In Vivo, In Vitro and In Silico Study of Cucurbita moschata Flower Extract: A Promising Source of Natural Analgesic, Anti-Inflammatory, and Antibacterial Agents
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening
2.1.1. Qualitative Screening
2.1.2. Quantitative Screening
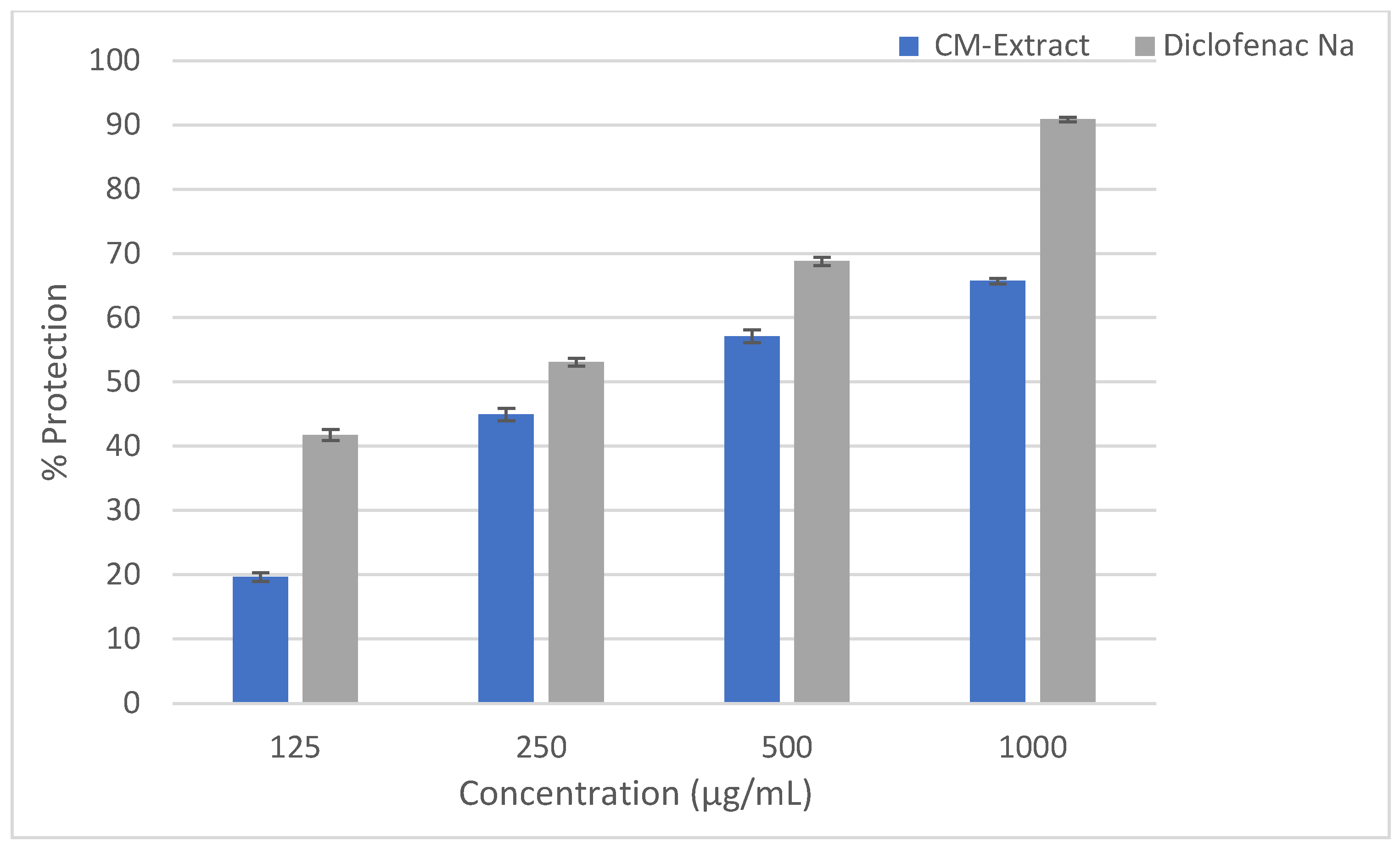
2.2. Anti-Inflammatory Activity
HRBC Membrane Stabilizing Assay
2.3. Analgesic Activity
Acetic Acid-Induced Writhing Test
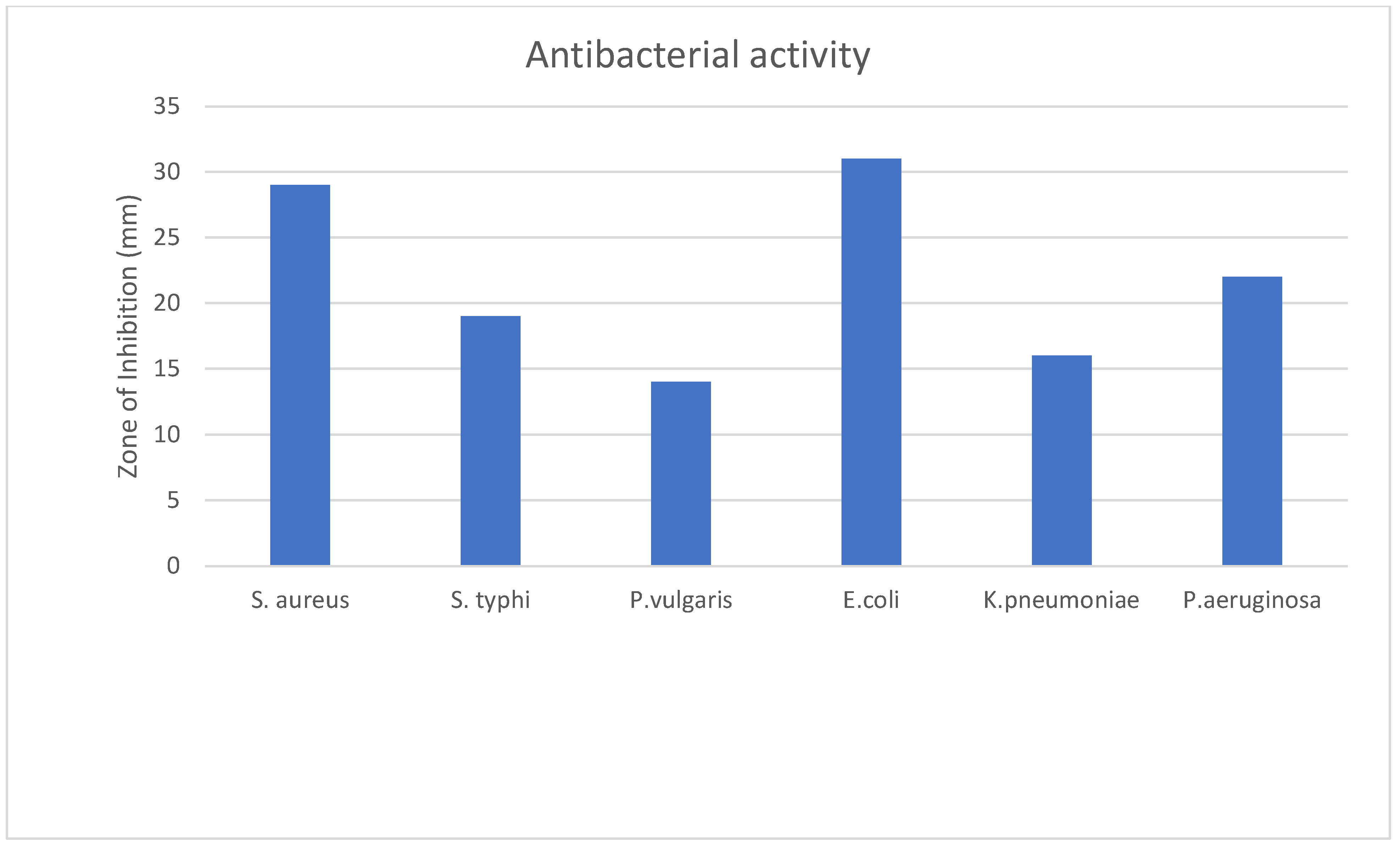
2.4. Antibacterial Activity
2.5. In Silico Study
2.5.1. ADME/T and Drug-Likeness Analysis
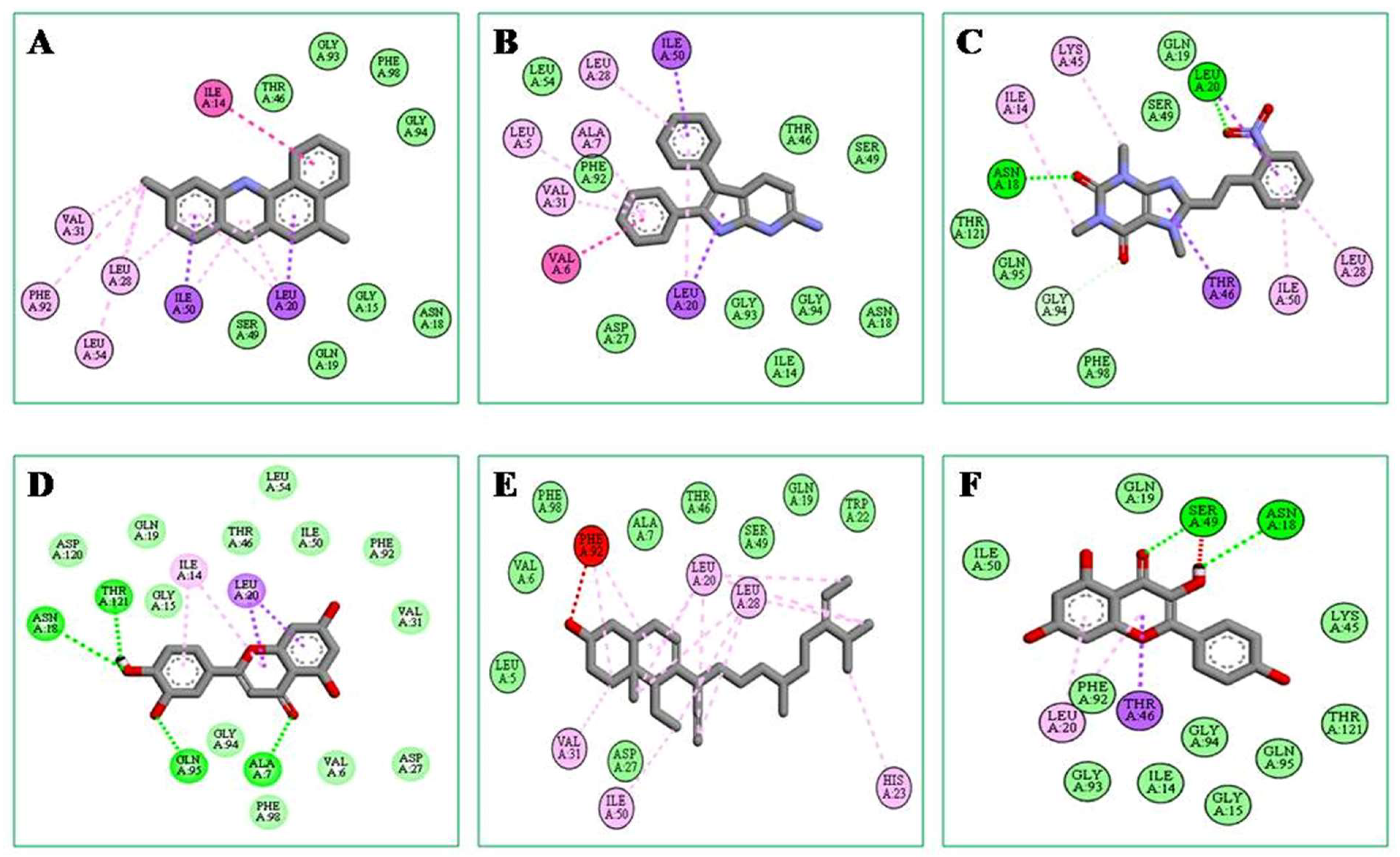
2.5.2. Molecular Docking and Post-Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Plant Extract
4.3. Experimental Animals
4.4. Phytochemical Screening
4.4.1. Qualitative Screening
4.4.2. Quantitative Screening
Total Phenolic Content
Total Flavonoid Content
4.5. Anti-Inflammatory Study
Human RBC Membrane Stabilization Assay
4.6. Analgesic Activity
Acetic Acid-Induced Writhing Test
4.7. Antibacterial Activity
Disc Diffusion Method
4.8. In Silico Study
4.8.1. Selection of Ligands
4.8.2. Validation of the Ligands as Potential Therapeutic Agents
4.8.3. Protein Preparation and Active Site Determination
4.8.4. Molecular Docking and Post-Docking Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, K.H.; Elavarasi, P. Definition of pain and classification of pain disorders. J. Adv. Clin. Res. Insights 2016, 3, 87–90. [Google Scholar] [CrossRef]
- Stanton-Hicks, M.; Jänig, W.; Hassenbusch Sa Haddox, J.; Boas, R.; Wilson, P. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain 1995, 63, 127–133. [Google Scholar] [CrossRef]
- Tadele, A.; Asres, K.; Melaku, D.; Mekonnen, W. In vivo anti-inflammatory and antinociceptive activities of the leaf extracts of Clematis simensis Fresen. Ethiop. Pharm. J. 2009, 27, 33–41. [Google Scholar] [CrossRef]
- Salzano, S. Redox Regulation of Inflammation and Immunity; University of Brighton: Boston, MA, USA, 2013. [Google Scholar]
- World Health Organization. Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Henschke, N.; Kamper, S.J.; Maher, C.G. The epidemiology and economic consequences of pain. Mayo Clin. Proc. 2015, 90, 139–147. [Google Scholar] [CrossRef]
