Abstract
Ingestion of polonium-210 from environmental media and food can cause serious health hazards (e.g., gastrointestinal symptoms, tumours, etc.) and has been a public health concern worldwide since the 2006 poisoning of Agent Litvinenko 210Po in Russia. With the development of uranium mining and applications of nuclear technology in recent decades, the radioactive hazards posed by 210Po to living organisms and the environment have become increasingly prominent. In order to strengthen the monitoring of environmental 210Po and protect both the environment and human health, a series of explorations on the methods of 210Po determination have been ongoing by researchers across the globe. However, previous reviews have focused on individual sample types and have not provided a comprehensive account of environmental, food, and biological samples that are closely related to human health. In this work, the sources, health hazards, chemical purification, and detection methods of trace 210Po in different sample types are systematically reviewed. In particular, the advantages and disadvantages of various pretreatment methods are compared, and relevant domestic and international standards are integrated, which puts forward a new direction for the subsequent establishment of rapid, simple, and efficient measurement methods.
1. Introduction
1.1. Properties of 210Po
Polonium is a metallic element with an atomic number of 84 that was discovered by the Curies in 1898 when processing uranium ore [1,2]. The element is soluble in concentrated sulfuric acid, nitric acid, and diluted hydrochloric acid, as well as several other solutions, and it can also form soluble salts with chloride, nitrate, and other inorganic anions. Polonium has 43 isotopes [3] that range from 186 to 227 relative atomic masses, and all of them are radioactive, though 208Po, 209Po, and 210Po are the most stable. Other isotopes have short half-lives. 210Po the most widely used isotope [4] and is a radionuclide with high chemical and radiological toxicity [5].
In nature, 210Po is an alpha radionuclide derived from the natural radionuclide 238U through a series of decays [6,7]. The main decay process occurs when238U undergoes a series of decays to 210Pb, 210Pb undergoes beta decay to 210Bi, 210Bi undergoes beta decay to 210Po, and finally 210Po undergoes alpha decay to produce the stable 206Pb (Figure 1) [6]. Polonium-210 has an alpha particle energy of 5.38 MeV and a half-life of 138.4 d [8], which is the longest half-life of all naturally occurring polonium radionuclides. It has high specific activity (1.66 × 1014 Bq/g) [9] and high volatility [10] and can easily form colloids and adsorb on metal surfaces.
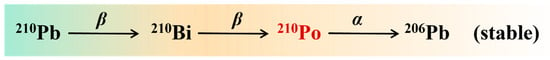
Figure 1.
The decay chain of 210Po [11].
1.2. Sources of 210Po
The sources of 210Po in the environment are divided into two main categories: natural and artificial sources. During anthropogenic activities such as uranium mining and hydrometallurgical processing, important decay substrates of the natural uranium system, such as 210Pb and 210Po are discharged with wastewater into the surface water and soil around mines, causing pollution of the hydrosphere and soil lithosphere [2]. Airborne 210Po comes mainly from natural factors such as the decay of 222Rn on the surface of the earth’s crust [12,13], resuspension and wind erosion of soils [14], and volcanic eruptions [15]. Human activities such as fossil fuel combustion, phosphoric acid production, and fertilizer use also increase atmospheric 210Po levels [16]. Atmospheric 210Po and 210Pb are rapidly adsorbed onto aerosol particles [17] and can migrate through aerosols over long distances and for long periods of time, eventually entering the water or settling to the soil surface as dry or wet depositions. Soil and atmospheric 210Po can also enter plant tissues by root uptake or surface adsorption. Eventually, the radionuclide 210Po can become enriched in the human body via our natural food chain (Figure 2).
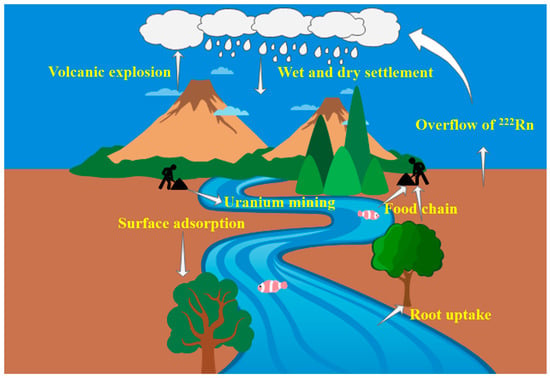
Figure 2.
Sources of 210Po in the environment and its life cycle in humans and the environment.
1.3. Health Risks of 210Po
Currently, 210Po has been found to be widely distributed in a variety of environmental and biological samples [1,18,19], such as groundwater [20,21,22], fish, and shellfish [20,23,24]. 210Po in environmental media can enter the body through inhalation, diet, drinking water, open wounds, and the food chain [17,25]. In fact, a significant proportion of an individual’s exposure to natural background radiation through ingestion of food and water comes from the radionuclide 210Po [26], which provides approximately 60% of the annual effective dose of all naturally ingested radionuclides [25].
Fifty to ninety percent of the 210Po that enters the body rapidly reaches the gastrointestinal tract [1]. After uptake by the gastrointestinal tract, 210Po is mainly bound and concentrated in red blood cells and plasma proteins [27]. The remainder reaches various organs via the bloodstream, is retained in the bones, lungs, kidneys, and liver, and is excreted in the form of excrement, urine, or sweat [6]. Because the alpha particles released by 210Po during decay are weak and easily shielded by the skin, the harm caused by external exposure to 210Po is negligible. However, once inside the body, it is extremely harmful, causing strong ionization in the body, destroying the genetic material of human cells, and triggering a series of serious biological effects [27]. Therefore, it is of great public health significance to enhance the monitoring of 210Po radioactivity levels in environmental media to prevent and mitigate 210Po hazards to both human health and the natural environment.
1.4. Analytical Method Reviews for 210Po
In the 21st century, a large number of scholars have conducted research on 210Po, especially after 2011; the number of research results on the keyword “210Po” on the Web of Science has doubled compared with previous years. The reason for this surge in research may be related to the Fukushima nuclear accident in 2011 (Figure 3). At present, however, there is no coherent review of the 210Po detection literature. To the best of our knowledge, only Matthews [6] and Thakur et al. [28] have reviewed the determination methods of 210Po in environmental samples. But there is still a lack of review of 210Po determination methods in food samples, air samples, and biological samples, as well as a better outlook and prospective prediction of future analytical methods for 210Po. A review of the 210Po method needs to cover a wide range of different sample types.
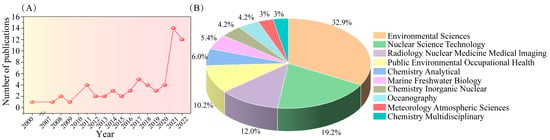
Figure 3.
(A) Number of publications on 210Po from 2000 to 2022. (B) Visualization of data for the main application areas of 210Po. Data extracted from Web of Science (April 2023).
In this paper, we systematically summarize the pretreatment, chemical separation and purification, preparation, and detection methods of 210Po in different environmental media, biological samples, and seafood. Table 1 presents the methods reported since the start of the 21st century for the detection of 210Po in environmental and biological samples. In general, the analytical procedure for determining whether 210Po is present in a sample is divided into four stages: pretreatment, chemical purification, deposition source preparation, and measurement, as shown in Figure 4 and described in detail later in the paper.

Table 1.
Overview of analytical methods developed for determining the quantity of 210Po in environmental and biological samples since 2000.
2. Sample Pretreatment
Due to the variety of sample types and quantities, different pretreatment methods are often used for 210Po testing depending on the specific sample type in question. The most crucial aspect of pretreatment is temperature control, which prevents polonium from volatilizing at high temperatures and resulting in a significantly reduced radiochemical yield [2,48]. Relevant studies have shown that various polonium compounds begin to volatilize at 100 °C, and when the temperature reaches 300 °C, 90% of the polonium can completely evaporate [10]. Chlorides, organic compounds, and chelates of polonium are particularly volatile, and all polonium complexes volatilize below 200 °C [49]. Consequently, temperature control is essential during the pretreatment of both environmental and biological samples. In addition, the time required for processing can be reduced by different pretreatment methods while achieving high yields.
2.1. Pretreatment of Environmental Samples
2.1.1. Pretreatment of Water Samples
Due to the relatively low levels of 210Po in natural environmental water samples, the volumes required to measure 210Po in water samples are usually large [50], so enrichment and concentration prior to testing are required. Currently, the treatment of water samples is often carried out using evaporation, co-precipitation, and chelation methods [26]. Due to its simple operation process when processing large-volume water samples, the direct evaporation method is preferred for concentrated environmental water samples [20,51]. However, evaporation is extremely time-consuming when processing large volumes of samples (>1 L), so the method is not suitable for radiological emergencies involving 210Po or samples containing large amounts of dissolved impurities [52].
The co-precipitation method is widely used to concentrate and enrich polonium in water samples due to the fact that it can save time compared with other methods, involves non-volatilization, and can remove interfering ions [6,28]. At present, the most popular methods for enriching 210Po in environmental water samples include iron hydroxide [Fe(OH)3] co-precipitation [22,53,54], manganese dioxide (MnO2) co-precipitation [55,56,57], and calcium phosphate [Ca3(PO4)2] co-precipitation [45].
Iron hydroxide [Fe(OH)3] co-precipitation, which involves first adding 209Po tracer to the water sample, uses iron hydroxide as a carrier to adsorb the carrier water 210Po and 209Po, dissolving them both in hydrochloric acid, and then adding ascorbic acid to reduce Fe3+ to Fe2+, followed by self-deposition for source production. When iron hydroxide is used for preconcentration, the solvent extraction step must be performed using an extractant such as diisopropyl ether to remove iron ions from the sample solution [58], as large amounts of iron ions can interfere with solvent extraction, affect the purification of polonium by extraction chromatography, and even affect the auto-deposition of polonium.
