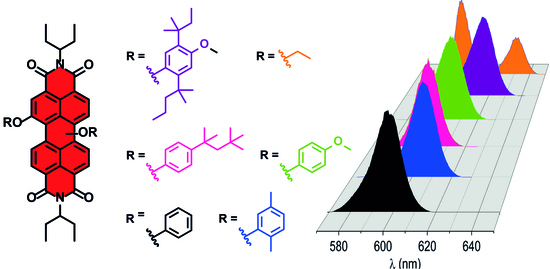
Effect of Different Substitutions at the 1,7-Bay Positions of Perylenediimide Dyes on Their Optical and Laser Properties
Abstract
:1. Introduction
2. Results and Discussion
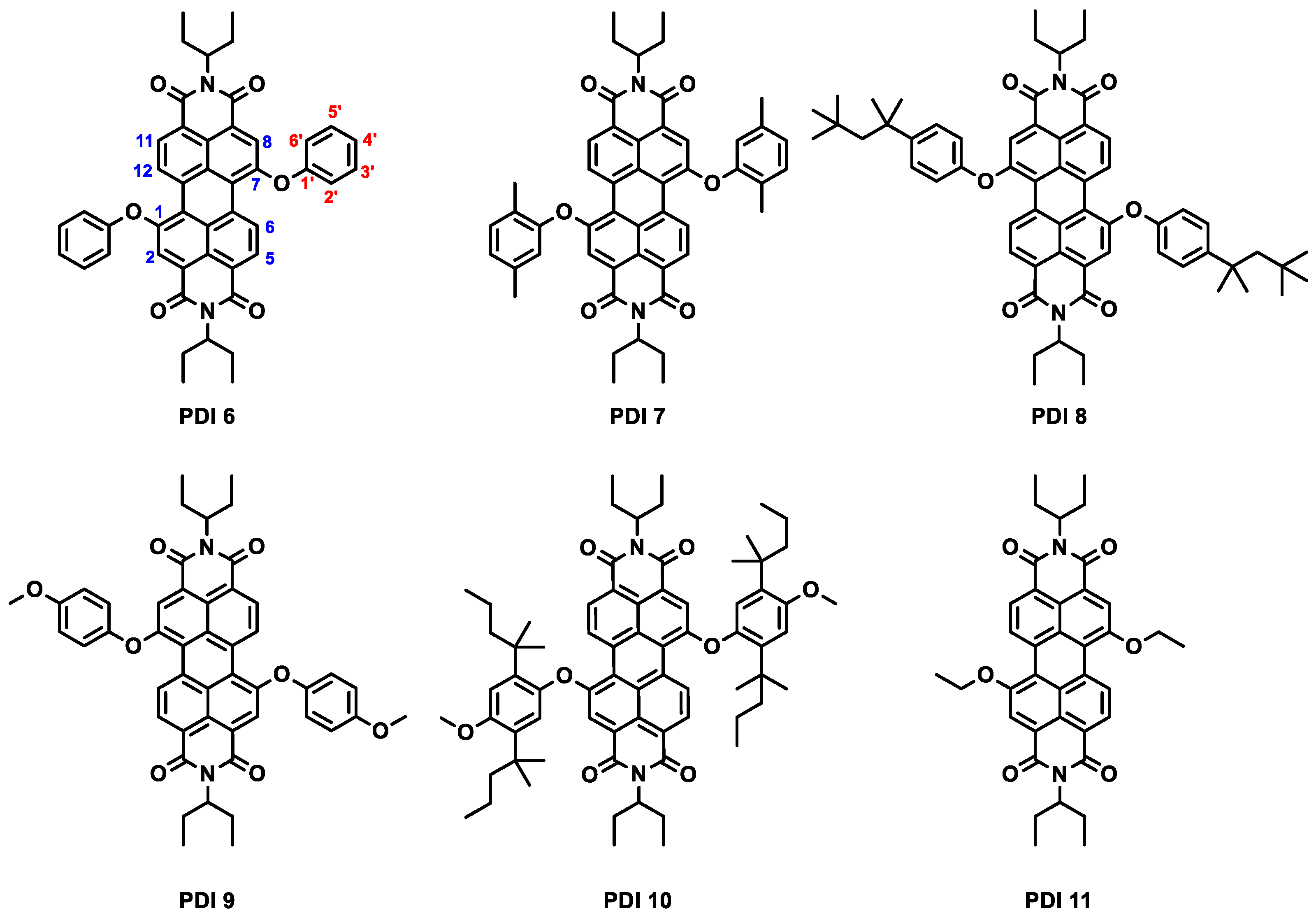
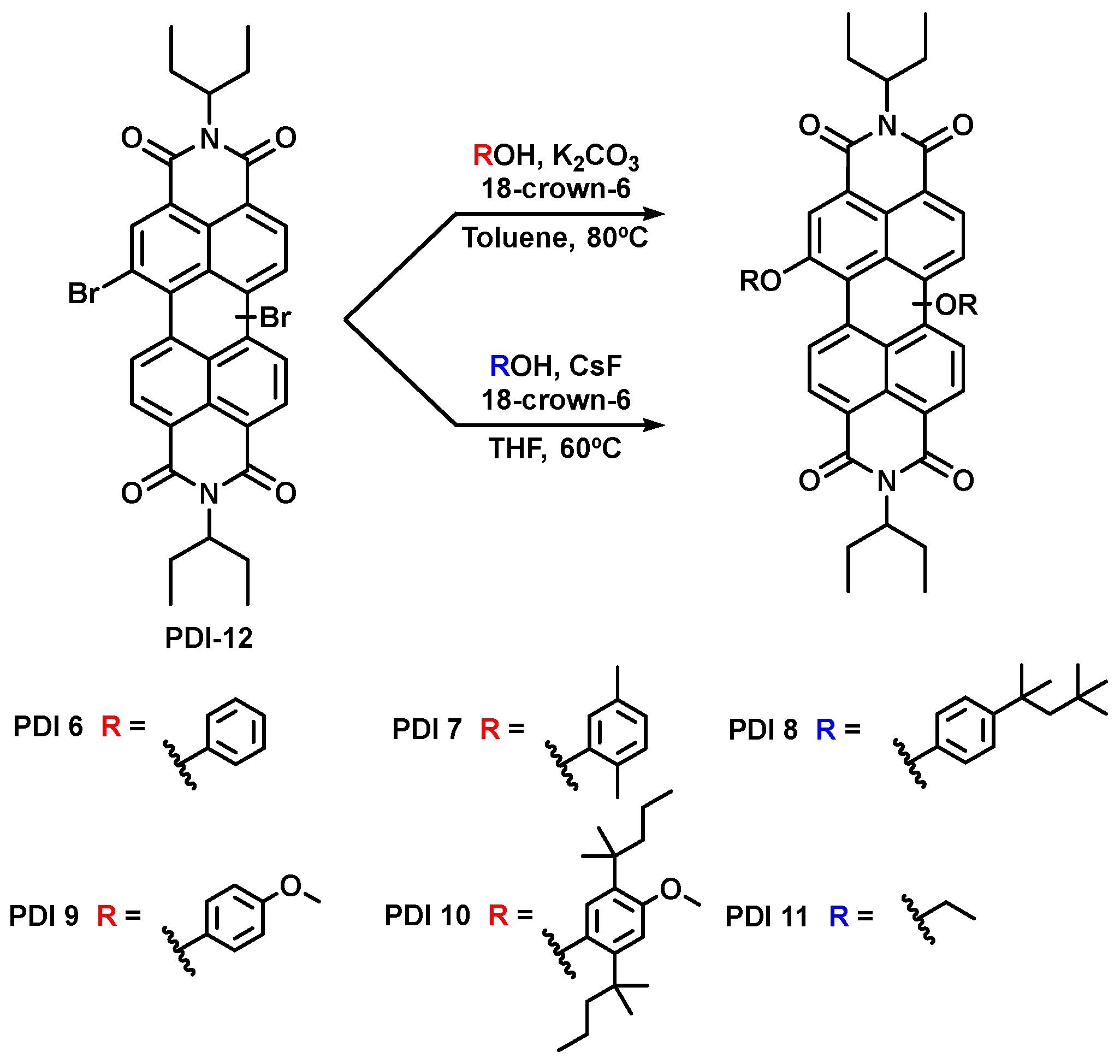
2.1. Preparation of Perylenediimide Derivatives
2.2. Optical Properties of PDIs in Solution
2.3. Optical Properties of PDIs in PS Thin Films
2.3.1. Properties of Highly Diluted Films
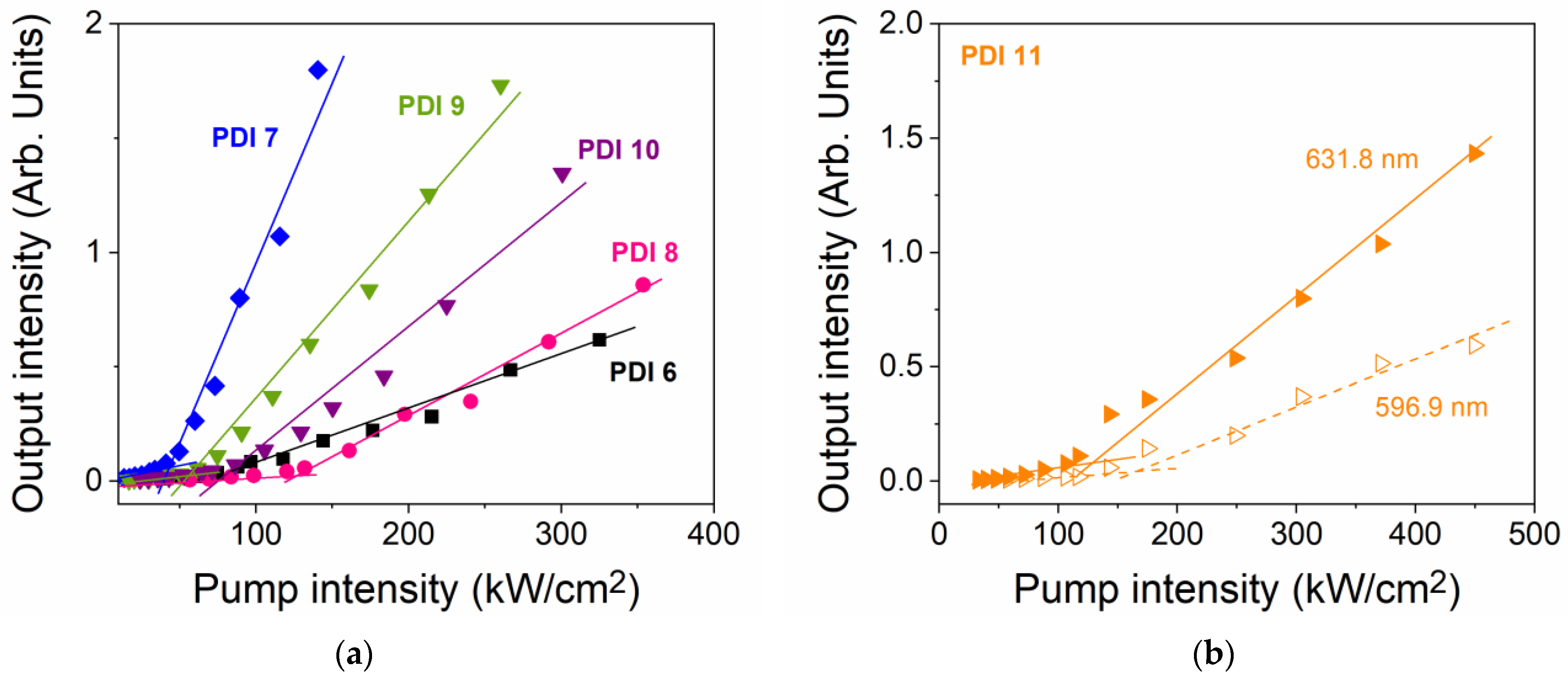
