The Double-Cross of Benzotriazole-Based Polymers as Donors and Acceptors in Non-Fullerene Organic Solar Cells
Abstract
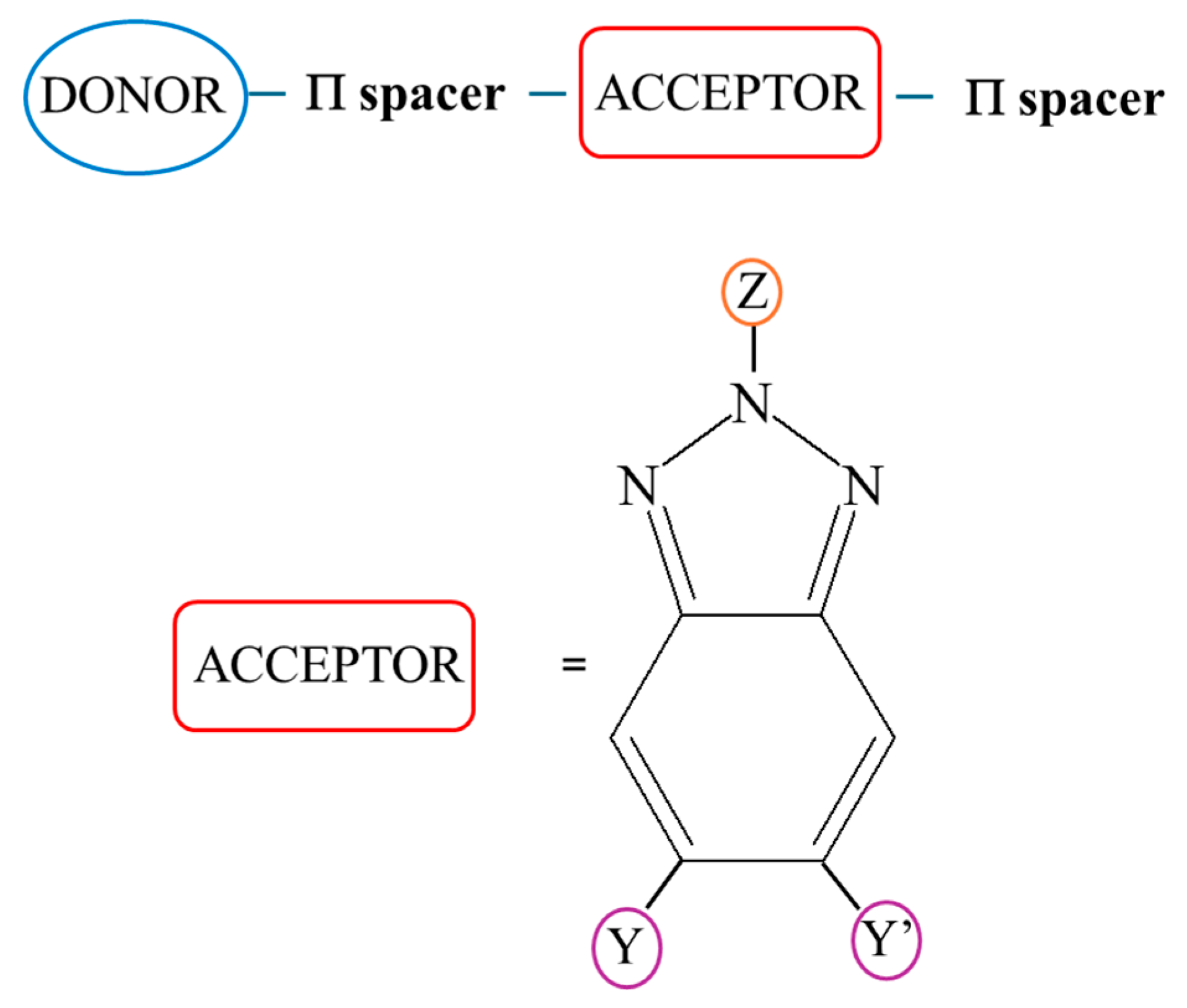
:1. Introduction
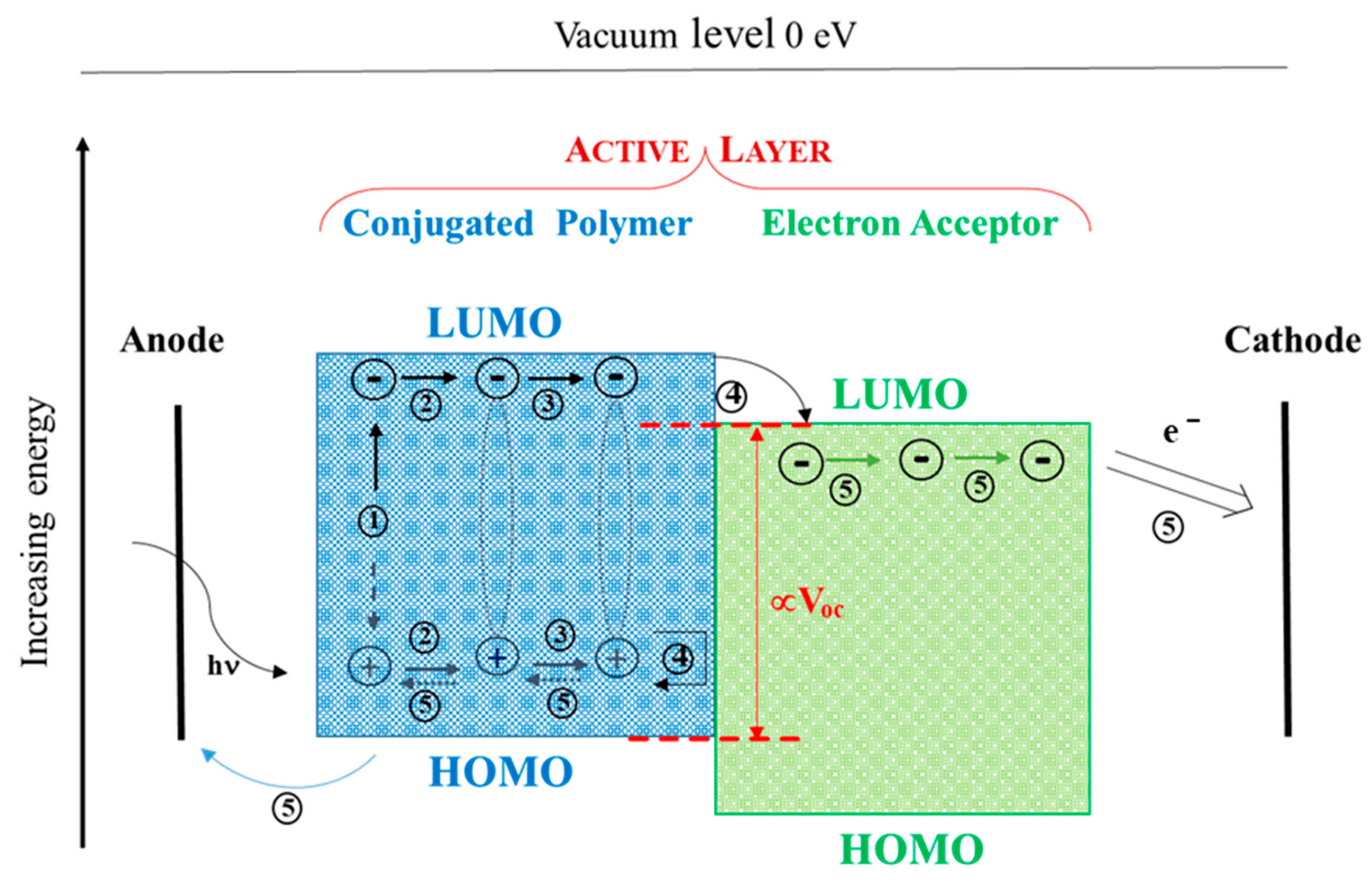
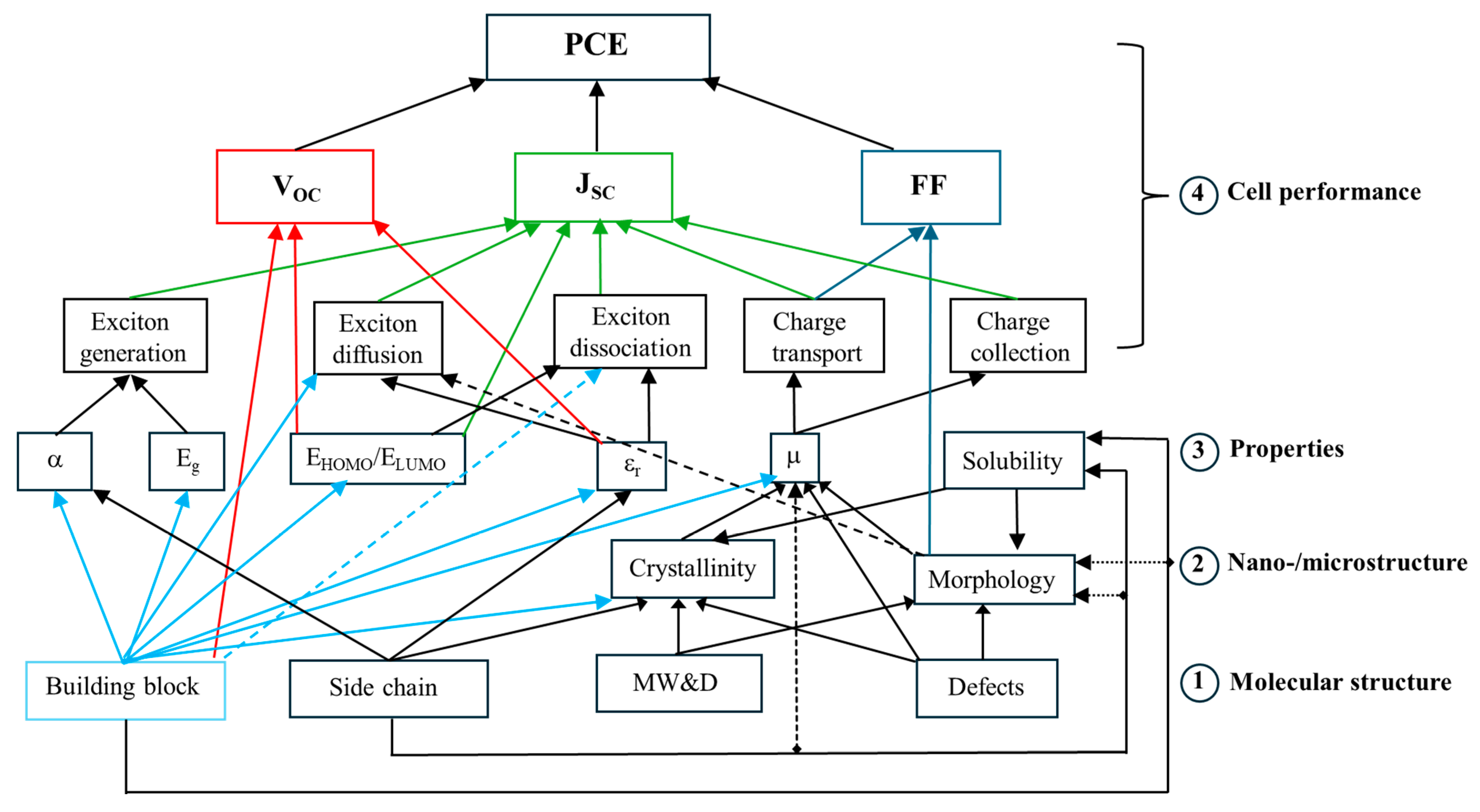
2. D-A Conjugated Polymers
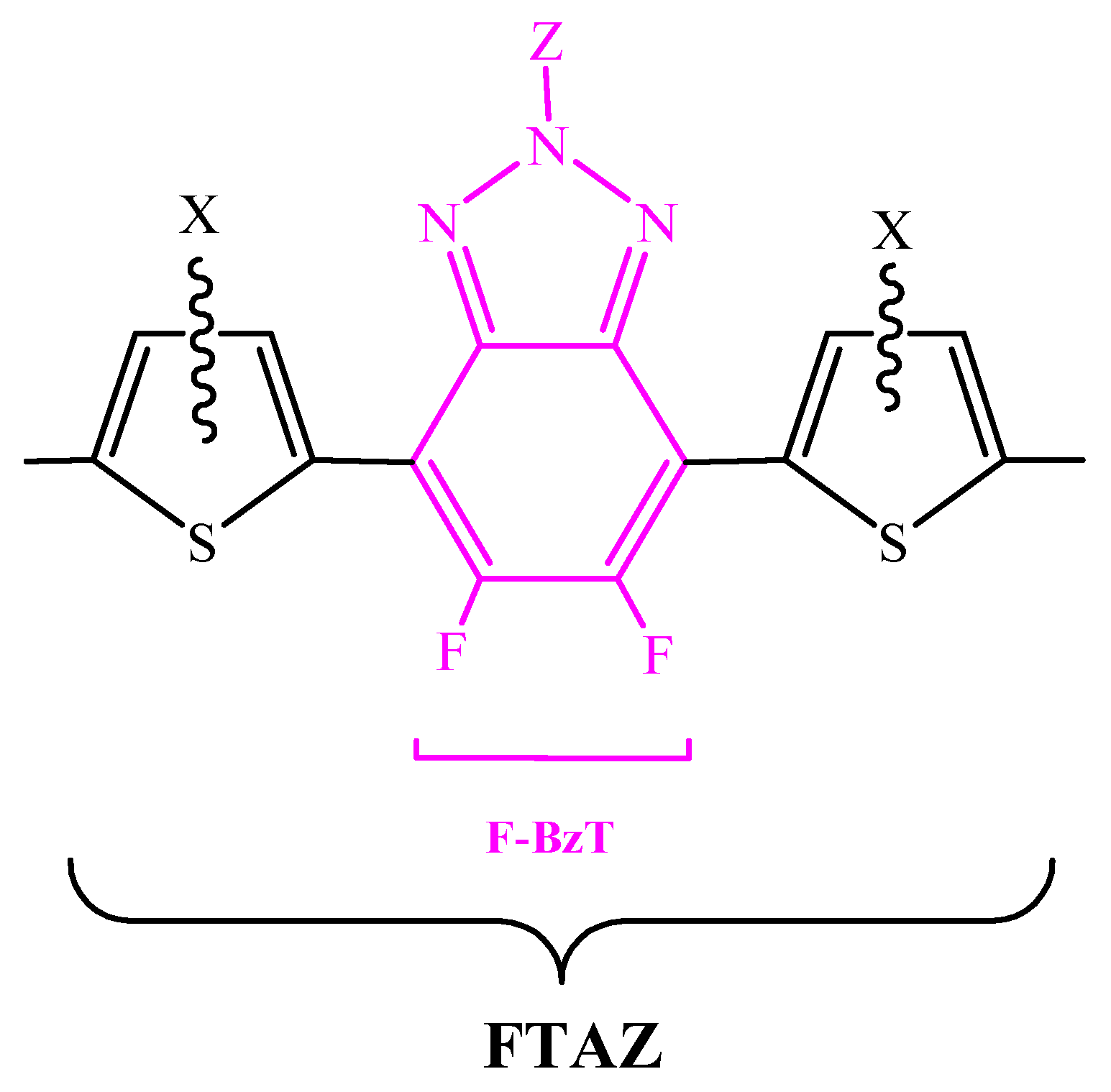
2.1. F-BzT-Based Conjugated Polymers with Thiophene Bridge
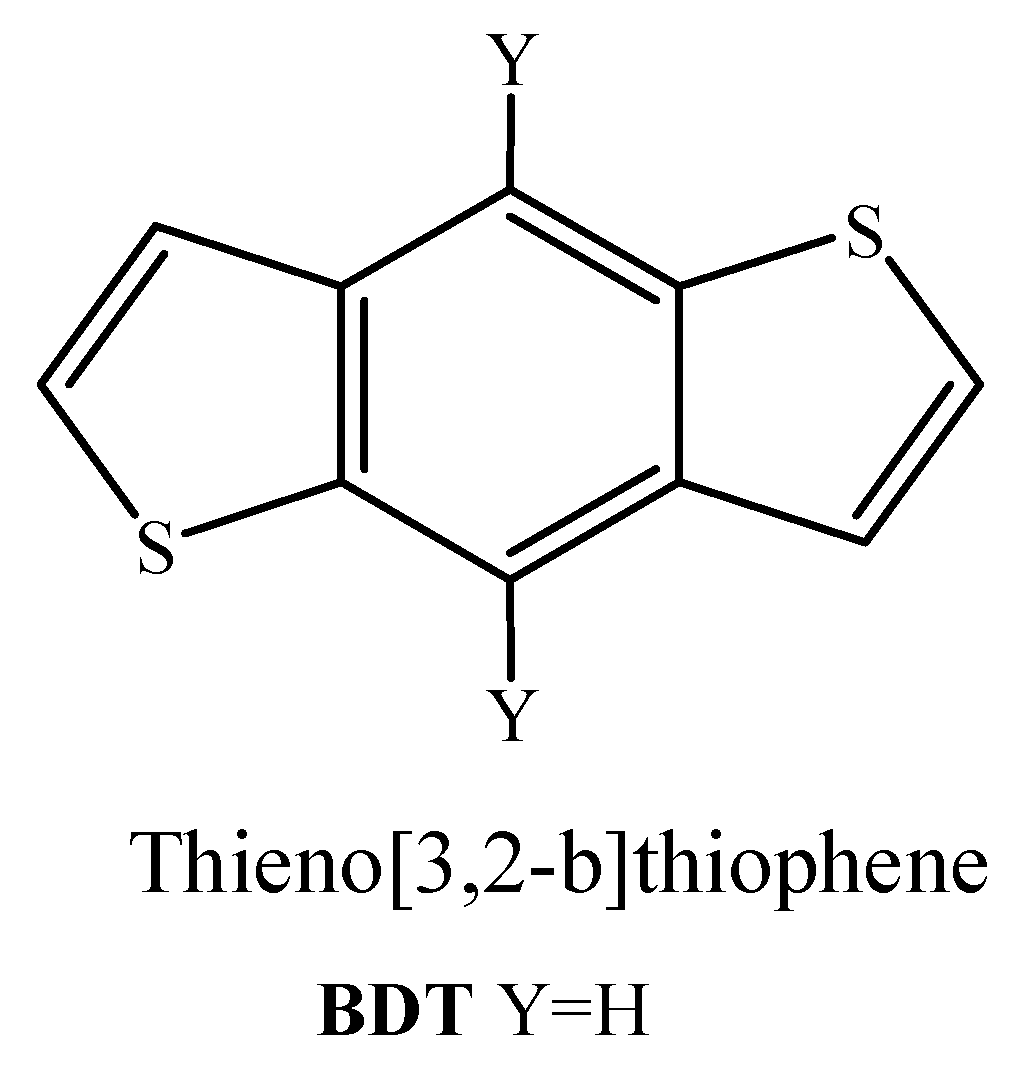
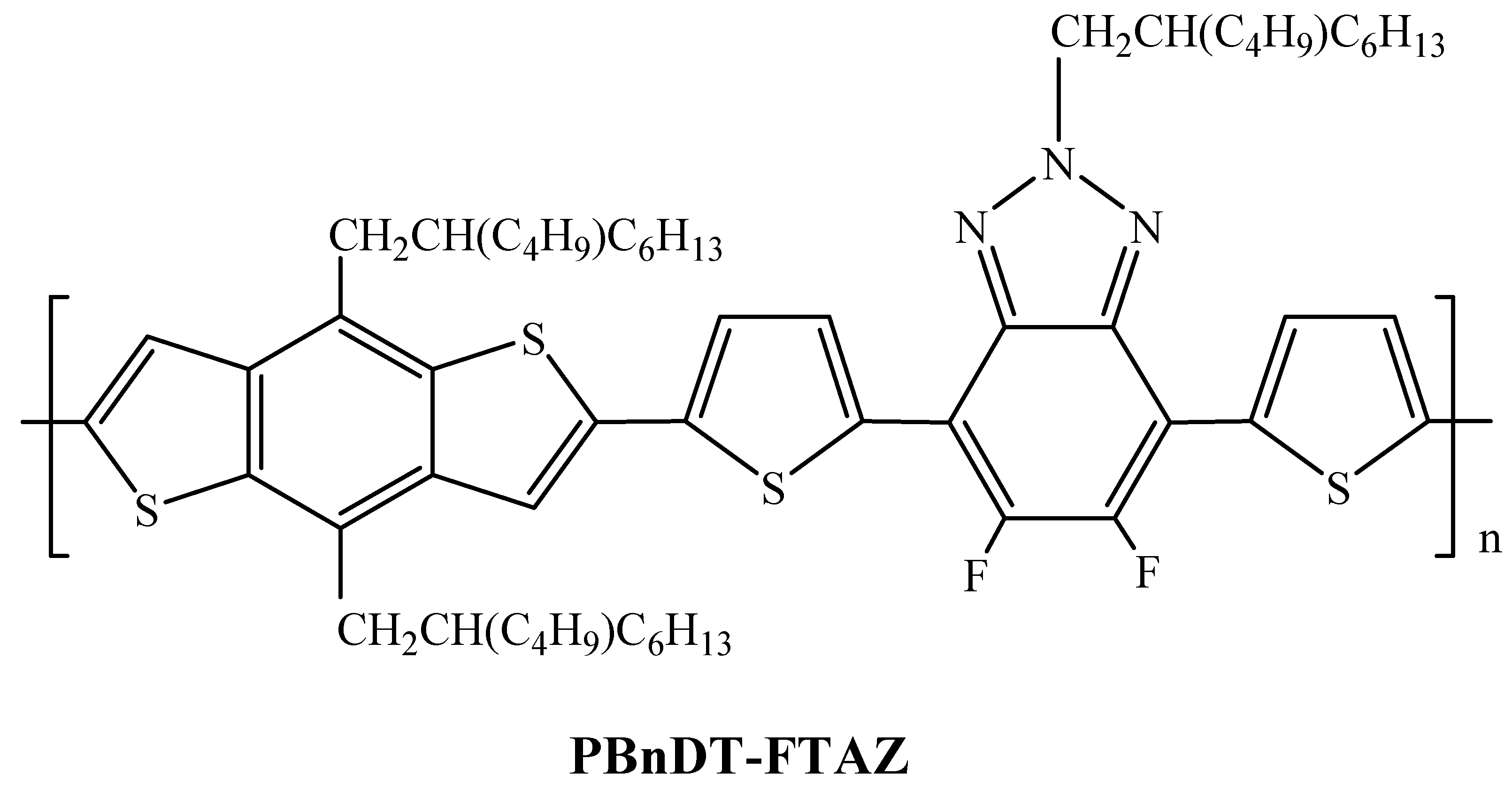
2.1.1. FTAZ-Conjugated Polymers with BDT as Donor Moiety
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PBnDT-FTAZ | SF-PDI2 | 2.3 | [25] | |
| FTAZ c | IDIC | 13.03 | [34] | |
| FTAZ c | IDCIC | 9.64 (13.58) d | 9.09 ± 0.34 (13.10 ± 0.26) d | [35] |
| FTAZ c | ITIC 1 | 8.54 | 8.32 ± 0.19 | [36] |
| FTAZ c | ITIC 2 | 11.0 | 10.6 ± 0.2 | [36] |
| FTAZ c | ITIC-Th | 8.88 | 8.67 ± 0.15 | [37] |
| FTAZ c | ITIC-Th 1 | 12.1 | 11.9 ± 0.1 | [37] |
| FTAZ c | INIC | 7.7 | 7.5 | [38] |
| FTAZ c | INIC1 | 10.1 | 9.9 | [38] |
| FTAZ c | INIC2 | 10.8 | 10.6 | [38] |
| FTAZ c | INIC3 | 11.5 | 11.2 | [38] |
| FTAZ c | IHIC 2 | 7.45 | 7.30 ± 0.18 | [39] |
| FTAZ c | IOIC 2 | 11.2 (12.1) e | 11.1 ± 0.1 (12.1± 0.2) e | [39] |
| FTAZ c | IDIC | 12.5 | 12.1 ± 0.4 e | [40] |
| FTAZ c | IT-M | 11.89 (12.22) f | [41] | |
| PBnDT-FTAZ | IT-M | 12.0 g | 11.7 ± 0.3 | [42] |
| OTAZ h | IT-M | 4.1 | 3.8 ± 0.2 | [42] |
| F OTAZ i | IT-M | 5.7 | 5.2 ± 0.4 | [42] |
| 4′-FT-FTAZ j | ITIC-Th1 | 10.3 | [28] |
2.1.2. FTAZ-Conjugated Polymers with Other Donor Moieties
2.1.3. BzT-Based Conjugated Polymers with Other Bridges
2.2. Condensed Ring BzT-Based Conjugated Polymers
3. BzT-Based Acceptor Polymers
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Crociani, L. Conjugated Polymers as Active Layers in Organic Solar Cells. In New Advances in Materials Technologies Experimental Characterizations, Theoretical Modeling, and Field Practices; Pourhashemi, H.H.A.A., Abraham, A.R., Haghi, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2024. [Google Scholar]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells—Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Bai, Y.; Xue, L.W.; Wang, H.Q.; Zhang, Z.G. Research Advances on Benzotriazole-based Organic Photovoltaic Materials. Acta Chim. Sin. 2021, 79, 820–852. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wu, Y.; Geng, Y.F.; Zhou, E.J.; Zhong, Y.F. Managing Challenges in Organic Photovoltaics: Properties and Roles of Donor/Acceptor Interfaces. Adv. Funct. Mater. 2022, 32, 2206707. [Google Scholar] [CrossRef]
- Manninen, V.; Niskanen, M.; Hukka, T.I.; Pasker, F.; Claus, S.; Höger, S.; Baek, J.; Umeyama, T.; Imahori, H.; Lemmetyinen, H. Conjugated donor-acceptor (D-A) copolymers in inverted organic solar cells—A combined experimental and modelling study. J. Mater. Chem. A Mater. 2013, 1, 7451–7462. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Feng, D.Q.; Wu, Y.; Tsai, S.T.; Li, G.; Ray, C.; Yu, L.P. Highly Efficient Solar Cell Polymers Developed via Fine-Tuning of Structural and Electronic Properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- He, K.Q.; Kumar, P.; Yuan, Y.; Li, Y.N. Wide bandgap polymer donors for high efficiency non-fullerene acceptor based organic solar cells. Mater. Adv. 2021, 2, 115–145. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Mori, T.; Michinobu, T. Rational Design of High-Mobility Semicrystalline Conjugated Polymers with Tunable Charge Polarity: Beyond Benzobisthiadiazole-Based Polymers. Adv. Funct. Mater. 2017, 27, 1604608. [Google Scholar] [CrossRef]
- Hacioglu, S.O.; Unlu, N.A.; Aktas, E.; Hizalan, G.; Yildiz, E.D.; Cirpan, A.; Toppare, L. A triazoloquinoxaline and benzodithiophene bearing low band gap copolymer for electrochromic and organic photovoltaic applications. Synth. Met. 2017, 228, 111–119. [Google Scholar] [CrossRef]
- Pollit, A.A.; Bridges, C.R.; Seferos, D.S. Evidence for the Chain-Growth Synthesis of Statistical π-Conjugated Donor-Acceptor Copolymers. Macromol. Rapid Commun. 2015, 36, 65–70. [Google Scholar] [CrossRef]
- Chen, S.; Kong, L.Q.; Ban, C.L.; Zhao, J.S.; Du, H.M. Synthesis and Characterization of Four Random copolymers Containing Fluorene as Electron Donors and Benzotriazole, Benzothiadiazole, Pyrido 3,4-b pyrazine as Electron Acceptors. Int. J. Electrochem. Sci. 2018, 13, 9964–9980. [Google Scholar] [CrossRef]
- Casey, A.; Green, J.P.; Tuladhar, P.S.; Kirkus, M.; Han, Y.; Anthopoulos, T.D.; Heeney, M. Cyano substituted benzotriazole based polymers for use in organic solar cells. J. Mater. Chem. A Mater. 2017, 5, 6465–6470. [Google Scholar] [CrossRef]
- Banal, J.L.; Subbiah, J.; Graham, H.; Lee, J.K.; Ghiggino, K.P.; Wong, W.W.H. Electron deficient conjugated polymers based on benzotriazole. Polym. Chem. 2013, 4, 1077–1083. [Google Scholar] [CrossRef]
- Yum, S.; An, T.K.; Wang, X.; Lee, W.; Uddin, M.A.; Kim, Y.J.; Nguyen, T.L.; Xu, S.; Hwang, S.; Park, C.E.; et al. Benzotriazole-Containing Planar Conjugated Polymers with Noncovalent Conformational Locks for Thermally Stable and Efficient Polymer Field-Effect Transistors. Chem. Mater. 2014, 26, 2147–2154. [Google Scholar] [CrossRef]
- Lin, H.R.; Chen, S.S.; Li, Z.K.; Lai, J.Y.L.; Yang, G.F.; McAfee, T.; Jiang, K.; Li, Y.K.; Liu, Y.H.; Hu, H.W.; et al. High-Performance Non-Fullerene Polymer Solar Cells Based on a Pair of Donor-Acceptor Materials with Complementary Absorption Properties. Adv. Mater. 2015, 27, 7299–7304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Z.; Wang, J.Y.; Zhang, Z.G.; Bai, H.T.; Li, Y.F.; Zhu, D.B.; Zhan, X.W. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Zhan, T.; Zhang, Y.; Li, J.Y.; Wu, Y.F.; He, Z.C.; Cai, P.; Duan, C.H.; Huang, F.; Cao, Y. Wide Bandgap Conjugated Polymers Based on Difluorobenzoxadiazole for Efficient Non-Fullerene Organic Solar Cells. Macromol. Rapid Commun. 2022, 43, 2200591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Kong, X.L.; Sun, G.P.; Li, Y.F. Organic small molecule acceptor materials for organic solar cells. Escience 2023, 3, 15. [Google Scholar] [CrossRef]
