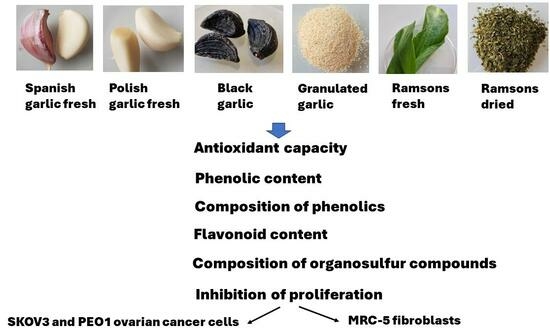
Comparison of Antioxidant and Antiproliferative Effects of Various Forms of Garlic and Ramsons
Abstract
:1. Introduction
2. Results
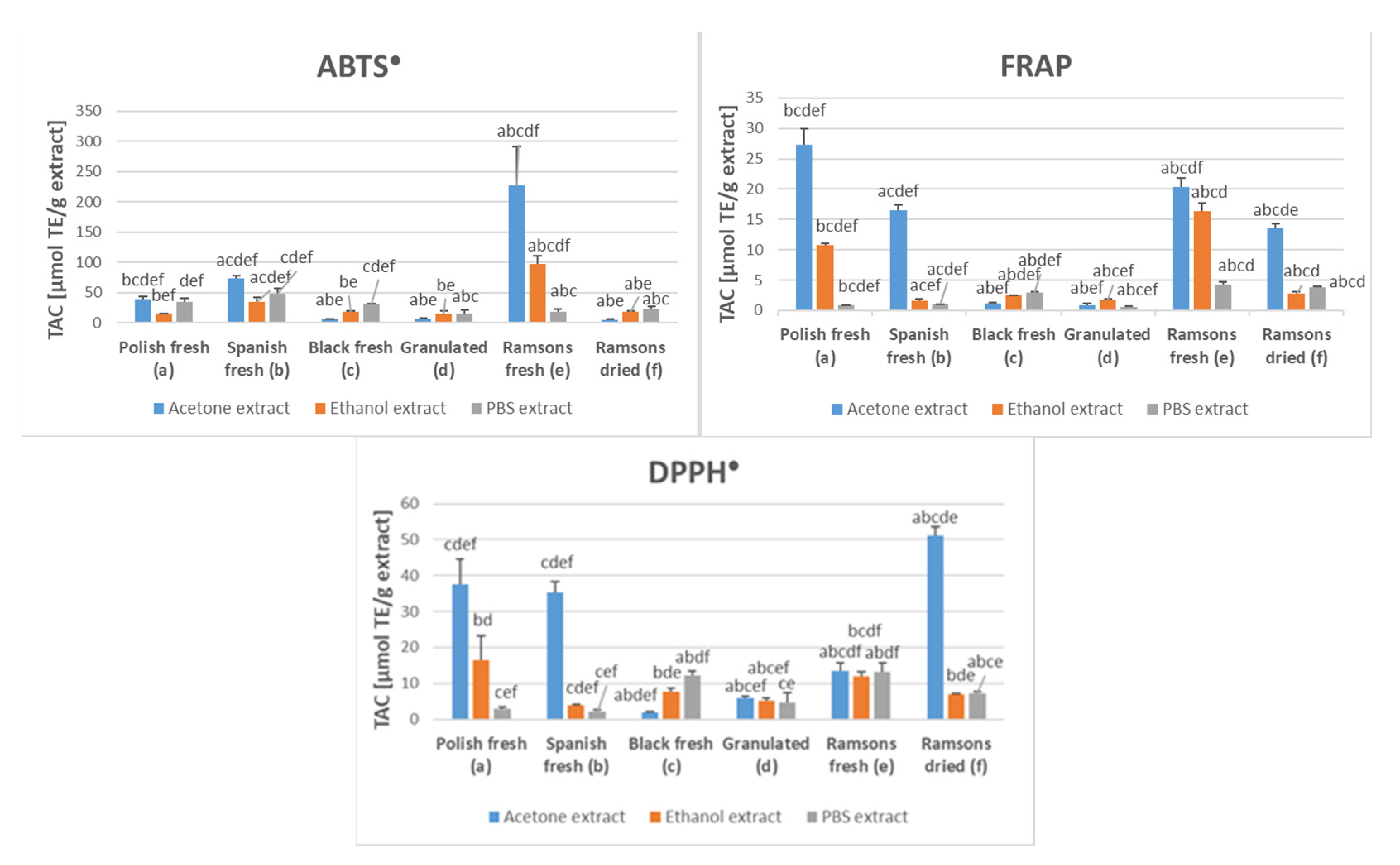
2.1. Antioxidant Capacity of Garlic Extracts
2.2. Garlic Content of Organosulfur Compounds
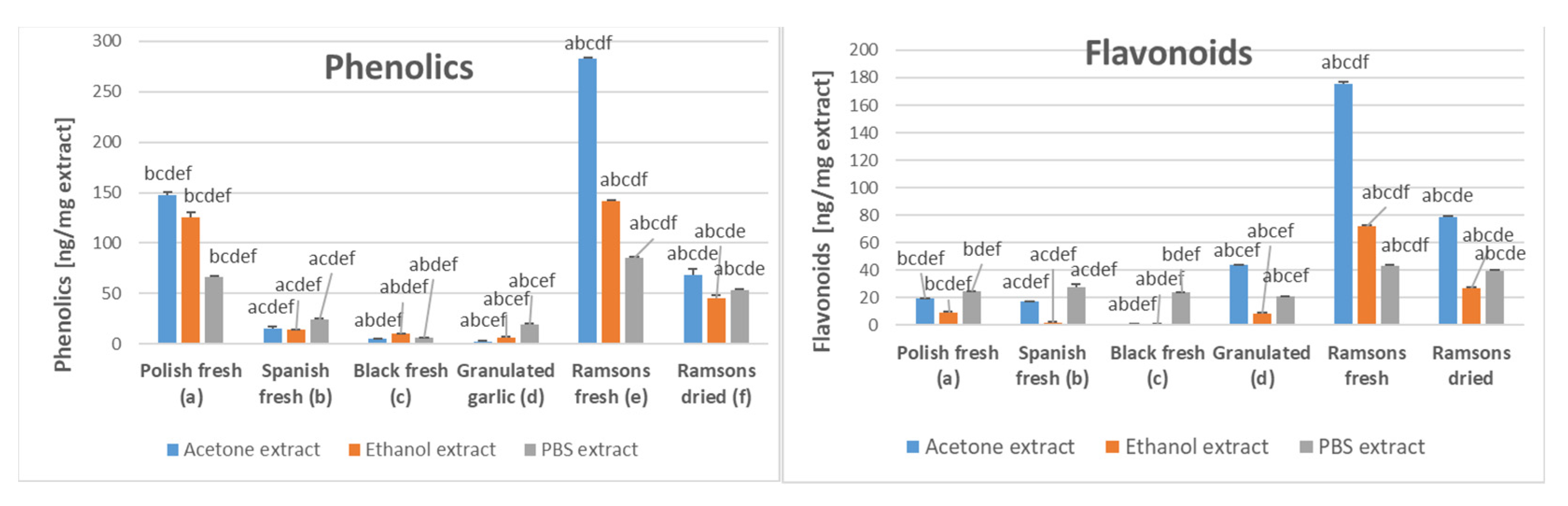
2.3. Ramsons Content of Phenolics
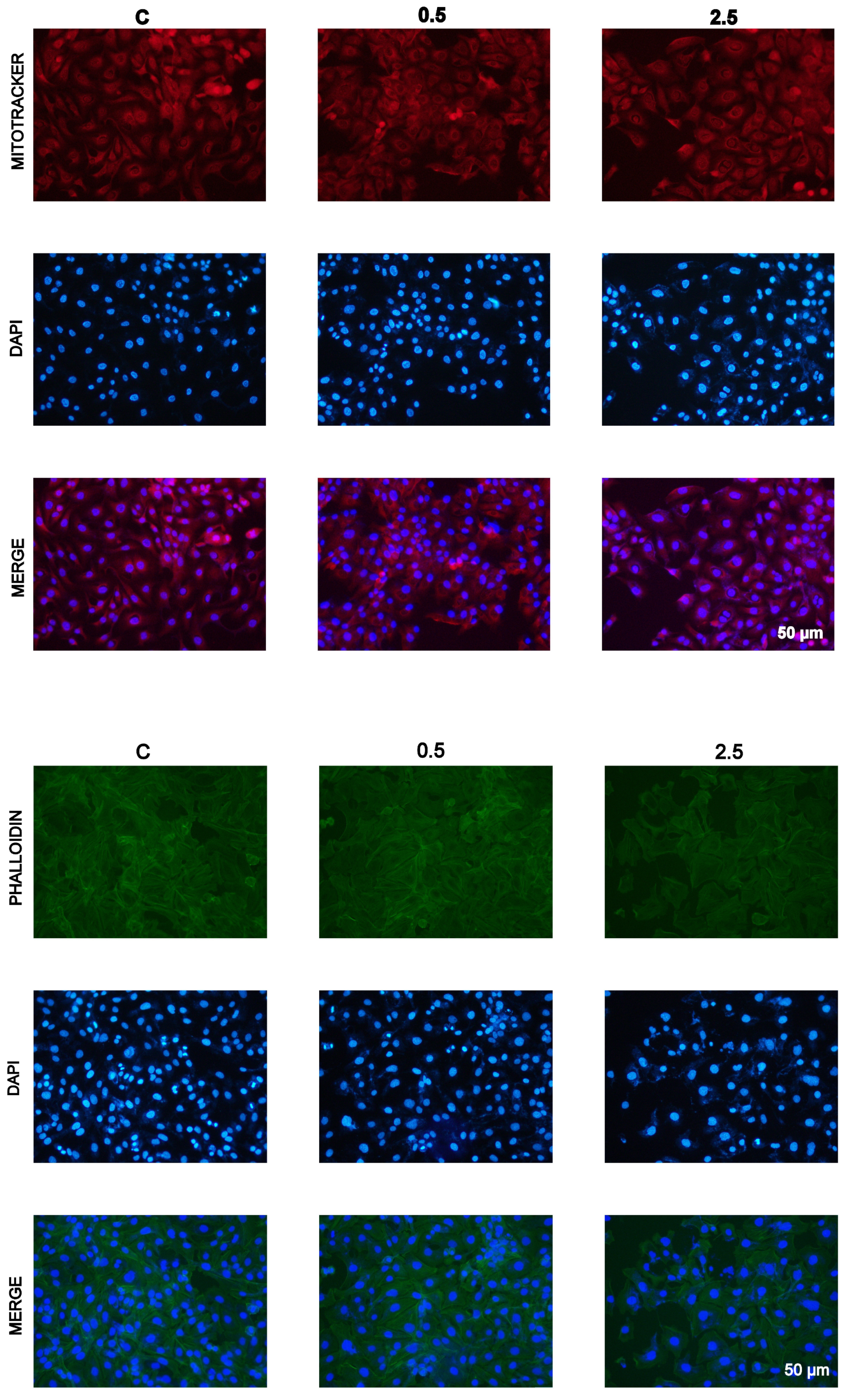
2.4. Inhibition of Cell Proliferation In Vitro by Garlic Extracts
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Garlic Samples
4.3. Preparation of Garlic Extracts
4.4. Assays of Antioxidant Activity
4.4.1. ABTS• Decolorization Assay
4.4.2. DPPH• Scavenging Assay
4.4.3. FRAP Assay
4.4.4. Calculation of Antioxidant Capacity
4.5. Estimation of Polyphenol Content
4.6. Estimation of Flavonoid Content
4.7. Determination of Organosulfur Compounds by UPLC-PDA-MS/MS
4.8. Cell Culture
4.9. Estimation of Cytotoxicity
4.10. Staining with Atto-488-Phalloidin, Mitotracker, and DAPI
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef]
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of garlic and their anticancer and antioxidant activity: A review of the epidemiologic and experimental evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef]
- Shouk, R.; Abdou, A.; Shetty, K.; Sarkar, D.; Eid, A.H. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr. Res. 2014, 34, 106–115. [Google Scholar] [CrossRef]
- Ibraheem, L.J.; Saeed, N.A.H.A.A. Advantages of garlic in treating hypertension: A review article. Eurasian Med. Res. Period. 2023, 19, 28–37. [Google Scholar]
- Dehghani, S.; Alipoor, E.; Salimzadeh, A.; Yaseri, M.; Hosseini, M.; Feinle-Bisset, C.; Hosseinzadeh-Attar, M.J. The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis. Phytomedicine 2018, 15, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural hydrogen sulfide donors from allium sp. as a nutraceutical approach in type 2 diabetes prevention and therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef]
- Sohn, C.W.; Kim, H.; You, B.R.; Kim, M.J.; Kim, H.J.; Lee, J.Y.; Sok, D.E.; Kim, J.H.; Lee, K.J.; Kim, M.R. High temperature- and high pressure-processed garlic improves lipid profiles in rats fed high cholesterol diets. J. Med. Food. 2012, 15, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.W.; Ying, T.; Kim, W.K. The effects of black garlic (Allium sativum) extracts on lipid metabolism in rats fed a high fat diet. Nutr. Res. Pract. 2015, 9, 30–36. [Google Scholar] [CrossRef]
- Papu, S.; Jaivir, S.; Sweta, S.; Singh, B.R. Medicinal values of garlic (Allium sativum L.) in human life: An overview. Greener J. Agric. Sci. 2014, 4, 265–280. [Google Scholar]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef] [PubMed]
- Pârvu, M.; Moţ, C.A.; Pârvu, A.E.; Mircea, C.; Stoeber, L.; Roşca-Casian, O.; Ţigu, A.B. Allium sativum extract chemical composition, antioxidant activity and antifungal effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa causing onychomycosis. Molecules 2019, 24, 3958. [Google Scholar] [CrossRef]
