholar] [CrossRef]
- Li, X.; Zhao, D.; Guo, Z.; Li, T.; Qili, M.; Xu, B.; Qian, M.; Liang, H.; E, X.; Chege Gitau, S.; et al. Overexpression of SerpinE2/protease nexin-1 Contribute to Pathological Cardiac Fibrosis via increasing Collagen Deposition. Sci. Rep. 2016, 6, 37635. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Shanmugam, M.K.; Sethi, G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011, 303, 9–20. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, G.; Song, Y.; Wang, L.; Tu, L.; Zhang, L.; Zhang, C. Celastrol-Induced Suppression of the MiR-21/ERK Signalling Pathway Attenuates Cardiac Fibrosis and Dysfunction. Cell. Physiol. Biochem. 2016, 38, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.B.; Zhang, Y.; Wu, D.D.; Cui, J.G.; Liu, L.; Wang, P.W.; Wang, W.J.; Zhu, W.L.; Chen, Y.; Zhang, T. Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacol. Sin. 2017, 38, 331–341. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Feng, K.; Zhao, Y.; Tao, R.; Xu, H.; Tang, Y. Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J. Ethnopharmacol. 2020, 249, 112404. [Google Scholar] [CrossRef]
- Noratto, G.D.; Angel-Morales, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from acai (Euterpe oleracea Mart.) and red muscadine grape (Vitis rotundifolia) protect human umbilical vascular Endothelial cells (HUVEC) from glucose- and lipopolysaccharide (LPS)-induced inflammation and target microRNA-126. J. Agric. Food Chem. 2011, 59, 7999–8012. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Zhang, X.; Yan, H.; Li, S.; Zhu, W.; Fan, F.; Zhu, J. Xiaoxianggou attenuates atherosclerotic plaque formation in endogenous high Ang II ApoE(−/−) mice via the inhibition of miR-203 on the expression of Ets-2 in endothelial cells. Biomed. Pharmacother. 2016, 82, 173–179. [Google Scholar] [CrossRef]
- Yin, S.; Bai, W.; Li, P.; Jian, X.; Shan, T.; Tang, Z.; Jing, X.; Ping, S.; Li, Q.; Miao, Z.; et al. Berberine suppresses the ectopic expression of miR-133a in endothelial cells to improve vascular dementia in diabetic rats. Clin. Exp. Hypertens. 2019, 41, 708–716. [Google Scholar] [CrossRef]
- Hua, J.Y.; He, Y.Z.; Xu, Y.; Jiang, X.H.; Ye, W.; Pan, Z.M. Emodin prevents intima thickness via Wnt4/Dvl-1/beta-catenin signaling pathway mediated by miR-126 in balloon-injured carotid artery rats. Exp. Mol. Med. 2015, 47, e170. [Google Scholar] [CrossRef]
- Kwok, H.H.; Chan, L.S.; Poon, P.Y.; Yue, P.Y.; Wong, R.N. Ginsenoside-Rg1 induces angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression through miR-23a. Toxicol. Appl. Pharmacol. 2015, 287, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hibender, S.; Franken, R.; van Roomen, C.; Ter Braake, A.; van der Made, I.; Schermer, E.E.; Gunst, Q.; van den Hoff, M.J.; Lutgens, E.; Pinto, Y.M.; et al. Resveratrol Inhibits Aortic Root Dilatation in the Fbn1C1039G/+ Marfan Mouse Model. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1618–1626. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Guo, S.; Song, N.; Wang, J.; Jia, L.; Zhu, A. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int. Immunopharmacol. 2019, 70, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Fu, A.; Spiro, C.; Lee, H.C. Proteomic Analysis of Vascular Endothelial Cells-Effects of Laminar Shear Stress and High Glucose. J. Proteom. Bioinform. 2009, 2, 445. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2017, 100, 1–19. [Google Scholar] [CrossRef]