- Chen, S.S.; Liu, Y.H.; Zhang, L.; Chow, P.C.Y.; Wang, Z.; Zhang, G.Y.; Ma, W.; Yan, H. A Wide-Bandgap Donor Polymer for Highly Efficient Non-fullerene Organic Solar Cells with a Small Voltage Loss. J. Am. Chem. Soc. 2017, 139, 6298–6301. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Y.H.; Wang, Y.C.; Wang, X.C.; Cui, W.; Wen, S.G.; Zheng, N.; Sun, M.L.; Yang, R.Q. Fuse the π-Bridge to Acceptor Moiety of Donor-π-Acceptor Conjugated Polymer: Enabling an All-Round Enhancement in Photovoltaic Parameters of Nonfullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 31087–31095. [Google Scholar] [CrossRef]
- Du, M.Z.; Tang, A.L.; Yu, J.G.; Geng, Y.F.; Wang, Z.T.; Guo, Q.; Zhong, Y.F.; Lu, S.R.; Zhou, E.R. Benzotriazole-Based D-p-A-Type Photovoltaic Polymers Break Through 17% Efficiency. Adv. Energy Mater. 2023, 13, 2302429. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liang, Z.Z.; Li, X.M.; Qin, J.C.; Ren, M.L.; Yang, C.Y.; Bao, X.C.; Xia, Y.J.; Li, J.F. Self-doping n-type polymer as a cathode interface layer enables efficient organic solar cells by increasing built-in electric field and boosting interface contact. J. Mater. Chem. C Mater. 2019, 7, 11152–11159. [Google Scholar] [CrossRef]
- Atli, G.Ö.; Yilmaz, E.A.; Aslan, S.T.; Udum, Y.A.; Toppare, L.; Çirpan, A. Synthesis and characterization of optical, electrochemical and photovoltaic properties of selenophene bearing benzodithiophene based alternating polymers. J. Electroanal. Chem. 2020, 862, 114014. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.G.; Xue, L.W.; Min, J.; Zhang, J.Q.; Wei, Z.X.; Li, Y.F. All-Polymer Solar Cells Based on Absorption-Complementary Polymer Donor and Acceptor with High Power Conversion Efficiency of 8.27%. Adv. Mater. 2016, 28, 1884–1890. [Google Scholar] [CrossRef]
- Bauer, N.; Zhang, Q.Q.; Zhao, J.B.; Ye, L.; Kim, J.H.; Constantinou, I.; Yan, L.; So, F.; Ade, H.; Yan, H.; et al. Comparing non-fullerene acceptors with fullerene in polymer solar cells: A case study with FTAZ and PyCNTAZ. J. Mater. Chem. A Mater. 2017, 5, 4886–4893. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Wang, Z.; Zhang, J.Q.; Zhang, H.; Lu, K.; Guo, F.Y.; Wei, Z.X.; Yang, Y.L.; Zhao, L.C.; Zhang, Y. Wide-Bandgap Conjugated Polymers Based on Alkylthiofuran-Substituted Benzo 1,2-b:4,5-b′ difuran for Efficient Fullerene-Free Polymer Solar Cells. Macromolecules 2018, 51, 2498–2505. [Google Scholar] [CrossRef]
- Rech, J.J.; Yan, L.; Peng, Z.X.; Dai, S.X.; Zhan, X.W.; Ade, H.; You, W. Utilizing Difluorinated Thiophene Units To Improve the Performance of Polymer Solar Cells. Macromolecules 2019, 52, 6523–6532. [Google Scholar] [CrossRef]
- Bauer, N.; Zhang, Q.Q.; Rech, J.J.; Dai, S.X.; Peng, Z.X.; Ade, H.; Wang, J.Y.; Zhan, X.W.; You, W. The impact of fluorination on both donor polymer and non-fullerene acceptor: The more fluorine, the merrier. Nano Res. 2019, 12, 2400–2405. [Google Scholar] [CrossRef]
- Tang, A.L.; Zhang, Q.Q.; Du, M.Z.; Li, G.Q.; Geng, Y.F.; Zhang, J.Q.; Wei, Z.X.; Sun, X.N.; Zhou, E.J. Molecular Engineering of D-π-A Copolymers Based on 4,8-Bis(4-chlorothiophen-2-yl)benzo 1,2-b:4,5-b′ dithiophe ne (BDT-T-Cl) for High-Performance Fullerene-Free Organic Solar Cells. Macromolecules 2019, 52, 6227–6233. [Google Scholar] [CrossRef]
- Bin, H.J.; Angunawela, I.; Ma, R.J.; Nallapaneni, A.; Zhu, C.H.; Leenaers, P.J.; Saes, B.W.H.; Wienk, M.M.; Yan, H.; Ade, H.; et al. Effect of main and side chain chlorination on the photovoltaic properties of benzodithiophene-alt-benzotriazole polymers. J. Mater. Chem. C Mater. 2020, 8, 15426–15435. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, Y.; Na, Y.H.; Baek, N.S.; Jung, J.W.; Choi, Y.; Cho, N.; Kim, T.D. Efficient Inverted Solar Cells Using Benzotriazole-Based Small Molecule and Polymers. Polymers 2021, 13, 393. [Google Scholar] [CrossRef]
- Dai, T.T.; Li, X.D.; Lei, P.; Tang, A.L.; Geng, Y.F.; Zeng, Q.D.; Zhou, E.J. Modulating the molecular orientation of linear benzodifuran-based isomeric polymers by exchanging the positions of chlorine and fluorine atoms. Nano Energy 2022, 99, 107413. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.Q.; Zhou, H.X.; You, W. Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer-Fullerene Solar Cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, Y.Q.; Zhu, J.S.; Zhang, Q.Q.; Xu, R.P.; Li, C.; Zhang, Y.X.; Huang, J.S.; Zhan, X.W.; You, W.; et al. Polymer Solar Cells with 90% External Quantum Efficiency Featuring an Ideal Light- and Charge-Manipulation Layer. Adv. Mater. 2018, 30, 1706083. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhao, F.W.; Xin, J.M.; Rech, J.J.; Wei, Z.X.; Ma, W.; You, W.; Li, B.; Jiang, L.; Li, Y.F.; et al. A Fused Ring Electron Acceptor with Decacyclic Core Enables over 13.5% Efficiency for Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1802050. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, W.; Wang, X.H.; Wu, Y.; Zhang, Q.Q.; Yan, C.Q.; Ma, W.; You, W.; Zhan, X.W. Enhancing Performance of Nonfullerene Acceptors via Side-Chain Conjugation Strategy. Adv. Mater. 2017, 29, 1702125. [Google Scholar] [CrossRef]
- Zhao, F.W.; Dai, S.X.; Wu, Y.Q.; Zhang, Q.Q.; Wang, J.Y.; Jiang, L.; Ling, Q.D.; Wei, Z.X.; Ma, W.; You, W.; et al. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29, 1700144. [Google Scholar] [CrossRef]
- Dai, S.X.; Zhao, F.W.; Zhang, Q.Q.; Lau, T.K.; Li, T.F.; Liu, K.; Ling, Q.D.; Wang, C.R.; Lu, X.H.; You, W.; et al. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Ke, Z.F.; Zhang, Q.Q.; Wang, J.Y.; Dai, S.X.; Wu, Y.; Xu, Y.; Lin, Y.Z.; Ma, W.; You, W.; et al. Naphthodithiophene-Based Nonfullerene Acceptor for High-Performance Organic Photovoltaics: Effect of Extended Conjugation. Adv. Mater. 2018, 30, 1704713. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Zhao, F.W.; Prasad, S.K.K.; Chen, J.D.; Cai, W.Z.; Zhang, Q.Q.; Chen, K.; Wu, Y.; Ma, W.; Gao, F.; et al. Balanced Partnership between Donor and Acceptor Components in Nonfullerene Organic Solar Cells with >12% Efficiency. Adv. Mater. 2018, 30, 1706363. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, L.; Gadisa, A.; Zhang, Q.Q.; Rech, J.J.; You, W.; Ade, H. Revealing the Impact of F4-TCNQ as Additive on Morphology and Performance of High-Efficiency Nonfullerene Organic Solar Cells. Adv. Funct. Mater. 2019, 29, 1806262. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Chen, Z.; Zhang, Q.Q.; Fei, Z.P.; Henry, R.; Heeney, M.; O’Connor, B.T.; You, W.; Ade, H. Sequential Deposition of Organic Films with Eco-Compatible Solvents Improves Performance and Enables Over 12%-Efficiency Nonfullerene Solar Cells. Adv. Mater. 2019, 31, 1808153. [Google Scholar] [CrossRef] [PubMed]