- Calati, R.; Bakhiyi, C.L.; Artero, S.; Ilgen, M.; Courtet, P. The impact of physical pain on suicidal thoughts and behaviors: Meta-analyses. J. Psychiatr. Res. 2015, 71, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Geremew, H.; Shibeshi, W.; Tamiru, W.; Engdawork, E. Experimental evaluation of analgesic and anti-inflammatory activity of 80% methanolic leaf extract of Moringa stenopetala Bak. F. in mice. Ethiop. Pharm. J. 2015, 31, 15–26. [Google Scholar] [CrossRef]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Castellsague, J.; Varas-Lorenzo, C.; Rodríguez, L.A.G. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am. J. Kidney Dis. 2005, 45, 531–539. [Google Scholar] [CrossRef]
- Varışlı, B.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Ayna, A.; Genç, A.; Taysı, S. Chrysin mitigates diclofenac-induced hepatotoxicity by modulating oxidative stress, apoptosis, autophagy and endoplasmic reticulum stress in rats. Mol. Biol. Rep. 2022, 50, 433–442. [Google Scholar] [CrossRef]
- Özbolat, S.N.; Ayna, A. Chrysin Suppresses HT-29 Cell Death Induced by Diclofenac through Apoptosis and Oxidative Damage. Nutr. Cancer 2020, 73, 1419–1428. [Google Scholar] [CrossRef]
- Andres, T. (Ed.) Diversity in tropical pumpkin (Cucurbita moschata): Cultivar Origin and History. In Progress in Cucurbit Genetics and Breeding Research, Proceedings of Cucurbitaceae; Palacký University in Olomouc: Olomouc, Czech Republic, 2004; pp. 113–118. [Google Scholar]
- Men, X.; Choi, S.-I.; Han, X.; Kwon, H.-Y.; Jang, G.-W.; Choi, Y.-E.; Park, S.-M.; Lee, O.-H. Physicochemical, nutritional and functional properties of Cucurbita moschata. Food Sci. Biotechnol. 2021, 30, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Valenzuela, N.; Maróstica-Junior, M.R.; de Jesús Zazueta-Morales, J.; Gallegos-Infante, J.A. Physicochemical, technological properties, and health-benefits of Cucurbita moschata Duchense vs. Cehualca. Food Res. Int. 2011, 44, 2587–2593. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Secondary Metabolites and Other Bioactive Compounds in Cucurbita pepo L. and Cucurbita moschata Pumpkin Cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef]