Due to the cumbersome nature of iron hydroxide co-precipitation and its use of toxic or flammable organic reagents, researchers have explored other methods of preconcentration [59,60]. Co-precipitation using MnO2 is widely used since with this method no interfering ions are present, and the method is particularly suitable for the pre-enrichment of polonium in large volumes (>1 L) of water samples [28]. Lee et al. [52] concentrated polonium nuclides with manganese dioxide precipitation and purified them by solvent extraction. 209Po tracer was added to their tap water sample and stirred well. Then, KMnO4 and MnCl2 were added, and the solution was adjusted to a pH of 9 with 25% NH4OH. The sample solution processed by this method could then be directly used for radiochemical separation. In contrast to the traditional MnO2 co-precipitation method, their method required no evaporation step, thus saving a lot of experimental time.
The calcium phosphate co-precipitation method first adds calcium nitrate and ammonium biphosphate to an aqueous sample, adjusts the pH to 10 with ammonium hydroxide, and then forms a co-precipitation with the resulting calcium phosphate [61]. Maxwell et al. [45] used a new rapid method developed at the Savannah River National Laboratory (SRNL) to measure 210Po in water samples in which they used rapid calcium phosphate co-precipitation for sample pretreatment, separation, and purification using N,N,N′,N′-tetra-n-octyldiglycolamide (DGA) resin, and then micro-precipitation of 210Po using bismuth phosphate that they counted using alpha spectrometry. This new method allows rapid detection in a short time with excellent removal of interfering ions, and its high chemical yield (>95%) and excellent alpha peak resolution make it suitable for both emergencies and routine water analysis. 210Po in water can also be precipitated by a chelating agent (ammonium cobalt dithiopyrrolidine disulfate, CO-APDC), which has been shown to be a prerequisite not only for quantitative extraction of polonium from samples [26] but also for direct extraction of polonium from water samples [26,62,63,64].
In summary, the co-precipitation method is prized by a wide range of researchers for its ability to pretreat large volumes of water samples. Its advantages are primarily time-saving, much less loss of polonium due to volatilization than evaporation, and the strong ability to remove interfering ions. Nonetheless, co-precipitation is labour-intensive as the treatment steps are cumbersome and require constant supervision by the experimenter.
2.1.2. Pretreatment of Air Samples
The range of activity concentrations in ground-level air for 210Po is 0.03–0.3 Bq/m3 [13], and the background radiation received by inhalation of 210Po accounts for more than 70% of the average total dose of radiation to the human body [65]. 210Po entering the body poses considerable health risks by damaging the internal structure of the body and thereby increasing the risk of cancer. However, there is currently no national standard for the determination of 210Po in the air in China. The main challenge for the detection of 210Po in air samples is the question of how to separate 210Po from aerosols and establish a hydrochloric acid self-deposition system [65]. The most prominent existing research idea on this subject is to first convert the 210Po in the sample into Po2+ or Po4+ in the particle state [66], digest and destroy the organic matter adsorbed in the aerosol with concentrated nitric acid and concentrated sulfuric acid, and then leach it several times with concentrated hydrochloric acid to form a hydrochloric acid system where self-deposition can occur.
However, the high-temperature acid digestion methods normally used for many solid samples can result in the loss of polonium through volatilization unless carried out in specially designed containers such as Kjeldahl flasks [67]. Khaing et al. [68] used alkaline melting to treat 210Po in air filter samples, where the main step was to dissolve glass or cellulose air filters with sodium hydroxide and hydrogen peroxide, followed by extraction chromatography to separate 210Po. Unfortunately, the recovery yield of this method was low (<60%), indicating that there was still organic material that was fully digested. Maxwell et al. [32] therefore investigated a new method for recovery and measurement of 210Po on air filters after fusion of the air filter with sodium nitrate or potassium nitrate and sodium hydroxide (1:1). In a rapid sample, polonium was rapidly separated using iron hydroxide co-precipitation and DGA resin separation. This method has the advantages of high chemical yields and effective removal of sample matrix interference [32].
In addition to being present in aerosols, 210Po can also be found in the fumes produced by burning tobacco. Studies have shown that more than half of the radioactive substances emitted by burning cigarettes are released into the air, and even nonsmokers (second-hand smokers) can inhale these substances [69]. The smoke inhaled by smokers transports about 32% of the 210Po in each cigarette to the smoker’s lungs [70,71] and adsorbs it in the bronchi, where it is not easy to remove and poses a hidden danger to health [69]. In the past decade, many scholars have studied radionuclides in tobacco, especially the exploration of 210Po [72,73,74]. One group ground tobacco taken from cigarettes into a homogeneous powder and acid digested it with HNO3, HClO4, and HF [73]. Berthet et al. [75] washed 210Po from flue gas with three consecutive 1 M HCl wash bottles, F1 to F3. Independent analysis of each flask fraction showed that F1 retained 70% of the total 210Po activity in the flue gas of conventional cigarettes, F2 retained about 20%, and F3 retained about 10%. These results show that the acid digestion method reduced the volatilization of polonium caused by high-temperature combustion and better retained 210Po in tobacco and smoke.
2.1.3. Pretreatment of Solid Samples
For solid environmental samples such as sediment, soil, and sludge, the pre-treatment process includes both physical and chemical treatment. The samples are first dried, ground, and sieved to obtain a homogenized sample. Chemical treatment involves the dissolution of the sample itself as well as the destruction of organic matter and is usually achieved by any of three methods: wet ashing in an open system, acid digestion in a pressure vessel, or microwave digestion [6]. The traditional wet ashing method uses a mixture of HCl, HF, HNO3, and HClO4 in different proportions, heated at different temperatures for different times, to destroy the organic material in the sample. This method has several disadvantages, however, such as time-consuming steps and the risk of external sample contamination, the formation of insoluble salts during sample evaporation, and the loss of polonium from volatilization during heating [76].
The melting method is also commonly used for the complete dissolution of solid samples (e.g., soils, sediments, rocks, and solid biological samples containing refractory matrices) [28]. It is carried out by heating the sample with various molten mixtures (e.g., hydroxides, peroxides, carbonates, bisulphates, pyrosulphates, alkali metal borates, fluoride-pyrosulphates, etc.) in graphite, nickel, zirconium, or platinum crucibles [32,35] above a burner or in a muffle furnace until the molten mixture is completely melted and clarified. After cooling, the molten mass is dissolved in diluted nitric or hydrochloric acid. This method is more aggressive than acid digestion and is able to dissolve insoluble substances such as silicates, resulting in a more homogeneous and pure solution that contains 210Po.
2.2. Pretreatment of Biological Samples
In radiological monitoring and risk assessment, biological samples such as urine, blood, and hair are often analyzed for 210Po. For such samples, similar pre-treatment methods to those used for environmental samples can be used, with the appropriate method selection depending on the sample type. Urine is a complex matrix that contains many organic compounds and some inorganic salts [77]. The main problem with urine samples is the possible presence of suspended particles that may be retained by filters, resulting in poor recovery and/or spectral resolution [21]. To combat this, Manickam et al. [1] used a combination of concentrated nitric and hydrochloric acids for the acid digestion of urine. However, the digestion was not allowed to dry, thus avoiding polonium loss due to volatilization and greatly reducing the total sample preparation time. After cooling, concentrated NH4OH was added to raise the pH to approximately 9. MnCl2 and KMnO4 were then added, and Po was co-precipitated with the formed MnO2. In emergency situations, this method can prepare and measure samples within 24 h. In another study, Guérin et al. [21] used KBrO3 to oxidize and filter urine samples and found that samples oxidized with KBrO3 were filtered faster than those without the oxidant.
Both blood and hair are pretreated with a wet digestion process similar to urine. The organic matter in the blood is destroyed with a mixture of concentrated nitric acid [78] or 1:1 concentrated nitric acid and 20% H2O2 oxidation, and polonium ions are released into the solution [79]. After evaporation, the dry residue is dissolved in dilute hydrochloric acid for subsequent treatment [80]. For the pretreatment of hair samples, the main difference is that the collected hair is repeatedly washed with detergent so that it does not contain foreign matter. Finally, the hair is rinsed with distilled water, dried for 4–5 h, and stored in polyethylene bags for further processing [81].
2.3. Pretreatment of Food Samples
With its surface particle chemistry and high affinity for proteins [9,82], 210Po is readily adsorbed to the surface of phytoplankton in the marine environment [4] and is further transferred to subsequent trophic levels. Protein-rich organisms, therefore, typically have high concentrations of 210Po activity [83]. Since ingestion is the most important route of 210Po entry into the human body, fish products are the main contributors to 210Po dietary intake [18]. The trophic levels of the aquatic food chain have a strong bioconcentration and biomagnification effect on 210Po, especially in marine organisms, which makes high-protein seafood the main source of human 210Po [79].
Currently, there are three methods of dissolution for biological samples: dry digestion, wet digestion, and microwave dissolution [84]. For sample dissolution, the wet digestion method has significant advantages over the traditional dry digestion method. First, the wet ashing method can effectively dissolve impurities in the sample and can operate at a lower temperature, thus increasing the efficiency of the process. In addition, the wet digestion method avoids the losses associated with the volatilization of polonium due to heating in the traditional dry ashing method, thus ensuring the accuracy of the sample. However, the efficiency of wet digestion is much lower than that of microwave digestion for samples that contain high amounts of oils [18].
The vast majority of analyses of 210Po in aquatic products in China are currently carried out by wet ashing [85,86]. The wet ashing method involves the addition of strong oxidants (e.g., concentrated nitric acid, concentrated sulfuric acid, perchloric acid, potassium permanganate, hydrogen peroxide, etc.) to the sample and heating the sample to decompose and oxidize the organic material completely [87]. After this, the components to be measured are transformed into their ionic states within the digestion solution. Dong et al. [85] analyzed 210Po levels in typical seafood from the Yellow Sea waters of China and proposed a rapid disposal of collected seafood to avoid interference from other factors. Their samples had to be kept at a low temperature (<10 °C) at all times until pretreatment, when they were first thawed, at which time the edible parts were removed, cleaned, weighed, dried, and ground. Once ground into a homogeneous powder, the samples were placed in refrigeration pending analysis. Each biological sample was dried and then added to a standard solution of 209Po tracer of known activity, and the organic matter was destroyed using a ‘tri-acid wet ashing’ method with nitric acid, hydrogen peroxide, and perchloric acid. In this case, the authors advise that the final solution should be colourless and transparent when the organic matter in the sample is sufficiently digested [85], but the oil and grease components in aquatic samples are often difficult to digest thoroughly, and incomplete digestion can seriously reduce the efficiency of the 210Po self-deposition.