- Su, W.Y.; Li, G.W.; Fan, Q.P.; Zhu, Q.L.; Guo, X.; Chen, J.; Wu, J.N.; Ma, W.; Zhang, M.J.; Li, Y.F. Nonhalogen solvent-processed polymer solar cells based on chlorine and trialkylsilyl substituted conjugated polymers achieve 12.8% efficiency. J. Mater. Chem. A Mater. 2019, 7, 2351–2359. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Z.G.; Zhang, S.Y.; Li, Y.F. Conjugated Side-Chain-Isolated D-A Copolymers Based on Benzo 1,2-b:4,5-b′ dithiophene-alt-dithienylbenzotr iazole: Synthesis and Photovoltaic Properties. Chem. Mater. 2012, 24, 3247–3254. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.G.; Bin, H.J.; Xue, L.W.; Yang, Y.K.; Wang, C.; Liu, F.; Russell, T.P.; Li, Y.F. High-Efficiency Nonfullerene Polymer Solar Cells with Medium Bandgap Polymer Donor and Narrow Bandgap Organic Semiconductor Acceptor. Adv. Mater. 2016, 28, 8288–8295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Liu, H.F.; Huai, Z.X.; Yang, S.P. Wide Band Gap and Highly Conjugated Copolymers Incorporating 2-(Triisopropylsilylethynyl)thiophene-Substituted Benzodithiophene for Efficient Non-Fullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 28828–28837. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, G.Y.; Li, X.M.; Wang, H.; Li, Y.H.; Jiang, H.X.; Zheng, N.; Yang, R.Q. Side-Chain-Promoted Benzodithiophene-based Conjugated Polymers toward Striking Enhancement of Photovoltaic Properties for Polymer Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 42747–42755. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Q.; Wang, B.B.; Miao, W.T.; Zhang, G.P.; Zhou, Y.; Guo, P.Z.; Xia, Y.J. p-nitrophenol-terminated alkyl side chain substituted polymer as high dielectric constant polymer additive enables efficient organic solar cells. Opt. Mater. 2022, 127, 112347. [Google Scholar] [CrossRef]
- Li, Z.Y.; Fan, B.B.; He, B.T.; Ying, L.; Zhong, W.K.; Liu, F.; Huang, F.; Cao, Y. Side-chain modification of polyethylene glycol on conjugated polymers for ternary blend all-polymer solar cells with efficiency up to 9.27%. Sci. China-Chem. 2018, 61, 427–436. [Google Scholar] [CrossRef]
- Bin, H.J.; Zhang, Z.G.; Gao, L.; Chen, S.S.; Zhong, L.; Xue, L.W.; Yang, C.; Li, Y.F. Non-Fullerene Polymer Solar Cells Based on Alkylthio and Fluorine Substituted 2D-Conjugated Polymers Reach 9.5% Efficiency. J. Am. Chem. Soc. 2016, 138, 4657–4664. [Google Scholar] [CrossRef]
- Fan, Q.P.; Su, W.Y.; Meng, X.Y.; Guo, X.; Li, G.D.; Ma, W.; Zhang, M.J.; Li, Y.F. High-Performance Non-Fullerene Polymer Solar Cells Based on Fluorine Substituted Wide Bandgap Copolymers Without Extra Treatments. Solar Rrl 2017, 1, 1700020. [Google Scholar] [CrossRef]
- Lin, Z.K.; Zhang, L.; Tu, S.L.; Wang, W.; Ling, Q.D. Highly thermally stable all-polymer solar cells enabled by photo-crosslinkable bromine-functionalized polymer donors. Solar Energy 2020, 201, 489–498. [Google Scholar] [CrossRef]
- Xue, L.W.; Yang, Y.K.; Xu, J.Q.; Zhang, C.F.; Bin, H.J.; Zhang, Z.G.; Qiu, B.B.; Li, X.J.; Sun, C.K.; Gao, L.; et al. Side Chain Engineering on Medium Bandgap Copolymers to Suppress Triplet Formation for High-Efficiency Polymer Solar Cells. Adv. Mater. 2017, 29, 1703344. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Xiao, B.; Chen, F.; Zhang, J.Q.; Wei, Z.X.; Zhou, E.J. The Introduction of Fluorine and Sulfur Atoms into Benzotriazole-Based p-Type Polymers to Match with a Benzotriazole-Containing n-Type Small Molecule: “The Same-Acceptor-Strategy” to Realize High Open-Circuit Voltage. Adv. Energy Mater. 2018, 8, 1801582. [Google Scholar] [CrossRef]
- Liu, Z.T.; Gao, Y.R.; Dong, J.; Yang, M.L.; Liu, M.; Zhang, Y.; Wen, J.; Ma, H.B.; Gao, X.; Chen, W.; et al. Chlorinated Wide-Bandgap Donor Polymer Enabling Annealing Free Nonfullerene Solar Cells with the Efficiency of 11.5%. J. Phys. Chem. Lett. 2018, 9, 6955–6962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, C.X.; Wang, J.C.; Tang, A.L.; Zhang, B.; Liu, X.F.; Chen, X.G.; Wei, Z.X.; Zhou, E.J. Introducing methoxy or fluorine substitutions on the conjugated side chain to reduce the voltage loss of organic solar cells. J. Mater. Chem. C Mater. 2021, 9, 11163–11171. [Google Scholar] [CrossRef]
- Zhou, J.L.; Cong, P.Q.; Chen, L.; Zhang, B.; Geng, Y.F.; Tang, A.L.; Zhou, E.J. Gradually modulating the three parts of D-π-A type polymers for high-performance organic solar cells. J. Energy Chem. 2021, 62, 532–537. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.T.; Oh, J.; Yuan, Z.Y.; Yang, C.; Hu, Y.; Zhao, X.H.; Chen, Y.W. Halogen-free donor polymers based on dicyanobenzotriazole for additive-free organic solar cells. Chem. Eng. J. 2022, 442, 136068. [Google Scholar] [CrossRef]
- Liao, J.X.; Weng, F.B.; Zeng, L.X.; Huang, Z.E.; Zheng, P.J.; Xu, G.B.; Zhang, Z.J.; Wang, W.J.; Zhuang, G.J.; Zhao, H.B.; et al. Simple Approach for Synthesizing a Fluorinated Polymer Donor Enables Promoted Efficiency in Polymer Solar Cells. ACS Appl. Energy Mater. 2022, 5, 14250–14261. [Google Scholar] [CrossRef]
- Negash, A.; Genene, Z.; Eachambadi, R.T.; Kesters, J.; Van den Brande, N.; D’Haen, J.; Penxten, H.; Abdulahi, B.A.; Wang, E.G.; Vandewal, K.; et al. Diketopyrrolopyrrole-based terpolymers with tunable broad band absorption for fullerene and fullerene-free polymer solar cells. J. Mater. Chem. C Mater. 2019, 7, 3375–3384. [Google Scholar] [CrossRef]
- Pan, F.; Sun, C.K.; Bin, H.J.; Angunawela, I.; Lai, W.B.; Meng, L.; Ade, H.; Li, Y.F. Side-chain engineering of medium bandgap polymer donors for efficient polymer solar cells. Org. Electron. 2020, 78, 105603. [Google Scholar] [CrossRef]