- Prommaban, A.; Kuanchoom, R.; Seepuan, N.; Chaiyana, W. Evaluation of Fatty Acid Compositions, Antioxidant, and Pharmacological Activities of Pumpkin (Cucurbita moschata) Seed Oil from Aqueous Enzymatic Extraction. Plants 2021, 10, 1582. [Google Scholar] [CrossRef]
- de Oliveira, M.L.M.; Nunes-Pinheiro, D.C.S.; Bezerra, B.M.O.; Leite, L.O.; Tomé, A.R.; Girão, V.C.C. Topical anti-inflammatory potential of pumpkin (Cucurbita pepo L.) seed oil on acute and chronic skin inflammation in mice. Acta Sci. Vet. 2013, 41, 1–9. [Google Scholar]
- Apu, A.S.; Bhuyan, S.H.; Prova, S.S.; Muhit, M.A. Anti-inflammatory activity of medicinal plants native to Bangladesh: A review. J. Appl. Pharm. Sci. 2012, 2, 7–10. [Google Scholar]
- Seraj, S.; Jahan, F.; Chowdhury, A.; Monjur-Ekhuda, M.; Khan, M.; Aporna, S.; Jahan, R.; Samarrai, W.; Islam, F.; Khatun, Z.; et al. Tribal formulations for treatment of pain: A study of the Bede community traditional medicinal practitioners of Porabari Village in Dhaka District, Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 26–34. [Google Scholar] [CrossRef]
- Islam, R.; Kabir, M.F.; Alam, R.; Dhar, R.; Rana, M.N.; Islam, E.; Parvin, M.S.; Hossain, A. Sedative, membrane stability, cytotoxic and antioxidant properties of methanol extract of leaves of Protium serratum Wall. Asian Pac. J. Trop. Dis. 2014, 4, S928–S933. [Google Scholar] [CrossRef]
- Chou, C.T. The Anti-inflammatory Effect of an Extract of Tripterygium wilfordii Hook F on Adjuvant-induced Paw Oedema in Rats and Inflammatory Mediators Release. Phytother. Res. Int. J. Devoted Med. Sci. Res. Plants Plant Prod. 1997, 11, 152–154. [Google Scholar]
- Chakrabarty, N.; Chung, H.J.; Alam, R.; Emon, N.U.; Alam, S.; Kabir, M.F.; Islam, M.M.; Hong, S.T.; Sarkar, T.; Sarker, M.M.; et al. Chemico-Pharmacological Screening of the Methanol Extract of Gynura nepalensis D.C. Deciphered Promising Antioxidant and Hepatoprotective Potentials: Evidenced from in vitro, in vivo, and Computer-Aided Studies. Molecules 2022, 27, 3474. [Google Scholar] [CrossRef] [PubMed]