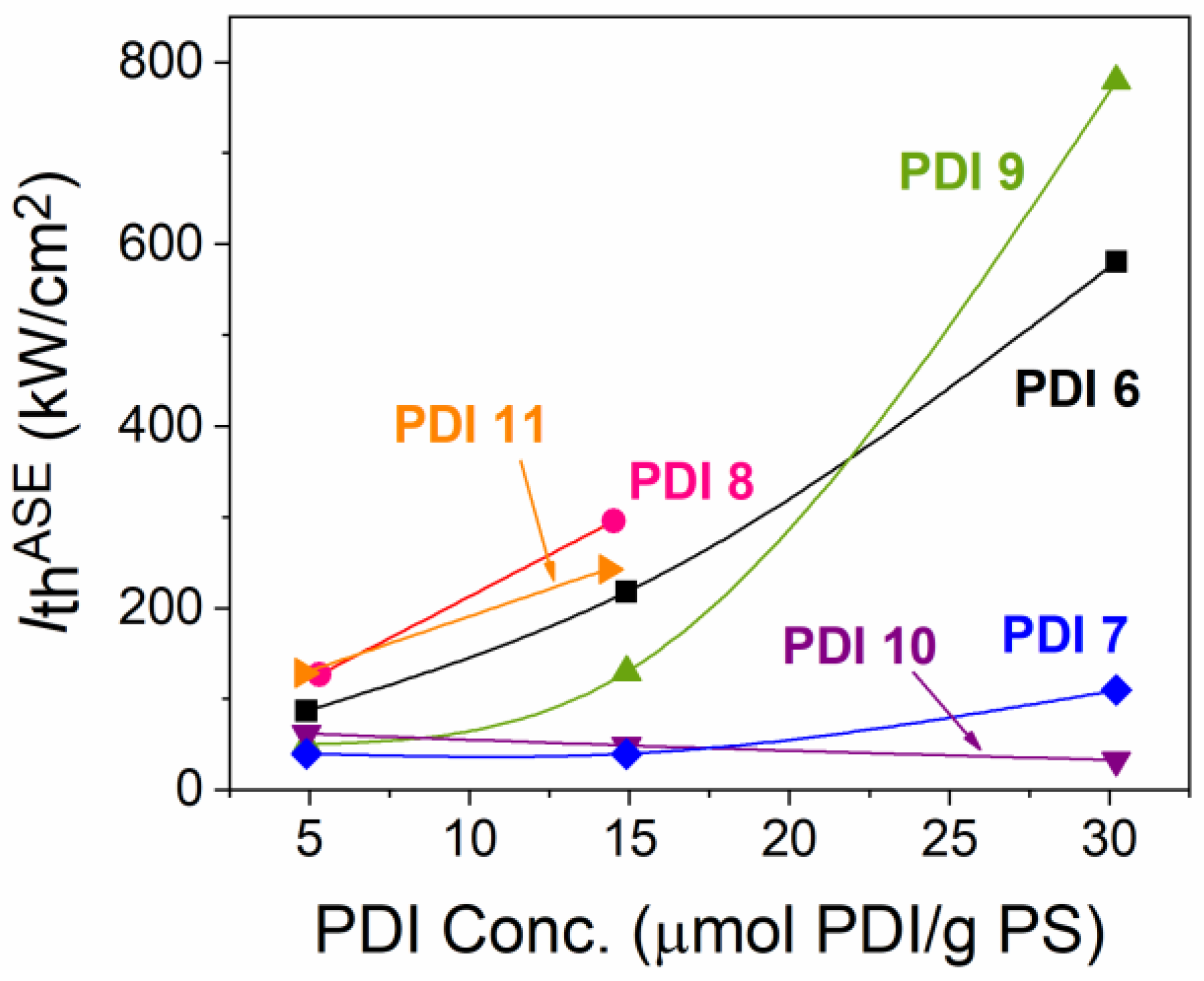
2.3.2. Dye-Concentration Dependence of the Properties
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis of Perylenediimide Derivatives PDI 6–PDI 11
- 1H NMR (300 MHz, CD2Cl4): δ = 9.62 (dd, 2H, J = 8.4 Hz, 2xH-PDI), 8.62 (dd, 2H, J = 8.4 Hz, 2xH-PDI), 8.33 (s, 2H, H-PDI), 7.52 (m, 4H, 2xH-Ph), 7.25 (m, 6H, 2xH-Ph), 4.99 (m, 2H, 2xN-CH(CH2-CH3)2), 2.18 (m, 4H, 2xN-CH(CH2-CH3)2), 1.94 (m, 4H, 2xN-CH(CH2-CH3)2), 0.93 (t, 12H, 2xN-CH(CH2-CH3)2) ppm; 13C NMR (75 MHz, CD2Cl4, 60 °C): δ. 164.11, 156.51, 155.46, 155.28, 155.16, 133.54, 131.31, 130.94, 129.50, 129.08, 128.03, 125.62, 125.56, 125.51, 124.15, 124.04, 122.00, 120.09, 119.96, 58.20, 57.96, 57.73, 25.27, 11.77 ppm; IR (KBr) ν: 2950, 2932, 2868, 1701 (C=O imide), 1654 (C=O imide), 1596, 1485, 1409, 1328, 1258, 1211, 1047, 925, 803, 768, 692 cm−1; UV-vis (chloroform), λmax (log ε): 404 (3.8), 510 (4.4) 542 nm (4.6); MS (MALDI-TOF) m/z: calcd for C46H38N2O6 714.2724 [M]−, found 714.2244 [M]−.