- He, E.F.; Lu, Y.; Zheng, Z.; Guo, F.Y.; Gao, S.Y.; Zhao, L.C.; Zhang, Y. Halogenation on benzo 1,2-b:4,5-b′ difuran polymers for solvent additive-free non-fullerene polymer solar cells with efficiency exceeding 11%. J. Mater. Chem. C Mater. 2020, 8, 139–146. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Kuklin, S.A.; Khokhlov, A.R.; Konstantinov, I.O.; Nekrasova, N.V.; Xie, Z.Y.; Sharma, G.D. Synthesis of new 2,6-bis(6-fluoro-2-hexyl-2H-benzotriazol-4-yl)-4,4-bis(2-ethylhex yl)-4H-silolo 3,2-b:4,5-b′ dithiophene based D-A conjugated terpolymers for photovoltaic application. Polymer 2017, 133, 195–204. [Google Scholar] [CrossRef]
- Wang, X.C.; Tang, A.L.; Yang, J.; Du, M.Z.; Li, J.F.; Li, G.Q.; Guo, Q.; Zhou, E.R. Tuning the intermolecular interaction of A2-A1-D-A1-A2type non-fullerene acceptors by substituent engineering for organic solar cells with ultrahighVOCof ∼1.2 V. Sci. China-Chem. 2020, 63, 1666–1674. [Google Scholar] [CrossRef]
- Dai, T.T.; Tang, A.L.; Wang, J.C.; He, Z.H.; Li, X.D.; Guo, Q.; Chen, X.G.; Ding, L.M.; Zhou, E.R. The Subtle Structure Modulation of A2-A1-D-A1-A2 Type Nonfullerene Acceptors Extends the Photoelectric Response for High-Voltage Organic Photovoltaic Cells. Macromol. Rapid Commun. 2022, 43, 2100810. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.H.; Xie, Y.P.; Chen, L.; Tan, Y.; Huang, S.R.; An, Y.K.; Ryu, H.S.; Meng, X.C.; Liao, X.F.; Huang, B.; et al. Fluorobenzotriazole (FTAZ)-Based Polymer Donor Enables Organic Solar Cells Exceeding 12% Efficiency. Adv. Funct. Mater. 2019, 29, 9. [Google Scholar] [CrossRef]
- Li, X.S.; Weng, K.K.; Ryu, H.S.; Guo, J.; Zhang, X.N.; Xia, T.; Fu, H.T.; Wei, D.H.; Min, J.; Zhang, Y.; et al. Non-Fullerene Organic Solar Cells Based on Benzo 1,2-b:4,5-b′ difuran-Conjugated Polymer with 14% Efficiency. Adv. Funct. Mater. 2020, 30, 1906809. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F.; Li, J.F.; Xiao, Y.Z.; Zuo, K.Y.; Tang, A.L.; Zhang, B.; Zhou, E.J. Modulating the middle and end-capped units of A2-A1-D-A1-A2 type non-fullerene acceptors for high VOC organic solar cells. Org. Electron. 2021, 95, 106195. [Google Scholar] [CrossRef]
- Liao, J.X.; Weng, F.B.; Zheng, P.J.; Xu, G.B.; Zeng, L.X.; Huang, Z.G.; Deng, T.J.; Pang, Y.Q.; Wu, S.Y.; Chen, J.H.; et al. Enhanced efficiency of polymer solar cells via simple fluorination on the π-bridge of polymer donors. Org. Electron. 2022, 108, 106611. [Google Scholar] [CrossRef]
- Qiu, B.B.; Chen, S.S.; Li, H.N.; Luo, Z.H.; Yao, J.; Sun, C.K.; Li, X.J.; Xue, L.W.; Zhang, Z.G.; Yang, C.D.; et al. A Simple Approach to Prepare Chlorinated Polymer Donors with Low-Lying HOMO Level for High Performance Polymer Solar Cells. Chem. Mater. 2019, 31, 6558–6567. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Cai, F.F.; Yuan, J.; Wei, Q.Y.; Zhou, L.Y.; Qiu, B.B.; Hu, Y.B.; Li, Y.F.; Peng, H.J.; Zou, Y.P. A new non-fullerene acceptor based on the combination of a heptacyclic benzothiadiazole unit and a thiophene-fused end group achieving over 13% efficiency. Phys. Chem. Chem. Phys. 2019, 21, 26557–26563. [Google Scholar] [CrossRef]
- Yang, Y.K.; Zhang, Z.G.; Bin, H.J.; Chen, S.S.; Gao, L.; Xue, L.W.; Yang, C.; Li, Y.F. Side-Chain Isomerization on an n-type Organic Semiconductor ITIC Acceptor Makes 11.77% High Efficiency Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. [Google Scholar] [CrossRef]
- Fan, Q.P.; Su, W.Y.; Guo, X.; Wang, Y.; Chen, J.; Ye, C.N.; Zhang, M.J.; Li, Y.F. Side-chain engineering for efficient non-fullerene polymer solar cells based on a wide-bandgap polymer donor. J. Mater. Chem. A Mater. 2017, 5, 9204–9209. [Google Scholar] [CrossRef]
- Huang, H.; Bin, H.J.; Peng, Z.X.; Qiu, B.B.; Sun, C.K.; Liebman-Pelaez, A.; Zhang, Z.G.; Zhu, C.H.; Ade, H.; Zhang, Z.J.; et al. Effect of Side-Chain Engineering of Bithienylbenzodithiophene-alt-fluorobenzotriazole-Based Copolymers on the Thermal Stability and Photovoltaic Performance of Polymer Solar Cells. Macromolecules 2018, 51, 6028–6036. [Google Scholar] [CrossRef]
- Shi, S.W.; Chen, X.F.; Liu, X.B.; Wu, X.F.; Liu, F.; Zhang, Z.G.; Li, Y.F.; Russell, T.P.; Wang, D. 3D Structural Model of High-Performance Non-Fullerene Polymer Solar Cells as Revealed by High-Resolution AFM. ACS Appl. Mater. Interfaces 2017, 9, 24451–24455. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Xiao, B.; Wang, Y.M.; Gao, F.; Tajima, K.; Bin, H.J.; Zhang, Z.G.; Li, Y.F.; Wei, Z.X.; Zhou, E.J. Simultaneously Achieved High Open-Circuit Voltage and Efficient Charge Generation by Fine-Tuning Charge-Transfer Driving Force in Nonfullerene Polymer Solar Cells. Adv. Funct. Mater. 2018, 28, 1704507. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, Y.F.; Tang, A.L.; Wang, X.C.; Sun, Y.M.; Zhou, E.J. Changing the π-bridge from thiophene to thieno 3,2-b thiophene for the D-π-A type polymer enables high performance fullerene-free organic solar cells. Chem. Commun. 2019, 55, 6708–6710. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Xu, X.P.; Li, R.P.; Yu, L.Y.; Meng, L.; Wang, Y.L.; Li, Y.; Peng, Q. Asymmetric Siloxane Functional Side Chains Enable High-Performance Donor Copolymers for Photovoltaic Applications. ACS Appl. Mater. Interfaces 2020, 12, 17772–17780. [Google Scholar] [CrossRef]
- Bai, Y.L.; Yu, R.N.; Bai, Y.M.; Zhou, E.; Hayat, T.; Alsaedi, A.; Tan, Z. Ternary blend strategy in benzotriazole-based organic photovoltaics for indoor application. Green Energy Environ. 2021, 6, 920–928. [Google Scholar] [CrossRef]
- Li, Z.J.; Xu, X.F.; Zhang, W.; Meng, X.Y.; Genene, Z.; Ma, W.; Mammo, W.; Yartsev, A.; Andersson, M.R.; Janssen, R.A.J.; et al. 9.0% power conversion efficiency from ternary all-polymer solar cells. Energy Environ. Sci. 2017, 10, 2212–2221. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, N. Complementary light absorption and efficient exciton dissociation lead to efficient and excellent ternary polymer solar cells. J. Mater. Chem. A Mater. 2020, 8, 3211–3221. [Google Scholar] [CrossRef]