- Parameswari, P.; Devika, R.; Vijayaraghavan, P. In vitro anti-inflammatory and antimicrobial potential of leaf extract from Artemisia nilagirica (Clarke) Pamp. Saudi J. Biol. Sci. 2019, 26, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Adeneye, A.A.; Alabi, T.E. Analgesic activity of the aqueous seed extract of Hunteria umbellata (K. Schum.) Hallier f. in rodents. Indian J. Exp. Biol. 2011, 49, 698–703. [Google Scholar]
- Bentley, G.; Newton, S.; Starr, J. Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br. J. Pharmacol. 1983, 79, 125–134. [Google Scholar] [CrossRef]
- Ahmed, F.; Hossain, M.H.; Rahman, A.A.; Shahid, I.Z. Antinociceptive and sedative effects of the bark of Cerbera odollam Gaertn. Adv. Tradit. Med. 2006, 6, 344–348. [Google Scholar]
- Zulfiker, A.; Rahman, M.M.; Hossain, M.K.; Hamid, K.; Mazumder, M.; Rana, M.S. In vivo analgesic activity of ethanolic extracts of two medicinal plants-Scoparia dulcis L. and Ficus racemosa Linn. Biol. Med. 2010, 2, 42–48. [Google Scholar]
- Deraedt, R.; Jouquey, S.; Delevallée, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Ghani, Z.D.F.A.; Nor, R.N.S.R.M.; Gopalan, H.K.; Sulaiman, M.R.; Jais, A.M.M.; Somchit, M.N.; Kader, A.A.; Ripin, J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef]
- Sultana, N.; Chung, H.-J.; Emon, N.U.; Alam, S.; Taki, T.I.; Rudra, S.; Tahamina, A.; Alam, R.; Ahmed, F.; Al Mamun, A. Biological Functions of Dillenia pentagyna Roxb. Against Pain, Inflammation, Fever, Diarrhea, and Thrombosis: Evidenced From in vitro, in vivo, and Molecular Docking Study. Front. Nutr. 2022, 9, 911274. [Google Scholar] [CrossRef]
- Sengupta, R.; Sheorey, S.D.; Hinge, M.A. Analgesic and anti-inflammatory plants: An updated review. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 114–119. [Google Scholar]
- Nostro, A.; Cannatelli, M.A.; Crisafi, G.; Musolino, A.D.; Alonzo, V. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 2004, 30, 772–777. [Google Scholar]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K.; Singh, A. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2005, 91, 519–524. [Google Scholar] [CrossRef]