- Wu, X.; Santos, R.R.; Fink-Gremmels, J. Analyzing the antibacterial effects of food ingredients: Model experiments with allicin and garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci. Nutr. 2015, 3, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2017, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Varshney, A.; Nandru, R.; Kushwaha, S.; Prasad, V.; Dasgupta, S. Essential oils and extracts of commonly used medicinal plants to fight against emerging and re-emerging infectious diseases including COVID-19. J. Med. Plants 2023, 11, 11–24. [Google Scholar] [CrossRef]
- Sarhan, A.T.; Abdulabbas, M.A.; Abd Al-Hussein, Q.; Huda, Z.; Mohammed, A. Antimicrobial Activities of Medicinal Plant on the Oral Diseases. Int. J. Pharm. BioMed. Sci. 2023, 3, 147–151. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, A.; Dey, P.; Chauhan, A.K.; Rashid, S.; Chen, K.T.; Sharma, R. Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: An updated review. Front. Nutr. 2023, 10, 1120377. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Yaraee, R.; Rahmati, B.; Hakimzadeh, H.; Shams, J.; Jalali-Nadoushan, M.R. In vitro cytotoxic effect of garlic extract on malignant and nonmalignant cell lines. Immunopharmacol. Immunotoxicol. 2011, 33, 603–608. [Google Scholar] [CrossRef]
- Modem, S.; Dicarlo, S.E.; Reddy, T.R. Fresh garlic extract induces growth arrest and morphological differentiation of MCF7 breast cancer cells. Genes Cancer 2012, 3, 177–186. [Google Scholar] [CrossRef]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude garlic extract inhibits cell proliferation and induces cell cycle arrest and apoptosis of cancer cells in vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, F.; Wang, Q.W.; Wang, J.; Yang, K.; Hu, R.R.; Liu, H.C.; Wang, H.Y.; Wang, Y.S. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef]
- Su, C.C.; Chen, G.W.; Tan, T.W.; Lin, J.G.; Chung, J.G. Crude extract of garlic induced caspase-3 gene expression leading to apoptosis in human colon cancer cells. In Vivo 2006, 20, 85–90. [Google Scholar] [PubMed]
- Shin, S.S.; Song, J.H.; Hwang, B.; Noh, D.H.; Park, S.L.; Kim, W.T.; Park, S.S.; Kim, W.J.; Moon, S.K. HSPA6 augments garlic extract-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; Implication for cell cycle dysregulation, signaling pathway alteration, and transcription factor-associated MMP-9 regulation. PLoS ONE 2017, 12, e0171860. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Davis, S.; Rice, M.; Tapp, H.; Saxon, B.; Revesz, T. Garlic compounds selectively kill childhood pre-B acute lymphoblastic leukemia cells in vitro without reducing T-cell function: Potential therapeutic use in the treatment of ALL. Biologics 2008, 2, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Sun, H.; Liu, J.; Lin, Y.; Zhu, Z.; Han, X.; Sun, X.; Li, X.; Zhang, H.; Tang, Z. Effects of garlic oil on pancreatic cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 5905–5910. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Wu, M.; Han, R.Q.; Zhang, X.F.; Wang, X.S.; Liu, A.M.; Zhou, J.Y.; Lu, Q.Y.; Zhang, Z.F.; Zhao, J.K. Raw garlic consumption as a protective factor for lung cancer, a population-based case-control study in a Chinese population. Cancer Prev. Res. 2013, 6, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Chokkalingam, A.P.; Gao, Y.T.; Madigan, M.P.; Deng, J.; Gridley, G.; Fraumeni, J.F., Jr. Allium vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer Inst. 2002, 94, 1648–1651. [Google Scholar] [CrossRef]