Microwave digestion is a pretreatment technique in which a substance sample is heated directly by both molecular polarization and ionic conductivity, causing the surface layer of the solid sample to break down rapidly, creating a new surface for the solvent to interact with, and allowing the sample to be completely digested within minutes [88]. Szarlowicz et al. [89] reported that for smaller masses (0.1–0.2 g) of sediment samples, microwave digestion using concentrated HNO3 + HCl gave acceptable recoveries of polonium and did not require the use of HF, effectively reducing both processing time and cost. Although the microwave digestion technique is a promising method, it is only capable of rapid and accurate detection of very small amounts of organic matter (typically 0.5 g dry, 2–3 g wet), so for larger masses of samples (20–25 g, wet weight), it may be necessary to split them into multiple digestion vessels to obtain the sensitivity required to measure low levels of 210Po [90].
In order to solve the above problems, Baki et al. [18] used the Digiprep HT250–10 digestion system for the pretreatment of fish. This system consists of a graphite heating block that accommodates ten tall cylindrical digestion tubes (300 mL, glass, 5 cm deep) that house heat to provide more uniform heating. The system can efficiently dissolve fish samples (20 g, wet weight) into a clear liquid solution in less than 7 h, and the digestion ends without any visible fat residue. Although microwave digestion technology such as this is characterized by simple operation, rapid and complete decomposition, low reagent consumption, low loss of volatile elements, and low blanking and is known as a “green chemical reaction technology” [67], the major drawback of this method is that it does not allow the processing of large numbers of samples (>5 g) at one time [58].
The advantages and disadvantages of all of the above pretreatment methods for environmental, biological, and food samples are detailed in Figure 5.
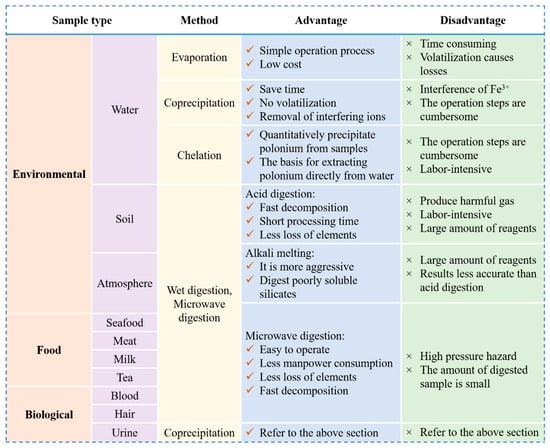
Figure 5.
The advantages and disadvantages of the different methods used to pretreat samples.
3. Chemical Purification
After sample pretreatment is completed, the source preparation of 210Po can be carried out directly. Direct deposition source preparation has the advantage of saving a lot of time in practical analysis, but for some environmental samples, direct deposition may be affected by other ions, resulting in a thicker deposition source or reduced Po recovery [6,91]. Depending on the deposition conditions, deposition of 210Pb and 210Bi may result when the sample has a high 210Pb/210Po and/or 210Bi/210Po ratio. Such samples need to be chemically separated and extracted first. The main chemical separation methods commonly used are discussed below and their advantages and disadvantages are summarized in Figure S1.
3.1. Solvent Extraction
Solvent extraction, also known as liquid-liquid extraction, is an effective method for separating, enriching, and extracting useful substances from solutions, and it is frequently used for the separation of radionuclides. The principle is to add an immiscible (or slightly immiscible) solvent to a liquid mixture and use the different solubilities of its components in the solvent to achieve separation or extraction [92]. Polonium has four stable chemical valence states, namely −2, +2, +4, and +6, with the +4 valence state being the most stable in most solutions [4] and in chloride solutions in the PoCl62− state [93] in particular. Using this property, polonium can be extracted from acidic solutions with a variety of extractants. Isopropyl ether, methyl isobutyl ketone, diisopropyl ketone, and tributyl phosphate can all be used to extract polonium from acidic solutions [6].
Diethylammonium diethyl dithiocarbamate (DDTC) has also been used to extract Po from HCl solutions into chlorinated hydrocarbon solutions (CHCl3, CCl4, or CH3CCl3) [91,94], and trioctylphosphine oxide (TOPO) is an excellent extractant for polonium in hydrochloric acid media that allows for essentially 100% of polonium to be extracted over a wide range of hydrochloric acid concentrations [95]. A major disadvantage of this method, however, is the generation of mixed radioactive waste during the separation process and the problem of third-phase formation [28].
3.2. Ion Exchange Chromatography
Ion exchange is a method of separating metal ions in solution by exchanging ions on an ion exchanger with ions in the solution [96]. The main process of this ion resin exchange includes six steps: preselection, pretreatment, column loading, resin exchange, resin elution, and resin regeneration [97]. Ion exchange chromatography is one of the most common methods used for 210Po purification and separation, and separation of polonium on anion exchange columns is usually performed in HCl media since polonium is strongly retained in 0.05–12 M HCl anion exchange resins such as Dowex-1, Dowex-2, and Bio-rad AG1-X4. Indeed, polonium has a high retention capacity in HCl media throughout the acidic range [28]. However, in HNO3 media, this retention capacity is very low.
Interestingly, a method for the chemical separation of Au from fission products using anion exchange was developed by Douglas et al. This method demonstrated that Po could be eluted in the same fraction as Au with minimal interference from other elements using only a small volume of reagent [98]. This is more desirable than previous studies that used ion exchange and large elution volumes [99,100,101]. Moreover, the anion exchange resin Bio-Rad AG1-X4 has been shown to adsorb Po in acids of 0.1–12 mol/L strength [6]. Dowex anion resins have been used for the separation of Po, Pb and Bi, and cation exchange resins such as Bio-Rad AG50Wx8 can also effectively adsorb 210Po from dilute HF, HNO3, or H2SO4 and elute with a 7 mol/L HCl solution [6].
3.3. Extraction Chromatography
Extraction chromatography, also known as extractive lamination, is a new separation method developed in the 1970s after ion exchange and solvent extraction. The basic principle is the combination of liquid-liquid extraction and chromatography to achieve separation according to the different distribution ratios of the components in the two phases. This is a new separation technique for the separation of inorganic substances using an extractant adsorbed on an inert support or polymerized with a resin as the stationary phase and an inorganic aqueous solution as the mobile phase [102]. Vajda et al. [103] first reported a method for separating polonium using a crown ether column. This method selectively retains polonium and lead from a 2 M HCl solution using bis-4,4′(5′)-t-butyl-cyclohexano-18-crown-6-ether and then strips polonium with 6 M HNO3 and lead with a 6 M HCl solution. Recently, extractive chromatography has been increasingly used for the separation and purification of 210Po, with Sr-Spec resin in particular, which can retain Po from acidic solutions with a recovery of about 70% [103]. The retention capacity for polonium on Sr-resin is about 100 in 0.5–1.0 M HNO3. Above this concentration, the retention of Po (IV) drops off rapidly [103,104]. Therefore, one must pay special attention to the acid concentration when using Sr resin.
DGA is another commonly used resin for separating polonium [105]. The presence of interfering ions such as Na+, K+, Mg2+, and Fe3+ in a sample has little effect on the retention of Po (IV) on DGA resin. However, the high cost of DGA is a major drawback to its use in conventional polonium analysis. Other solid extractants include extractive drench resins impregnated with di(2-ethylhexyl) phosphoric acid (HDEHP), which can be used to elute 210Po from dilute citric acid solutions [106]. Tri-n-butyl phosphate coated on a polytetrafluoroethylene support has also been found to extract Po well (100%) from a 6 mol/L hydrochloric acid solution but requires elution with a 2 mol/L HF solution [107].
In recent years, scholars have additionally proposed a new continuous extraction system for the continuous separation and enrichment of 210Po, uranium, and thorium isotopes. This system is composed of TRU resin, SR resin, and RA-01 and AG50Wx8 mixed columns (HRA resin) [42,43]. Compared with other methods, this new method reduces the processing time, amount of chemical reagent, and amount of resin needed [108], and it has already been applied to the separation and purification of water and slag samples.
Extraction chromatography combines the high selectivity of solvent extraction with the simple, multi-stage nature of column chromatography. The main advantages of this method are that the chromatographic resins have high selectivity for polonium, exchange kinetics faster than anion exchange, and are characterized by simple operation steps, easy sample processing, and low cost. More importantly, extraction chromatography can significantly reduce the volume of highly radioactive solutions and the amount of solid waste, as well as the harmful effects of radioactive waste on human health and the environment [54].
4. Source Preparation
The measurement of the radionuclide 210Po in water and food in HJ 813–2016 and GB 14883.5–2016 uses alpha spectrometry [109,110], which is one of the most popular methods in research. However, alpha spectrometry requires a homogeneous thin-layer source to obtain maximum resolution of the Po peak. Currently, there are three main methods for source preparation: self-deposition, electrodeposition, and micro-precipitation. We summarize the advantages and disadvantages of different methods to prepare sources in Figure S2.
4.1. Spontaneous Deposition
Due to its higher reduction potential than many metals, 210Po can undergo spontaneous electrochemical exchange with such metals [111]. Using this property, 210Po can be self-deposited onto metal surfaces to form a coating. Furthermore, ascorbic acid, sodium citrate, and hydroxylamine hydrochloride are used in the self-deposition process to reduce the effect of competing ions (e.g., Fe3+, Cr6+) present in some samples [28,112,113].