- Mahady, G.B. Medicinal plants for the prevention and treatment of bacterial infections. Curr. Opin. Investig. Drugs 2005, 6, 223–225. [Google Scholar] [CrossRef]
- Ooi, L.S.; Li, Y.; Kam, S.L.; Wang, H.; Wong, E.Y.; Ooi, V.E. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; KVassilatis, D.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Enneb, S.; Drine, S.; Bagues, M.; Triki, T.; Boussora, F.; Guasmi, F.; Nagaz, K.; Ferchichi, A. Phytochemical profiles and nutritional composition of squash (Cucurbita moschata D.) from Tunisia. S. Afr. J. Bot. 2020, 130, 165–171. [Google Scholar] [CrossRef]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; et al. Nutritional Value, Phytochemical Potential, and Therapeutic Benefits of Pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Ghani, A. Medicinal Plants of Bangladesh; The Asiatic Society of Bangladesh: Dhaka, Bangladesh, 2003; pp. 1–16. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, F.; Nazrin, T.T.; Salleh, M.; Hadi, N.N.; Ahmad, N.; Hamzah, A.A.; Yusof, Z.A.; Azman, I.N. Total phenolic content and total flavonoid content in Moringa oleifera seed. Galeri Waris. Sains 2017, 1, 23–25. [Google Scholar] [CrossRef]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Bin Emran, T.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytoscience 2020, 6, 59. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Gutmanas, A.; Alhroub, Y.; Battle, G.M.; Berrisford, J.M.; Bochet, E.; Conroy, M.J.; Dana, J.M.; Montecelo, M.A.F.; van Ginkel, G.; Gore, S.P.; et al. PDBe: Protein data bank in Europe. Nucleic Acids Res. 2014, 42, D285–D291. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Wolf, L.K. PyRx. C&EN 2009, 87, 31. [Google Scholar]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Protein–ligand docking: Current status and future challenges. Proteins Struct. Funct. Bioinform. 2006, 65, 15–26. [Google Scholar] [CrossRef]
| Phytochemical Constituents | Specific Tests | Inference |
|---|---|---|