- Gao, C.M.; Takezaki, T.; Ding, J.H.; Li, M.S.; Tajima, K. Protective effect of allium vegetables against both esophageal and stomach cancer: A simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Jpn. J. Cancer Res. 1999, 90, 614–621. [Google Scholar] [CrossRef]
- Hu, J.F.; Liu, Y.Y.; Yu, Y.K.; Zhao, T.Z.; Liu, S.D.; Wang, Q.Q. Diet and cancer of the colon and rectum: A case-control study in China. Int. J. Epidemiol. 1991, 20, 362–367. [Google Scholar] [CrossRef]
- Levi, F.; Pasche, C.; La Vecchia, C.; Lucchini, F.; Franceschi, S. Food groups and colorectal cancer risk. Br. J. Cancer 1999, 79, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Speciani, M.C.; Gargari, G.; Penagini, R.; Mutignani, M.; Ferraroni, M.; Natale, A.; Katsoulis, M.; Cintolo, M.; Leone, P.; Airoldi, A.; et al. Garlic consumption in relation to colorectal cancer risk and to alterations of blood bacterial DNA. Eur. J. Nutr. 2023, 62, 2279–2292. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef]
- Hansson, L.E.; Nyrén, O.; Bergström, R.; Wolk, A.; Lindgren, A.; Baron, J.; Adami, H.O. Diet and risk of gastric cancer—A population-based case-control study in Sweden. Int. J. Cancer 1993, 55, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Plummer, M.; Vivas, J.; Moreno, V.; De Sanjosé, S.; Lopez, G.; Oliver, W. A case-control study of gastric cancer in Venezuela. Int. J. Cancer 2001, 93, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chang, W.K.; Kim, M.K.; Lee, S.S.; Choi, B.Y. Dietary factors and gastric cancer in Korea: A case-control study. Int. J. Cancer 2002, 97, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, P.; Wu, Y.; Liu, D.; Ji, M.; Li, H.; Wang, Y. Association and mechanism of garlic consumption with gastrointestinal cancer risk: A systematic review and meta-analysis. Oncol. Lett. 2022, 23, 125. [Google Scholar] [CrossRef]
- Tanaka, S.; Haruma, K.; Kunihiro, M.; Nagata, S.; Kitadai, Y.; Manabe, N.; Sumii, M.; Yoshihara, M.; Kajiyama, G.; Chayama, K. Effects of aged garlic extract (AGE) on colorectal adenomas: A double-blinded study. Hiroshima J. Med. Sci. 2004, 53, 39–45. [Google Scholar]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136 (Suppl. S3), 821S–826S. [Google Scholar] [CrossRef]
- Kyo, E.; Uda, N.; Suzuki, A.; Kakimoto, M.; Ushijima, M.; Kasuga, S.; Itakura, Y. Immunomodulation and antitumor activities of Aged Garlic Extract. Phytomedicine 1998, 5, 259–267. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rybczynska-Tkaczyk, K.; Gawel-Beben, K.; Swieca, M.; Karas, M.; Jakuczyk, A.; Matysiak, M.; Binduga, U.E.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, X.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Drying of garlic slices (Allium sativum L.) and its effect on thiosulfinates, total phenolic compounds and antioxidant activity during infrared drying. J. Food Proc. Pres. 2017, 41, e12734. [Google Scholar] [CrossRef]