- Eren, E.; Yilmaz, N.; Aydin, O. Functionally defective high-density lipoprotein and paraoxonase: A couple for endothelial dysfunction in atherosclerosis. Cholesterol 2013, 2013, 792090. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Xing, W.; Su, F.; Liang, X.; Tian, F.; Gao, F.; Wang, S.; Zhang, H. Tetrahydroxystilbene Glycoside Improves Microvascular Endothelial Dysfunction and Ameliorates Obesity-Associated Hypertension in Obese ZDF Rats Via Inhibition of Endothelial Autophagy. Cell. Physiol. Biochem. 2017, 43, 293–307. [Google Scholar] [CrossRef]
- Wilkinson, E.L.; Sidaway, J.E.; Cross, M.J. Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells. J. Cell. Physiol. 2018, 233, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Ding, Y.; Dai, X.; Zhang, Z.; Li, Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017, 15, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tempel, D.; den Dekker, W.K.; Haasdijk, R.; Chrifi, I.; Bos, F.L.; Wagtmans, K.; van de Kamp, E.H.; Blonden, L.; Biessen, E.A.; et al. Ets2 determines the inflammatory state of endothelial cells in advanced atherosclerotic lesions. Circ. Res. 2011, 109, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, A.; Williams, H.; Lyon, C.A.; Taylor, V.; Swain, A.; Johnson, J.L.; George, S.J. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ. Res. 2011, 108, 427–436. [Google Scholar] [CrossRef]
- Chen, Y.; Lüttmann, F.F.; Schoger, E.; Schöler, H.R.; Zelarayán, L.C.; Kim, K.P.; Haigh, J.J.; Kim, J.; Braun, T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 2021, 373, 1537–1540. [Google Scholar] [CrossRef]
- Cowan, C.E.; Kohler, E.E.; Dugan, T.A.; Mirza, M.K.; Malik, A.B.; Wary, K.K. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ. Res. 2010, 107, 959–966. [Google Scholar] [CrossRef]
- Hale, A.T.; Tian, H.; Anih, E.; Recio, F.O., 3rd; Shatat, M.A.; Johnson, T.; Liao, X.; Ramirez-Bergeron, D.L.; Proweller, A.; Ishikawa, M.; et al. Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J. Biol. Chem. 2014, 289, 12016–12028. [Google Scholar] [CrossRef]
- Zhou, G.; Hamik, A.; Nayak, L.; Tian, H.; Shi, H.; Lu, Y.; Sharma, N.; Liao, X.; Hale, A.; Boerboom, L.; et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Investig. 2012, 122, 4727–4731. [Google Scholar] [CrossRef]
- Dambal, S.; Shah, M.; Mihelich, B.; Nonn, L. The microRNA-183 cluster: The family that plays together stays together. Nucleic Acids Res. 2015, 43, 7173–7188. [Google Scholar] [CrossRef] [PubMed]


| Natural Products (Extracts) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
| Averrhoa carambola free phenolic extract | miR-33↓ miR-34a↓ | 10, 20, 30 g/kg/d for 8 weeks | Gavage | db/db mice | / | ● Reduced liver TG; ● Inhibited the signal transduction of hepatic lipogenesis; ● Exhibited a potent hepatic steatosis-relieving effect. | [20] |
| Cerasus humilis polyphenol extract | miR-7a/b↓ | 40 μg/mL for 48 h; 250 g/kg/day for 12 weeks | Cell culture; gavage | 3T3-L1 pre-adipocyte cells; obese mice | Sirt1, Prdm16 | ● Reduced body weight; ● Improved abnormal serum lipid and glucose levels; ● Inhibited adipocyte differentiation; ● Reduced fat accumulation by mitigating fat deposition, inflammation, and oxidation. | [21] |