The self-deposited materials used in experiments are mainly silver flakes or silver foil made of metallic silver [114,115,116]. Due to the high cost of silver flakes, however, materials such as copper flakes [117], stainless steel flakes [81], and nickel flakes [118] have also been used instead of silver flakes. However, Li et al. [119] studied and compared these alternative self-deposited materials and concluded that silver flakes have better selectivity for Po, better resistance to other ions in solution, and better stability compared with copper flakes, for example. Another study of self-deposition found that the deposition rate of polonium on copper and nickel decreased as the concentration of hydrochloric acid increased, whereas the rate of self-deposition on silver did not change significantly with the concentration of hydrochloric acid [111]. The reason for this may be that copper and nickel are chemically more active than silver, and the higher the concentration of hydrochloric acid, the more the copper and nickel flakes will be corroded by the high concentration of hydrochloric acid, thus affecting the onset of self-deposition [50].
In terms of temperature selection for the self-deposition process in a water bath, Li et al. [119] found that the self-deposition recovery increased with increasing temperature and that the recovery tended to stabilize at a temperature of 70 °C to 96 °C, reaching 95.3% to 98.3%, which was in line with the ideal state. Du et al. [120] also found that the higher the temperature, the shorter the self-deposition time. When the self-deposition temperature was 80 °C and the self-deposition time was 2.5 h, the self-deposition time reached equilibrium and the α net count rate reached its maximum. Moreover, the self-deposition equilibrium time was maintained for a longer period. Karali’s research showed that at 0.5 M HCl, 70–75 °C for 3 h, the deposition efficiency of polonium on different metal discs could be ranked in the following order, from high to low: silver > nickel > stainless steel > copper [121]. Therefore, attention should be paid not only to the temperature of the water bath during the experiment to prevent the volatilization of 210Po due to high temperatures but also to the concentration of hydrochloric acid to avoid causing corrosion of the self-deposited metal.
4.2. Electrodeposition
Electrodeposition is also commonly used in the source production process for 210Po. In electrodeposition, polonium is electrochemically coated from an electrolyte solution onto a 10 mm diameter polished stainless steel disc for several hours at a constant current (250 mA) [122]. The mechanism of the electrodeposition process is controlled by Hansen’s theory of electrodeposition of lanthanide and actinide hydroxides at low current densities. High concentrations of hydroxyl ions are required near the cathode surface to precipitate hydroxides from very low mass concentrations of radionuclides in the electrolyte [2], and research has shown that direct electrodeposition of polonium from acidic solutions on carbon electrodes is 40–85% efficient [4]. Rieth et al. [123] studied the electrodeposition of 210Po in 0.1 M HNO3 solution on various electrode materials (Cu, Ag, Ti, Pd, and Ni in the form of 6 × 6 mm foils) and found that the highest deposition was achieved on Ni electrodes and the lowest on Pd electrodes. The electrodeposition results observed on Ni, Cu, and Ag were also satisfactory, indicating that they have potential in the preparation of 210Po source deposition as well.
4.3. Micro-Precipitation
In recent years, microprecipitation has become a desirable alternative to autodeposition for the preparation of polonium alpha counting sources. Compared with conventional autodeposition, micro-precipitation is much faster since it does not require heating and can therefore be used to process large sample volumes with high recoveries (80–95%) [28]. Although micro-precipitation can improve the yield of the autodeposition method in a shorter time without heating, it requires the chemical separation of Po from potentially interfering alpha-emitting nuclides [32]. Many studies have been carried out on different micro-precipitation methods for the preparation of 210Po counting sources, including copper sulphide [21], bismuth phosphate [124], and monolithic tellurium micro-precipitation [125].
4.3.1. Copper Sulphide (CuS) Micro-Precipitation
Due to the low solubility of PoS, the α counting source of 210Po can be rapidly prepared in HCl solution by CuS micro-precipitation technology, and the final recovery rate of this method can reach about 85% when treating water and urine samples [21]. Compared with conventional plating, CuS micro-precipitation is faster, can more easily process large batches, and gives high recoveries (80–95%) without a heating step. However, the recoveries are significantly lower when the molar concentration of HCl is higher than 1 mol. Miura et al. [30] used copper sulphide micro-precipitation to separate 210Po from Sr resin and electro-deposited 210Po on stainless steel sheets. The recovery of this method ranged from 56% to 99%, with a large variation interval as well as instability. Therefore, the copper sulfide (CuS) micro-precipitation method may have only limited potential as the deposition source for the preparation of Po-210.
4.3.2. Tellurium (Te) Micro-Precipitation
The tellurium micro-precipitation method uses hydrochloric acid to leach the polonium in the sample at a low temperature and uses the single Te precipitate produced by the reduction of Te by stannous chloride as a carrier to carry polonium. The α source of 210Po is then obtained by filtration, which was first proposed by Song et al. [125]. This method is faster than the traditional natural precipitation method and saves experimental time, while also allowing large sample volumes to be processed with >90% recovery throughout and without the heating step required for high recoveries, reducing the loss of 210Po due to heating and making the process both simpler and more efficient. Compared with copper sulphide micro-precipitation, tellurium micro-precipitation is more resistant to acidity and can better effect the rapid detection of 210Po in poorly soluble solid samples [126]. This is not only suitable for the measurement of polonium-210 in water samples but also for other environmental media such as soil and organisms [126]. However, it has been demonstrated that the reduction of Te(IV) to monomeric Te is incomplete in acidic systems in ascorbic acid and not in hydroxylamine hydrochloride. This phenomenon resulted in a very low recovery of polonium, almost close to zero [28].
4.3.3. Bismuth Phosphate (BiPO4) Micro-Precipitation
The bismuth phosphate micro-precipitation method was established to separate 210Po in urine, specifically [124]. The procedure involves adding concentrated phosphoric acid to a well-digested urine sample to adjust the pH, followed by bismuth nitrate for bismuth phosphate precipitation. Since solutions containing inorganic salts precipitate an average of about 90% in 50 mL or 500 mL of urine, some researchers expect that this method can be applied to larger volumes of urine. However, although this technique shortens the preparation time of the counting source, it does not provide sufficient selectivity against other alpha radionuclides and also requires a purification step, making it more cumbersome than other methods.
5. Radioactivity Measurements
There are three main methods for the detection of 210Po in environmental and biological media: alpha spectrometry, liquid scintillation counting, and large-area screen grid spectrometry. The principles, advantages, and disadvantages of each method are summarized in detail in the following subsections and Figure S3.
5.1. Alpha Spectrometry
Alpha spectrometry using a silicon surface potential or PIPS detector has become the method of choice for the determination of 210Po in environmental and bioassay samples [28,84]. The main principle of this method is to add an isotopic tracer of known activity to a self-deposited system, count it using an alpha spectrometer after the counting source has been prepared, and calculate the activity concentration of 210Po from the count rate. The most commonly used tracers are 208Po and 209Po. 208Po is often chosen as the tracer due to its better practicality. However, in recent years, 209Po has been increasingly used because it has a longer half-life and its alpha ray energy is further away from that of 210Po [127], which means that there are more easily identifiable peaks when measuring alpha energy spectra. Unfortunately, 209Po tracers are difficult to obtain commercially and expensive, making them unsuitable for the detection of bulk samples [120]. Alpha spectrometry has the advantages of lower detection limits, higher energy resolution, and lower cost compared with other methods, but tracer contamination of the detection probe and the need for a complex pretreatment process are two of its major drawbacks.
In China, total α counting is an earlier method used in the national standard for the determination of 210Po in water. The total alpha counting method uses manganese dioxide and hydroxide as carriers to carry 210Po off from the sample, uses silver or copper flakes as carriers in an acidic solution to make the source, and then determines the total alpha activity in the sample as the activity of 210Po. This method is similar to the procedure described above for alpha spectroscopy but does not require the addition of polonium-210 isotopes for tracing. The full recovery of the method ranges from about 40% to 80%. The main advantage of total alpha counting is that it allows rapid self-deposition of the source and near-field measurements without tracer and without separation of matrix elements (high detection efficiency due to the close distance between source and detector), but it is more difficult to achieve a correction for full recovery for each sample. At present, the total alpha counting method is still used in the determination of 210Po radioactive substances in national standard foods. However, a 210Po standard solution was added to calculate the recovery rate in the detection process.
5.2. Liquid Scintillation Counting
Liquid scintillation counting (LSC) is a direct measurement of alpha radionuclides using the pulse shape discrimination technique (PSA) provided by a specific LSA [84,128]. This method involves placing the sample solution in a glass or plastic scintillation bottle, adding a scintillation cocktail to configure it, and placing it in a liquid flash machine for testing [129]. Liquid scintillation counting has been used by some scholars to detect 210Po content in samples [103]. Its main advantage is that it eliminates contamination in the detector chamber and also has a high efficiency in detecting alpha particles [28,129]. However, the unoptimized LSC method has poor energy resolution and high detection limits, and the detection limit for the determination of 210Po is an order of magnitude higher than that of alpha spectrometry. The presence of other alpha emitters and beta counting is critical to interfering with the measurement. In addition, chemical yield monitoring using 209Po or other radioisotopes of polonium does not work in this case [128]. This method is therefore not very useful for measuring the activity of individual alpha-emitting nuclides [5]. Its observed lower detection limit was obtained by scholars who optimized recovery, efficiency, and background counting at 210Po [130,131].
The LSC method is also commonly used by researchers for the simultaneous determination of 210Po and 210Pb in samples [128,129]. In the case of simultaneous detection of 210Po and 210Pb, determining the appropriate PSA value is important to avoid misclassification of alpha and beta pulses. The study by Ozden et al. concluded that the optimal PSA level for 210Po is “10” [129]. In addition, in order to achieve satisfactory alpha/beta screening, the ratio of beta to alpha activity in the sample must not be too high, and for alpha activity measurement, a radioactivity ratio of β to α of less than 100 is generally required [132]. The LSC method avoids the step of separating polonium from the sample and uses alpha spectrometry for detection, saving experimental time and eliminating the cumbersome step of sample preparation.