- Babetto, A.C.; Freire, F.B.; Barrozo, M.A.S.; Freire, J.T. Drying of garlic slices: Kinetics and nonlinearity measures for selecting the best equilibrium moisture content equation. J. Food Engin. 2011, 107, 347–352. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bio-activity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1059–1062. [Google Scholar] [CrossRef]
- Voća, S.; Šic Žlabur, J.; Fabek Uher, S.; Peša, M.; Opačić, N.; Radman, S. Neglected potential of wild garlic (Allium ursinum L.)—Specialized metabolites content and antioxidant capacity of wild populations in relation to location and plant phenophase. Horticulturae 2021, 8, 24. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, B.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Fei, M.L.; Tong, L.I.; Wei, L.I.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Wangcharoen, W.; Morasuk, W. Effect of heat treatment on the antioxidant capacity of garlic. Maejo Int. J. Sci. Technol. 2009, 3, 60–70. [Google Scholar]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J. Food Proces. Preserv. 2017, 41, e12921. [Google Scholar] [CrossRef]
- Mahmutovic, O.; Tahirovic, I.; Copra, A.; Memic, M.; Ibragic, S.; Karic, L. Correlation of total secondary sulfur compounds, total phenols and antioxidant capacity in the Ramsons and Garlic. Br. J. Pharm. Res. 2014, 4, 2662–2669. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [PubMed]
- Škrovánková, S.; Mlček, J.; Snopek, L.; Planetová, T. Polyphenols and antioxidant capacity in different types of garlic. Potravin. Slovak J. Food Sci. 2018, 12, 267–272. [Google Scholar] [CrossRef]
- Furdak, P.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Extracts of common vegetables inhibit the growth of ovary cancer cells. Foods 2022, 11, 2518. [Google Scholar] [CrossRef] [PubMed]
- Oravetz, K.; Todea, A.V.; Balacescu, O.; Cruceriu, D.; Rakosy-Tican, E. Potential antitumor activity of garlic against colorectal cancer: Focus on the molecular mechanisms of action. Eur. J. Nutr. 2023, 62, 2347–2363. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front. Pharmacol. 2023, 14, 656. [Google Scholar] [CrossRef]
- Shekhar, S.; Prakash, P.; Singha, P.; Prasad, K.; Singh, S.K. Modeling and optimization of ultrasound-assisted extraction of bioactive compounds from Allium sativum leaves using response surface methodology and artificial neural network coupled with genetic algorithm. Foods 2023, 12, 1925. [Google Scholar] [CrossRef]
- Wongsa, P.; Bhuyar, P.; Tongkoom, K.; Spreer, W.; Müller, J. Influence of hot-air drying methods on the phenolic compounds/allicin content, antioxidant activity and α-amylase/α-glucosidase inhibition of garlic (Allium sativum L.). Eur. Food Res. Technol. 2023, 249, 523–535. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Rocha, J.P.M.; Coelho, A.D.; Oliveira, A.J.M.; Mendes, L.C.; Pereira, M.M.A.; Morais, L.C.; Forim, M.R.; et al. Solvent mixture optimization in the extraction of bioactive compounds and antioxidant activities from garlic (Allium sativum L.). Molecules 2021, 26, 6026. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, N.; Li, S.; Liu, W.; Li, M.; Zhang, M.; Chen, H. Biochemical composition, antioxidant activity and antiproliferative effects of different processed garlic products. Molecules 2023, 28, 804. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Ionita, P. The chemistry of DPPH· free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef]