| Citrus peel flavonoids | miR-33↓ miR-122↓ | 10 μg/mL for 0.5, 1, 3 and 6 h | Cell culture | Oleic acid-treated HepG2 cells | FAS, CPT1a | ● Attenuated intracellular lipid accumulation. | [22] |
| Coffee polyphenols | miR-122↑ | 2.5 × 10−4%; diet containing 0.5% or 1.0% coffee polyphenols for 15 weeks | Cell culture; diet | Hepa 1-6 cells; HFD-fed mice | SREBP1c | ● Activated AMPK; ● Enhanced energy metabolism; ● Reduced lipogenesis; ● Reduced body weight gain, abdominal and liver fat accumulation. | [23] |
| Ginger extract | miR-21↓ miR-132↓ | Diet containing 0.8% ginger extract for 10 weeks | Diet | HFD-fed rats | / | ● Lowered body weight and white adipose tissue mass; ● Reduced serum and hepatic lipid levels; ● Enhanced AMPK activity; ● Ameliorated obesity and inflammation. | [24] |
| Grape seed proanthocyanidins extract | miR-33a↓ miR-122↓ | 5, 25, 50 mg/kg for 3 weeks | Gavage | HFD–induced obese rats | ABCA1; FAS, PPARβ/δ | ● Hypolipidemic; ● Decreased total liver fat. | [16] |
| miR-33a↓ miR-122↓ | 5, 15, 25, 50 mg/kg for 3 weeks | Gavage | Healthy Wistar rats | ABCA1; FAS | ● Improved postprandial hyperlipemia; ● Increased liver cholesterol efflux to HDL formation; ● Reduced fatty acid synthesis. | [25] | |
| miR-33↓ miR-122↓ | 10, 25, 50, or 100 mg/L for 0.5, 1, 3, or 5 h; 250 mg/kg for 1 or 3 h | Cell culture; gavage | FAO cells; Wistar rats | ABCA1; FAS | ● Hypolipidemic; ● Reduced lipogenesis; ● Increased liver cholesterol efflux to HDL formation. | [26] | |
| miR-33a↓ miR-122↓ | 25 mg/kg for 3 weeks | Gavage | Dyslipidemic obese rats | ABCA1, CPT1a; FAS, PPARβ/δ | ● Improved dyslipidemia; ● Decreased total liver fat. | [27] | |
| miR-96↓ | 200 mg/kg/day for 180 days | Diet | HFD-fed mice | mTOR, FOXO1 | ● Decreased the weight gain, serum levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol but increased high-density lipoprotein cholesterol; ● Clearance of lipid accumulation. | [28] | |
| miR-33↓ miR-122↓ | 250 mg/kg once | Gavage | HFD-fed grass carp | / | ● Decreased TG accumulation by reducing de novo lipogenesis and enhancing lipolysis and β-oxidation. | [29] | |
| Green tea extract | miR-34a↓ miR-194↑ | 500 mg/kg for 12 weeks (5 days/week) | Gavage | HFD-fed mice | Sirt1, PPARα, INSIG2; HMGCS, APOA5 | ● Protected against NAFLD development by altering lipid metabolism, increasing gene expression involved in triglycerides and fatty acid catabolism, and decreasing uptake and lipid accumulation. | [30] |
| miR-335↓ | 500 mg/kg for 12 weeks (5 days/week) | Gavage | HFD-fed mice | FOXO1, GSK3β | ● Reduced weight gain, adiposity and inflammation; ● Increased energy expenditure; ● Improved insulin sensitivity. | [31] | |
| Guarana extract | miR-27b↓ miR-34b↓ miR-760↓ | 150 µg/mL for 48 h | Cell culture | 3T3-L1 pre-adipocyte cells | Wnt3a, Wnt1, Wnt10b | ● Anti-adipogenic effect. | [32] |
| Lychee pulp phenolics | miR-33↓ miR-122↓ | 500 mg/kg for 10 weeks | Gavage | HFD-fed mice | ABCA1, ABCG1, NPC1; FAS, ACC1, ACC2, SCD1, ACLY | ● Hypolipidemic; ● Repressed fatty acid synthesis and promoting fatty acid β-oxidation and cholesterol efflux in the liver; ● Decreased body fat accumulation; ● Ameliorated lipid metabolism. | [33] |