| Alkaloids | Mayer’s test | + |
| Hager’s test | + | |
| Wagner test | - | |
| Carbohydrates | Molisch’s test | + |
| Benedict’s test | - | |
| Fehling’s test | + | |
| Flavanoids | Alkaline reagent test | + |
| Phenols | Ferric chloride test | + |
| Saponins | Foam test | - |
| Tannins | Gelatin test | + |
| Glycosides | Liebermann’s test | + |
| Extracts | Total Phenolic Content (TPC) mg/g GAE | Total Flavonoids Content (TFC) mg/g GAE |
|---|---|---|
| CM extract | 18.16 ± 0.28 | 10.42 ± 0.10 |
| Groups | Treatment | Dose &Route | No. of Writhing | % Inhibition |
|---|---|---|---|---|
| G-I | 1% Tween 80 (Control) | 10 mL/kg; p.o | 23.5 ± 0.91 | NA |
| G-II | Diclofenac Na (Standard) | 50 mg/kg; p.o | 4.5 ± 0.61 | 80.85 |
| G-III | CM extract | 200 mg/kg; p.o | 17.5 ± 0.84 * | 25.53 |
| G-IV | CM extract | 400 mg/kg; p.o | 10.33 ± 0.56 * | 56.03 |
| Sample | Stephylococcus aureus | Salmonella 88typhi | Proteus vulgaris | E. coli | Klebsiella pneumoniae | Pseudomonas aeruginosa |
|---|---|---|---|---|---|---|
| Zone of Inhibition (mm) | ||||||
| CM Extract (1 mg/mL) | 29 ± 0.58 | 19.33 ± 0.88 | 14 ± 1.15 | 31 ± 1.16 | 16.33 ± 0.33 | 21.67 ± 1.45 |
| Ciprofloxacin (5 µg/mL) | 21.33 ± 0. | 31.67 ± 0.66 | 19.67 ± 1.76 | 25.33 ± 0.33 | 22.33 ± 0.89 | 23.33 ± 0.88 |
| Compounds Name | Absorption | Distribution | Metabolism | Excretion | Toxicity | Drug Likeliness | Bioavailability | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Water Solubility (log mol/L) | Intestinal Absorption (Human) (% Absorbed) | VDss (Human) (log L/kg) | BBB Permeability(log BB) | CYP3A4 Substrate | Total Clearance (log ml/min/kg) | AMES Toxicity | Hepato Toxicity | |||
| Protocatechuic acid | −2.07 | 71.17 | −1.29 | −0.683 | No | 0.551 | No | No | Yes | 0.56 |
| Vanillic acid | −1.84 | 78.15 | −1.74 | −0.38 | No | 0.719 | No | No | Yes | 0.85 |
| Caffeic acid | −2.33 | 69.41 | −1.09 | −0.647 | No | 0.508 | No | No | Yes | 0.56 |
| Syringic acid | −2.22 | 73.07 | −1.44 | −0.191 | No | 0.646 | No | No | Yes | 0.56 |
| Ferulic acid | −2.82 | 93.68 | −1.36 | −0.239 | No | 0.623 | No | No | Yes | 0.85 |