- Petrovic, V.; Nepal, A.; Olaisen, C.; Bachke, S.; Hira, J.; Søgaard, C.K.; Røst, L.M.; Misund, K.; Andreassen, T.; Melø, T.M.; et al. Anti-cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients 2018, 10, 450. [Google Scholar] [CrossRef]
- Azizzadeh, D.A.; Heshmati, M.; Ghaini, M.H. Garlic extract can induce apoptotic cell death in the human colon adenocarcinoma HT29 cell line. Iranian J. Pathol. 2010, 5, 126–131. [Google Scholar]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Food. 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Li, G.; Qiao, C.; Lin, R.; Pinto, J.; Osborne, M.; Tiwari, R. Antiproliferative effects of garlic constituents in cultured human breast-cancer cells. Oncol. Rep. 1995, 2, 787–791. [Google Scholar] [CrossRef]
- Iciek, M.; Kwiecień, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Tsubura, A.; Lai, Y.C.; Kuwata, M.; Uehara, N.; Yoshizawa, K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anti-Cancer Agents Med. Chem. 2011, 11, 249–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, J.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ha, M.; Gong, Y.; Xu, Y.; Dong, N.; Yuan, Y. Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol. Rep. 2010, 24, 1585–1592. [Google Scholar]
- Zhang, Q.; Yang, D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp. Ther. Med. 2019, 17, 1523–1528. [Google Scholar] [CrossRef]
- Maitisha, G.; Aimaiti, M.; An, Z.; Li, X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021, 48, 7261–7272. [Google Scholar] [CrossRef] [PubMed]
- Azadi, H.G.; Riazi, G.H.; Ghaffari, S.M.; Ahmadian, S.; Khalife, T.J. Effects of Allium hirtifolium (Iranian shallot) and its allicin on microtubule and cancer cell lines. Afr. J. Biotechnol. 2009, 8, 5030–5037. [Google Scholar]
- Xu, L.; Yu, J.; Zhai, D.; Zhang, D.; Shen, W.; Bai, L.; Cai, Z.; Yu, C. Role of JNK activation and mitochondrial bax translocation in allicin-induced apoptosis in human ovarian cancer SKOV3 cells. Evid. Based Complement. Alternat. Med. 2014, 2014, 378684. [Google Scholar] [CrossRef]
- Xu, Y.S.; Feng, J.G.; Zhang, D.; Zhang, B.; Luo, M.; Su, D.; Lin, N.M. S-allylcysteine, a garlic derivative, suppresses proliferation and induces apoptosis in human ovarian cancer cells in vitro. Acta Pharmacol. Sin. 2014, 35, 267–274. [Google Scholar] [CrossRef]
- Xu, Y.; Su, D.; Zhu, L.; Zhang, S.; Ma, S.; Wu, K.; Yuan, Q.; Lin, N. S-allylcysteine suppresses ovarian cancer cell proliferation by DNA methylation through DNMT1. J. Ovarian Res. 2018, 11, 39. [Google Scholar] [CrossRef]