| Mulberry fruit extract | miR-33↓ | Diet containing 0.4% mulberry fruit extract for 4 weeks | Diet | High cholesterol/cholic acid diet-fed rats | / | ● Promoted serum high-density lipoprotein cholesterol levels; ● Decreased serum and hepatic cholesterol, serum low-density lipoprotein cholesterol, and fecal bile acid levels. | [34] |
| Mulberry leaf extract | miR-34a↓ | 3 mg/mL for 24 h | Cell culture | Glucolipotoxicity-induced HepG2 cells | Sirt1 | ● Reduced liver fat accumulation; ● Decreased inflammatory responses and steatohepatitis; ● Exerted anti-glucolipotoxicity effects. | [35] |
| Moringa oleifera leaf extract | miR-21a↓ miR-103↓ miR-122↓ miR-34a↓ | 9.375 mg/d for 8 weeks | Gavage | HFD-fed mice | / | ● Improved ITT and decreased SREBP1c hepatic protein, while Sirt1 increased; ● Reduced insulin resistance, de novo lipogenesis, hepatic inflammation, and ER stress; ● Prevented progression of liver damage in a model of NASH. | [36] |
| Portulaca oleracea extract | miR-122↓ | 25, 50, 100 mg/kg/d for 7 days | Gavage | Acute alcoholic liver injury rats | / | ● Reduced the ethanol-elevated serum level of ALT, AST, ALP, and TG; ● Enhanced activities of SOD and GSH-Px; ● Decreased content of NO and MDA; ● Increased antioxidant capacity; ● Relieved the inflammatory injury; ● Improved the lipid metabolism disorder. | [37] |
| miR-33↓ miR-34a↓ | Diet containing 0.8% portulaca oleracea L. extract for 4 weeks | Diet | High-cholesterol diet-fed rats | / | ● Improved serum, liver, and fecal lipid profiles; ● Promoted cholesterol efflux and bile acid synthesis; ● Enhanced hepatic AMPK activity. | [38] | |
| Rosmarinus officinalis extract | miR let-7f-1↑ | 30 μg/mL for 35 days | Cell culture | Human primary omental pre-adipocytes and adipocytes | / | ● Decreased triglyceride accumulation; ● Increased glycerol release; ● Stimulated lipolytic activity in differentiating pre-adipocytes and mature adipocytes; ● Modulated the adipocyte life cycle at different levels. | [39] |
| Natural Products (Compounds) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
A-type ECG and EGCG dimers | miR-7a/b↑ | ECG dimer: 20 μg/mL for 1–8 days; ECGG dimer: 60 μg/mL for 1–8 days | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation; ● Blocked MCE process; ● Decreased the fluidity and hydrophobicity and increased the permeability of membrane. | [40] |
Curcumin | miR-17↓ | 2 μM or 10 μM for 6 h; | Cell culture | 3T3-L1 pre-adipocyte cells; HFD-fed mice | TCF7L2 | ● Inhibited adipocyte differentiation and adipogenesis; ● Stimulated the Wnt signaling pathway. | [41] |
Grape seed procyanidin B2 | miR-483↓ | 150 μg/mL for 48 h | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation. | [42] |
EGCG | miR-143↑ | 50 μM for 24 h | Cell culture | 3T3-L1 pre-adipocyte cells | MAPK7 | ● Inhibited 3T3-L1 cell growth. | [43] |
Lycopene | miR-21↑ | 50 μM for 24 h; diet containing 0.05% lycopene for 8 weeks | Cell culture; gavage | Hepa 1–6 cells; HFD-fed mice | FABP7 | ● Lowered body weight; ● Inhibited intracellular lipid accumulation; ● Protected against HFD-induced hepatic steatosis. | [44] |