- Bin, H.J.; Gao, L.; Zhang, Z.G.; Yang, Y.K.; Zhang, Y.D.; Zhang, C.F.; Chen, S.S.; Xue, L.W.; Yang, C.; Xiao, M.; et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 2016, 7, 13651. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.J.; Yang, Y.K.; Peng, Z.X.; Ye, L.; Yao, J.; Zhong, L.; Sun, C.K.; Gao, L.; Huang, H.; Li, X.J.; et al. Effect of Alkylsilyl Side-Chain Structure on Photovoltaic Properties of Conjugated Polymer Donors. Adv. Energy Mater. 2018, 8, 1702324. [Google Scholar] [CrossRef]
- Luo, Z.H.; Bin, H.J.; Liu, T.; Zhang, Z.G.; Yang, Y.K.; Zhong, C.; Qiu, B.B.; Li, G.H.; Gao, W.; Xie, D.J.; et al. Fine-Tuning of Molecular Packing and Energy Level through Methyl Substitution Enabling Excellent Small Molecule Acceptors for Nonfullerene Polymer Solar Cells with Efficiency up to 12.54%. Adv. Mater. 2018, 30, 1706124. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Guo, J.; Sun, C.K.; Wang, T.; Luo, Z.H.; Zhang, Z.H.; Jiao, X.C.; Tang, W.H.; Yang, C.L.; Li, Y.F.; et al. A universal layer-by-layer solution-processing approach for efficient non-fullerene organic solar cells. Energy Environ. Sci. 2019, 12, 384–395. [Google Scholar] [CrossRef]
- Sun, R.; Guo, J.; Wu, Q.; Zhang, Z.H.; Yang, W.Y.; Guo, J.; Shi, M.M.; Zhang, Y.H.; Kahmann, S.; Ye, L.; et al. A multi-objective optimization-based layer-by-layer blade-coating approach for organic solar cells: Rational control of vertical stratification for high performance. Energy Environ. Sci. 2019, 12, 3118–3132. [Google Scholar] [CrossRef]
- Adil, M.A.; Zhang, J.Q.; Wang, Y.H.; Yu, J.D.; Yang, C.; Lu, G.H.; Wei, Z.X. Regulating the phase separation of ternary organic solar cells via 3D architectured AIE molecules. Nano Energy 2020, 68, 104271. [Google Scholar] [CrossRef]
- Li, Z.J.; Xu, X.P.; Zhang, G.J.; Yu, T.; Li, Y.; Peng, Q. Highly Efficient Non-Fullerene Polymer Solar Cells Enabled by Wide Bandgap Copolymers With Conjugated Selenyl Side Chains. Solar Rrl 2018, 2, 1800186. [Google Scholar] [CrossRef]
- Bin, H.J.; Zhong, L.; Zhang, Z.G.; Gao, L.; Yang, Y.K.; Xue, L.W.; Zhang, J.; Zhang, Z.J.; Li, Y.F. Alkoxy substituted benzodithiophene-alt-fluorobenzotriazole copolymer as donor in non-fullerene polymer solar cells. Sci. China-Chem. 2016, 59, 1317–1322. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, L.; Rech, J.J.; Hu, J.; Zhang, Q.Q.; You, W. Green-Solvent-Processed Conjugated Polymers for Organic Solar Cells: The Impact of Oligoethylene Glycol Side Chains. ACS Appl. Polym. Mater. 2019, 1, 804–814. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.Y.; Zhang, K.L.; Zhu, T.T.; Zhong, Y.Q.; Li, F.; Li, Y.H.; Sun, M.L.; Yang, R.Q. Efficient fullerene-free solar cells with wide optical band gap polymers based on fluorinated benzotriazole and asymmetric benzodithiophene. J. Mater. Chem. A Mater. 2017, 5, 21650–21657. [Google Scholar] [CrossRef]
- Wen, S.G.; Chen, W.C.; Huang, G.Y.; Shen, W.F.; Liu, H.Z.; Duan, L.R.; Zhang, J.; Yang, R.Q. 2D expanded conjugated polymers with non-fullerene acceptors for efficient polymer solar cells. J. Mater. Chem. C Mater. 2018, 6, 1753–1758. [Google Scholar] [CrossRef]
- Li, J.F.; Liang, Z.Z.; Li, X.M.; Li, H.D.; Wang, Y.F.; Qin, J.C.; Tong, J.F.; Yan, L.H.; Bao, X.C.; Xia, Y.J. Insights into Excitonic Dynamics of Terpolymer-Based High-Efficiency Nonfullerene Polymer Solar Cells: Enhancing the Yield of Charge Separation States. ACS Appl. Mater. Interfaces 2020, 12, 8475–8484. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, F.; Zhu, T.T.; Li, Y.H.; Yu, L.M.; Yang, R.Q.; Sun, M.L. 1 V high open-circuit voltage fluorinated alkoxybiphenyl side-chained benzodithiophene based photovoltaic polymers. Synth. Met. 2019, 257, 116182. [Google Scholar] [CrossRef]
- Li, W.B.; Li, G.D.; Guo, X.; Wang, Y.; Guo, H.; Xu, Q.Q.; Zhang, M.J.; Li, Y.F. A trifluoromethyl substituted wide bandgap conjugated polymer for non-fullerene polymer solar cells with 10.4% efficiency. J. Mater. Chem. A Mater. 2018, 6, 6551–6558. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.E.; Zhu, T.; Liu, Z.Y.; Chen, L. Two Better Compatible and Complementary Light Absorption Polymer Donors Contributing Synergistically to High Efficiency and Better Thermally Stable Ternary Organic Solar Cells. ACS Appl. Energy Mater. 2022, 5, 5026–5035. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, Y.; Ma, R.J.; Luo, Z.H.; Liu, T.; Kang, S.H.E.; Yan, H.; Yuan, Z.Y.; Yang, C.; Chen, Y.W. Wide Band-gap Two-dimension Conjugated Polymer Donors with Different Amounts of Chlorine Substitution on Alkoxyphenyl Conjugated Side Chains for Non-fullerene Polymer Solar Cells. Chin. J. Polym. Sci. 2020, 38, 797–805. [Google Scholar] [CrossRef]
- Li, X.M.; Huang, G.Y.; Zheng, N.; Li, Y.H.; Kang, X.; Qiao, S.L.; Jiang, H.X.; Chen, W.C.; Yang, R.Q. High-Efficiency Polymer Solar Cells Over 13.9% With a High VOC Beyond 1.0V by Synergistic Effect of Fluorine and Sulfur. Solar Rrl 2019, 3, 1900005. [Google Scholar] [CrossRef]
- Xue, X.N.; Zheng, B.; Zhang, Y.; Zhang, M.; Wei, D.H.; Liu, F.; Wan, M.X.; Liu, J.; Chen, G.M.; Huo, L.J. Two Birds with One Stone: High Efficiency and Low Synthetic Cost for Benzotriazole-Based Polymer Solar Cells by a Simple Chemical Approach. Adv. Energy Mater. 2020, 10, 2002142. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhu, Z.X.; Li, H.F.; Fan, W.L.; Ning, K.H.; Su, C.; Ren, J.P.; Wang, L.X. Copolymers based on trialkylsilylethynyl-phenyl substituted benzodithiophene building blocks for efficient organic solar cells. New J. Chem. 2021, 45, 19818–19825. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, B.; Du, M.Z.; Dai, T.T.; Tang, A.L.; Guo, Q.; Zhou, E.J. Side-chain engineering of copolymers based on benzotriazole (BTA) and dithieno 2,3-d;2′,3′-d′ benzo 1,2-b;4,5-b′ dithiophenes (DTBDT) enables a high PCE of 14.6%. Nanotechnology 2021, 32, 22540. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Zhu, R.X.; Wang, Z.; Guo, F.Y.; Wei, Z.X.; Yang, Y.L.; Zhao, L.C.; Zhang, Y. An Asymmetrical Polymer Based on Thieno 2,3-f benzofuran for Efficient Fullerene-Free Polymer Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1888–1892. [Google Scholar] [CrossRef]