- Chu, Q.; Ling, M.T.; Feng, H.; Cheung, H.W.; Tsao, S.W.; Wang, X.; Wong, Y.C. A novel anticancer effect of garlic derivatives: Inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006, 27, 2180–2189. [Google Scholar] [CrossRef]
- Tang, F.Y.; Chiang, E.P.I.; Chung, J.G.; Lee, H.Z.; Hsu, C.Y. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J. Nutr. Biochem. 2009, 20, 1013–1020. [Google Scholar] [CrossRef]
- Ng, K.T.; Guo, D.Y.; Cheng, Q.; Geng, W.; Ling, C.C.; Li, C.X.; Liu, X.B.; Ma, Y.Y.; Lo, C.M.; Poon, R.T.; et al. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS ONE 2012, 7, e31655. [Google Scholar] [CrossRef]
- Sigounas, G.; Hooker, J.; Anagnostou, A.; Steiner, M. S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast, and prostate cancer cell lines. Nutr. Cancer. 1997, 27, 186–191. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, S.; Zhang, J.; Qu, X.; Jiang, S.; Zhong, Z.; Zhang, F.; Wong, Y.; Chen, H. Over-expression of survivin is a factor responsible for differential responses of ovarian cancer cells to S-allylmercaptocysteine (SAMC). Exp. Mol. Pathol. 2016, 100, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M. Alliin in garlic. Perish. Handl. Quart. Issue 2000, 102, 5–6. [Google Scholar]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, K.; Naparło, K.; Soszyński, M.; Bartosz, G.; Sadowska-Bartosz, I. Capsaicin toxicity to the yeast Saccharomyces cerevisiae is not due to oxidative stress but to disruption of membrane structure. Chem. Biol. Int. 2023, 374, 110407. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2214. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Pycia, K.; Pawłowska, A.; Kapusta, I.T. Phytochemical screening and bioactive properties of Juglans regia L. pollen. Antioxidants 2022, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
| Compound | Rt [min] | [M-H]+ m/z | |
|---|---|---|---|
| MS | MS/MS | ||
| 1. S-Ethylcysteine sulfoxide | 1.83 | 166 | 88 |
| 2. γ-Glu-allylcysteine | 2.34 | 291 | 145 |
| 3. γ-Glu-propenylcysteine | 2.75 | 291 | 145 |
| 4. γ-Glu-allylthiocysteine | 3.66 | 323 | 145 |
| 5. Allicin | 6.19 | 163 | 73 |
| 6. S-allylcysteine | 7.05 | 163 | 146 |
| Compound | Garlic | Ethanol Extract | PBS Extract |
|---|---|---|---|
| S-Ethylcysteine sulfoxide | Polish fresh | - | 0.56 ± 0.02 |
| Spanish fresh | 1.00 ± 0.04 | 2.31 ± 0.10 | |
| Black fresh | - | 9.78 ± 0.41 | |
| Granulated | - | - | |
| γ-Glu-allylcysteine | Polish fresh | - | 1.29 ± 0.07 |
| Spanish fresh | 11.26 ± 0.62 | 17.97 ± 0.99 | |
| Black fresh | - | - | |
| Granulated | - | 23.68 ± 1.30 | |
| γ-Glu-propenylcysteine | Polish fresh | 3.27 ± 0.11 | 14.06 ± 0.46 |
| Spanish fresh | 3.64 ± 0.12 | 5.25 ± 0.17 | |
| Black fresh | - | - | |
| Granulated | - | 92.25 ± 3.04 | |
| γ-Glu-allylthiocysteine | Polish fresh | - | - |
| Spanish fresh | 2.41 ± 0.11 | 5.59 ± 0.25 | |
| Black fresh | - | - | |
| Granulated | - | 12.49 ± 0.55 | |
| Allicin | Polish fresh | 7.24 ± 0.46 | 34.75 ± 2.23 |
| Spanish fresh | 19.35 ± 1.24 | 40.43 ± 2.59 | |
| Black fresh | - | - | |
| Granulated | - | 47.72 ± 3.05 | |
| S-allylcysteine | Polish fresh | 4.97 ± 0.14 | 4.80 ± 0.13 |
| Spanish fresh | 7.65 ± 0.21 | 7.40 ± 0.20 | |
| Black fresh | - | - | |