Nonivamide | miR let-7d↑ | 1 μM for 12 days | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ | ● Impaired adipogenesis; ● Reduced mean lipid accumulation; ● Activated TRPV1. | [45] |
Oleanolic acid | miR-98↑ | 10 mM for 6, 12, 24 h; 20 mg/kg for 4 weeks | Cell culture | HFD-fed mice; db/db mice | PGC1β | ● Hypolipidemic. | [46] |
Persimmon tannin  | miR-27↑ | 20, 40, or 60 μg/mL for 1–8 days | Cell culture | 3T3-L1 pre-adipocyte cells | PPARγ, C/EBPα | ● Inhibited pre-adipocyte differentiation; ● Reduced intracellular lipid accumulation; ● Delayed MCE process. | [47] |
Pseudoprotodioscin | miR-33a/b↓ | 25 μM for 24 h | Cell culture | Human HepG2 cells and THP-1 monocytic cells | SREBP1c, SREBP2 | ● Promoted the cholesterol effluxion. | [48] |
Resveratrol | miR-103↓ miR-107↓ miR-122↓ | 30 mg/kg for 6 weeks | Diet | Obesogenic diet-fed rats | SREBP1; SREBP1, CPT1a; FAS | ● Reduced obesogenic diet-induced hepatic steatosis; ● Activated AMPK. | [49] |
| miR-539↑ | 30 mg/kg for 6 weeks | Diet | Obesogenic diet-fed rats | SP1 | ● Inhibited de novo lipogenesis. | [50] | |
| miR-155↑ | 25 μM for 1–8 days | Cell culture | 3T3-L1 pre-adipocyte cells | CEBP/α | ● Inhibited adipogenesis. | [51] | |
Zerumbone | miR-46b↓ | 25 μM for 48 h; diet containing 0.025% zerumbone for 8 weeks | Cell culture; diet | 3T3-L1 fibroblasts; HFD-fed mice | Sirt1 | ● Induced AMPK activation and phosphorylation of acetyl-CoA carboxylase; ● Ameliorated diet-induced obesity and inhibited adipogenesis. | [52] |
| Natural Products (Extracts) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
| Alpinia oxyphylla extract | miR-let-7k, miR-378d: ↑; miR-129, miR-21a, miR-29c, miR-203, miR-7a: ↓ | 50 mg/kg/d for 8 weeks | Gavage | DB/DB and db-/db- mice | / | ● Lowered concentrations of blood glucose; ● Changed the expressions of specific miRNAs. | [76] |
| Blueberry anthocyanins extract | miR-182↓ | 200 mg/kg/d for 6 days | Gavage | STZ-induced diabetic rats | OGG1 | ● Restored the increase of apoptosis, ROS level, and ERS induced by high-concentration glucose. | [77] |
| Coreopsis tinctoria nutt extract | miR-192↓ miR-200b↓ | 300 mg/kg/d for 10 weeks | Gavage | db/db mice | ZEB2, PTEN | ● Decreased body weight, fasting blood glucose, and 24 h urinary albumin excretion; alleviated kidney damage; ● Modulated the activity of the PTEN/PI3K/AKT pathway to reduce the degree of renal fibrosis. | [78] |
| Crataegus persica extract | miR-126↓ | 300 mg/kg/d for 10 weeks | Gavage | Diabetic rats | Nrf2 | ● Decreased elevated levels of renal oxidative stress, glomerular filtration rate, insulin sensitivity, and pathological score. | [79] |
| Licorice flavonoid | miR-122↑ | 30 mg/kg for 5 weeks, 5 times per week | Gavage | HFD-fed mice | PTP1B | ● Reduced blood glucose; ● Restored IR and IRS1/2 tyrosine phosphorylation and insulin signaling; ● Abrogated hepatic insulin resistance induced by HFD diet. | [80] |
| Nigella sativa oil | miR-34a↓ miR-26b↓ | 2.0 mL for 21 days | Gavage | Diabetic rats | / | ● Suppressed oxidative stress; ● Improved insulin resistance and insulin signaling pathway. | [81] |
| Natural Products (Compounds) | Relevant miRNAs | Dose | Administration Methods | Experimental Models | Targets | Observed Effects | References |