- 1H NMR (300 MHz, CDCl3): δ = 9.70 (d, 2H, J = 8.4 Hz, 2xH-PDI), 8.60 (d, 2H, J = 8.4 Hz, 2xH-PDI), 8.17 (s, 2H, 2xH-PDI), 7.28 (d, 4H, J = 7.4 Hz, 2xH-Ph), 7.05 (d, 4H, J = 7.5 Hz, 2xH-Ph), 6.88 (s, 2H, 2xH-Ph), 5.08–4.92 (m, 2H, 2xN-CH(CH2-CH3)2), 2.33 (s, 6H, 2xPh-CH3), 2.28 (s, 6H, 2xPh-CH3), 2.24–2.12 (m, 4H, 2xN-CH(CH2-CH3)2), 1.98–1.80 (m, 4H, 2xN-CH(CH2-CH3)2), 0.89 (t, 12H, J = 7.5 Hz, 2xN-CH(CH2-CH3)2) ppm; 13C NMR (75 MHz, CD2Cl4, 60 ºC): δ 166.9, 166.6, 159.8, 158.7, 155.4, 155.3, 141.1, 136.6, 136.4, 134.9, 134.1, 132.8, 132.3, 131.9, 131.6, 131.1, 130.5, 129.8, 129.7, 129.6, 129.5, 127.8, 126.9, 126.8, 125.5, 125.2, 124.7, 123.9, 123.8, 123.3, 60.6, 32.5, 27.9, 23.9, 18.7, 14.3 ppm; IR (KBr) ν: 2958, 2917, 2872, 1699 (C=O imide), 1650 (C=O imide), 1593, 1503, 1405, 1327, 1262, 1200, 1111, 1053, 804, 751 cm−1; UV-vis (chloroform), λmax (log ε): 398 (3.89), 508 (4.53), 546 (4.71) nm; MS (MALDI-TOF) m/z: calcd for C50H46N2O6 770.3356 [M]−, found 770.3111 [M]−.
- 1H NMR (300 MHz, CDCl3): δ = 9.56 (dd, 2H, J = 8.4 Hz, 2xH-PDI), 8.59 (dd, 2H, J = 8.4 Hz, 2xH-PDI), 8.33 (s, 1H, H-PDI), 8.25 (s, 1H, H-PDI),7.46 (d, 4H, J = 8.9 Hz, 2xH-Ph), 7.09 (d, 4H, J = 8.8 Hz, 2xH-Ph), 5.00 (m, 2H, 2xN- CH(CH2-CH3)2), 2.18 (m, 4H, 2xN-CH(CH2-CH3)2), 1.90 (m, 4H, 2xN-CH(CH2-CH3)2), 1,76 (br, 4H, 2xPh-C(CH3)2-CH2-C(CH3)3), 1.42 (br, 12H, 2xPh-C(CH3)2-CH2-C(CH3)3), 0.89 (m, 12H, 2xN-CH(CH2-CH3)2), 0.79 (m, 18H, 2xPh-C(CH3)2-CH2-C(CH3)3) ppm; 13C NMR (75 MHz, CDCl3): δ 164.25, 163.77, 156.46, 155.37, 152.43, 152.32, 147.34, 147.22, 133.32, 133.24, 130.12, 129.21, 128.74, 128.19, 128.17, 127.66, 125.20, 123.87, 123.63, 122.34, 122.16, 118.91, 118.77, 57.77, 57.60, 57.42, 57.14, 38.44, 38.43, 38.42, 32.42, 32.41, 32.41, 31.81, 31.44, 29.67, 24.95, 22.66, 14.09, 11.32 ppm;. IR (KBr) ν: 2961, 2880, 1701 (C=O imide), 1666 (C=O imide), 1596, 1497, 1409, 1328, 1258, 1199, 1170, 1053, 1007, 925, 803, 756 cm−1; UV-vis (chloroform) λmax (log ε): 407 (4.0), 511 (4.5), 545 (4.7) nm; MS (MALDI-TOF) m/z: calcd for C62H70N2O6 938.5228 [M]−, found 938.5149 [M]−.
- 1H NMR (300 MHz, CDCl3): δ = 9.64 (d, 2H, J = 8.4 Hz, 2xH-PDI), 8.60 (d, 2H, J = 8.3 Hz, 2xH-PDI), 8.29 (s, 2H, 2xH-PDI), 7.14 (d, 4H, J = 9.1 Hz, 4xH-Ph), 7.00 (d, 4H, J = 9.1 Hz, 4xH-Ph), 5.07–4.97 (m, 2H, 2xN-CH(CH2-CH3)2), 3.86 (s, 6H, 2xO-CH3), 2.26–2.14 (m, 4H, J = 7.3 Hz, 2xN-CH(CH2-CH3)2), 1.96–1.82 (m, 4H, J = 7.3 Hz, 2xN-CH(CH2-CH3)2), 0.88 (t, 12H, J = 7.4 Hz, 2xN-CH(CH2-CH3)2) ppm; 13C NMR (75 MHz, CDCl3): δ = 157.2, 157.1, 156.2, 148.2, 133.4, 129.3, 128.8, 125.0, 123.1, 121.3, 121.2, 115.7, 57.6, 55.7, 29.7, 25.0, 11.3 ppm; IR (KBr) ν: 2966, 2913, 2876, 1691 (C=O imide), 1654 (C=O imide), 1593, 1499, 1405, 1323, 1249, 1200, 1033, 923, 806, 767 cm−1; UV-vis (chloroform) λmax (log ε): 408 (3.97), 514 (4.56), 550 (4.73) nm; MS (MALDI-TOF) m/z: calcd for C48H42N2O6 774.2941 [M]−, found 774.2883 [M]−.