- Wang, D.X.; Dou, K.K.; Cui, W.; Li, F.; Jing, X.; Yu, L.M.; Sun, M.L. VOC enhancement of thienobenzofuran and benzotriazole backboned photovoltaic polymer by side chain sulfuration or fluoridation. Dye. Pigment. 2021, 184, 108775. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Chen, H.G.; Kim, S.; Chen, Y.T.; Zhang, Y.; Yang, M.Q.; Chen, Z.L.; Yang, C.D.; Wu, H.B.; Gao, X.; et al. Benzo 1,2-b:4,5-b’ Difuran-Based Polymer for Organic Solar Cells with 17.5% Efficiency via Halogenation-Mediated Aggregation Control. Adv. Energy Mater. 2023, 13, 2204394. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Wang, Z.; Zhang, J.Q.; Zhang, H.; Lu, K.; Guo, F.Y.; Yang, Y.L.; Zhao, L.C.; Wei, Z.X.; Zhang, Y. Two-dimensional benzo 1,2-b:4,5-b” difuran-based wide bandgap conjugated polymers for efficient fullerene-free polymer solar cells. J. Mater. Chem. A Mater. 2018, 6, 4023–4031. [Google Scholar] [CrossRef]
- Zhu, R.X.; Wang, Z.; Gao, Y.Y.; Zheng, Z.; Guo, F.Y.; Gao, S.Y.; Lu, K.; Zhao, L.C.; Zhang, Y. Chain Engineering of Benzodifuran-Based Wide-Bandgap Polymers for Efficient Non-Fullerene Polymer Solar Cells. Macromol. Rapid Commun. 2019, 40, 1900227. [Google Scholar] [CrossRef]
- Bin, H.J.; Zhong, L.; Yang, Y.K.; Gao, L.; Huang, H.; Sun, C.K.; Li, X.J.; Xue, L.W.; Zhang, Z.G.; Zhang, Z.J.; et al. Medium Bandgap Polymer Donor Based on Bi(trialkylsilylthienyl-benzo 1,2-b:4,5-b′ -difuran) for High Performance Nonfullerene Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1700746. [Google Scholar] [CrossRef]
- Zheng, Z.; He, E.F.; Lu, Y.; Yin, Y.L.; Pang, X.C.; Guo, F.Y.; Gao, S.Y.; Zhao, L.C.; Zhang, Y. Benzo 1,2-b:4,5-b’ difuran Polymer-Based Non-Fullerene Organic Solar Cells: The Roles of Non-Fullerene Acceptors and Molybdenum Oxide on Their Ambient Stabilities and Processabilities. ACS Appl. Mater. Interfaces 2021, 13, 15448–15458. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.L.; Li, X.M.; Wang, H.; Zhang, B.Y.; Li, Z.; Zhao, J.; Chen, W.C.; Yang, R.Q. Temperature-Dependent and Aggregation-Breaking Strategy for Benzodifuran-Constructed Organic Solar Cells. Solar Rrl 2019, 3, 1900159. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Shen, Z.T.; Tan, F.R.; Yue, G.T.; Liu, R.; Wang, Z.J.; Qu, S.C.; Wang, Z.G.; Zhang, W.F. Novel benzo 1,2-b:4,5-b′ difuran-based copolymer enables efficient polymer solar cells with small energy loss and high VOC. Nano Energy 2020, 76, 104964. [Google Scholar] [CrossRef]
- Gao, X.M.; Su, Z.H.; Qu, S.C.; Zhang, W.Z.; Gao, Y.Y.; He, S.H.; Wang, Z.J.; Shang, L.W.; Dong, G.H.; Yue, G.T.; et al. Efficient and moisture-resistant organic solar cells via simultaneously reducing the surface defects and hydrophilicity of an electron transport layer. J. Mater. Chem. C Mater. 2021, 9, 13500–13508. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Xiao, Z.; Cui, M.H.; Saidaminov, M.I.; Tan, F.R.; Shang, L.W.; Li, W.P.; Qin, C.C.; Ding, L.M. Asymmetric Π-Bridge Engineering Enables High-Permittivity Benzo 1,2-B:4,5-b′ Difuran-Conjugated Polymer for Efficient Organic Solar Cells. Adv. Mater. 2023, 36, 2306373. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Chen, F.; Xiao, B.; Yang, J.; Li, J.F.; Wang, X.C.; Zhou, E.J. Utilizing Benzotriazole and Indacenodithiophene Units to Construct Both Polymeric Donor and Small Molecular Acceptors to Realize Organic Solar Cells With High Open-Circuit Voltages Beyond 1.2 V. Front. Chem. 2018, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Xu, X.P.; Lee, Y.W.; Woo, H.Y.; Bi, Z.Z.; Ma, W.; Li, Y.; Peng, Q. Dithienothiapyran: An Excellent Donor Block for Building High-Performance Copolymers in Nonfullerene Polymer Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Jiang, K.; Yang, G.F.; Lai, J.Y.L.; Ma, T.X.; Zhao, J.B.; Ma, W.; Yan, H. Donor polymer design enables efficient non-fullerene organic solar cells. Nat. Commun. 2016, 7, 13094. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Bin, H.J.; Angunawela, I.; Jia, Z.R.; Qiu, B.B.; Sun, C.K.; Li, X.J.; Zhang, Z.J.; Ade, H.; Li, Y.F. Effect of Replacing Thiophene by Selenophene on the Photovoltaic Performance of Wide Bandgap Copolymer Donors. Macromolecules 2019, 52, 4776–4784. [Google Scholar] [CrossRef]
- Unlu, N.A.; Hacioglu, S.O.; Hizalan, G.; Yildiz, D.E.; Toppare, L.; Cirpan, A. Benzodithiophene and Benzotriazole Bearing Conjugated Polymers for Electrochromic and Organic Solar Cell Applications. J. Electrochem. Soc. 2017, 164, G71–G76. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.Q.; Du, M.Z.; Li, G.Q.; Li, Z.J.; Huang, H.; Geng, Y.F.; Zhang, X.L.; Zhou, E.J. Benzotriazole-Based p-Type Polymers with Thieno 3,2-b thiophene π-Bridges and Fluorine Substituents To Realize High VOC. ACS Appl. Polym. Mater. 2019, 1, 906–913. [Google Scholar] [CrossRef]
- Lei, P.; Zhang, B.; Chen, Y.; Geng, Y.F.; Zeng, Q.D.; Tang, A.L.; Zhou, E.J. Gradual Fluorination on the Phenyl Side Chains for Benzodithiophene-Based Linear Polymers to Improve the Photovoltaic Performance. ACS Appl. Mater. Interfaces 2020, 12, 38451–38459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, P.; Geng, Y.F.; Meng, T.; Li, X.Y.; Zeng, Q.D.; Guo, Q.; Tang, A.L.; Zhong, Y.F.; Zhou, E.R. Selective fluorination on donor and acceptor for management of efficiency and energy loss in non-fullerene organic photovoltaics. Sci. China-Chem. 2023, 66, 1190–1200. [Google Scholar] [CrossRef]
- Fan, B.B.; Zhang, K.; Jiang, X.F.; Ying, L.; Huang, F.; Cao, Y. High-Performance Nonfullerene Polymer Solar Cells based on Imide-Functionalized Wide-Bandgap Polymers. Adv. Mater. 2017, 29, 1606396. [Google Scholar] [CrossRef]
- Fan, B.B.; Ying, L.; Wang, Z.F.; He, B.T.; Jiang, X.F.; Huang, F.; Cao, Y. Optimisation of processing solvent and molecular weight for the production of green-solvent-processed all-polymer solar cells with a power conversion efficiency over 9%. Energy Environ. Sci. 2017, 10, 1243–1251. [Google Scholar] [CrossRef]
- Xu, Y.L.; Yuan, J.Y.; Zhou, S.J.; Seifrid, M.; Ying, L.; Li, B.; Huang, F.; Bazan, G.C.; Ma, W.L. Ambient Processable and Stable All-Polymer Organic Solar Cells. Adv. Funct. Mater. 2019, 29, 1806747. [Google Scholar] [CrossRef]