|---|---|---|---|---|---|---|---|
Astragaloside IV | miR-138↓ | 25 mM for 48 h | Cell culture | High glucose cultured retinal pigment epithelial cells | Sirt1, Nrf2 | ● Alleviated high glucose-induced RPE cell damage; ● Increased Sirt1/Nrf2 activity and cellular antioxidant capacity; ● Alleviated ferroptosis; ● Decreased cell death. | [82] |
Curcumin | miR-206↑ | 15, 30, and 60 mg/kg/d for 6 weeks | Gavage | Fructose-fed rats | PTP1B | ● Improved insulin signaling; ● Improved glucose intolerance and insulin sensitivity. | [83] |
Dioscin | miR-34a↓ | 50, 100, and 200 ng/mL for 12 h; 10, 20, and 40 mg/kg for 12 days; 15, 30, and 60 mg/kg/d for 10 days | Cell culture; gavage | NRK-52E and HK-2 cells; Wistar rats; C57BL/6J mice | Sirt1 | ● Decreased the ROS levels; ● Suppressed oxidative stress. | [84] |
Genistein | miR-34a↓ | 1000 nM for 6 h | Cell culture | HUVECs | Sirt1 | ● Restrained ROS and MDA production; ● Ameliorated the inhibitory effect on SOD, CAT, GSH, GPx activity; ● Suppressed oxidative stress. | [85] |
Gypenoside A | miR-150↓ | 50 or 100 mg/kg/d for 12 weeks | Gavage | HFD-fed mice | PDX1 | ● Alleviated pancreatic impairments; ● Improved the dysfunction of β pancreatic cells. | [86] |
Oridonin | miR-1305, miR-152, miR-182, miR-29b, miR-4298, miR-939: ↑; miR-1246, miR-1268, miR-1290, miR-135a, miR-181b, miR-1973, miR-210, miR-30c, miR-30e, miR-3162, miR-4299, miR-572, miR-575, miR-630, miR-642b: ↓ | 5 μM for 24 h | Cell culture | H2O2-exposed HaCaT cells | / | ● Protected against H2O2-induced oxidative stress. | [87] |
Polydatin | miR-200a↑ | 10, 20, and 40 μM for 24 h; 7.5, 15, and 30 mg/kg/d for 7 weeks | Cell culture; gavage | High fructose-treated buffalo rat liver cells and HepG2 cells; fructose-fed rats | Keap1 | ● Alleviated hepatic oxidative stress, inflammation, and lipid deposition; ● Activated Keap1/Nrf2 pathway. | [88] |
Sodium tanshinone IIA sulfonate | miR-133a↓ | 10 mg/kg/d for 3 weeks | Gavage | STZ-induced diabetic mice | / | ● Improved perfusion recovery; ● Increased capillary densities; ● Decreased ROS level and increased GCH-1 protein level. | [89] |
Vaccarin | miR-34a↓ | 1 mg/kg/d for 4 weeks | Intraperitoneal injection | STZ/HFD-induced T2DM mice | / | ● Reduced blood glucose; ● Increased glucose and insulin tolerance; ● Relieved the disorder of lipid metabolism and oxidative stress; ● Improved endothelium-dependent vasorelaxation. | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, H.; Li, X.; Song, C.; Wang, L.; Wang, D. Micro-Executor of Natural Products in Metabolic Diseases. Molecules 2023, 28, 6202. https://doi.org/10.3390/molecules28176202
Liu J, Chen H, Li X, Song C, Wang L, Wang D. Micro-Executor of Natural Products in Metabolic Diseases. Molecules. 2023; 28(17):6202. https://doi.org/10.3390/molecules28176202
Chicago/Turabian StyleLiu, Jinxin, Huanwen Chen, Xiaoli Li, Chunmei Song, Li Wang, and Deguo Wang. 2023. "Micro-Executor of Natural Products in Metabolic Diseases" Molecules 28, no. 17: 6202. https://doi.org/10.3390/molecules28176202
APA StyleLiu, J., Chen, H., Li, X., Song, C., Wang, L., & Wang, D. (2023). Micro-Executor of Natural Products in Metabolic Diseases. Molecules, 28(17), 6202. https://doi.org/10.3390/molecules28176202