| Granulated | - | 4.97 ± 0.14 | |
| Total | Polish fresh | 15.48 ± 0.49 | 55.46 ± 2.28 |
| Spanish fresh | 45.31 ± 1.41 | 78.95 ± 2.80 | |
| Black fresh | - | 9.78 ± 0.41 | |
| Granulated | - | 176.14 ± 4.53 |
| Compound | Rt [min] | λmax [nm] | [M-H]+ m/z | |
|---|---|---|---|---|
| MS | MS/MS | |||
| Kaempferol 3-O-rutinoside-7-O-glucoside | 1.83 | 264, 347 | 166 | 88 |
| Coumaric acid glucoside | 2.34 | 312 | 291 | 145 |
| Kaempferol 3-O-glucoside-7-O-glucoside | 2.75 | 264, 345 | 291 | 145 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer I | 3.66 | 264, 347 | 323 | 145 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer II | 6.19 | 264, 346 | 163 | 73 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer III | 7.05 | 264, 338 | 163 | 145.73 |
| Kaempferol rutinoside-coumaroyl-glucoside-glucoside isomer I | 4.46 | 264, 315 | 1063 | 593, 447, 285 |
| Kaempferol rutinoside-coumaroyl-glucoside-glucoside isomer II | 4.56 | 266, 316 | 1063 | 593, 447, 285 |
| Kaempferol 3-O-rutinoside | 4.66 | 264, 338 | 593 | 285 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer I | 5.05 | 266, 316 | 901 | 593, 447, 285 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer II | 5.12 | 267, 316 | 901 | 593, 447, 285 |
| Kaempferol 3-O-rutinoside acetyl derivative | 5.27 | 264, 331 | 635 | 593, 285 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer III | 5.36 | 264, 316 | 901 | 593, 447, 285 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer acetyl derivat. I | 5.60 | 267, 315 | 943 | 901, 593, 285 |
| Kaempferol rutinoside-coumaroyl-glucoside acetyl derivative II | 5.72 | 264, 317 | 943 | 901, 593, 285 |
| Kaempferol-rutinoside-feruloyl-glucoside acetyl derivative | 5.77 | 264, 338 | 973 | 797, 593, 285 |
| Compound | Fresh | Dry | ||
|---|---|---|---|---|
| Ethanol Extract | PBS Extract | Ethanol Extract | PBS Extract | |
| Kaempferol 3-O-rutinoside-7-O-glucoside | 15.30 ± 0.79 | 44.12 ± 0.82 | 52.01 ± 0.97 | 373.9 ± 6.99 |
| Coumaric acid glucoside | 3.81 ± 0.15 | 1.31 ± 0.07 | 2.00 ± 0.10 | 53.96 ± 2.79 |
| Kaempferol 3-O-glucoside-7-O-glucoside | 4.20 ± 0.07 | 13.64 ± 0.54 | 23.24 ±0.92 | 94.34 ± 3.74 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer I | 3.88 ± 0.07 | 13.77 ± 0.21 | 9.03 ±0.14 | 92.77 ± 1.45 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer II | 5.37 ± 0.20 | 10.40 ± 0.18 | 18.59 ± 0.33 | 157.7 ± 2.77 |
| Kaempferol (acetyl)-glucoside-rhamnoside-glucoside isomer III | 0.44 ± 0.01 | 2.16 ± 0.08 | 2.86 ± 0.11 | 20.06 ± 0.76 |
| Kaempferol rutinoside-coumaroyl-glucoside-glucoside isomer I | 1.64 ± 0.02 | 7.21 ± 0.22 | 9.73 ± 0.29 | 15.05 ± 0.45 |
| Kaempferol rutinoside-coumaroyl-glucoside-glucoside isomer II | 2.32 ± 0.11 | 16.18 ± 0.21 | 19.56 ± 0.25 | 73.45 ± 0.94 |
| Kaempferol 3-O-rutinoside | 4.10 ± 0.01 | 4.81 ± 0.23 | 5.71 ± 0.27 | 39.52 ± 1.88 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer I | 1.28 ± 0.03 | 6.13 ± 0.01 | 9.14 ± 0.02 | 7.15 ± 0.02 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer II | 2.91 ± 0.17 | 20.20 ± 0.40 | 29.60 ± 0.59 | 41.26 ± 0.82 |