As one of the LSC, photonic electron rejection alpha liquid scintillation (PERALS®) spectroscopy is an emerging method for determining 210Po in environmental samples by combining the chemical separation of liquid-liquid extraction with the measurement of α activity in water-immiscible scintillators [28]. Compared with alpha spectrometry, alpha liquid scintillation with β/γ rejection (PERALS®) sample preparation is both fast and sensitive and can be used for the detection of native 210Po [133]. Case and McDowell applied liquid scintillation to the measurement of polonium-210 in various types of samples, including ore, tailings, and environmental samples [134]. From a 7 M phosphoric acid-0.01 M hydrochloric acid solution, a 0.20 M trioctylphosphine oxide solution (together with scintillators) was extracted in toluene to concentrate polonium-210 and separate it from interfering elements such as iron. Polonium-210 was then measured by counting alpha radiation at 5.3 MeV with a photon/electron suppression alpha liquid scintillation spectrometer [134]. However, the PERALS® system offers poor resolution, and its main interfering nuclides are 228Th and 239Pu. This means that a separation of 210Po from other nuclides is required [28,133]. Previous studies have shown that Polex, TNOA, and TOPO liquid scintillants are also effective in extracting 210Po from spiked water samples without the need for 238U or 234U co-extraction steps [134].
When using the PERALS® method to determine the content of 210Po in water, an appropriate amount of phosphoric acid must be added to the pre-enriched water sample, and other acids must be removed by evaporation. After this, one must add tracer, hydrochloric acid, and extraction scintillation solution before shaking well and letting stand while waiting for the extraction to complete. After the organic layer and the water layer are clearly separated, the transparent liquid of the organic phase layer can be reserved, and argon gas can then be sprayed on the counting tube to prevent oxidation from affecting the final sample. The final processed sample can then be placed on a liquid scintillation counter. Although the detection limit for liquid scintillation alpha counting measurements is low, the solution containing 210Po still needs to be transparent enough for light collection [84]. In addition, the PERALS® method requires fewer water samples, can allow direct measurement without complex chemical treatment, and is more convenient to operate than other methods. The main disadvantage of PERALS®, however, is that commercial-grade phosphoric acid contains a large amount of 210Pb and 210Po as impurities, so high-purity-grade phosphoric acid [28] is required.
5.3. Large-Area Screen Grid Spectrometry
The main step of the large-area screen grid ionization chamber measurement method is to put the pretreated sample into a beaker, add distilled water, and then use an ultrasonic cleaner to crush the sample into about 1 micron particles. Then, the sample is spread in a vacuum drying oven to make a large-area sample source [84,135]. This prepared sample source can then be measured in a large-area α grid ionization chamber. After microwave digestion of 0.5 g of seafood, Li et al. [136] used large-area screen grid spectrometry and found that the detection efficiency of 210Po was about 30%. The advantage of this method is that the detection source area is large, and a lower detection limit can thus be obtained [136]. Furthermore, the sample preparation process is simple, the sample is not easy to cross-contaminate, it loses almost no nuclides, and the total α in the sample and the activity of more than 10 α radionuclides, including 210Po, can be measured at the same time [127]. However, the requirements for the particles after the sample crushing and grinding treatment are high: the diameter needs to be below 1 micron, and the particle uniformity must be good enough [84]. At present, this method has not been widely used because of the instability of the thickness, uniformity, and firmness of the large-area sources.
6. Domestic and International Standards
A series of standard determination methods have been published for 210Po in environmental and food samples. The standard methods for 210Po in environmental and food samples are listed in Table 2. According to the detection methods used in domestic and international standards, alpha spectroscopy is the most widely used method, which involves the most extensive types of samples tested and has had the most studies carried out on it. Therefore, its determination technology is the most mature.

Table 2.
Standard method for the determination of 210Po in environmental and food samples at home and abroad.
7. Conclusions and Perspectives
This article reviewed the analytical methods for detecting 210Po in environmental media and biological samples that have been in use since the 21st century and summarized the advantages and disadvantages of these current sample preparation, chemical separation, sedimentation source preparation, and 210Po detection methods. At present, pretreatment methods primarily include evaporation, co-precipitation, chelation, wet digestion, and microwave digestion. The most widely used of these is wet digestion, which efficiently dissolves organic matter in samples at lower temperatures with a fast digestion process and low loss of elements. In addition, many new methods have emerged, such as microwave acid digestion, which also shows good decomposition efficiency. Furthermore, crown ether extraction chromatography is gradually replacing solvent extraction due to its high resolution. Among the various methods for measuring 210Po, alpha spectrometry is widely regarded as the most practical analytical technique for quantifying radioactive polonium isotopes. Photonic electron rejection in liquid alpha spectroscopy is also gaining traction due to its simple and rapid sample preparation. Liquid scintillation counting and large-area screen grid spectroscopy, on the other hand, are less commonly used by researchers, mainly because of the high pre-treatment requirements.
Traditional detection methods have complex pretreatment steps, and the whole experiment typically takes a long time, which is not suitable for rapid detection during radiological emergencies. At present, the main bottleneck is how to reach the detection limit of trace 210Po in environmental and biological samples through quick and convenient pretreatment. Some literature reports that polonium can be automatically deposited in dilute hydrochloric acid systems containing ascorbic acid, hydroxylamine hydrochloride, etc., without any prior chemical separation, but this is only applicable to individual samples (e.g., biological samples, sediments), and other researchers have raised the difficulties of this approach. Future research will focus on improving the efficiency of the pretreatment, chemical separation, and source preparation stages. In terms of pretreatment, rapid treatment of a large number of insoluble samples needs to be explored. Although the preparation of the photon electron-rejecting liquid alpha spectroscopy is simple and fast, the phosphoric acid used in this method is often contaminated with 210Pb and 210Po impurities. The main improvement in alpha spectroscopy is to avoid contamination of the detector with tracer without extending the time interval between source preparation and determination. Therefore, urgent research is needed into a fast, efficient, and low-pollution alternative to the traditional method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176268/s1, Figure S1: The advantages and disadvantages of the different chemical purification for 210Po; Figure S2: The advantages and disadvantages of the different source preparation for 210Po; Figure S3: The advantages and disadvantages of the different radioactivity measurements for 210Po.
Author Contributions
Writing—original draft preparation, L.Z. and R.W.; writing—review and editing, L.Z. and R.W.; supervision, H.R. and P.W., funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zhejiang Health Science and Technology Plan (China, No. 2021KY613, 2022RC120, 2022KY130, 2022KY132, 2023KY643) and the Project of South Zhejiang Institute of Radiation Medicine and Nuclear Technology (China, No. ZFY-2021-K-003, ZFY-2022-K-001, ZFY-2022-K-006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Manickam, E.; Sdraulig, S.; O’Brien, R. Improved and Rapid Radiochemical Method for the Determination of Polonium-210 in Urine. Aust. J. Chem. 2010, 63, 38–46. [Google Scholar] [CrossRef]
- Sethy, N.K.; Sutar, A.K.; Rath, P.; Jha, V.N.; Ravi, P.M. Tripathi. A review of radio chemical analysis and estimation of 210Po in soil matrices. J. Radiat. Res. Appl. Sci. 2015, 8, 590–596. [Google Scholar]
- Brookhaven National Laboratory. NNDC|National Nuclear Data Center. Available online: https://www.nndc.bnl.gov/ (accessed on 6 June 2023).
- Figgins, P. The radiochemistry of polonium. In National Academies of Sciences Nuclear Science Series 3037.S; Atomic Energy Commission; U.S. Department of Commerce: Springfield, MO, USA, 1961. [Google Scholar]