- 1H NMR (300 MHz, CDCl3): δ = 9.77 (d, 2H, J = 8.8 Hz, 2xH-PDI), 8.57 (d, 2H, J = 8.4 Hz, 2xH-PDI), 8.15 (s, 2H, 2xH-PDI), 6.93 (s, 2H, 2xH-Ph), 6.77 (s, 2H, 2xH-Ph), 5.04–4.94 (m, 2H, 2xN-CH(CH2-CH3)2), 3.91 (s, 6H, 2xO-CH3), 2.34–2.10 (m, 4H, 2xN-CH(CH2-CH3)2), 1.94–1.79 (m, 4H, 2xN-CH(CH2-CH3)2), 1.68–1.11 (m, 40H, 2xPh-(C(CH3)2-(CH2)2-CH3)2), 0.98–0.85 (m, 24H, 2xN-CH(CH2-CH3)2.+ 2xPh-(C(CH3)2-(CH2)2-CH3)2) ppm; 13C NMR (75 MHz, CD2Cl4): δ = 164.1, 163.2, 158.0, 156.7, 155.5, 155.4, 146.2, 146.0, 138.3, 138.2, 136.8, 134.1, 133.7, 131.0, 129.5, 129.3, 127.9, 127.8, 127.7, 126.6, 124.7, 123.8, 123.6, 123.1, 122.8, 122.3, 122.0, 121.3, 121.1, 112.6, 58.2, 57.5, 55.6, 45.2, 43.4, 38.0, 37.7, 28.8, 28.6, 28.1, 28.0, 27.9, 25.0, 24.9, 18.4, 18.3, 18.2, 14.7, 14.6, 11.3, 11.1, 11.0 ppm; IR (KBr) ν: 2945, 2872, 1699 (C=O imide), 1662 (C=O imide), 1593, 1499, 1401, 1319, 1266, 1176, 1115, 1053, 812, 743 cm−1; UV-vis (chloroform) λmax (log ε): 523 (4.45), 563 (4.62) nm; MS (MALDI-TOF) m/z: calcd for C72H90N2O8 1110.6697 [M]−; found 1110.6758 [M]−.
- 1H NMR (300 MHz, CDCl3) δ = 9.62 (d, 2H, J = 8.4 Hz, 2xH-PDI), 8.68–8.55 (dd, 2H, J = 8.3 Hz, 2xH-PDI), 8.48 (s, 1H, H-PDI), 8.37 (s, 1H, H-PDI), 5.07 (m, 2H, 2xN-CH(CH2-CH3)2), 4.58 (m, 4H, 2xO-CH2-CH3), 2.28 (m, 4H, J = 7.4 Hz, 2xN-CH(CH2-CH3)2), 1.94 (m, 4H, J = 7.4 Hz, 2xN-CH(CH2-CH3)2), 1.71 (t, 6H, J = 6.9 Hz, 2xO-CH2-CH3), 0.93 (t, 12H, 2xN-CH(CH2-CH3)2) ppm; 13C NMR (75 MHz, CDCl3): δ = 157.82, 156.66, 134.29, 133.83, 130.79, 129.30, 128.59, 127.98, 127.22, 123.88, 123.59, 121.50, 120.75, 117.70, 66.11, 57.60, 29.68, 25.05, 15.08, 11.32 ppm;. IR (KBr) ν: 2967, 2938, 2874, 1701 (C=O imide), 1660 (C=O imide), 1590, 1526, 1415, 1328, 1269, 1205, 1065, 1013, 808, 750 cm−1; UV-vis (chloroform) λmax (log ε): 402 (3.8), 531 (4.5), 568 (4.7) nm; MS (MALDI-TOF) m/z: calcd for C38H38N2O6 618.2724 [M]−, found 618.2609 [M]−.
3.3. Thin Film Fabrication
3.4. Optical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anni, M.; Lattante, S. Organic Lasers: Fundamentals, Developments, and Applications; Pan Stanford Publishing: Singapore, 2018. [Google Scholar]
- Grivas, C.; Pollnau, M. Organic solid-state integrated amplifiers and lasers. Laser Photonics Rev. 2012, 6, 419–462. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- Chenais, S.; Forget, S. Recent advances in solid-state organic lasers. Polym. Int. 2012, 61, 390–406. [Google Scholar] [CrossRef]
- Baldo, M.A.; Holmes, R.J.; Forrest, S.R. Prospects for electrically pumped organic lasers. Phys. Rev. B 2002, 66, 035321. [Google Scholar] [CrossRef]
- Clark, J.; Lanzani, G. Organic photonics for communications. Nat. Photonics 2010, 4, 438–446. [Google Scholar] [CrossRef]
- Vannahme, C.; Klinkhammer, S.; Lemmer, U.; Mappes, T. Plastic lab-on-a-chip for fluorescence excitation with integrated organic semiconductor lasers. Opt. Express 2011, 19, 8179–8186. [Google Scholar] [CrossRef]
- Heydari, E.; Buller, J.; Wischerchoff, E.; Laschewsky, A.; Döring, S.; Stumpe, J. Label-Free Biosensor Based on an All-Polymer DFB Laser. Adv. Opt. Mater. 2014, 2, 137–141. [Google Scholar] [CrossRef]
- Wang, Y.; Morawska, P.O.; Kanibolotsky, A.L.; Skabara, P.J.; Turnbull, G.A.; Samuel, I.D.W. LED pumped polymer laser sensor for explosives. Laser Photonics Rev. 2013, 7, L71–L76. [Google Scholar] [CrossRef]
- Morales-Vidal, M.; Boj, P.G.; Quintana, J.A.; Villalvilla, J.M.; Retolaza, A.; Merino, S.; Díaz-García, M.A. Distributed feedback lasers based on perylenediimide dyes for label-free refractive index sensing. Sens. Actuators B 2015, 220, 1368–1375. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Q.; Zou, J.-H.; Zhu, X.-H.; Li, A.-Y.; Li, J.-W.; Wu, S.; Peng, J.; Cao, Y.; Xia, R.; et al. Electroluminescence and Laser Emission of Soluble Pure Red Fluorescent Molecular Glasses Based on Dithienylbenzothiadiazole. Adv. Funct. Mater. 2009, 19, 2978–2986. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Xu, T.; Liu, W.; Wang, R.; Yu, H.; Jiang, X.-F.; Ma, Y. Low Optical Loss Amplified Spontaneous Emission and Lasing in a Solution-Processed Organic Semiconductor. Adv. Opt. Mater. 2019, 7, 1900701. [Google Scholar] [CrossRef]