- Du, H.J.; An, K.; Wang, R.; Yin, Z.P.; Peng, F.; Lüer, L.; Brabec, C.J.; Ying, L.; Li, N. Achieving High External Quantum Efficiency for ITIC-Based Organic Solar Cells with Negligible Homo Energy Offsets. Adv. Energy Mater. 2023, 14, 2301965. [Google Scholar] [CrossRef]
- Zhu, C.G.; Li, Z.Y.; Zhong, W.K.; Peng, F.; Zeng, Z.M.Y.; Ying, L.; Huang, F.; Cao, Y. Constructing a new polymer acceptor enabled non-halogenated solvent-processed all-polymer solar cell with an efficiency of 13.8%. Chem. Commun. 2021, 57, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.B.; Ying, L.; Zhu, P.; Pan, F.L.; Liu, F.; Chen, J.W.; Huang, F.; Cao, Y. All-Polymer Solar Cells Based on a Conjugated Polymer Containing Siloxane-Functionalized Side Chains with Efficiency over 10%. Adv. Mater. 2017, 29, 1703906. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Ying, L.; Zhu, P.; Zhong, W.K.; Li, N.; Liu, F.; Huang, F.; Cao, Y. A generic green solvent concept boosting the power conversion efficiency of all-polymer solar cells to 11%. Energy Environ. Sci. 2019, 12, 157–163. [Google Scholar] [CrossRef]
- Li, M.J.; Fan, B.B.; Zhong, W.K.; Zeng, Z.M.Y.; Xu, J.K.; Ying, L. Rational Design of Conjugated Polymers for d-Limonene Processed All-polymer Solar Cells with Small Energy Loss. Chin. J. Polym. Sci. 2020, 38, 791–796. [Google Scholar] [CrossRef]
- Fan, B.B.; Zhang, D.F.; Li, M.J.; Zhong, W.K.; Zeng, Z.M.Y.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% efficiency for single-junction organic solar cells. Sci. China-Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Fan, B.B.; Zeng, Z.M.Y.; Zhong, W.K.; Ying, L.; Zhang, D.F.; Li, M.J.; Peng, F.; Li, N.; Huang, F.; Gao, Y. Optimizing Microstructure Morphology and Reducing Electronic Losses in 1 cm2 Polymer Solar Cells to Achieve Efficiency over 15%. ACS Energy Lett. 2019, 4, 2466–2472. [Google Scholar] [CrossRef]
- Nie, Q.L.; Tang, A.L.; Cong, P.Q.; Chen, L.; Zhang, Q.Q.; Ji, H.R.; Li, G.Q.; Guo, Q.; Zhou, E.J. Wide Band Gap Photovoltaic Polymer Based on Pyrrolo 3,4-f benzotriazole-5,7-dione (TzBI) with Ultrahigh VOC Beyond 1.25 V. J. Phys. Chem. C 2020, 124, 19492–19498. [Google Scholar] [CrossRef]
- Zheng, N.N.; Mahmood, K.; Zhong, W.K.; Liu, F.; Zhu, P.; Wang, Z.F.; Xie, B.M.; Chen, Z.M.; Zhang, K.; Ying, L.; et al. Improving the efficiency and stability of non-fullerene polymer solar cells by using N2200 as the Additive. Nano Energy 2019, 58, 724–731. [Google Scholar] [CrossRef]
- Li, M.J.; Zeng, Z.M.Y.; Fan, B.B.; Zhong, W.K.; Zhang, D.F.; Zhang, X.N.; Zhang, Y.; Ying, L.; Huang, F.; Cao, Y. Tailoring the side chain of imide-functional benzotriazole based polymers to achieve internal quantum efficiency approaching 100%. J. Mater. Chem. A Mater. 2020, 8, 23519–23525. [Google Scholar] [CrossRef]
- An, K.; Zhong, W.K.; Peng, F.; Deng, W.Y.; Shang, Y.; Quan, H.L.; Qiu, H.; Wang, C.; Liu, F.; Wu, H.B.; et al. Mastering morphology of non-fullerene acceptors towards long-term stable organic solar cells. Nat. Commun. 2023, 14, 2688. [Google Scholar] [CrossRef]
- Yang, T.Y.; Liao, C.T.; Duan, Y.W.; Xu, X.P.; Deng, M.; Yu, L.Y.; Li, R.P.; Peng, Q. Tradeoff between Intermolecular Interaction and Backbone Disorder by High Molecular Dipole Block for Improving Blend Morphology of Polymer Solar Cells. Adv. Funct. Mater. 2022, 32, 2208950. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Zhu, J.S.; Lau, T.K.; Cheng, P.; Lu, X.H.; Zhan, X.W.; Chen, X.G. Constructing D-A copolymers based on thiophene-fused benzotriazole units containing different alkyl side-chains for non-fullerene polymer solar cells. J. Mater. Chem. C Mater. 2017, 5, 8179–8186. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.C.; Yang, Y.; Zhan, X.W.; Chen, X.G. Fluorinated Thieno 2′,3′:4,5 benzo 1,2-d 1,2,3 triazole: New Acceptor Unit To Construct Polymer Donors. ACS Omega 2018, 3, 13894–13901. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Chen, X.G.; Zhou, J.L.; Tang, A.L.; Geng, Y.F.; Guo, Q.; Zhou, E.J. Modulation of Three p-Type Polymers Containing a Fluorinated-Thiophene-Fused-Benzotriazole Unit To Pair with a Benzotriazole-Based Non-fullerene Acceptor for High Voc Organic Solar Cells. Macromolecules 2019, 52, 8625–8630. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.C.; Wang, W.; Yang, Y.; Zhan, X.W.; Chen, X.G. Impact of an electron withdrawing group on the thiophene-fused benzotriazole unit on the photovoltaic performance of the derived polymer solar cells. Dye. Pigment. 2019, 166, 381–389. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhong, C.; Cai, G.L.; Li, Y.W.; Wang, J.Y.; Lu, H.; Jia, B.Y.; Lu, X.H.; Lin, Y.Z.; Zhan, X.W.; et al. Subtle structural modification of thiophene-fused benzotriazole unit to simultaneously improve the JSC and VOC of OSCs. J. Mater. Chem. C Mater. 2024, 12, 1860–1869. [Google Scholar] [CrossRef]
- Tang, D.S.; Wan, J.H.; Xu, X.P.; Lee, Y.W.; Woo, H.Y.; Feng, K.; Peng, Q. Naphthobistriazole-based wide bandgap donor polymers for efficient non-fullerene organic solar cells: Significant fine-tuning absorption and energy level by backbone fluorination. Nano Energy 2018, 53, 258–269. [Google Scholar] [CrossRef]
- Feng, K.; Yuan, J.; Bi, Z.Z.; Ma, W.; Xu, X.P.; Zhang, G.J.; Peng, Q. Low-Energy-Loss Polymer Solar Cells with 14.52% Efficiency Enabled by Wide-Band-Gap Copolymers. IScience 2019, 12, 1–12. [Google Scholar] [CrossRef]
- He, Z.; Dai, T.; Ji, M.; Tang, A.; Wang, H.; Zhou, E. Fused Benzotriazole A-Unit Constructs a D-π-A Polymer Donor for Efficient Organic Photovoltaics. ACS Macro Lett. 2023, 12, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.T.; Li, Y.X.; Yu, J.W.; Wu, Z.A.; Fan, Q.P.; Lin, F.; Woo, H.Y.; Gao, F.; Zhu, Z.L.; Jen, A.K.Y. High Efficiency (15.8%) All-Polymer Solar Cells Enabled by a Regioregular Narrow Bandgap Polymer Acceptor. J. Am. Chem. Soc. 2021, 143, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.X.; Wang, K.; Li, M.J.; Peng, Z.Y.; Liu, H.L.; Huang, M.H.; Zhao, B. A-A Strategy Enables Desirable Performance of All-Polymer Solar Cells Fabricated with Nonhalogenated Solvents. ACS Appl. Mater. Interfaces 2023, 15, 48255–48263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.F.; Tang, A.L.; Geng, Y.F.; Guo, Q.; Zhou, E.J. Utilizing Benzotriazole-Fused DAD-Type Heptacyclic Ring to Construct n-Type Polymer for All-Polymer Solar Cell Application. ACS Appl. Energy Mater. 2021, 4, 4217–4223. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.G.; Yuan, J.; Zou, J.; Li, J.; Guan, H.L.; Zou, Y.P. A new low-bandgap polymer acceptor based on benzotriazole for efficient all-polymer solar cells. J. Cent. South. Univ. 2021, 28, 1919–1931. [Google Scholar] [CrossRef]