- Nathwani, A.C.; Down, J.F.; Goldstone, J.; Yassin, J.; Dargan, P.I.; Virchis, A.; Gent, N.; Lloyd, D.; Harrison, J.D. Polonium-210 poisoning: A first-hand account. Lancet 2016, 388, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.M.; Kim, C.K.; Martin, P. Determination of 210Po in environmental materials: A review of analytical methodology. Appl. Radiat. Isot. 2007, 65, 267–279. [Google Scholar] [CrossRef]
- Barbosa Gonzalez, N.R.; Ramos Rincon, J.M. Determination of polonium—210 (210Po) in food and water: A review (2014–2019). Rev. Investig. Y Apl. Nucl. 2021, 5, 26–43. [Google Scholar] [CrossRef]
- Hansen, V.; Mosbech, A.; Søgaard-Hansen, J.; Riget, F.F.; Merkel, F.R.; Linnebjerg, J.F.; Schulz, R.; Zubrod, J.P.; Eulaers, I. 210Po and 210Pb activity concentrations in Greenlandic seabirds and dose assessment. Sci. Total Environ. 2020, 712, 136548. [Google Scholar] [CrossRef]
- Skwarzec, B.; Fabisiak, J. Bioaccumulation of polonium 210Po in marine birds. J. Environ. Radioact. 2007, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Blanchard, R.L. The thermal volatilisation of caesium-137, polonium-210 and lead-210 from in vivo labelled samples. Analyst 1969, 94, 441–446. [Google Scholar] [CrossRef]
- Vesterbacka, P.; Ikaheimonen, T.K. Optimization of Pb-210 determination via spontaneous deposition of Po-210 on a silver disk. Anal. Chim. Acta 2005, 545, 252–261. [Google Scholar] [CrossRef]
- Schmidt, V.; Hamel, P. Measurements of deposition velocity of radon decay products for examination of the correlation between air activity concentration of radon and the accumulated Po-210 surface activity. Sci. Total Environ. 2001, 272, 189–194. [Google Scholar] [CrossRef]
- Persson, B.R.R.; Holm, E. Polonium-210 and lead-210 in the terrestrial environment: A historical review. J. Environ. Radioact. 2011, 102, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.R.R.; Holm, E. 7Be, 210Pb, and 210Po in the surface air from the Arctic to Antarctica. J. Environ. Radioact. 2014, 138, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Reagan, M.; Tepley Iii, F.; Gill, J.B.; Wortel, M.; Garrison, J. Timescales of degassing and crystallization implied by 210Po-210Pb-226Ra disequilibria for andesitic lavas erupted from Arenal volcano. Journal of Volcanology and Geothermal Research. J. Volcanol. Geotherm. Res. 2006, 157, 135–146. [Google Scholar] [CrossRef]
- Dlugosz-Lisiecka, M. The sources and fate of 210Po in the urban air: A review. Environ. Int. 2016, 94, 325–330. [Google Scholar] [CrossRef]
- Olszewski, G.; Borylo, A.; Skwarzec, B. The radiological impact of phosphogypsum stockpile in Wislinka (northern Poland) on the Martwa Wisla river water. J. Radioanal. Nucl. Chem. 2016, 307, 653–660. [Google Scholar] [CrossRef]
- Sadi, B.B.; Chen, J.; Kochermin, V.; Tung, G.; Chiorean, S. A faster sample preparation method for determination of polonium-210 in fish. J. Radioanal. Nucl. Chem. 2016, 308, 843–850. [Google Scholar] [CrossRef]
- Feroz Khan, M.; Godwin Wesley, S.; Rajan, M.P. Polonium-210 in marine mussels (bivalve molluscs) inhabiting the southern coast of India. J. Environ. Radioact. 2014, 138, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Irlweck, K. Determination of 210Pb, 210Bi and 210Po in natural drinking water. J. Radioanal. Nucl. Chem. 2001, 249, 191–196. [Google Scholar] [CrossRef]
- Guérin, N.; Dai, X. Determination of 210Po in Drinking Water and Urine Samples Using Copper Sulfide Microprecipitation. Anal. Chem. 2014, 86, 6026–6031. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, C.H.; Song, K.; Kim, C.K.; Martin, P. Determination of Polonium Nuclides in a Water Sample with Solvent Extraction Method. Bull. Korean Chem. Soc. 2010, 31, 2488–2492. [Google Scholar] [CrossRef][Green Version]
- Kristan, U.; Planinšek, P.; Benedik, L.; Falnoga, I.; Stibilj, V. Polonium-210 and selenium in tissues and tissue extracts of the mussel Mytilus galloprovincialis (Gulf of Trieste). Chemosphere 2015, 119, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orellana, E.L.N.C.J. Influence of submarine groundwater discharge on 210Po and 210Pb bioaccumulation in fish tissues. J. Environ. Radioact. 2016, 155–156, 46–54. [Google Scholar] [CrossRef]
- Charles, M. UNSCEAR report 2000: Sources and effects of ionizing radiation. United Nations Scientific Comittee on the Effects of Atomic Radiation. J. Radiol. Prot. 2001, 21, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Wildgust, M.A.; Mcdonald, P.; White, K.N. Temporal changes of 210Po in temperate coastal waters. Sci. Total Environ. 1998, 214, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guy, S.; Gaw, S.; Beaven, S.; Pearson, A.J. Dose assessment for polonium-210 (Po-210) in New Zealand shellfish. J. Environ. Radioact. 2022, 242, 106788. [Google Scholar] [CrossRef]
- Thakur, P.; Ward, A.L. 210Po in the environment: Insight into the naturally occurring polonium isotope. J. Radioanal. Nucl. Chem. 2020, 323, 27–49. [Google Scholar] [CrossRef]
- Vreček, P.; Benedik, L.; Pihlar, B. Determination of 210Pb and 210Po in sediment and soil leachates and in biological materials using a Sr-resin column and evaluation of column reuse. Appl. Radiat. Isot. 2004, 60, 717–723. [Google Scholar] [CrossRef]
- Miura, T.; Kawabe, K.; Kirita, H. Determination of 210Po in Reagent Samples by Alpha-Ray Spectrometry Using Extraction Chromatographic Resin. J. Radioanal. Nucl. Chem. 2000, 246, 327–330. [Google Scholar] [CrossRef]
- Martínez, J.; de Los Cobos, M.; Peñalver, A.; Tarancon, A.; Gimenez, I.; Bagan, H.; Aguilar, C.; Borrull, F. Simultaneous determination of 210Pb and 90Sr and 210Po isolation in sludge samples using a plastic scintillation resin. Appl. Radiat. Isot. 2023, 192, 110601. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Mcalister, D.R.; Sudowe, R. Novel rapid oxidizing fusion method to determine Polonium-210 in air filters. Appl. Radiat. Isot. 2019, 153, 108833. [Google Scholar] [CrossRef]
- Khater, A.E.M. Polonium-210 budget in cigarettes. J. Environ. Radioact. 2004, 71, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Desideri, D.; Meli, M.A.; Roselli, C. Natural and artificial radioactivity determination of some medicinal plants. J. Environ. Radioact. 2010, 101, 751–756. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Mcalister, D.R.; Sudowe, R. Rapid method to determine Polonium-210 in urban matrices. Appl. Radiat. Isot. 2019, 148, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ghanbar-Moghaddam, B.; Fathivand, A. Study of Polonium-210 in Persian cigarette and tobacco crops. Radiat. Prot. Dosim. 2020, 191, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Macklin Rani, L.; Jeevanram, R.K.; Kannan, V.; Govindaraju, M. Estimation of Polonium-210 activity in marine and terrestrial samples and computation of ingestion dose to the public in and around Kanyakumari coast, India. J. Radiat. Res. Appl. Sci. 2014, 7, 207–213. [Google Scholar] [CrossRef]
- Blanchet-Chouinard, G.; Larivière, D. Rapid determination of 210Pb and 210Po by sequential cloud point extraction for environmental monitoring. Anal. Methods 2022, 14, 199–202. [Google Scholar] [CrossRef]
- Connan, O.; Boust, D.; Billon, G.; Solier, L.; Rozet, M.; Bouderbala, S. Solid partitioning and solid-liquid distribution of 210Po and 210Pb in marine anoxic sediments: Roads of Cherbourg at the northwestern France. J. Environ. Radioact. 2009, 100, 905–913. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, Y.; Ha, W.; Jo, M.K.; Park, S.; Kim, J.-m. Improved urine analysis for polonium, natural uranium, and thorium isotopes and background survey in collected samples of normal people. J. Radioanal. Nucl. Chem. 2019, 322, 1871–1875. [Google Scholar] [CrossRef]
- Dalencourt, C.; Chabane, M.N.; Tremblay-Cantin, J.C.; Lariviere, D. A rapid sequential chromatographic separation of U- and Th-decay series radionuclides in water samples. Talanta 2020, 207, 120282. [Google Scholar] [CrossRef]
- Dalencourt, C.; Tremblay-Cantin, J.; Larivière, D. Development of a radiochemical sequential procedure for the quantification of Th- and U-decay series elements in mining residues. J. Radioanal. Nucl. Chem. 2020, 326, 1597–1607. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Baskaran, K.V.; Rao, D.D.; Sathyapriya, R.; Annamalai, S.K.; Kuruva, J.K.; Hari, S. Ingestion of polonium (210Po) via dietary sources in high background radiation areas of south India. Int. J. Radiat. Biol. 2014, 90, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Casacuberta, N.; Traversa, F.L.; Masqué, P.; Garcia-Orellana, J.; Anguita, M.; Gasa, J.; Garcia-Tenorio, R. Distribution and biokinetic analysis of 210Pb and 210Po in poultry due to ingestion of dicalcium phosphate. Sci. Total Environ. 2010, 408, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.L.; Culligan, B.K.; Hutchison, J.B.; Utsey, R.C.; McAlister, D.R. Rapid determination of 210Po in water samples. J. Radioanal. Nucl. Chem. 2013, 298, 1977–1989. [Google Scholar] [CrossRef]