- Taghipour, N.; Tanriover, I.; Dalmases, M.; Whitworth, G.L.; Graham, C.; Saha, A.; Özdemir, O.; Kundu, B.; Pruneri, V.; Aydin, K.; et al. Ultra-Thin Infrared Optical Gain Medium and Optically-Pumped Stimulated Emission in PbS Colloidal Quantum Dot LEDs. Adv. Funct. Mater. 2022, 32, 2200832. [Google Scholar] [CrossRef]
- Goldberg, I.; Annavarapu, N.; Leitner, S.; Elkhouly, K.; Han, F.; Verellen, N.; Kuna, T.; Qiu, W.; Rolin, C.; Genoe, J.; et al. Multimode Lasing in All-Solution-Processed UV-Nanoimprinted Distributed Feedback MAPbI3 Perovskite Waveguides. ACS Photonics 2023, 10, 1591–1600. [Google Scholar] [CrossRef]
- Anni, M.; Lattante, S. Amplified Spontaneous Emission Optimization in Regioregular Poly(3-hexylthiophene) (rrP3HT):poly(9,9-dioctylfluorene-co-benzothiadiazole) (F8BT) Thin Films through Control of the Morphology. J. Phys. Chem. C 2015, 119, 21620–21625. [Google Scholar] [CrossRef]
- Muñoz-Mármol, R.; Zink-Lorre, N.; Villalvilla, J.M.; Boj, P.G.; Quintana, J.A.; Vázquez, C.; Anderson, A.; Gordon, M.J.; Sastre-Santos, Á.; Fernández-Lázaro, F.; et al. Influence of Blending Ratio and Polymer Matrix on the Lasing Properties of Perylenediimide Dyes. J. Phys. Chem. C 2018, 122, 24896–24906. [Google Scholar] [CrossRef]
- Mhibik, O.; Forget, S.; Ott, D.; Venus, G.; Divliansky, I.; Glebov, L.; Chénais, S. An ultra-narrow linewidth solution-processed organic laser. Light Sci. Appl. 2016, 5, e16026. [Google Scholar] [CrossRef]
- Ramírez, M.G.; Pla, S.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Díaz-García, M.A.; Fernández-Lázaro, F.; Sastre-Santos, Á. 1,7-Bay-Substituted Perylenediimide Derivative with Outstanding Laser Performance. Adv. Opt. Mater. 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Adamow, A.; Sznitko, L.; Chrzumnicka, E.; Stachera, J.; Szukalski, A.; Martynski, T.; Mysliwiec, J. The ultra-photostable and electrically modulated Stimulated Emission in perylene-based dye doped liquid crystal. Sci. Rep. 2019, 9, 2143. [Google Scholar] [CrossRef]
- Sabatini, R.P.; Zhang, B.; Gupta, A.; Leoni, J.; Wong, W.W.H.; Lakhwani, G. Molecularly isolated perylene diimides enable both strong exciton–photon coupling and high photoluminescence quantum yield. J. Mater. Chem. C 2019, 7, 2954–2960. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Navarro-Fuster, V.; Calzado, E.M.; Boj, P.G.; Quintana, J.A.; Villalvilla, J.M.; Díaz-García, M.A.; Trabadelo, V.; Juarros, A.; Retolaza, A.; Merino, S. Highly photostable organic distributed feedback laser emitting at 573 nm. Appl. Phys. Lett. 2010, 97, 171104. [Google Scholar] [CrossRef]
- Ramírez, M.G.; Morales-Vidal, M.; Navarro-Fuster, V.; Boj, P.G.; Quintana, J.A.; Villalvilla, J.M.; Retolaza, A.; Merino, S.; Díaz-García, M.A. Improved performance of perylenediimide-based lasers. J. Mater. Chem. C 2013, 1, 1182–1191. [Google Scholar] [CrossRef]
- Calzado, E.M.; Villalvilla, J.M.; Boj, P.G.; Quintana, J.A.; Gómez, R.; Segura, J.L.; Díaz-García, M.A. Effect of Structural Modifications in the Spectral and Laser Properties of Perylenediimide Derivatives. J. Phys. Chem. C 2007, 111, 13595–13605. [Google Scholar] [CrossRef]
- Miasojedovas, A.; Kazlauskas, K.; Armonaite, G.; Sivamurugan, V.; Valiyaveettil, S.; Grazulevicius, J.V.; Juršėnas, S. Concentration effects on emission of bay-substituted perylene diimide derivatives in a polymer matrix. Dye. Pigment. 2012, 92, 1285–1291. [Google Scholar] [CrossRef]