| trans-sinapic acid | −2.87 | 93.06 | −1.11 | −0.247 | No | 0.718 | No | No | Yes | 0.85 |
| Tyrosol | −1.15 | 85.26 | −0.11 | −0.218 | No | 0.283 | No | No | Yes | 0.56 |
| Luteolin | −3.09 | 81.13 | 1.15 | −0.907 | No | 0.495 | No | No | Yes | 0.55 |
| Kaempferol | −3.04 | 74.29 | 1.274 | −0.939 | No | 0.477 | No | No | Yes | 0.56 |
| 3,4-dihydro-2H-pyran-2-yl)methanamine | −0.06 | 96.57 | 0.27 | −0.229 | No | 1.038 | No | No | Yes | 0.56 |
| 5,10-dimethylbenzo[c]acridine- | −5.61 | 99.45 | 0.13 | 0.645 | Yes | 0.851 | Yes | Yes | Yes | 0.56 |
| 2,3-diphenyl-1H-pyrrolo [2,3-b]pyridin-6-amine | −3.41 | 94.96 | −0.463 | 0.464 | Yes | 0.542 | Yes | Yes | Yes | 0.85 |
| Oxacyclotridecan-2-one | −2.759 | 95.30 | 0.142 | 0.408 | No | 1.345 | No | No | Yes | 0.56 |
| (E)-1,3,7-trimethyl-8-(2-nitrostyryl)-3,7-dihydro-1H-purine-2,6-dione | −3.209 | 83.45 | 0.073 | −1.362 | Yes | 0.045 | Yes | Yes | Yes | 0.55 |
| Avenasterol | −6.715 | 94.64 | 0.179 | 0.764 | Yes | 0.613 | No | No | Yes | 0.55 |
| Compounds Name | Docking Score | Non-Bonding Interaction | ||
|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Bond | Van Der Waals | ||
| 3,4-dihydro-2H-pyran-2-yl)methanamine- | −10.1 | ALA A:7, ASN A:18 | LEU A:28, LEU A:54, VAL A:31, PHE A:92 | GLY A:15, ASN A:18, GLN A: 19, THR A:46, SER A:49, GLY A:93, GLY A:94, PHE A:98 |
| 2,3-diphenyl-1H-pyrrolo[2,3-b]pyridin-6-amine | −10 | THR A:21, VAL A:31 | LEU A:5, ALA A:7, LEU A:28, VAL A:31 | ILE A: 14, ASN A:18, ASP A:27, THR A:46, SER A: 49, LEU A:54, PHE A:92, GLY A:93, GLY A:94 |
| Oxacyclotridecan-2-one | −9.1 | ASN A:18, LEU A:20 | ILE A:14, LEU A:28, LYS A:45, ILE A:50 | GLN A:19, SER A:49, GLN A:95, PHE A:98, THR A:121 |
| Luteolin | −8.7 | ALA A:7, ASN A:18, GLN A:95, THR A:21 | ILE A:14, LEU A:20 | VAL A:6, GLY A:15, GLN A:19, ASP A:27, VAL A:31, THR A:46, ILE A:50, LEU A:54, PHE A:92, GLY A:94, PHE A:98, ASP A:120 |
| (E)-1,3,7-trimethyl-8-(2-nitrostyryl)-3,7-dihydro-1H-purine-2,6-dione | −8.6 | LEU A:20, LEU A:28, HIS A:23, VAL A:31, ILE A:50 | LEU A:5, VAL A:6, ALA A:7, GLN A:19, TRP A:22, ASP A:27, THR A:46, SER A:49, PHE A:98 | |