- Fonollosa, E.; Peñalver, A.; Aguilar, C.; Borrull, F. Polonium-210 levels in different environmental samples. Environ. Sci. Pollut. Res. 2015, 22, 20032–20040. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P.; Oliveira, J.M. Bioassay of 210Po in human urine and internal contamination of man. J. Radioanal. Nucl. Chem. 2009, 280, 359–362. [Google Scholar] [CrossRef]
- Srivastava, A.; Tuli, V.; Scherer, U.W. Study of radiotoxic 210Po in indian tobacco using liquid scintillation spectrometry. Radiochim. Acta 2018, 106, 787–792. [Google Scholar] [CrossRef]
- Mabuchi, H. On the volatility of some polonium compounds. J. Inorg. Nucl. Chem. 1963, 25, 657–660. [Google Scholar] [CrossRef]
- Zhong, Q.; Puigcorbé, V.; Sanders, C.; Du, J. Analysis of 210Po, 210Bi, and 210Pb in atmospheric and oceanic samples by simultaneously auto-plating 210Po and 210Bi onto a nickel disc. J. Environ. Radioact. 2020, 220–221, 106301. [Google Scholar] [CrossRef]
- Martin, P.; Ryan, B. Natural-series radionuclides in traditional aboriginal foods in tropical northern Australia: A review. Sci. World J. 2004, 4, 77–95. [Google Scholar] [CrossRef][Green Version]
- Zhou, Z.; Ren, H.; Zhou, L.; Wang, P.; Lou, X.; Zou, H.; Cao, Y. Recent Development on Determination of Low-Level 90Sr in Environmental and Biological Samples: A Review. Molecules 2023, 28, 90. [Google Scholar] [CrossRef]
- Benedik, L.; Vasile, M.; Spasova, Y.; Wätjen, U. Sequential determination of 210Po and uranium radioisotopes in drinking water by alpha-particle spectrometry. Appl. Radiat. Isot. 2009, 67, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Frances, I.; Mantero, J.; Manjon, G.; Diaz, J.; Garcia-Tenorio, R. 210Po and 238U isotope concentrations in commercial bottled mineral water samples in Spain and their dose contribution. Radiat. Prot. Dosim. 2013, 156, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Skwarzec, B.; Strumińska-Parulska, D.I.; Boryło, A.; Kabat, K. Polonium, uranium and plutonium radionuclides in aquatic and land ecosystem of Poland. J. Environ. Sci. Health Part A 2012, 47, 479–496. [Google Scholar] [CrossRef]
- Skwarzec, B. Radionuclides of 210Po, 234U and 238U in drinking bottled mineral water in Poland. J. Radioanal. Nucl. Chem. 2003, 256, 361–364. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Byrakdar, M.E.; Mamish, S.; Al-Haleem, M.A. Determination of natural radioactivity in Euphrates river. J. Radioanal. Nucl. Chem. 2004, 261, 349–355. [Google Scholar] [CrossRef]
- Biggin, C.D.; Cook, G.T.; Mackenzie, A.B.; Pates, J.M. Time-efficient method for the determination of 210Pb, 210Bi, and 210Po activities in seawater using liquid scintillation spectrometry. Anal. Chem. 2002, 74, 671–677. [Google Scholar] [CrossRef]
- Narita, H.; Harada, K.; Burnett, W.C.; Tsunogai, S.; Mccabe, W.J. Determination of 210Pb, 210Bi and 210Po in natural waters and other materials by electrochemical separation. Talanta 1989, 36, 925–929. [Google Scholar] [CrossRef]
- Tokieda, T.; Narita, H.; Harada, K.; Tsunogai, S. Sequential and rapid determination of Po-210, Bi-210 and Pb-210 in natural waters. Talanta 1994, 41, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Haridasan, P.P.; Paul, A.C.; Desai, M.V.M. Natural radionuclides in the aquatic environment of a phosphogypsum disposal area. J. Environ. Radioact. 2001, 53, 155–165. [Google Scholar] [CrossRef]
- Gasco, C.; Anton, M.P.; Delfanti, R.; González, A.M.; Meral, J.; Papucci, C. Variation of the activity concentrations and fluxes of natural (210Po, 210Pb) and anthropogenic (239,240Pu, 137Cs) radionuclides in the Strait of Gibraltar (Spain). J. Environ. Radioact. 2002, 62, 241–262. [Google Scholar] [CrossRef]
- Shannon, L.V.; Orren, M.J. A rapid method for the determination of polonium-210 and lead-210 in sea water. Anal. Chim. Acta 1970, 52, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Shannon, L.V.; Cherry, R.D.; Orren, M.J. Polonium-210 and lead-210 in the marine environment. Geochim. Cosmochim. Acta 1970, 34, 701–711. [Google Scholar] [CrossRef]
- Yan, G.; Cho, H.; Lee, I.; Kim, G. Significant emissions of 210Po by coal burning into the urban atmosphere of Seoul, Korea. Atmos. Environ. 2012, 54, 80–85. [Google Scholar] [CrossRef]
- Jiaxing, L.; Tao, Y.; Jingshun, P. Exploration of analytical methods for the analysis of polonium-210 in aerosol samples. Technol. Innov. Appl. 2013, 13, 21–22. [Google Scholar]
- Planinšek, P.; Benedik, L.; Smodiš, B. Comparison of various dissolution techniques for determination of Po-210 in biological samples. Appl. Radiat. Isot. 2013, 81, 53–56. [Google Scholar] [CrossRef]
- Khaing, H.; Thakur, P. Rapid sequential separation method for 210Po and actinides in air filter samples. J. Radioanal. Nucl. Chem. 2017, 314, 1383–1392. [Google Scholar] [CrossRef]
- Karagueuzian, H.S.; White, C.; Sayre, J.; Norman, A. Cigarette smoke radioactivity and lung cancer risk. Nicotine Tob. Res. 2012, 14, 79–90. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US); Office on Smoking and Health (US). Office on, and Health, Reports of the Surgeon General, in The Health Consequences of Smoking: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK45031/ (accessed on 8 June 2023).
- Skwarzec, B.; Ulatowski, J.; Struminska, D.I.; Boryło, A. Inhalation of 210Po and 210Pb from cigarette smoking in Poland. J. Environ. Radioact. 2001, 57, 221–230. [Google Scholar] [CrossRef]
- Little, J.B.; Radford, E.J.; Mccombs, H.L.; Hunt, V.R. Distribution of polonium-210 in pulmonary tissues of cigarette smokers. N. Engl. J. Med. 1965, 273, 1343–1351. [Google Scholar] [CrossRef]
- Kubalek, D.; Serša, G.; Štrok, M.; Benedik, L.; Jeran, Z. Radioactivity of cigarettes and the importance of 210Po and thorium isotopes for radiation dose assessment due to smoking. J. Environ. Radioact. 2016, 155–156, 97–104. [Google Scholar] [CrossRef]
- Radford, E.J.; Hunt, V.R. Polonium-210: A volatile radioelement in cigarettes. Science 1964, 143, 247–249. [Google Scholar] [CrossRef]
- Berthet, A.; Butty, A.; Rossier, J.; Sadowski, I.J.; Froidevaux, P. 210Po and 210Pb content in the smoke of Heated Tobacco Products versus Conventional Cigarette smoking. Sci. Rep. 2022, 12, 10314. [Google Scholar] [CrossRef]
- Bermejo-Barrera, P.; Moreda-Piñeiro, A.; Bermejo-Barrera, A. Sample pre-treatment methods for the trace elements determination in seafood products by atomic absorption spectrometry. Talanta 2001, 57, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Putnam, D.F. Composition and Concentrative Properties of Human Urine; NASA: Washington, DC, USA, 1971. [Google Scholar]
- Shabana, E.I.; Elaziz, M.A.A.; Al-Arifi, M.N.; Al-Dhawailie, A.A.; Al-Bokari, M.A. Evaluation of the contribution of smoking to total blood polonium-210 in Saudi population. Appl. Radiat. Isot. 2000, 52, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Shahul Hameed, P.; Shaheed, K.; Somasundaram, S.S.N. A study on distribution of natural radionuclide polonium-210 in a pond ecosystem. J. Biosci. 1997, 22, 627–634. [Google Scholar] [CrossRef]
- Boryło, A.; Skwarzec, B.; Romańczyk, G.; Siebert, J. Polonium 210Po activities in human blood of patients with ischaemic heart disease from Gdańsk in Poland. J. Radioanal. Nucl. Chem. 2013, 298, 1685–1691. [Google Scholar] [CrossRef]
- Rathi, C.R.; Ross, E.M.; Wesley, S.G. Polonium-210 activity in human hair samples and factors affecting its accumulation. Iran. J. Radiat. Res. 2011, 9, 41–47. [Google Scholar]
- Fowler, S.W. 210Po in the marine environment with emphasis on its behaviour within the biosphere. J. Environ. Radioact. 2011, 102, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Polonium (210Po) and lead (210Pb) in marine organisms and their transfer in marine food chains. J. Environ. Radioact. 2011, 102, 462–472. [Google Scholar] [CrossRef]
- Menghui, H.; Yulian, L.; Ling, J. Research progress on the 210Po of water and foodstuff samples. China Med. Equip. 2018, 15, 145–149. [Google Scholar]
- Xinfang, D.; Ling, C.; Jingshun, P.; Jianchao, W.; Shuli, Z.; Zhonggang, C.; Ziqiang, P. 210Po Level in Five Kinds of Typical Aquatic Products From the Yellow Sea of China. J. Nucl. Radiochem. 2018, 40, 67–73. [Google Scholar]
- Pengxiang, L.; Zhou, L.; Jing, Z.; Zequan, G.; Ruijun, W.; Qinnan, S.; Yuhu, H.; Liping, M. Contents of 210Po in some aquatic organisms and its distribution in different parts of shrimp’s bodies. Radiat. Prot. 2018, 38, 15–18. [Google Scholar]
- Jia, G.; Belli, M.; Blasi, M.; Marchetti, A.; Rosamilia, S.; Sansone, U. 210Pb and 210Po determination in environmental samples. Appl. Radiat. Isot. 2000, 53, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Siqi, W.; Yixin, H. The Application Progress of Microwave Technology in the Analysis of the Sample Digestion and Extraction. 2016, Volume 43, pp. 118–119. Available online: www.gdchem.com (accessed on 16 May 2023).
- Szarlowicz, K. Optimization of the radiochemical procedure of 210Po determination in small amounts of sediment samples. Int. J. Environ. Sci. Technol. 2019, 16, 5735–5740. [Google Scholar] [CrossRef]
- Brown, J.; Gjelsvik, R.; Holm, E.; Roos, P.; Saxen, R.; Outola, I. Filling Knowledge Gaps in Radiation Protection Methodologies for Non-Human Biota Final Summary Report. Nordic Nuclear Safety Research, 200920. Available online: https://www.osti.gov/etdeweb/biblio/953256 (accessed on 6 June 2023).