- Cerdán, L.; Costela, A.; Duran-Sampedro, G.; García-Moreno, I.; Calle, M.; Juan-y-Seva, M.; de Abajo, J.; Turnbull, G.A. New perylene-doped polymeric thin films for efficient and long-lasting lasers. J. Mater. Chem. 2012, 22, 8938–8947. [Google Scholar] [CrossRef]
- Muñoz-Mármol, R.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Zink-Lorre, N.; Sastre-Santos, Á.; Aragó, J.; Ortí, E.; Baronas, P.; Litvinas, D.; et al. Effect of Substituents at Imide Positions on the Laser Performance of 1,7-Bay-Substituted Perylenediimide Dyes. J. Phys. Chem. C 2021, 125, 12277. [Google Scholar] [CrossRef]
- Ramalingam, A.; Palanisamy, P.K.; Masilamani, V.; Sivaram, B.M. Dual amplified spontaneous emission from7-amino-4-methyl coumarin dye. J. Photochem. Photobiol. A Chem. 1989, 49, 89–96. [Google Scholar] [CrossRef]
- Vijila, C.; Ramalingam, A.; Palanisamy, P.K.; Masilamani, V. Role of dipole moment of solvents in formation and stabilization of the TICT states in Coumarin 445 under nitrogen laser excitation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 491–497. [Google Scholar] [CrossRef]
- Ibnaouf, K.H. Amplified spontaneous emission spectra of poly(9,9-dioctylfluorenyl-2,7-diyl) under pulsed laser excitation. Synth. Met. 2015, 209, 534–543. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.H.; Alsalhi, M.S.; Masilamani, V. Laser from the dimer state of a conjugated polymer(PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Liu, B.; Lin, J.; Liu, F.; Yu, M.; Zhang, X.; Xia, R.; Yang, T.; Fang, Y.; Xie, L.; Huang, W. A highly crystalline and wide-bandgap polydiarylfluorene with β-phase conformation toward stable electroluminescence and dual amplified spontaneous emission. ACS Appl. Mater. Interfaces 2016, 8, 21648–21655. [Google Scholar] [CrossRef] [PubMed]
- Anni, M. Dual band amplified spontaneous emission in the blue in poly(9,9-dioctylfluorene) thin films with phase separated glassy and β-phases. Opt. Mater. 2019, 96, 109313. [Google Scholar] [CrossRef]
- Ibnaouf, K.H. Dimer and excimer states of a conjugated polymer poly(9,9-di-n-octylfluorenyl-2,7-diyl) in thin films. Opt. Quatum Electron. 2017, 49, 405. [Google Scholar] [CrossRef]
- Baronas, P.; Kreiza, G.; Adomenas, P.; Adomeniene, O.; Kazlauskas, K.; Ribierre, J.C.; Adachi, C.; Juršenas, S. Low-threshold light amplification in bifluorene single crystals: Role of the trap states. ACS Appl. Mater. Interfaces 2018, 10, 2768–2775. [Google Scholar] [CrossRef]
- Muñoz-Mármol, R.; Bonal, V.; Paternò, G.M.; Ross, A.M.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Scotognella, F.; D’Andrea, C.; Sardar, S.; et al. Dual Amplified Spontaneous Emission and Lasing from Nanographene Films. Nanomaterials 2020, 10, 1525. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective Bromination of Perylene Diimides under Mild Conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic Carboxylic Imide Structures as Structure Elements of High Stability. Novel Developments in Perylene Dye Chemistry. Heterocycles 1995, 40, 477–500. [Google Scholar] [CrossRef]
- Langhals, H. Control of the Interactions in Multichromophores: Novel Concepts. Perylene Bis-imides as Components for Larger Functional Units. Helv. Chim. Acta 2005, 88, 1309–1343. [Google Scholar] [CrossRef]
- Würthner, F. Bay-substituted perylene bisimides: Twisted fluorophores for supramolecular chemistry. Pure Appl. Chem. 2006, 78, 2341–2349. [Google Scholar] [CrossRef]
- Hermann, A.; Müllen, K. From Industrial Colorants to Single Photon Sources and Biolabels: The Fascination and Function of Rylene Dyes. Chem. Lett. 2006, 35, 978–985. [Google Scholar] [CrossRef]
- Zink-Lorre, N.; Font-Sanchis, E.; Sastre-Santos, Á.; Fernández-Lázaro, F. Fluoride-mediated alkoxylation and alkylthio-functionalization of halogenated perylenediimides. Org. Chem. Front. 2017, 4, 2016–2021. [Google Scholar] [CrossRef]