| Kaempferol | −8.4 | SER A:49, ASN A:18 | LEU A:20, THR A:46 | ILE A:14, GLY A:15, GLN A:19, LYS A:45, ILE A:50, PHE A:92, GLY A:93, GLY A:94, GLN A:95, THR A:121 |
| Avenasterol | −7.8 | THR A:46 | LEU A:20, VAL A:31, LYS A:45, ILE A:50, PHE A:92 | LEU A:5, VAL A:6, ALA A:7, ILE A:14, GLY A:15, ASP A:27, LEU A:28, GLY A:93, GLY A:94, GLN S:95, THR A:96, THR A:121 |
| Diclofenac | −8.0 | ALA A:7, ILE A:50, VAL A:31 | LEU A:28, GLY A:93, PHE A:98, GLY A:15 | VAL A:6, PHE A:92, GLY A:94 |
| Celecoxib | −9.5 | ARG A:89, ARG A:482, SER A:322, GLN A:161 | VAL A:492, ALA A:496, GLY A:495 | , LEU A:321, TYR A:354, VAL A:318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.R.; Alam, R.; Chung, H.-J.; Eva, T.A.; Kabir, M.F.; Mamurat, H.; Hong, S.-T.; Hafiz, M.A.; Hossen, S.M.M. In Vivo, In Vitro and In Silico Study of Cucurbita moschata Flower Extract: A Promising Source of Natural Analgesic, Anti-Inflammatory, and Antibacterial Agents. Molecules 2023, 28, 6573. https://doi.org/10.3390/molecules28186573
Hossain MR, Alam R, Chung H-J, Eva TA, Kabir MF, Mamurat H, Hong S-T, Hafiz MA, Hossen SMM. In Vivo, In Vitro and In Silico Study of Cucurbita moschata Flower Extract: A Promising Source of Natural Analgesic, Anti-Inflammatory, and Antibacterial Agents. Molecules. 2023; 28(18):6573. https://doi.org/10.3390/molecules28186573
Chicago/Turabian StyleHossain, Md. Rabiul, Rashedul Alam, Hea-Jong Chung, Taslima Akter Eva, Mohammed Fazlul Kabir, Husnum Mamurat, Seong-Tshool Hong, Md. Al Hafiz, and S. M. Moazzem Hossen. 2023. "In Vivo, In Vitro and In Silico Study of Cucurbita moschata Flower Extract: A Promising Source of Natural Analgesic, Anti-Inflammatory, and Antibacterial Agents" Molecules 28, no. 18: 6573. https://doi.org/10.3390/molecules28186573
APA StyleHossain, M. R., Alam, R., Chung, H.-J., Eva, T. A., Kabir, M. F., Mamurat, H., Hong, S.-T., Hafiz, M. A., & Hossen, S. M. M. (2023). In Vivo, In Vitro and In Silico Study of Cucurbita moschata Flower Extract: A Promising Source of Natural Analgesic, Anti-Inflammatory, and Antibacterial Agents. Molecules, 28(18), 6573. https://doi.org/10.3390/molecules28186573