- Clayton, R.F.; Bradley, E.J. A cost effective method for the determination of 210Po and 210Pb in environmental materials. Sci. Total Environ. 1995, 173–174, 23–28. [Google Scholar] [CrossRef]
- Shen, Q. Study on the Process of Recovering Zinc Fromthe Leaching Residue of Low Grade Zinc Oxide Ore and Its Industrialization; Kunming University of Scienceand Technology: Kunming, China, 2007. [Google Scholar]
- Ansoborlo, E.; Berard, P.; Den Auwer, C.; Leggett, R.; Menetrier, F.; Younes, A.; Montavon, G.; Moisy, P. Review of chemical and radiotoxicological properties of polonium for internal contamination purposes. Chem. Res. Toxicol. 2012, 25, 1551–1564. [Google Scholar] [CrossRef]
- Martin, P.; Hancock, G. Routine Analysis of Naturally Occurring Radionuclides in Environmental Samples by Alpha-Particle Spectrometry; Australian Government Publishing Service: Canberra, Australia, 1992. [Google Scholar]
- Kwon, E.; Chae, J.; Kim, Y. Determination of 210Pb by measurement of 210Pb and its progenies using a liquid scintillation counter. J. Radioanal. Nucl. Chem. 2019, 322, 1431–1436. [Google Scholar] [CrossRef]
- Sag, Y.; Kutsal, T. Determination of the biosorption heats of heavy metal ions on Zoogloea ramigera and Rhizopus arrhizus. Biochem. Eng. J. 2000, 6, 145–151. [Google Scholar] [CrossRef]
- Yafang, S.; Wen, Z.; Baichuan, H.; Le, W.; Hui, L. Research Progress in Chemical Removal of Iron in Hydrometallurgy. Multipurp. Util. Miner. Resour. 2021, 229, 114–119. [Google Scholar]
- Douglas, M.; Friese, J.I.; Greenwood, L.R.; Farmer, O.T.; Thomas, M.L.; Maiti, T.C.; Finn, E.C.; Garofoli, S.J.; Gassman, P.L.; Huff, M.M.; et al. Separation and quantification of chemically diverse analytes in neutron irradiated fissile materials. J. Radioanal. Nucl. Chem. 2009, 282, 63–68. [Google Scholar] [CrossRef]
- Seiner, B.N.; Morley, S.M.; Beacham, T.A.; Haney, M.M.; Gregory, S.; Metz, L. Effects of digestion, chemical separation, and deposition on Po-210 quantitative analysis. J. Radioanal. Nucl. Chem. 2014, 302, 673–678. [Google Scholar] [CrossRef]
- Reischmann, F.J.; Trautmann, N.; Herrmann, G. Chemistry at Low Concentrations: Polonium at a Level of 108 to 105 Atoms. Radiochim. Acta 1984, 36, 139–144. [Google Scholar] [CrossRef]
- Pacer, R.A. The role of Cherenkov and liquid scintilation counting in evaluating the anion-exchange separation of 210Pb-210Bi-210Po. J. Radioanal. Chem. 1983, 77, 19–28. [Google Scholar] [CrossRef]
- Chunfa, L.; Huaping, N.; Yunfen, J.; Yong, L. Status and prospects for the separation and purification of rare earths by extraction chromatography. Chin. J. Process Eng. 2006, 6 (Suppl. S1), 128–132. [Google Scholar]
- Vajda, N.; Larosa, J.; Zeisler, R.; Danesi, P.; Kis-Benedek, G. A novel technique for the simultaneous determination of 210Pb and 210Po using a crown ether. J. Environ. Radioact. 1997, 37, 355–372. [Google Scholar] [CrossRef]
- Philip Horwitz, E.; Chiarizia, R.; Dietz, M.L. A novel strontium-selective extraction chromatographic resin. Solvent Extr. Ion Exch. 1992, 10, 313–336. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Mcalister, D.R.; Bond, A.H.; Barrans, R.E. Novel Extraction of Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications. Solvent Extr. Ion Exch. 2005, 23, 319–344. [Google Scholar] [CrossRef]
- Ordoñez-Regil, E.; Iturbe, G. J L. Isolation and electroplating of 210Po. J. Radioanal. Nucl. Chem. 1993, 175, 47–53. [Google Scholar] [CrossRef]
- El Afifi, E.M.; Borai, E.H. Performance characteristics of sequential separation and quantification of lead-210 and polonium-210 by ion exchange chromatography and nuclear spectrometric measurements. J. Environ. Qual. 2006, 35, 568–574. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Gómez, D.C.P.; Alonso, J.J.; Perez-Moreno, S.M.; Ramos-Lerate, I.; Ruiz, M.C.; Bolivar, J.P. A new methodology based on TRU resin to measure U-, Th-isotopes and 210Po by alpha-particle spectrometry. Talanta 2023, 253, 123972. [Google Scholar] [CrossRef]
- GB 14883.5-2016. Measurement of 210Po in Food Samples. Chinese Standard. Standard Press of China: Beijing, China, 2016.
- HJ 813-2016. Analysis of Polonium-210 in Water. Chinese Standard. Standard Press of China: Beijing, China, 2016.
- Yungang, L.; Changlin, X.; Shuzheng, S. Self-deposition studies of polonium. At. Energy Sci. Technol. 1983, 6, 712–717. [Google Scholar]
- Smith, J.D.; Hamilton, T.F. Improved technique for recovery and measurement of polonium-210 from environmental materials. Anal. Chim. Acta 1984, 160, 69–77. [Google Scholar] [CrossRef]
- Jia, G.; Torri, G.; Petruzzi, M. Distribution coefficients of polonium between 5% TOPO in toluene and aqueous hydrochloric and nitric acids. Appl. Radiat. Isot. 2004, 61, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P.; Oliveira, J.M.; Alberto, G.; Vives i Batlle, J. Allometric relationships of 210Po and 210Pb in mussels and their application to environmental monitoring. Mar. Pollut. Bull. 2010, 60, 1734–1742. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Carvalho, F.P. Sequential extraction procedure for determination of uranium, thorium, radium, lead and polonium radionuclides by alpha spectrometry in environmental samples. Czechoslov. J. Phys. 2006, 56, 545–555. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Malta, M. Radionuclides in deep-sea fish and other organisms from the North Atlantic Ocean. ICES J. Mar. Sci. 2011, 68, 333–340. [Google Scholar] [CrossRef]
- Henricsson, F.; Ranebo, Y.; Holm, E.; Roos, P. Aspects on the analysis of 210Po. J. Environ. Radioact. 2011, 102, 415–419. [Google Scholar] [CrossRef]
- Blanchet-Chouinard, G.; Larivière, D. Determination of polonium-210 in environmental samples using diglycolamide-based cloud point extraction coupled to alpha spectrometry analysis. Appl. Radiat. Isot. 2021, 168, 109549. [Google Scholar] [CrossRef]
- Pengxiang, L.I.; Jing, Z.; Zhou, L.; Ruijun, W.; Wujing, Y.; Li, B.; Yuhu, H. A Method for Determination of 210Po in Biological Samples by using 209Po as Tracter. Sichuan Environ. 2018, 37, 41–45. [Google Scholar]
- DU Baohua, P.S.; Rizhong, W. Determination Method of 210Po in Geological Samples. Uranium Min. Metall. 2019, 38, 64–69. [Google Scholar]
- Karali, T.; Ölmez, S.; Yener, G. Study of spontaneous deposition of 210Po on various metals and application for activity assessment in cigarette smoke. Appl. Radiat. Isot. 1996, 47, 409–411. [Google Scholar] [CrossRef]
- Crespo, M.T. A review of electrodeposition methods for the preparation of alpha-radiation sources. Appl. Radiat. Isot. 2012, 70, 210–215. [Google Scholar] [CrossRef]
- Rieth, U.; Hummrich, H.; Kratz, J. Electrodeposition of Po-210 on Various Electrode Materials. 2023. Available online: https://www.researchgate.net/profile/Ulrich-Rieth/publication/267806707_Electrodeposition_of_Po-210_on_various_electrode_materials/links/00b495268e179db21b000000/Electrodeposition-of-Po-210-on-various-electrode-materials.pdf (accessed on 6 June 2023).
- Hölgye, Z. Coprecipitation of polonium with bismuth phosphate. J. Radioanal. Nucl. Chem. 2007, 274, 647–649. [Google Scholar] [CrossRef]
- Song, L.; Ma, Y.; Wang, Y.; Yang, Y.; Luo, M.; Dai, X. Method of Polonium Source Preparation Using Tellurium Microprecipitation for Alpha Spectrometry. Anal. Chem. 2017, 89, 13651–13657. [Google Scholar] [CrossRef]
- Chunming, F.; Dong, J.; Di, L.; Yao Fang, D.; Yu Ling, Z.; Hai Tao, W.; Di, S. Rapid Determination of Polonium-210 in Environmental Samples by Tellurium Coprecipitation Alpha Spectrometry. China Port Sci. Technol. 2021, 3, 4–9. [Google Scholar]
- Menghui, H. Evaluation of the Radioactivity Level of 210Po in Aerosol, Surface Soil and Drinking Water in Tianjin; Peking Union Medical College Hospital: Beijing, China, 2021. [Google Scholar]
- Kong, X.; Yin, L.; Ji, Y. Simultaneous determination of 210Pb and 210Po in seafood samples using liquid scintillation counting. J. Environ. Radioact. 2021, 231, 106553. [Google Scholar] [CrossRef]
- Ozden, B.; Vaasma, T.; Kiisk, M.; Tkaczyk, A.H. A modified method for the sequential determination of 210Po and 210Pb in Ca-rich material using liquid scintillation counting. J. Radioanal. Nucl. Chem. 2017, 311, 365–373. [Google Scholar] [CrossRef]
- Stojković, I.; Tenjović, B.; Nikolov, J.; Todorovic, N. Radionuclide, scintillation cocktail and chemical/color quench influence on discriminator setting in gross alpha/beta measurements by LSC. J. Environ. Radioact. 2015, 144, 41–46. [Google Scholar] [CrossRef]
- Devol, T.A.; Theisen, C.D.; Diprete, D.P. Effect of quench on alpha/beta pulse shape discrimination of liquid scintillation cocktails. Health Phys. 2007, 92 (Suppl. S5), S105–S111. [Google Scholar] [CrossRef]
- Weiyu, H.; Yihao, L. Determination of thorium-uranium in samples by flash alpha spectrometry of extracted liquids. Nucl. Tech. 1994, 1, 47–50. [Google Scholar]
- Véronneau, C.; Aupiais, J.; Dacheux, N. Selective determination of polonium by photon electron rejecting alpha liquid scintillation (PERALS® System). Anal. Chim. Acta 2000, 415, 229–238. [Google Scholar] [CrossRef]
- Case, G.N.; Mcdowell, W.J. An improved sensitive assay for polonium-210 by use of a background-rejecting extractive liquid-scintillation method. Talanta 1982, 29, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Shutang, L.; Dating, Y.; Yulian, L.; Yihua, X. Methods for preparation of large area low level alpha radiation sources and determination of samples. J. Nucl. Radiochem. 1989, 3, 149–155. [Google Scholar]
- Yucheng, L. A Method for the Determination of 210Po in Seafood Samples by α Energy Spectrometry in a Large Area Grid Ionization Chamber; Chinese Center for Disease Control and Prevention: Beijing, China, 2015. [Google Scholar]
- ISO/DIS 13161: 2020. Water Quality-Polonium 210-Test Method Using Alpha Spectrometry. International Standard Organization: Geneva, Switzerland, 2020.
- A Procedure for Determination of Po-210 in Water Samples by Alpha Spectrometry; International Atomic Energy Agency: Vienna, Austria, 2010. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/IAEA-AQ-12_web.pdf (accessed on 8 June 2023).
- A Procedure for the Sequential Determination of Radionuclides in Phosphogypsum; International Atomic Energy Agency: Vienna, Austria, 2014.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).