- Czajkowski, M.; Duda, Ł.; Czarnocki, S.J.; Szukalska, A.B.; Guzik, M.; Myśliwiec, J.; Skoren’ski, M.; Potaniec, B.; Cybińska, J. Novel highly luminescent diketofurofuran dye in liquid crystal matrices for thermal sensors and light amplification. J. Mater. Chem. C 2023, 11, 4426–4438. [Google Scholar] [CrossRef]
- Quintana, J.A.; Villalvilla, J.M.; Morales-Vidal, M.; Boj, P.G.; Zhu, X.; Ruangsupapichat, N.; Tsuji, H.; Nakamura, E.; Díaz-García, M.A. An efficient and color-tunable solution-processed organic thin-film laser with a polymeric top-layer resonator. Adv. Opt. Mater. 2017, 5, 1700238. [Google Scholar] [CrossRef]
- Morales-Vidal, M.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Yan, Q.; Lin, N.T.; Zhu, X.; Ruangsupapichat, N.; Casado, J.; Tsuji, H.; et al. Carbon-bridged oligo(p-phenylenevinylene)s for photostable and broadly tunable, solution-processable thin film organic lasers. Nat. Commun. 2015, 6, 8458. [Google Scholar] [CrossRef] [PubMed]


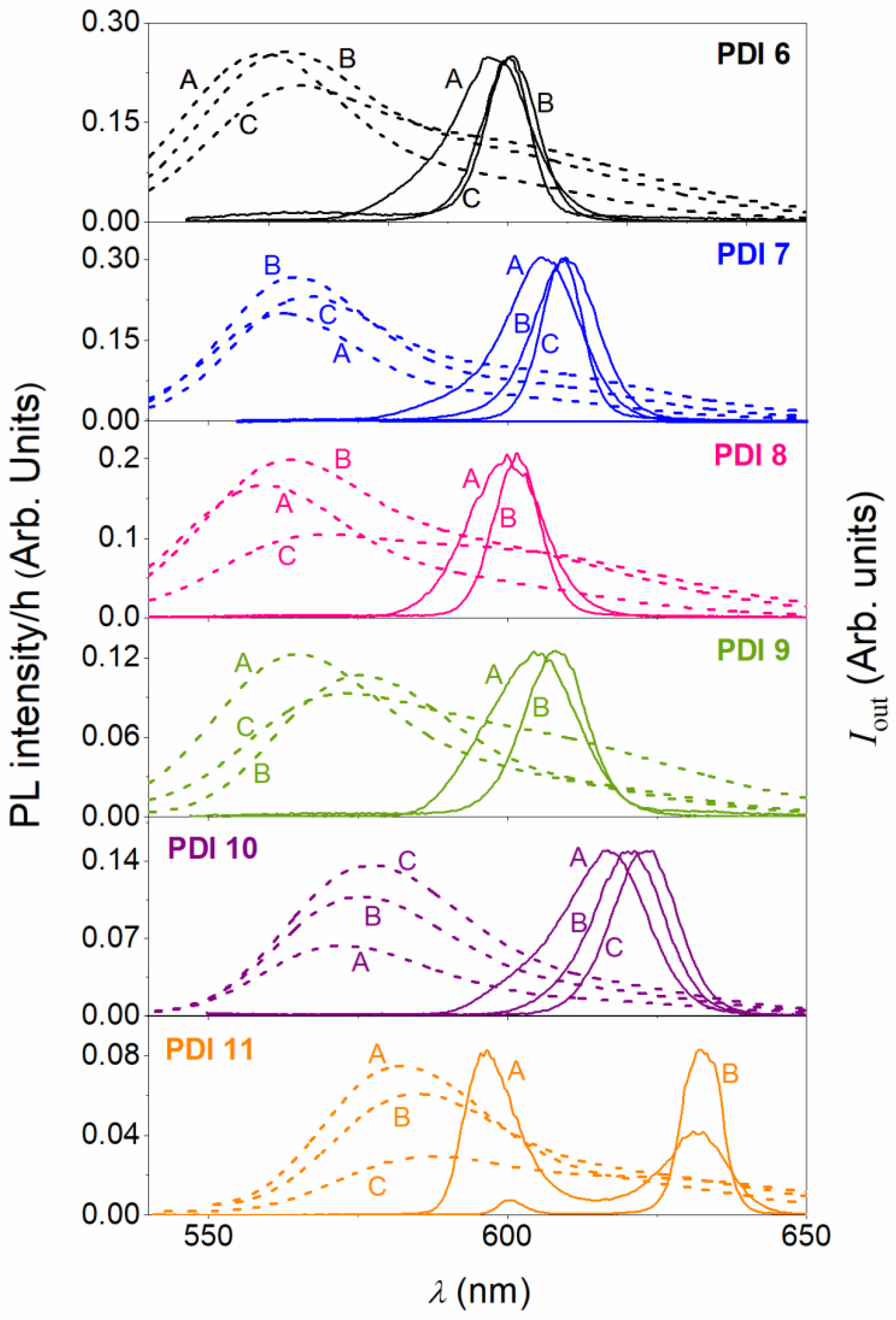
| Compound | (1,6)/(1,7) a | λABS maxb (nm) | λPLb (nm) | ϕPLc |
|---|---|---|---|---|
| PDI 6 | 22/78 | 542 | 568 | 0.45 |
| PDI 7 | 25/75 | 546 | 566 | 0.43 |
| PDI 8 | 27/73 | 545 | 573 | 0.3 |
| PDI 9 | 11/89 | 550 | 577 | 0.48 |
| PDI 10 | 24/76 | 563 | 596 | 0.14 |
| PDI 11 | 23/77 | 568 | 591 | 0.2 |
| Compound | PDI Conc. (μmol PDI/g PS) a | λABSb (nm) | λPL 0-0c (nm) | α (λPump) d (cm−1) | λASE e (nm) | FHWMASE f (nm) | IthASE g (kW/cm2) |
|---|---|---|---|---|---|---|---|
| PDI 6 | 5.3 | 501, 538 | 560 | 643 | 596.7 | 16.4 | 87 |
| 14.5 | 504, 538 | 563 | 1804 | 599.1 | 11.0 | 218 | |
| 29.5 | 505, 538 | 565 | 2620 | 598.6 | 8.2 | 581 | |
| PDI 7 | 4.9 | 510, 547 | 562 | 436 | 606.3 | 16.2 | 40 |
| 14.9 | 510, 547 | 564 | 1069 | 609.7 | 13.9 | 40 | |
| 30.2 | 511, 547 | 567 | 2205 | 609.0 | 9.0 | 110 | |
| PDI 8 | 4.9 | 507, 543 | 560 | 372 | 599.8 | 14.2 | 127 |
| 14.7 | 509, 543 | 564 | 1216 | 601.4 | 9.1 | 296 | |
| 30.7 | 510, 543 | 569 | 2306 | ||||
| PDI 9 | 4.9 | 510, 547 | 565 | 429 | 604.7 | 19.5 | 50 |
| 14.9 | 512, 547 | 575 | 1115 | 608.4 | 13.1 | 130 | |
| 30.2 | 512, 547 | 573 | 2191 | 8.8 | 780 | ||
| PDI 10 | 4.9 | 518, 557 | 572 | 329 | 616.8 | 18.9 | 62 |
| 14.9 | 518, 557 | 576 | 1123 | 620.4 | 15.5 | 49 | |
| 30.2 | 518, 557 | 578 | 1681 | 623.3 | 14.5 | 33 | |
| PDI 11 | 4.8 | 527, 569 | 582 | 305 | 596.9/631.8 | 10.6/13.8 | 167/129 |
| 14.3 | 528, 569 | 585 | 993 | 632.7 | 7.7 | 243 | |
| 31.1 | 529, 569 | 587 | 2058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zink-Lorre, N.; Ramírez, M.G.; Pla, S.; Boj, P.G.; Quintana, J.A.; Villalvilla, J.M.; Sastre-Santos, Á.; Fernández-Lázaro, F.; Díaz-García, M.A. Effect of Different Substitutions at the 1,7-Bay Positions of Perylenediimide Dyes on Their Optical and Laser Properties. Molecules 2023, 28, 6776. https://doi.org/10.3390/molecules28196776
Zink-Lorre N, Ramírez MG, Pla S, Boj PG, Quintana JA, Villalvilla JM, Sastre-Santos Á, Fernández-Lázaro F, Díaz-García MA. Effect of Different Substitutions at the 1,7-Bay Positions of Perylenediimide Dyes on Their Optical and Laser Properties. Molecules. 2023; 28(19):6776. https://doi.org/10.3390/molecules28196776
Chicago/Turabian StyleZink-Lorre, Nathalie, Manuel G. Ramírez, Sara Pla, Pedro G. Boj, José A. Quintana, José M. Villalvilla, Ángela Sastre-Santos, Fernando Fernández-Lázaro, and María A. Díaz-García. 2023. "Effect of Different Substitutions at the 1,7-Bay Positions of Perylenediimide Dyes on Their Optical and Laser Properties" Molecules 28, no. 19: 6776. https://doi.org/10.3390/molecules28196776
APA StyleZink-Lorre, N., Ramírez, M. G., Pla, S., Boj, P. G., Quintana, J. A., Villalvilla, J. M., Sastre-Santos, Á., Fernández-Lázaro, F., & Díaz-García, M. A. (2023). Effect of Different Substitutions at the 1,7-Bay Positions of Perylenediimide Dyes on Their Optical and Laser Properties. Molecules, 28(19), 6776. https://doi.org/10.3390/molecules28196776