| Kaempferol 3-O-rutinoside acetyl derivative | 1.14 ± 0.00 | 1.99 ± 0.11 | 9.31 ± 0.53 | 73.30 ± 4.17 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer III | 2.50 ± 0.02 | 4.16 ± 0.01 | 8.61 ± 0.03 | 10.69 |
| Kaempferol rutinoside-coumaroyl-glucoside isomer acetyl derivative I | 0.41 ± 0.00 | 3.18 ± 0.02 | 7.63 ± 0.05 | 30.78 |
| Kaempferol rutinoside-coumaroyl-glucoside acetyl derivative II | 1.96 ± 0.04 | 5.00 ± 0.06 | 4.65 ± 0.06 | 14.32 |
| Kaempferol-rutinoside-feruloyl-glucoside acetyl derivative | 2.14 ± 0.02 | 0.59 ± 0.01 | 1.62 ± 0.04 | 27.89 |
| Total | 51.83 ± 0.54 | 151.5 ± 1.60 | 213.2 ± 4.59 | 1126 ± 27.46 |
| Garlic/Extract/Cell Line | PEO1 | SKOV3 | MRC-5 | |
|---|---|---|---|---|
| Polish fresh | Acetone extract | 6.7 | 6.0 | 7.2 |
| Ethanol extract | 12.0 | 12.4 | 19.2 | |
| PBS extract | ND | 21.8 | 121.5 | |
| Acetone extract | 12.1 | 39.0 | 24.1 | |
| Spanish fresh | Ethanol extract | 40.1 | 134.4 | 453.7 |
| PBS extract | 0.71 | 61.6 | 42.0 | |
| Acetone extract | 420.5 | 650.4 | 696.4 | |
| Black fresh | Ethanol extract | 113.0 | 127.2 | 71.7 |
| PBS extract | 341.6 | 581.9 | 2159 | |
| Acetone extract | 51.7 | 71.7 | 832.8 | |
| Granulated | Ethanol extract | 78.2 | 45.0 | 1166 |
| PBS extract | 3.8 | 33.7 | 125.7 | |
| Acetone extract | 8.1 | 21.1 | 14.0 | |
| Ramsons fresh | Ethanol extract | 12.2 | 13.0 | 15.8 |
| PBS extract | 6.2 | 29.2 | 29.4 | |
| Ramsons dried | Acetone extract | 12.2 | 15.3 | 17.7 |
| Ethanol extract | 50.3 | 56.3 | 70.4 | |
| PBS extract | 32.2 | 197.2 | ND | |
| Correlation Coefficient | Flavonoid Content | TAC/ABTS● | TAC/FRAP | TAC/DPPH● | IC50/PEO1 | IC50/SKOV3 | IC50/MRC-5 |
|---|---|---|---|---|---|---|---|
| Phenolic content | 0.78 *** | 0.77 *** | 0.74 *** | 0.27 | −0.38 | −0.39 | −0.44 |
| Flavonoid content | 0.81 *** | 0.55 * | 0.05 | −0.23 | −0.21 | −0.23 | |
| TAC/ABTS● | 0.55 * | 0.05 | −0.23 | −0.21 | −0.23 | ||
| TAC/FRAP | 0.73 ** | −0.35 | −0.35 | −0.38 | |||
| TAC/DPPH● | −0.28 | −0.27 | −0.26 | ||||
| IC50/PEO1 | 0.97 *** | 0.69 ** | |||||
| IC50/SKOV3 | 0.69 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furdak, P.; Pieńkowska, N.; Kapusta, I.; Bartosz, G.; Sadowska-Bartosz, I. Comparison of Antioxidant and Antiproliferative Effects of Various Forms of Garlic and Ramsons. Molecules 2023, 28, 6512. https://doi.org/10.3390/molecules28186512
Furdak P, Pieńkowska N, Kapusta I, Bartosz G, Sadowska-Bartosz I. Comparison of Antioxidant and Antiproliferative Effects of Various Forms of Garlic and Ramsons. Molecules. 2023; 28(18):6512. https://doi.org/10.3390/molecules28186512
Chicago/Turabian StyleFurdak, Paulina, Natalia Pieńkowska, Ireneusz Kapusta, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2023. "Comparison of Antioxidant and Antiproliferative Effects of Various Forms of Garlic and Ramsons" Molecules 28, no. 18: 6512. https://doi.org/10.3390/molecules28186512
APA StyleFurdak, P., Pieńkowska, N., Kapusta, I., Bartosz, G., & Sadowska-Bartosz, I. (2023). Comparison of Antioxidant and Antiproliferative Effects of Various Forms of Garlic and Ramsons. Molecules, 28(18), 6512. https://doi.org/10.3390/molecules28186512