- Fu, H.T.; Li, Y.X.; Wu, Z.; Lin, F.R.; Woo, H.Y.; Jen, A.K.Y. Side-Chain Substituents on Benzotriazole-Based Polymer Acceptors Affecting the Performance of All-Polymer Solar Cells. Macromol. Rapid Commun. 2022, 43, 2200062. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Duan, X.P.; Qiao, J.W.; Li, S.L.; Cai, Y.H.; Zhang, J.Q.; Zhang, Y.; Hao, X.T.; Sun, Y.M. Benzotriazole-Based Polymer Acceptor for High-Efficiency All-Polymer Solar Cells with High Photocurrent and Low Voltage Loss. Adv. Energy Mater. 2023, 13, 2203044. [Google Scholar] [CrossRef]
- Morsy, M.A.; Saleh, K. Graded-Index Active Layer for Efficiency Enhancement in Polymer Solar Cell. Energies 2023, 16, 3933. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, L.Y.; Meng, L.X.; Zhang, C.; Duan, X.P.; Man, Y.H.; Jee, M.H.; Han, L.L.; Pan, Y.Y.; Wei, D.H.; et al. Rational Design of Near-Infrared Polymer Acceptors Using Steric Hindrance Strategy for High-Performance Organic Solar Cells. Adv. Funct. Mater. 2024, 9, 2316090. [Google Scholar] [CrossRef]
- Zhao, X.B.; Wang, T.; Wang, W.; Sun, R.; Wu, Q.; Shen, H.; Xia, J.L.; Wang, Y.; Zhang, M.J.; Min, J. Polymerized small-molecule acceptors based on vinylene as π-bridge for efficient all-polymer solar cells. Polymer 2021, 230, 124104. [Google Scholar] [CrossRef]
- Luo, M.; Chen, Y.C.; Zhang, L.J.; Yuan, D.; Zhang, Z.S.; Kong, L.C.; Chen, J.W. Benzotriazole-Featured Polymer Acceptors Toward As-Cast All-Polymer Solar Cells with a Remarkable Short-Circuit Current Density. ACS Appl. Polym. Mater. 2022, 4, 9116–9124. [Google Scholar] [CrossRef]
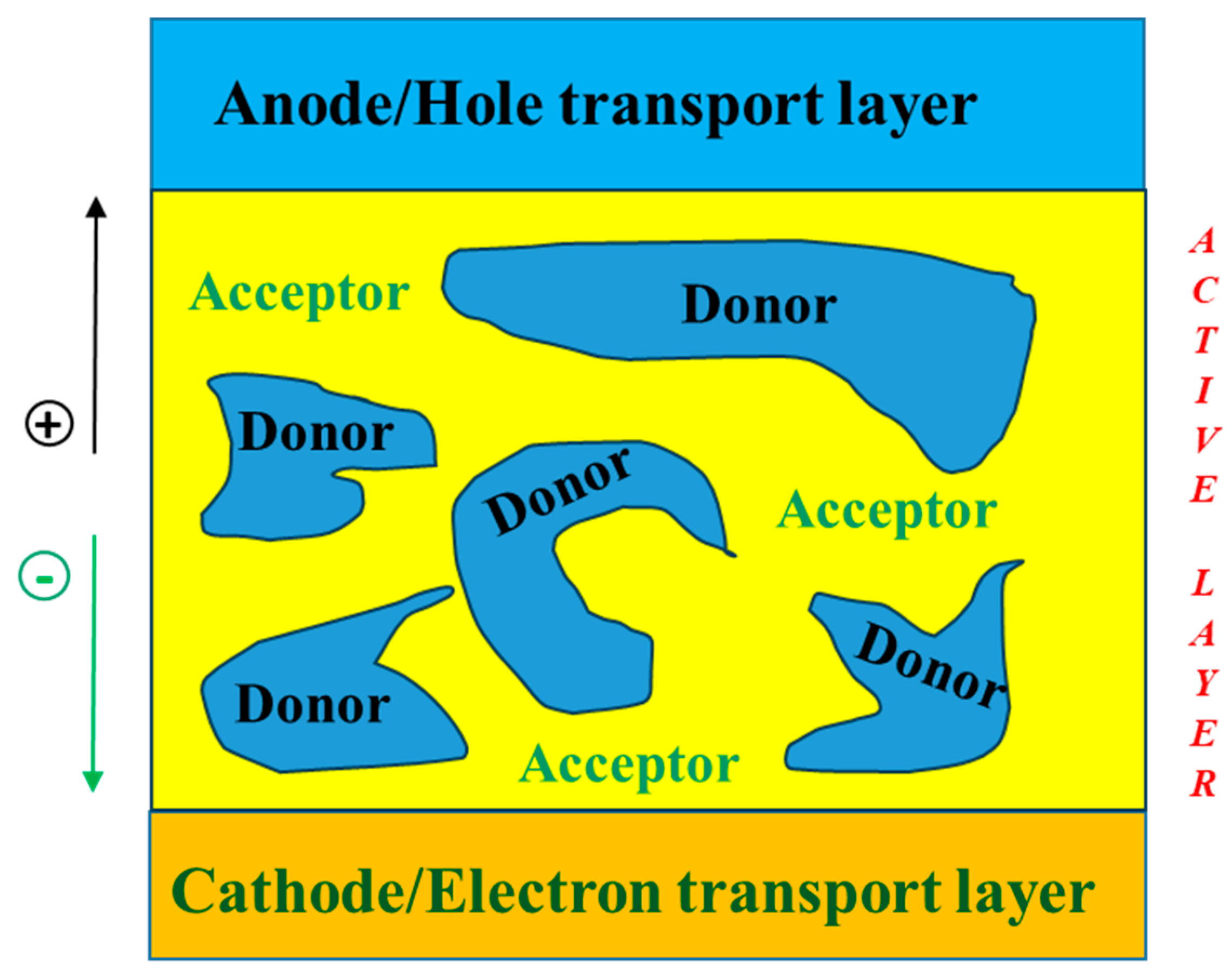
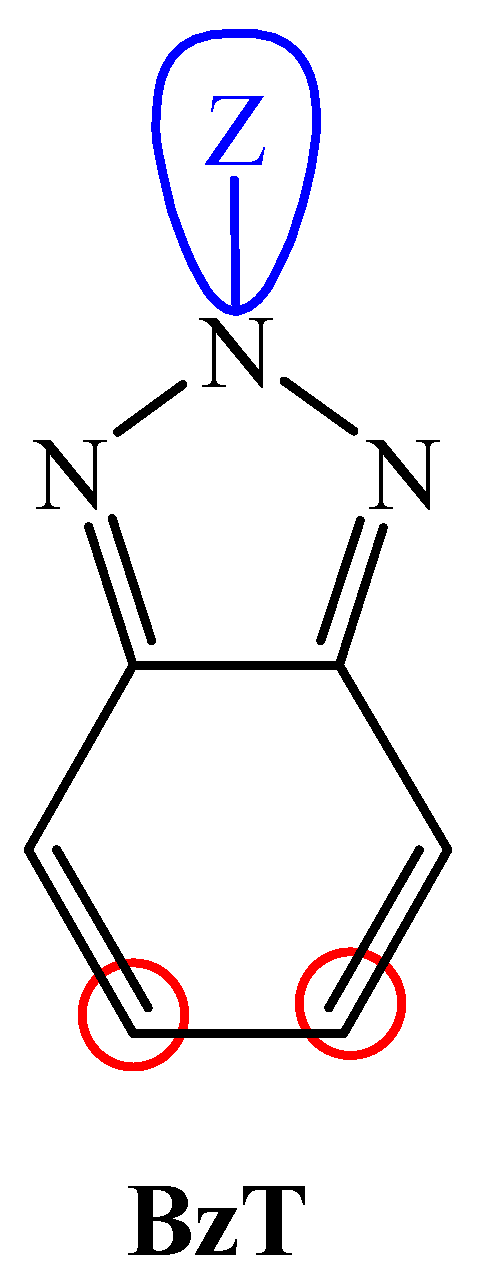

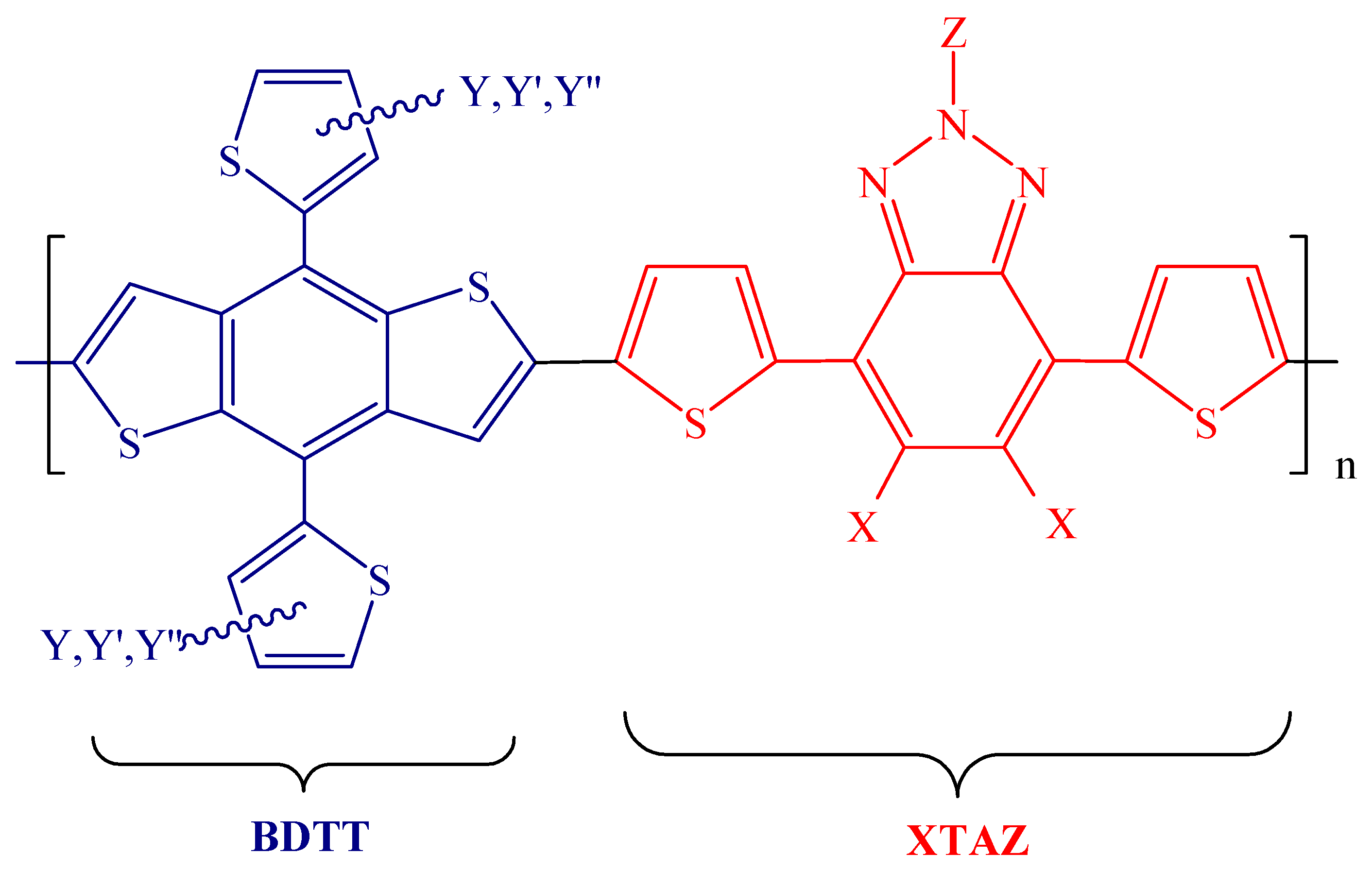
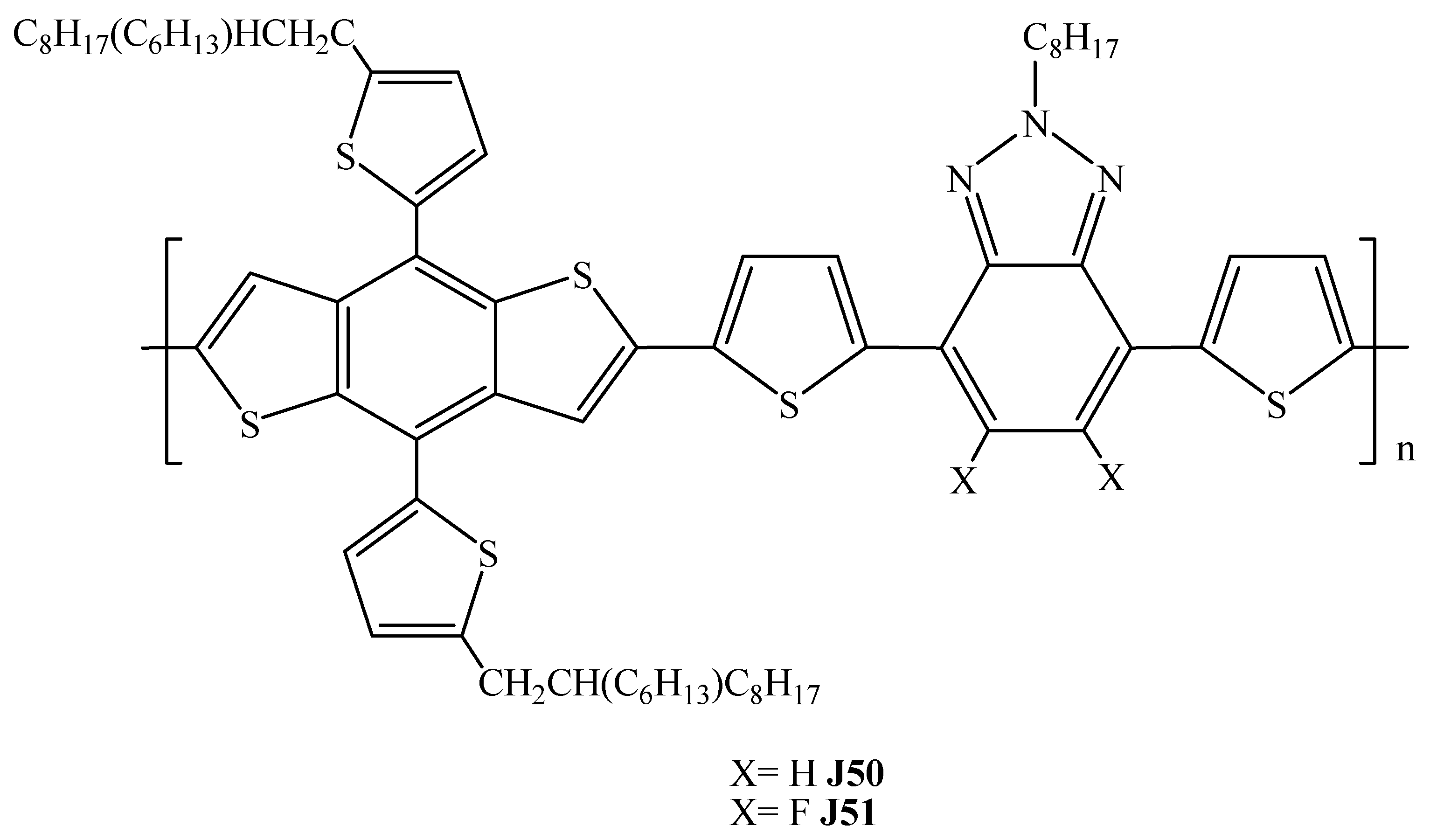
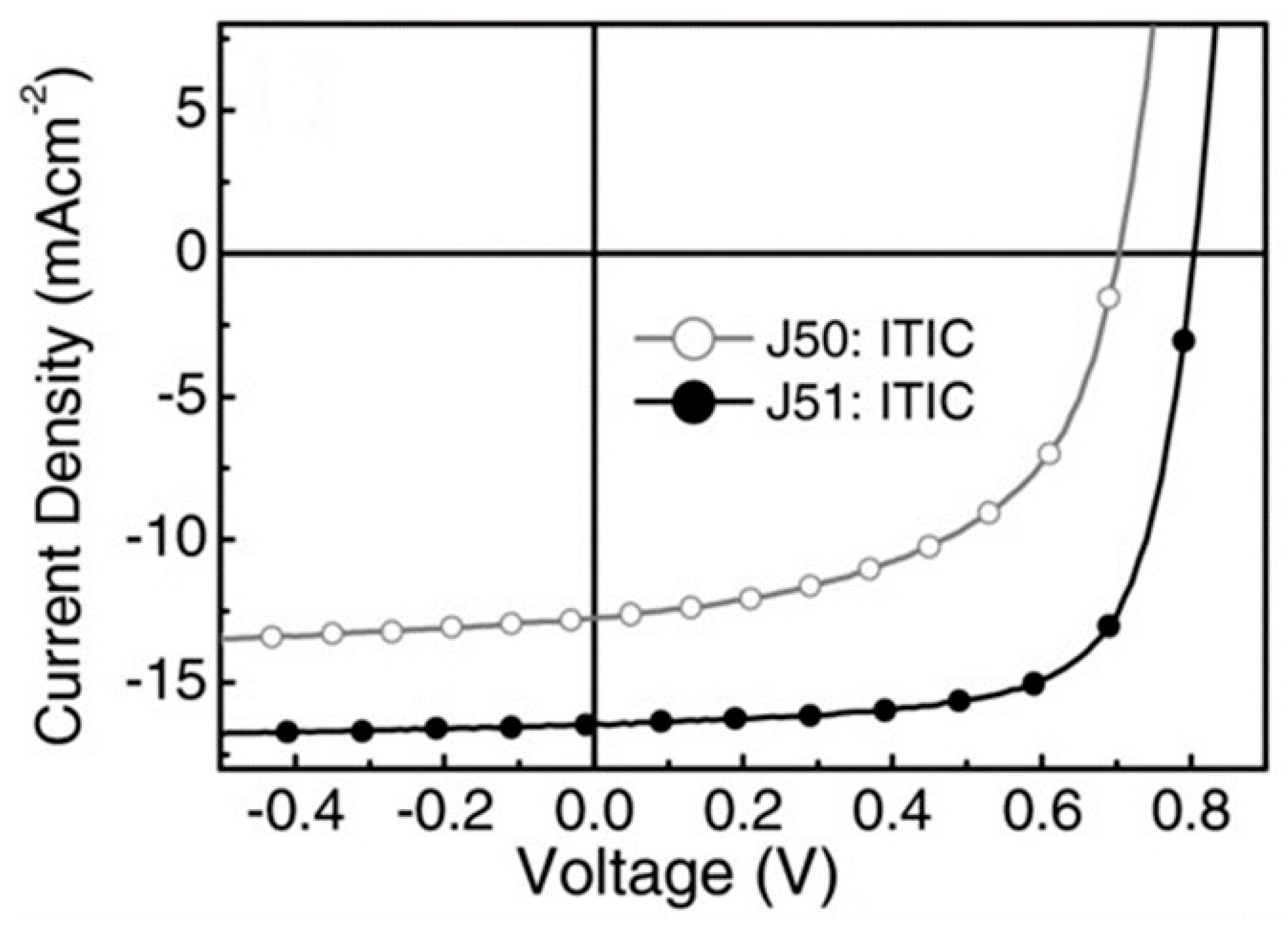
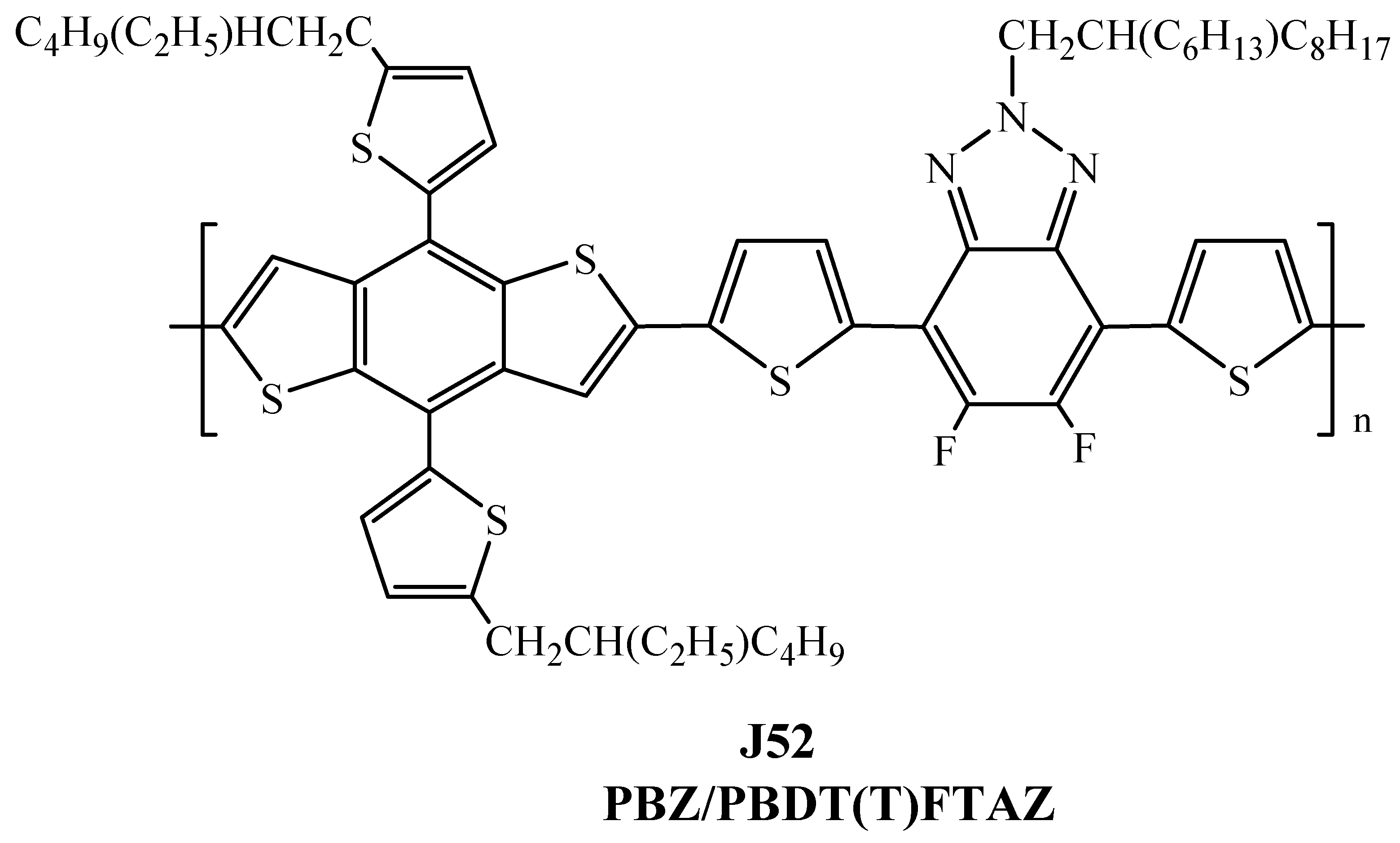

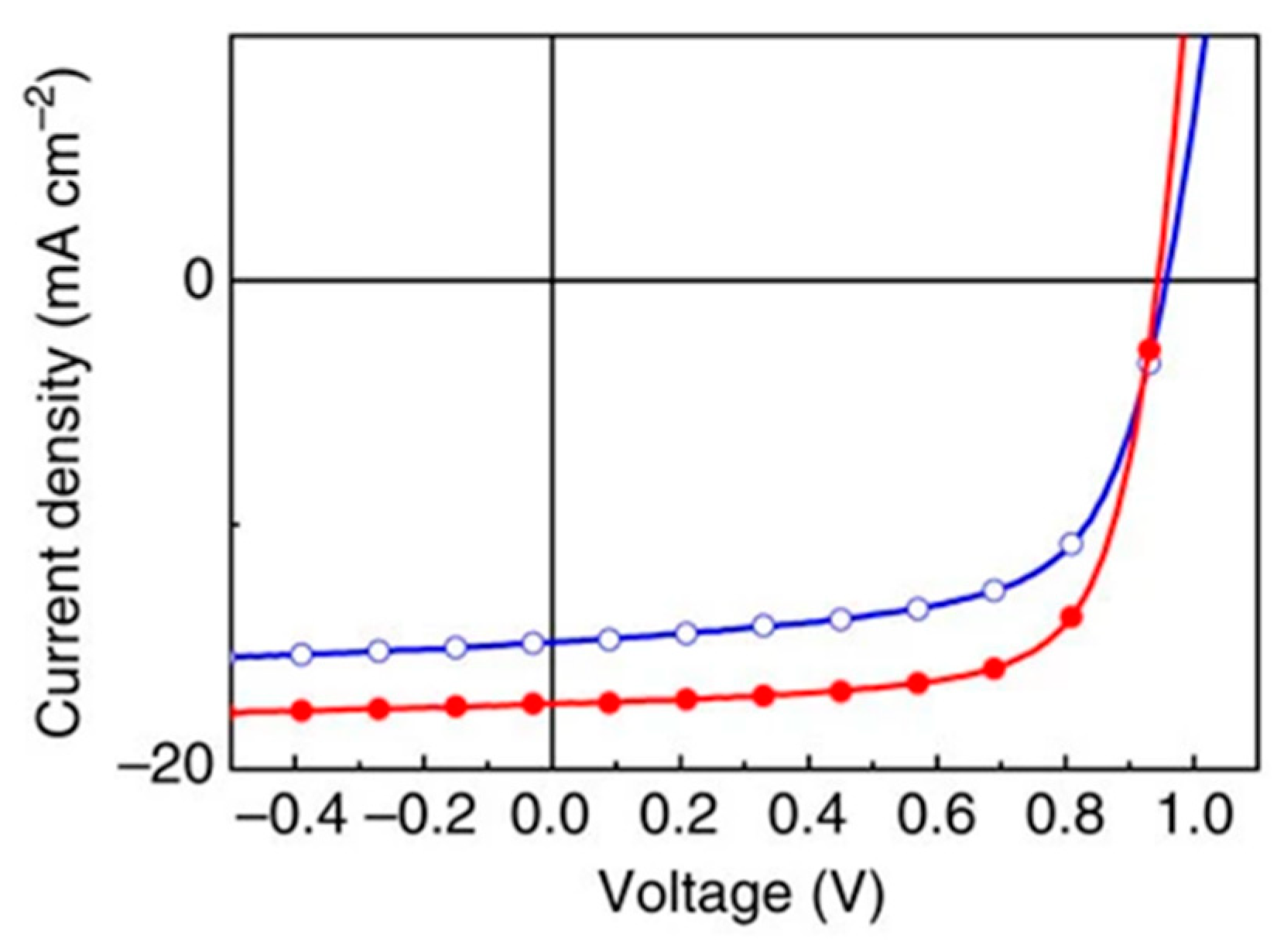
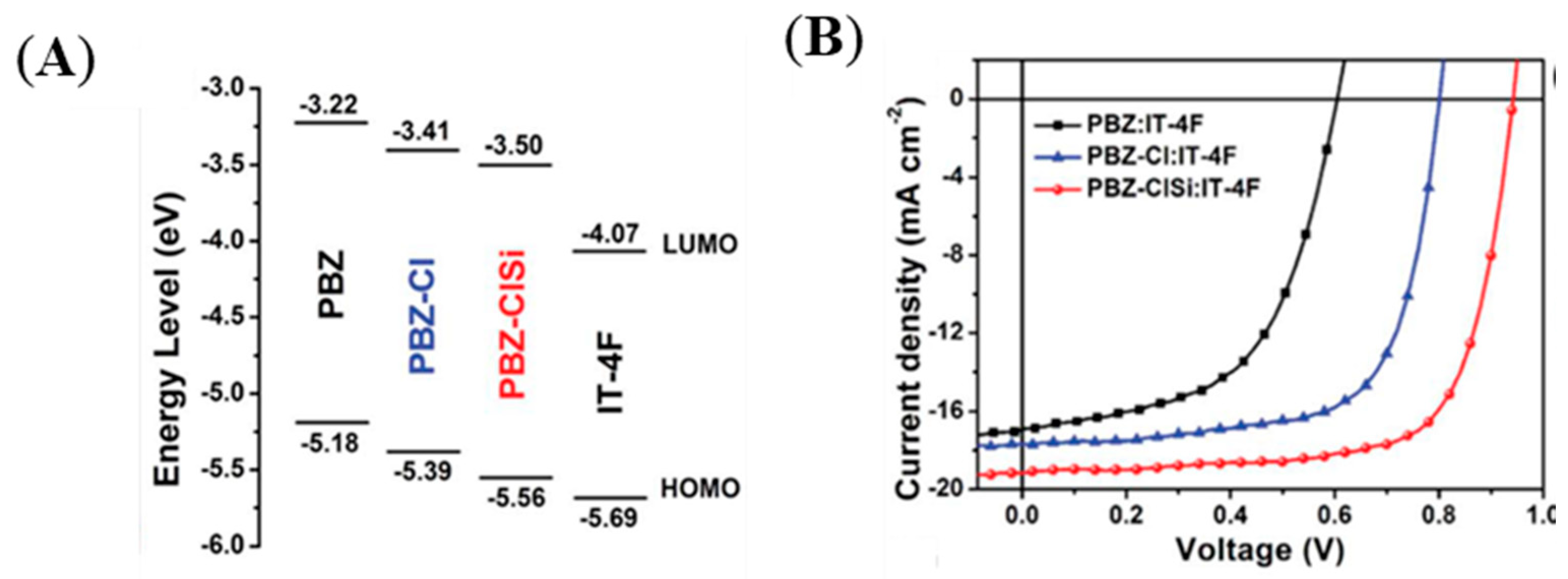

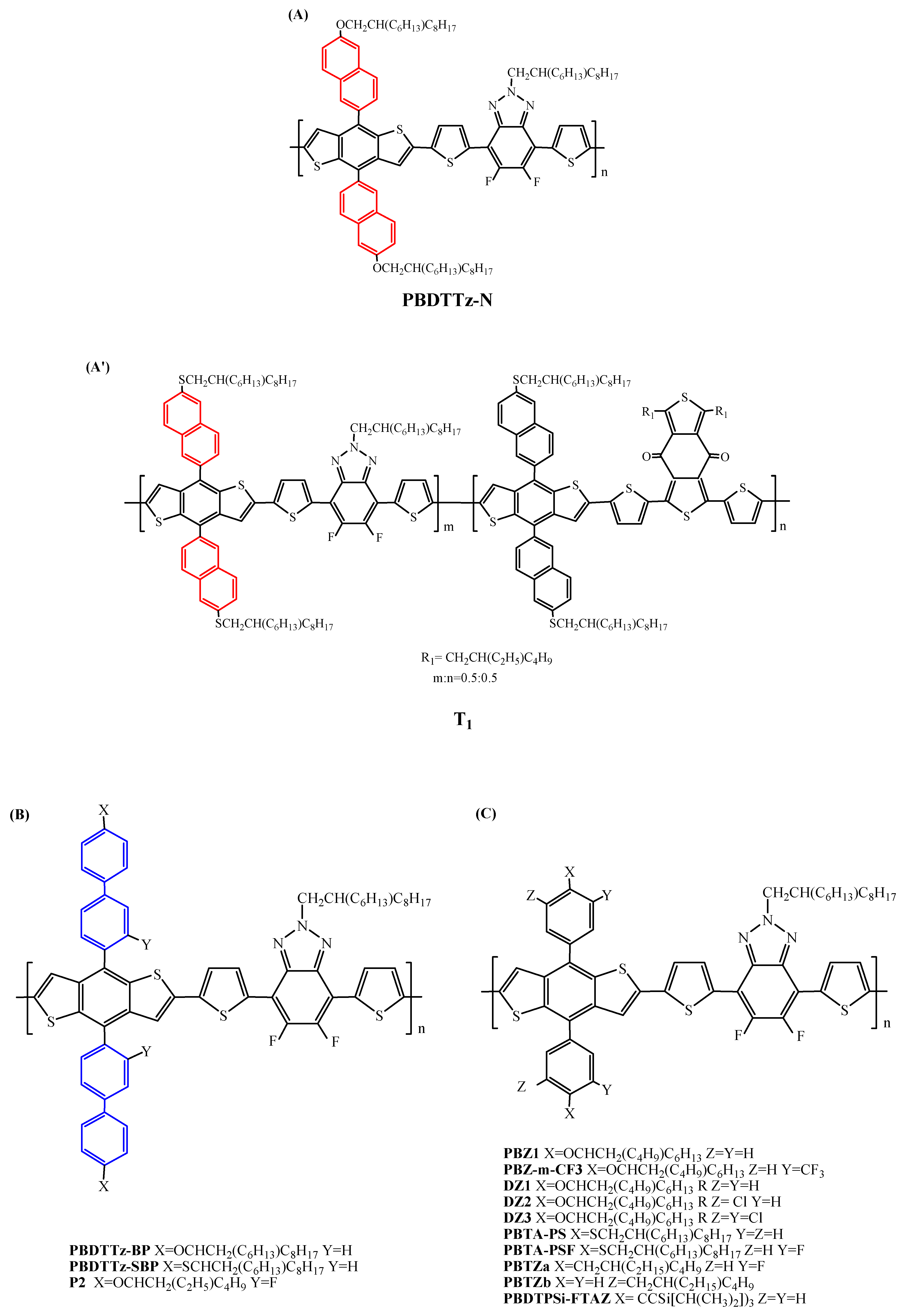
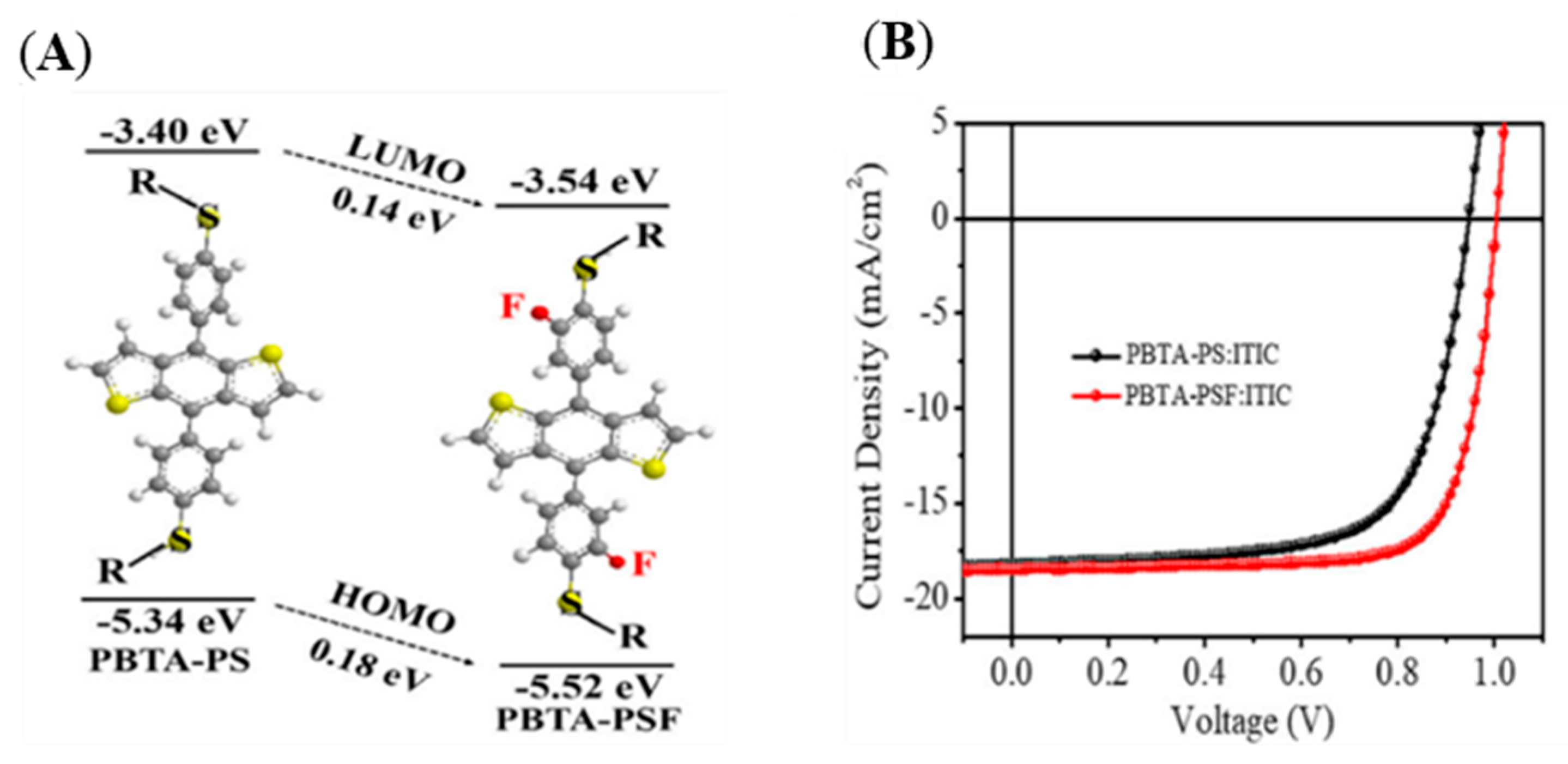
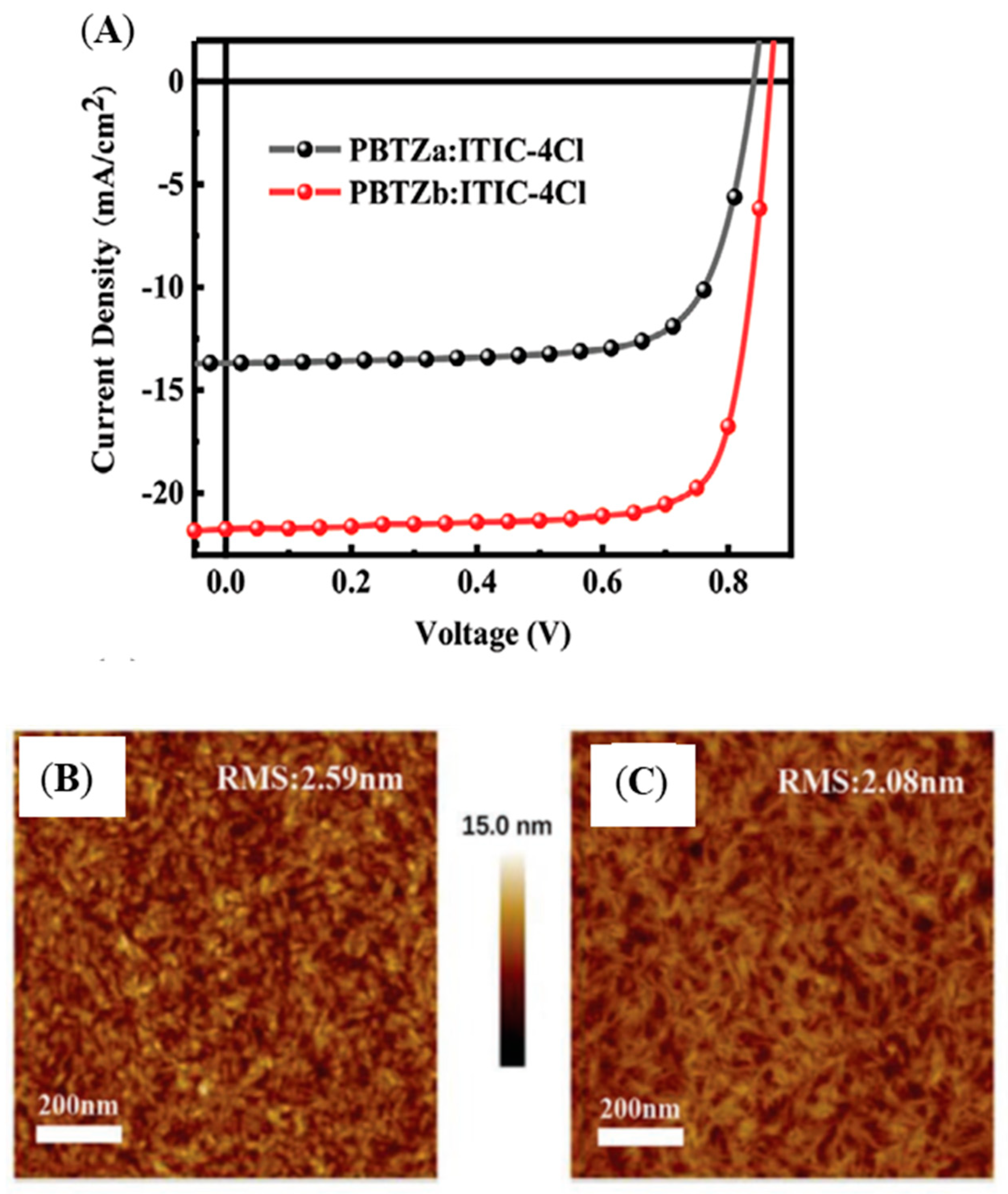
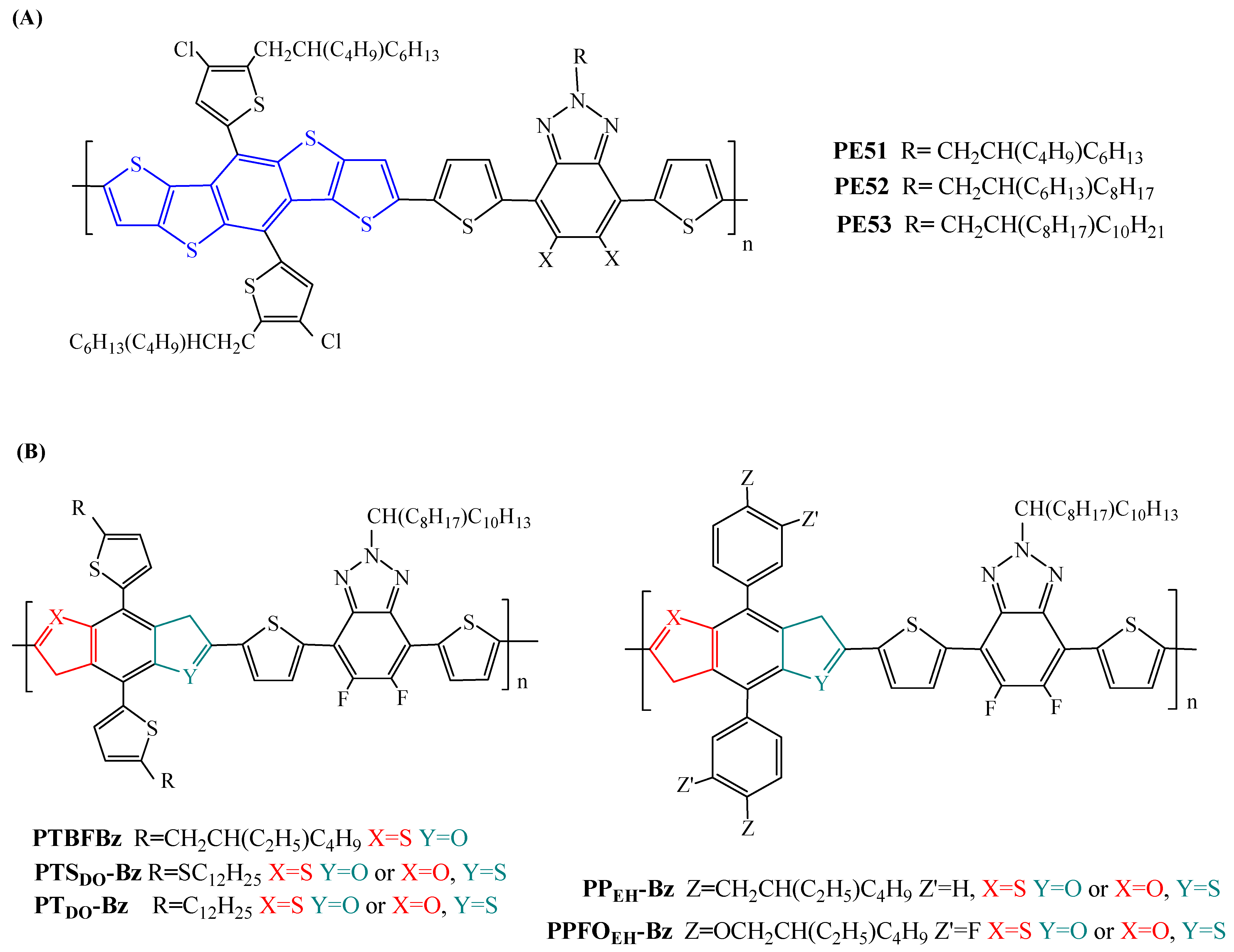
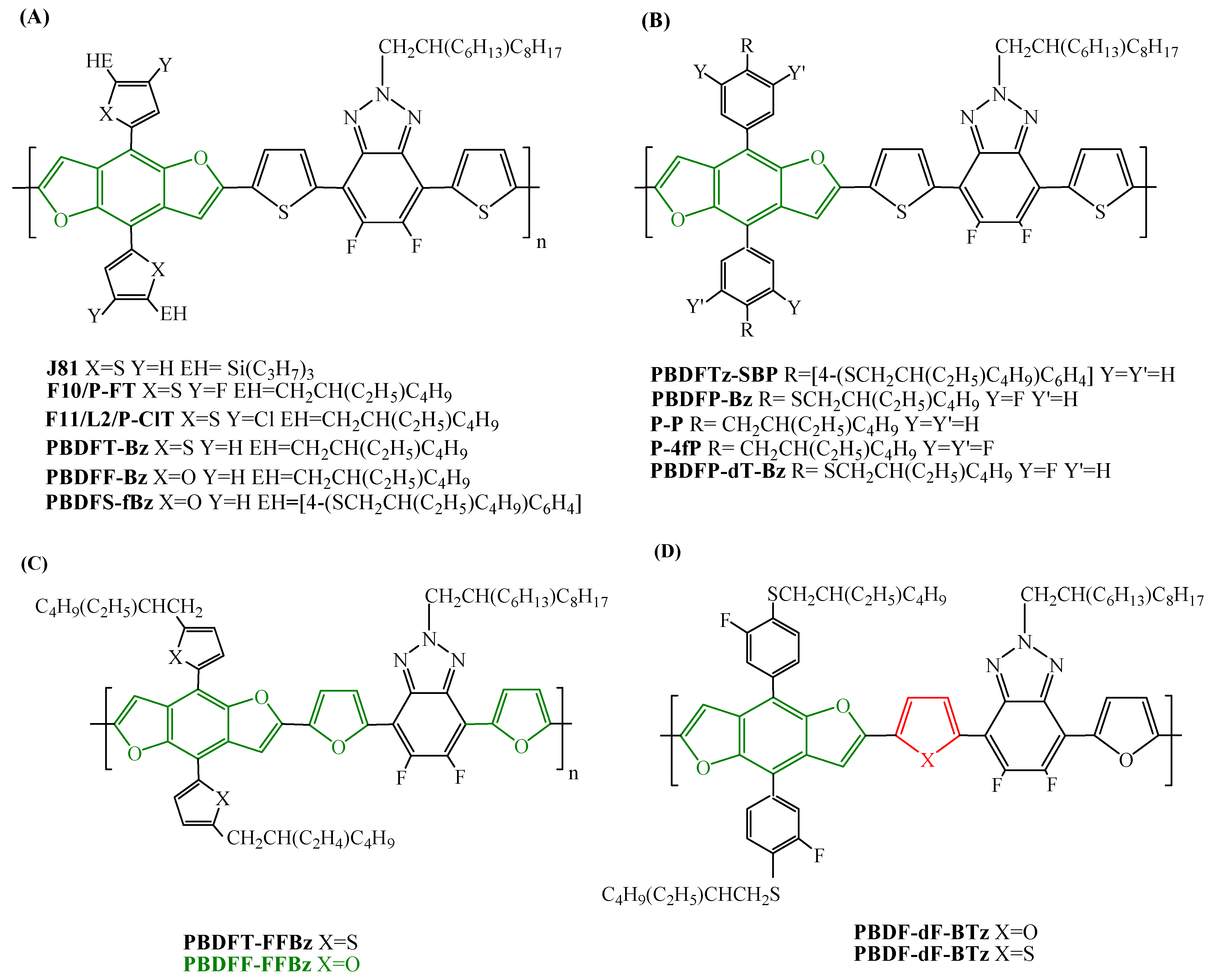


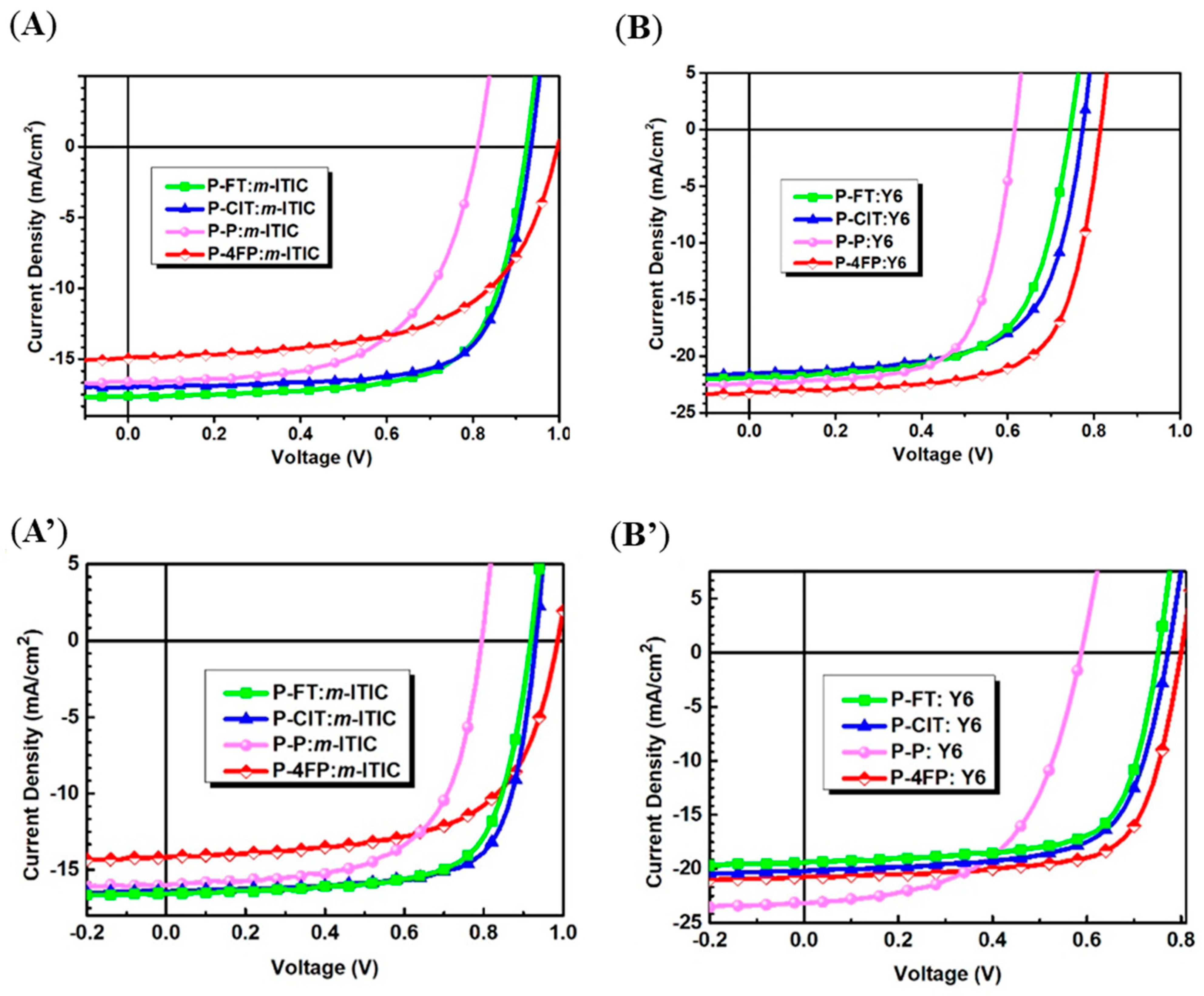
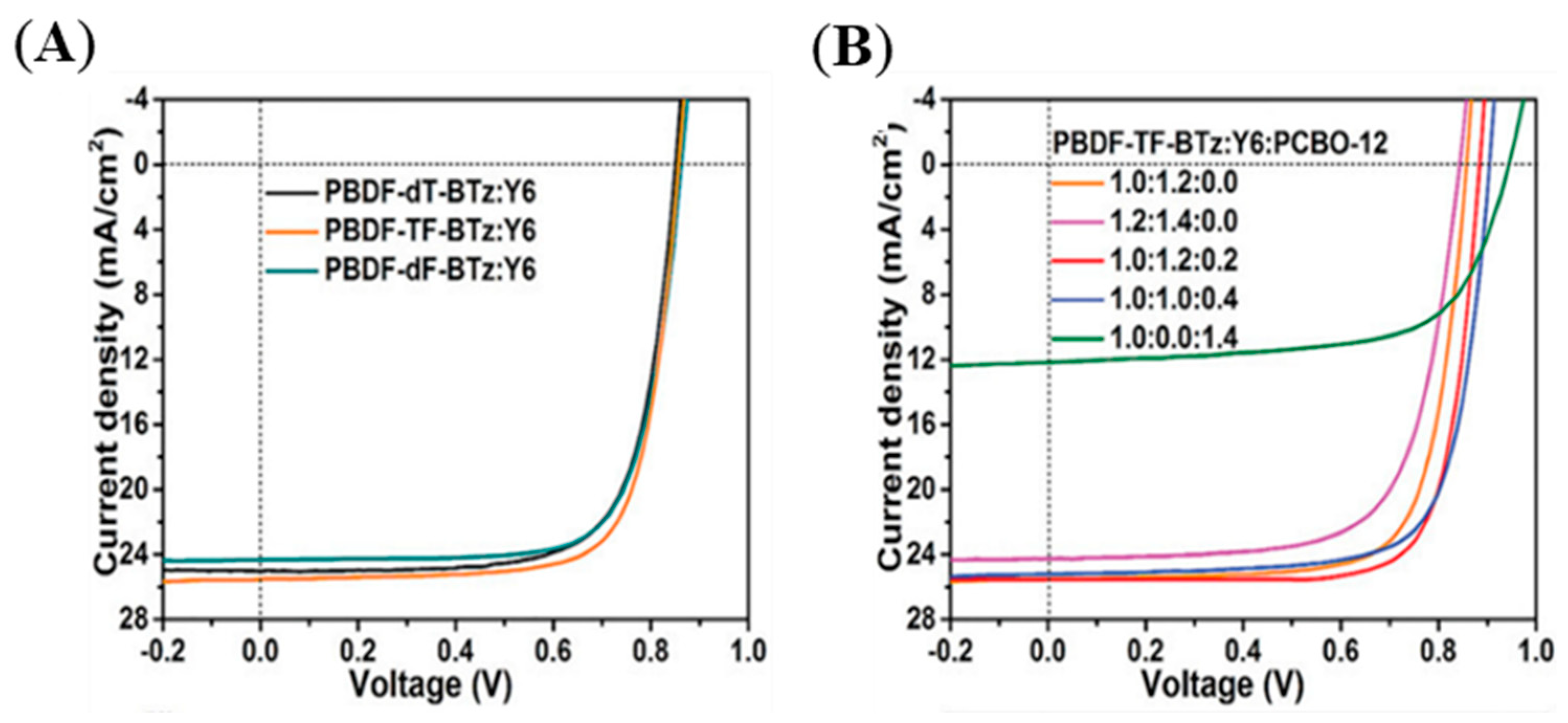

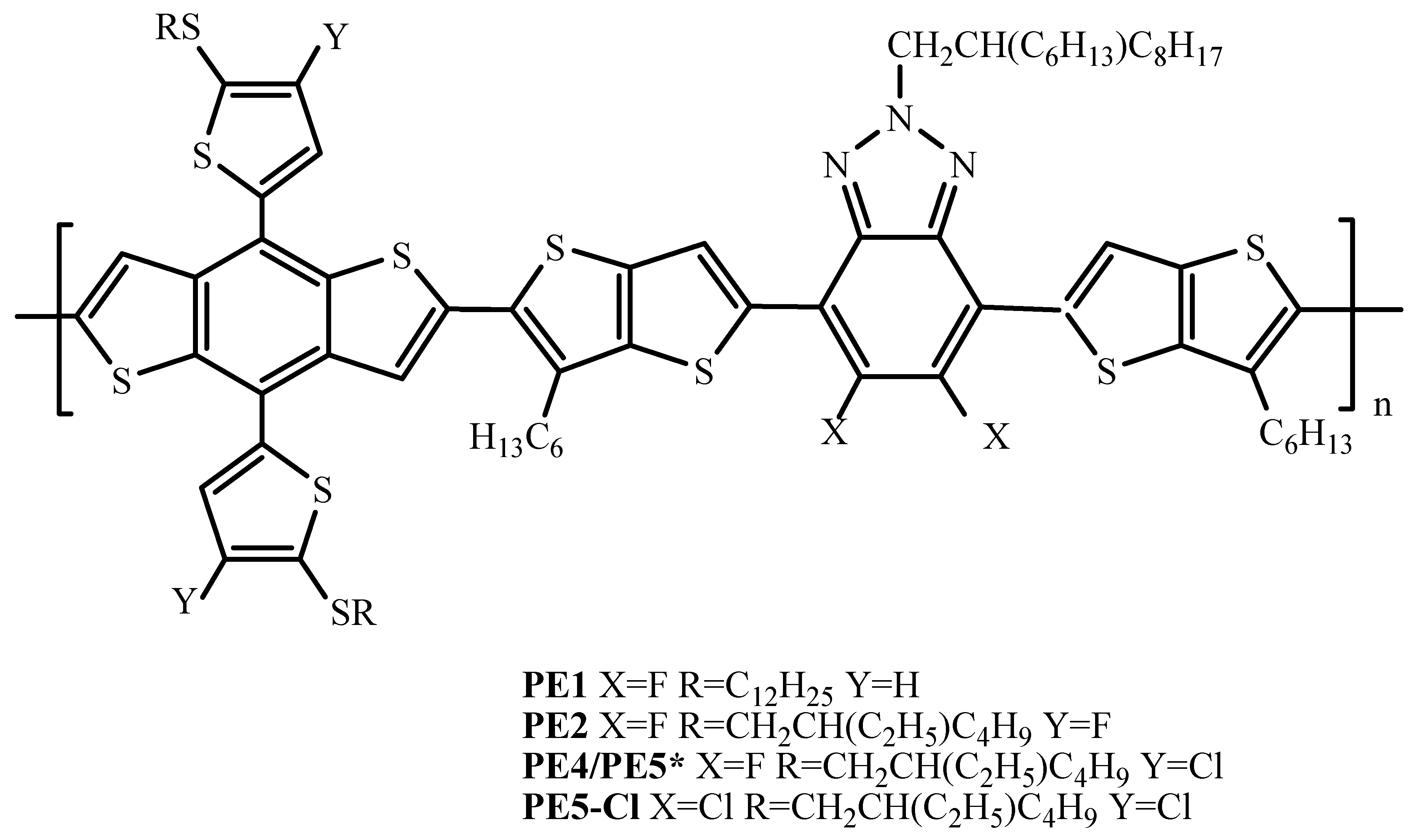
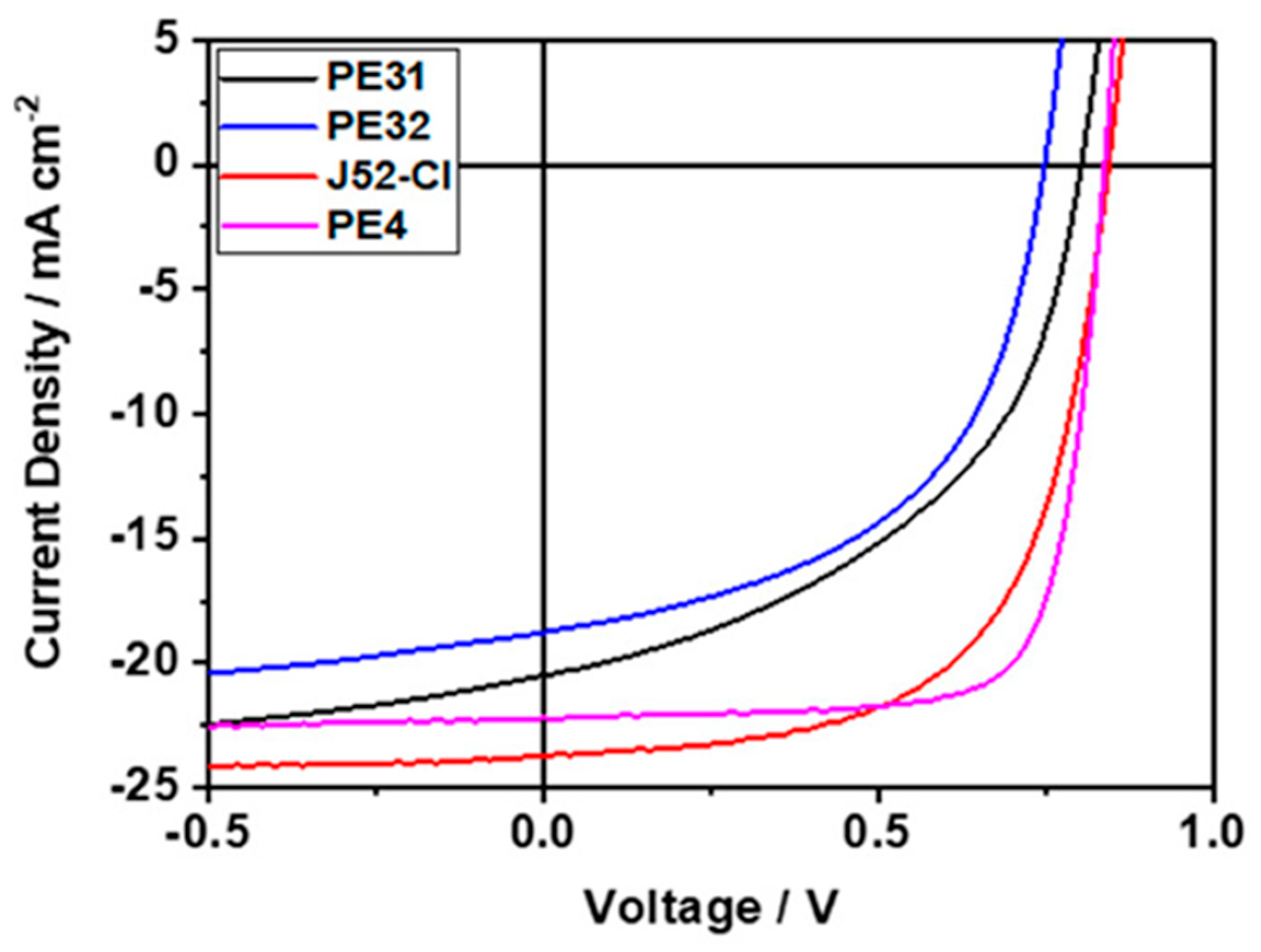
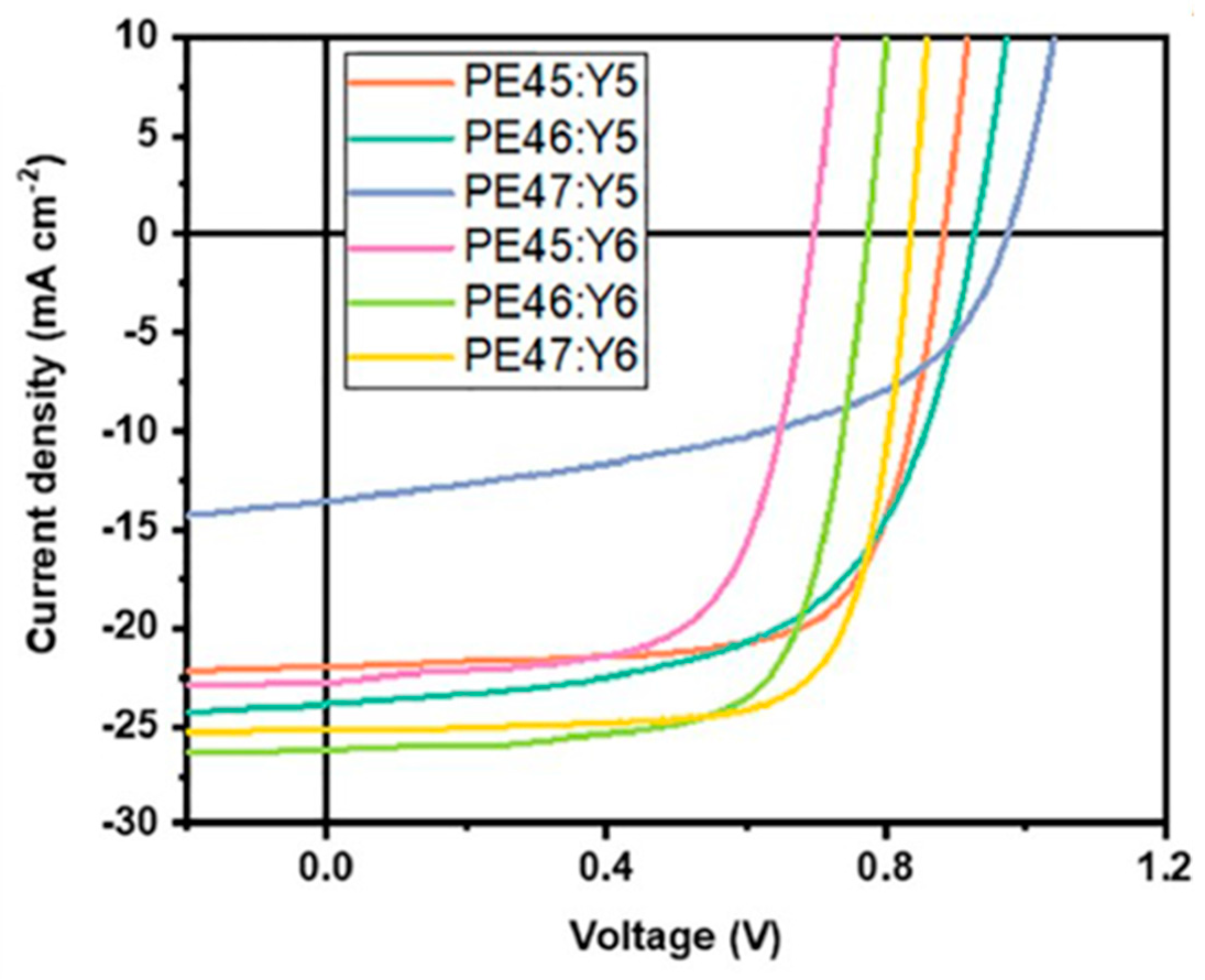

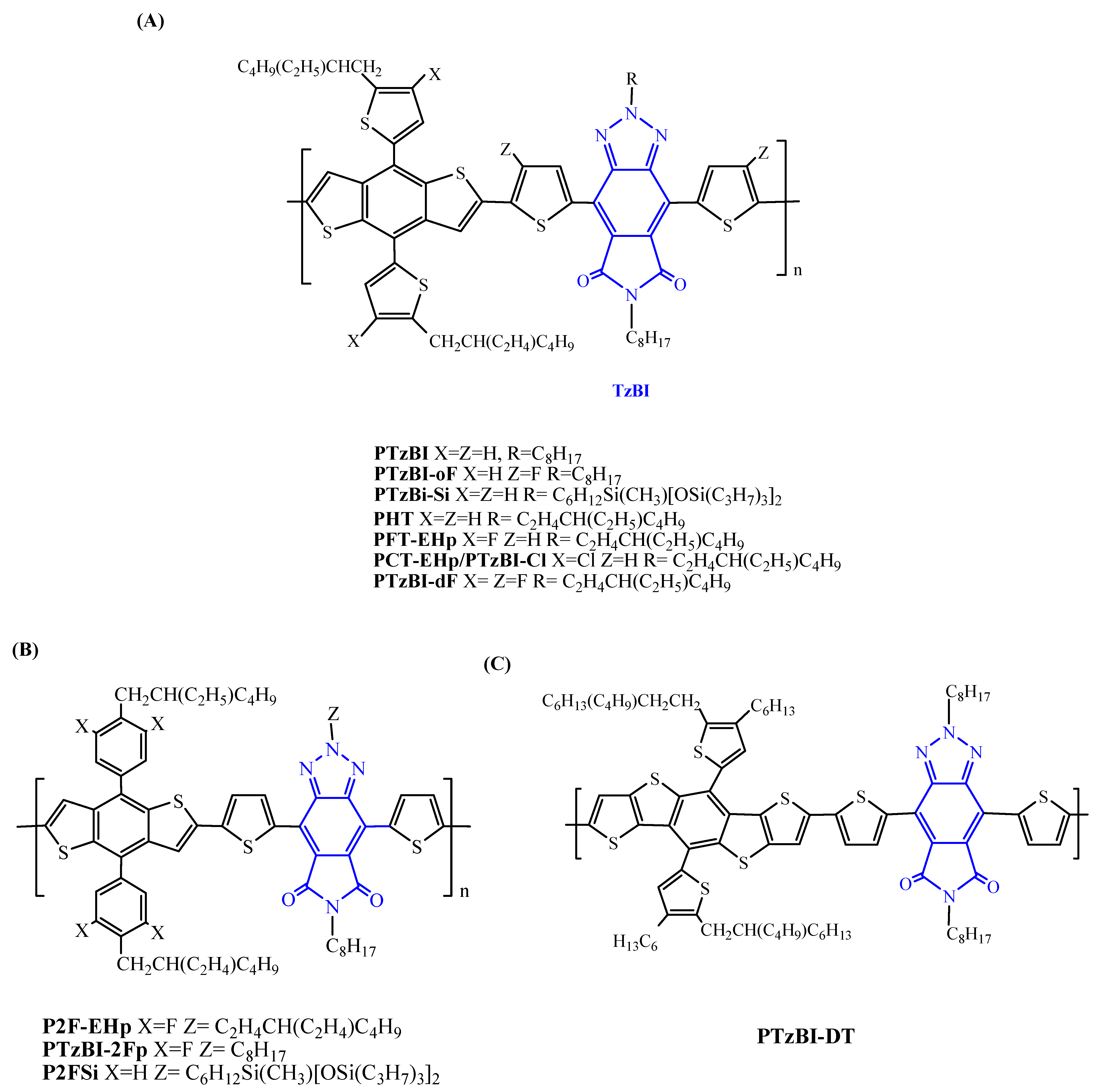
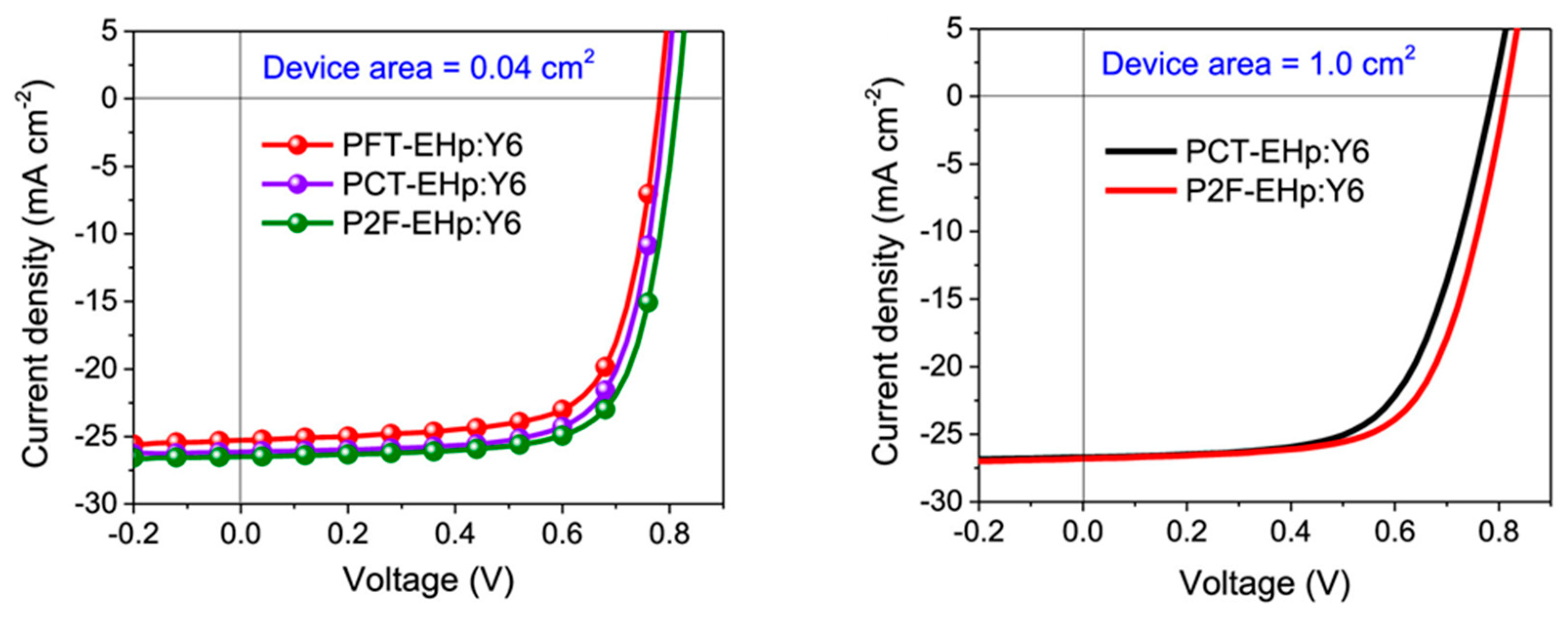
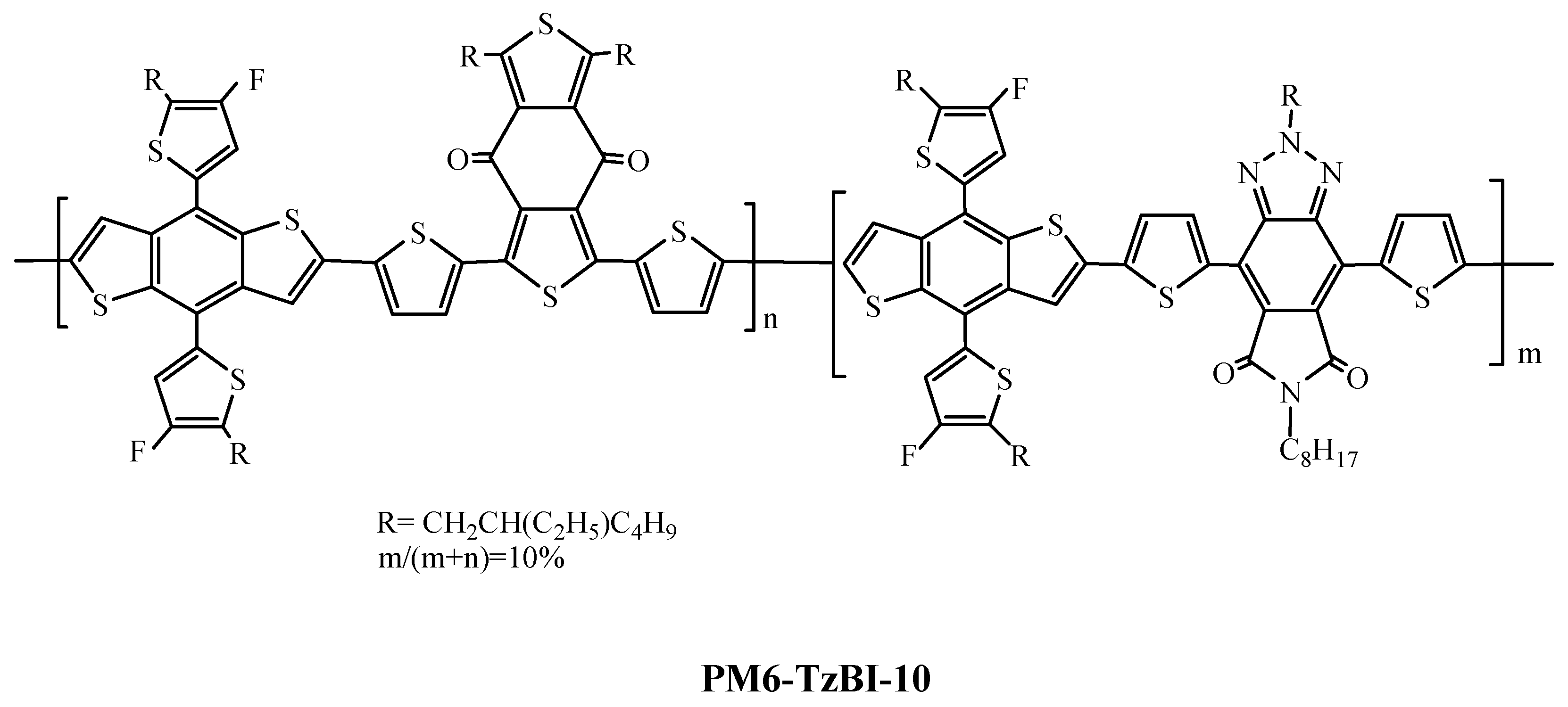

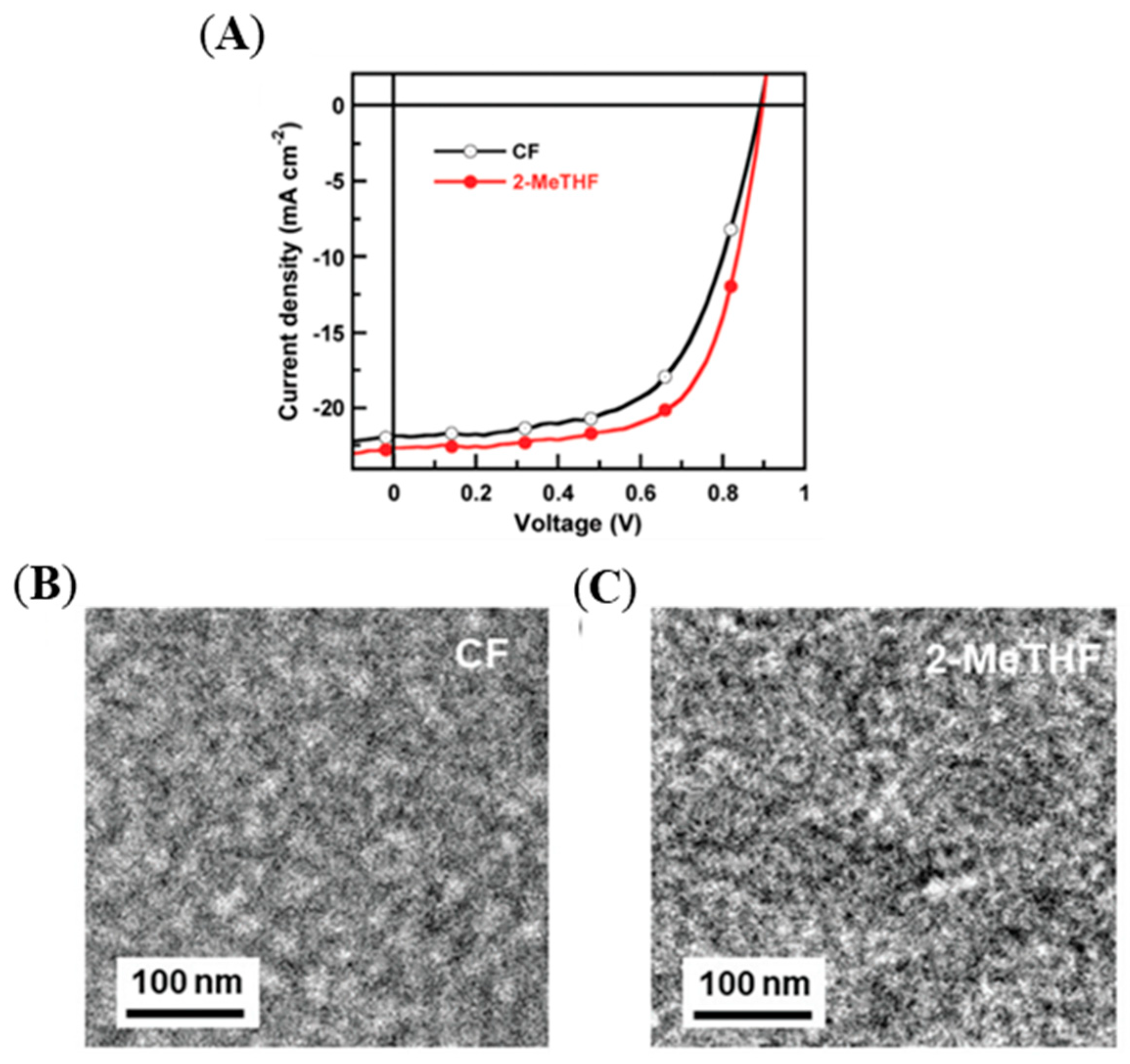
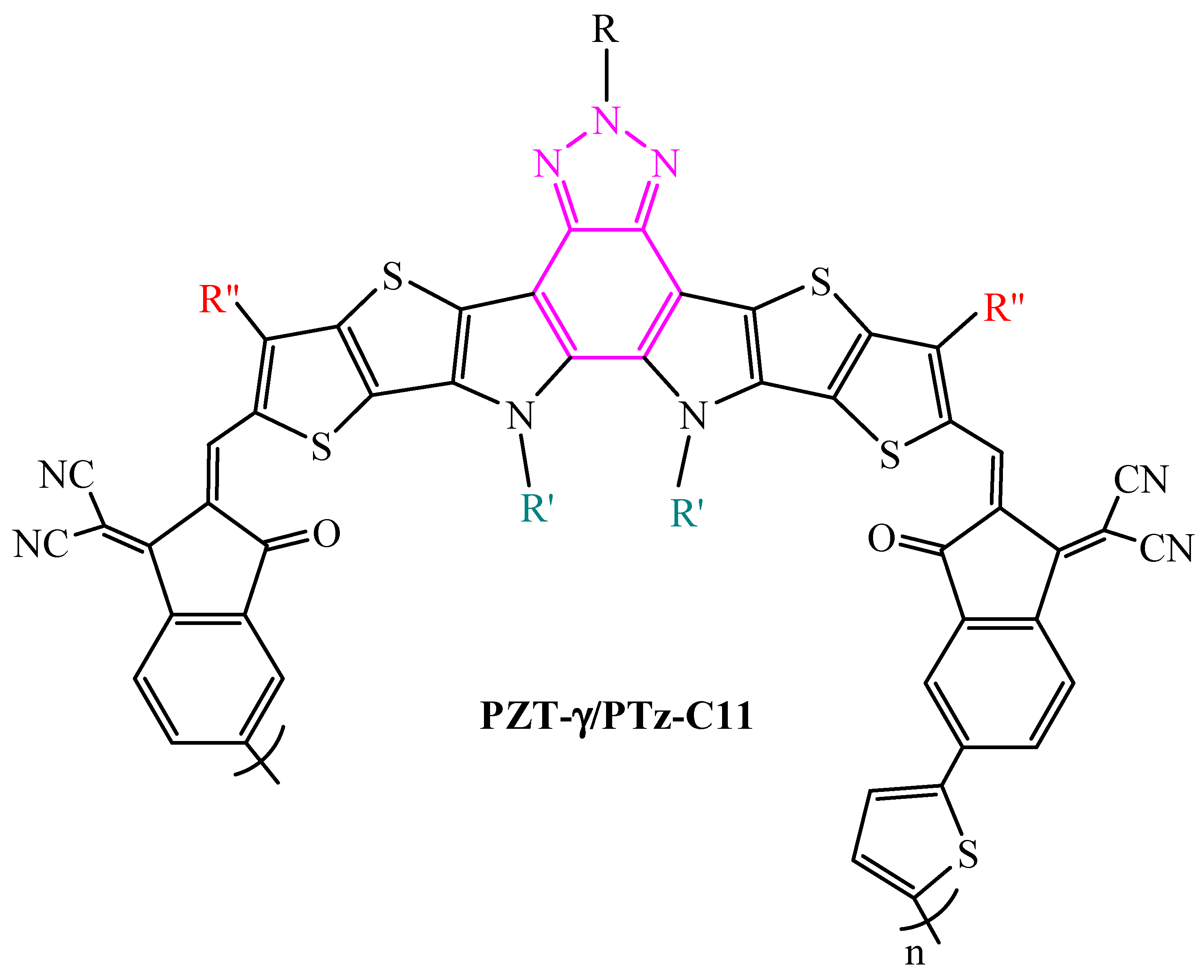
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| J52 | ITIC | 5.18 (5.51) c | 4.85 ± 0.26 (5.26 ± 0.18) c | [50] |
| PBZ (J52) | ITIC | 8.00 | 7.5 ± 0.3 | [51] |
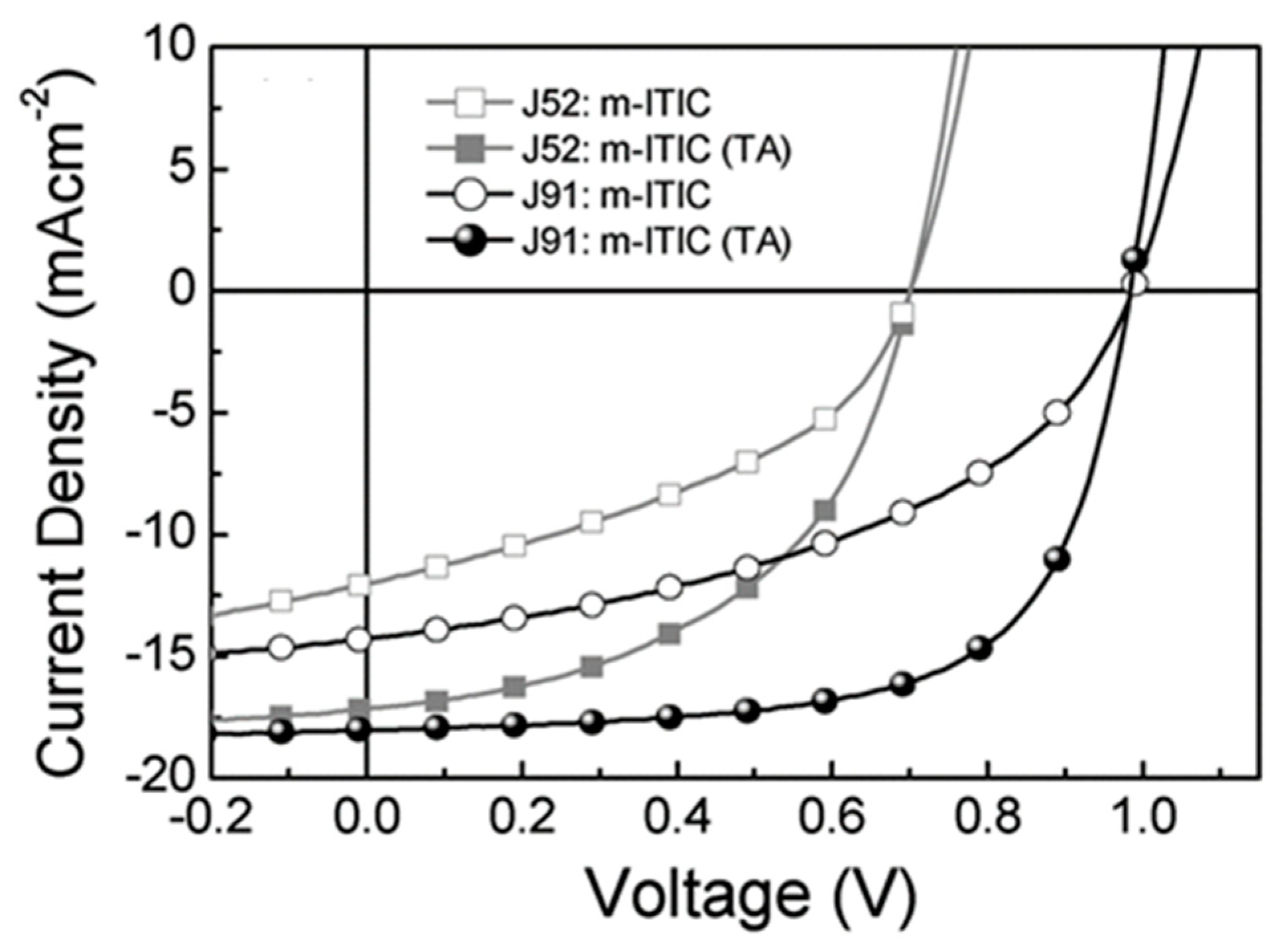
| J52 | m-ITIC | 3.45 (5.98) c | 5.89 ± 0.12 c | [53] |
| J52 | BTA13 | 7.82 | 7.63 ± 0.015 | [54] |
| J52 | ITIC | 3.78 | 3.01 | [55] |
| PBDT(T)FTAZ (J52) | N2200 | 4.95 (5.83) d (6.14) c | 4.71 (5.55) d (5.95)c | [52] |
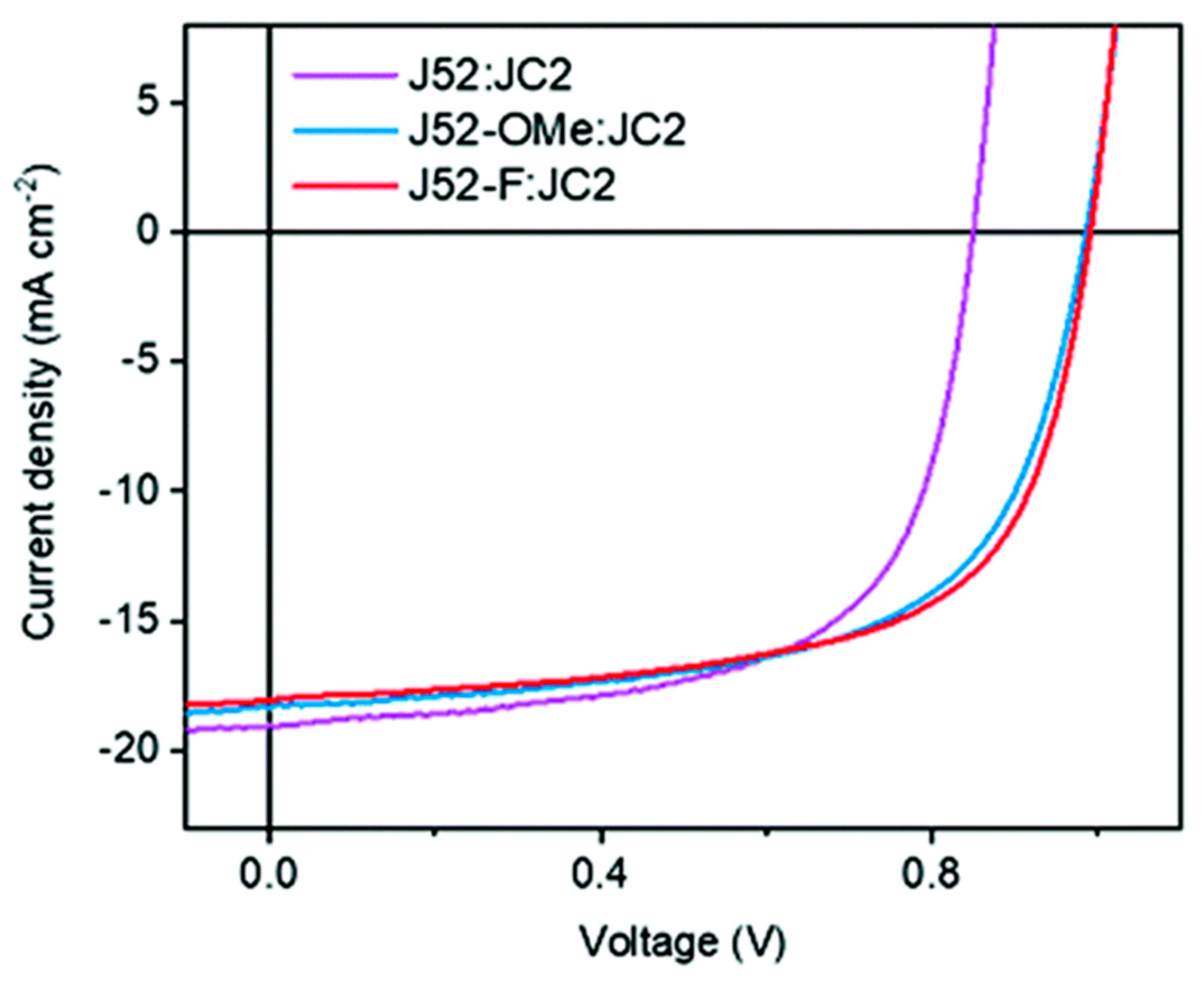
| J52 | JC2 | 10.27 | 10.12 ± 0.09 | [56] |
| J52 | Y6 | 7.15 | [57] | |
| J52 | Y6 | 6.02 | [58] | |
| J52 | IT-4F | 6.4 | [43] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| J91 | m-ITIC | 6.05 (11.63) c | 11.32 ± 0.21 c | [53] |
| J52-F(PFBZ) | ITIC | 10.4 | 9.8 ± 0.4 | [51] |
| J52-F | BTA13 | 8.36 | 8.28 ± 0.11 | [54] |
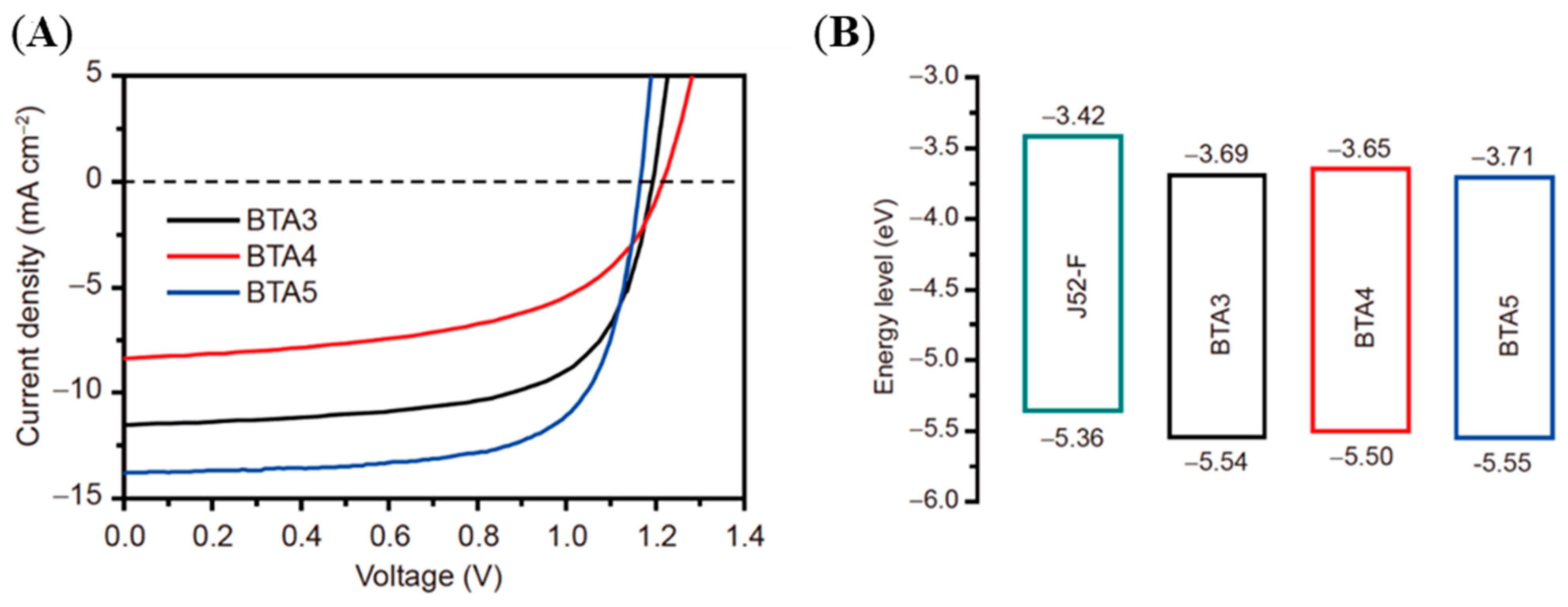
| J52-F | BTA3 | 9.04 | 8.68 | [64] |
| J52-F | BTA4 | 5.61 | 5.34 | [64] |
| J52-F | BTA5 | 11.26 | 10.95 | [64] |
| J52-F | BTA3b | 9.81 | 9.72 ± 0.09 | [65] |
| J52-F | J14 | 10.33 | 10.29 ± 0.04 | [65] |
| J52-Cl | ITIC | 11.53 | 10.9 | [55] |
| J52-Cl | Y6 | 12.31 | 12.31 ± 0.06 | [29] |
| J52-Cl | IT-4F | 9.7 | 9.3 | [43] |
| L68 (J52-Cl) | IT-4F | 9.30 | 9.1 ± 0.2 | [66] |
| L68 (J52-Cl) | TTPT-T-4F | 12.72 | 12.42 ± 0.27 | [67] |
| J52-Cl | JC2 | 11.44 | 11.21 ± 0.18 | [56] |
| J52-Cl | BTA1 | 0.6 | [68] | |
| J52-Cl | BTA11 | 0.13 | [68] | |
| J52-Cl | BTA3 | 10.5 | [68] | |
| J52-Cl | BTA13 | 7.16 | [68] | |
| J52-Cl | BTA7 | 5.78 | [68] | |
| J52-Cl | BTA17 | 5.18 | [68] | |
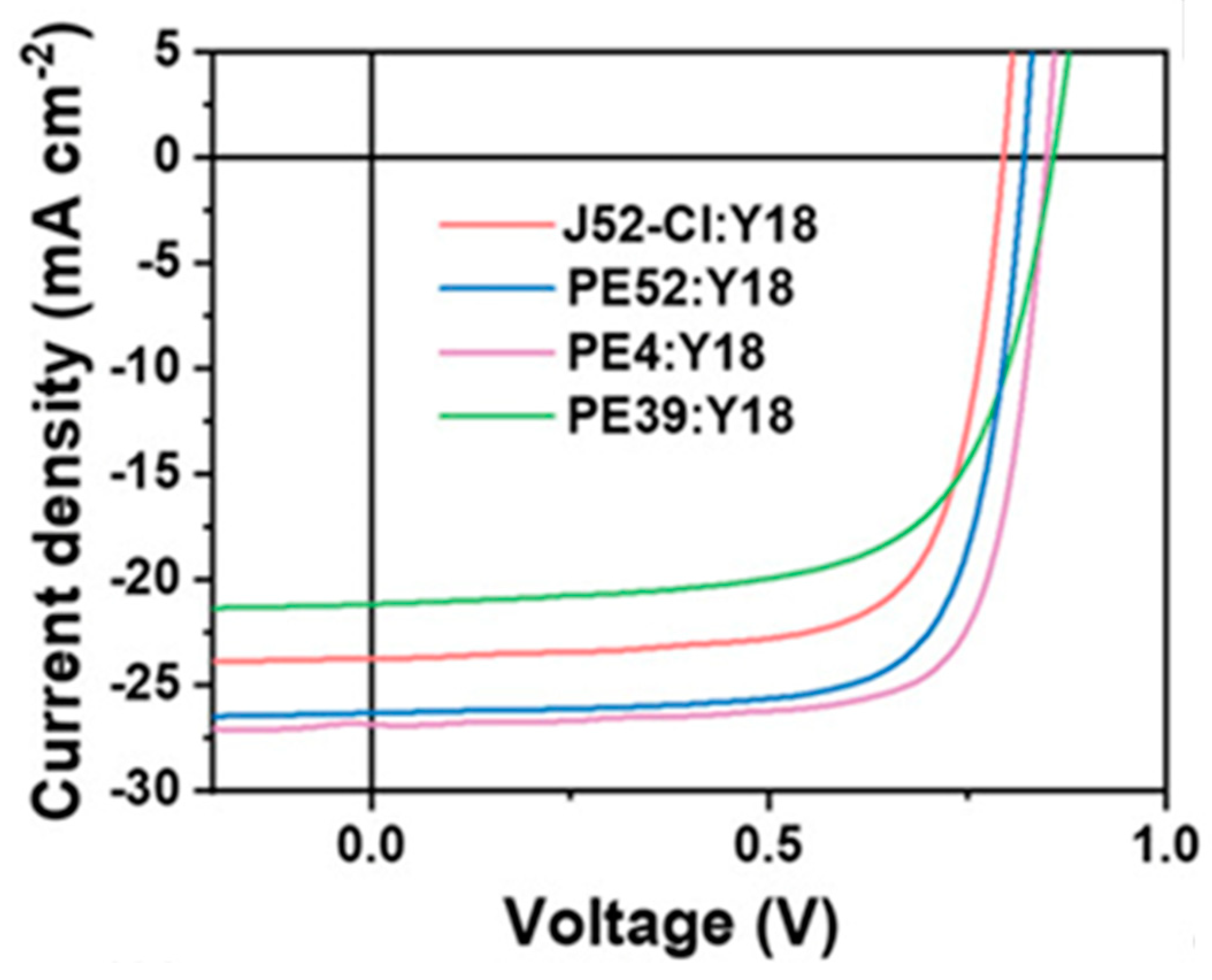
| J52-Cl | Y18 | 13.64 | [21] | |
| J52-Ome d | JC2 | 11.18 | 10.92 ± 0.16 | [56] |
| L24 | IT-4F | 1.33 | 1.3 ± 0.1 | [66] |
| L810 | IT-4F | 12.1 | 11.7 ± 0.4 | [66] |
| J52FTh | ITIC | 12.23 (13.32) c | [69] | |
| J52ClF | IT-4F | 13.73 (14.59) c | 13.49 ± 0.24 (14.32 ± 0.27) c | [59] |
| J11 | m-ITTC | 10.16 (12.32) c | 10.01 ± 0.24 (11.81 ± 0.21) | [70] |
| J12 | m-ITTC | 8.31 (8.74) c | 7.99 ± 0.13 (8.31 ± 0.25) | [70] |
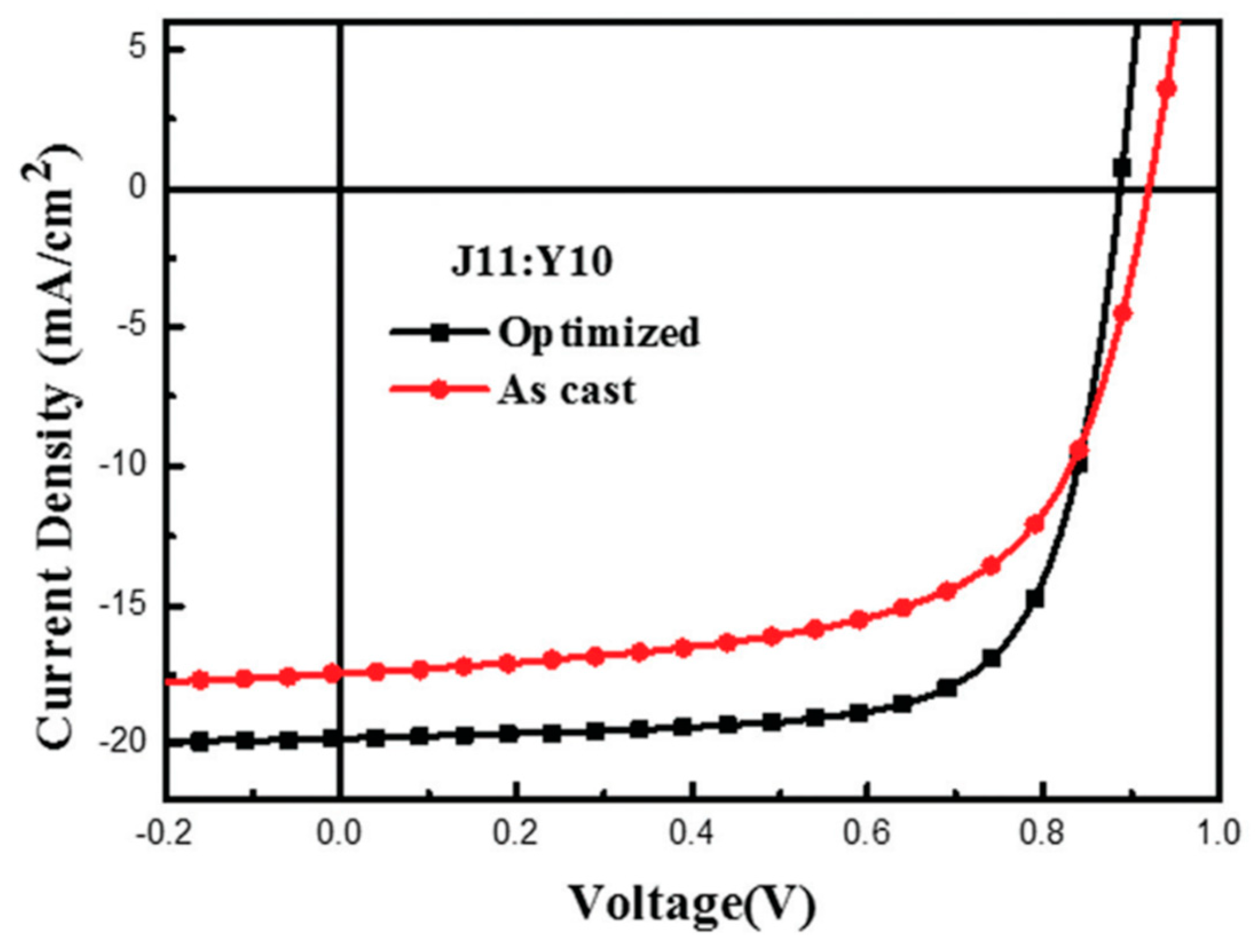
| J11 | Y10 | 11.37 (13.46) e | 11.17 ± 0.2 (13.26 ± 0.2) | [71] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| J60 | ITIC | 5.17 (8.97) c | 5.07 ± 0.14 (8.67 ± 0.31) c | [50] |
| J61 | ITIC | 9.15 (9.53) c | 8.93 ± 0.26 (9.22 ± 0.24) c | [50] |
| J61 | ITIC | 10.57 | 10.28 ± 0.15 | [72] |
| J61 | ITIC | 9.32 c | [75] | |
| J61 | m-ITIC | 11.77 | 11.49 ± 0.16 | [72] |
| PSBZ | ITIC | 9.7 (10.5) c | 9.2 ± 0.4 (10.0 ± 0.3) c | [73] |
| J62 | ITIC | 9.09 (10.81) c | 8.87 ± 0.19 (10.68 ± 0.11) c | [74] |
| J63 | ITIC | 7.38 (8.13) c | 7.24 ± 0.13 (8.02 ± 0.09) c | [74] |
| J64 | ITIC | 7.51 (8.59) c | 7.23 ± 0.19 (8.36 ± 0.18) c | [74] |
| J61 | BTA1 | 0.26 | 0.26 ± 0.003 | [76] |
| J61 | BTA2 | 3.02 | 2.98 ± 0.04 | [76] |
| J61 | BTA3 | 8.25 | 8.03 ± 0.14 | [76] |
| J52-FS | BTA13 | 3.84 | 3.83 ± 0.02 | [54] |
| J52-FS | Y6 | 10.58 | 10.14 ± 0.40 | [77] |
| J52-Cl-S | Y6 | 10.48 | 10.23 ± 0.24 | [57] |
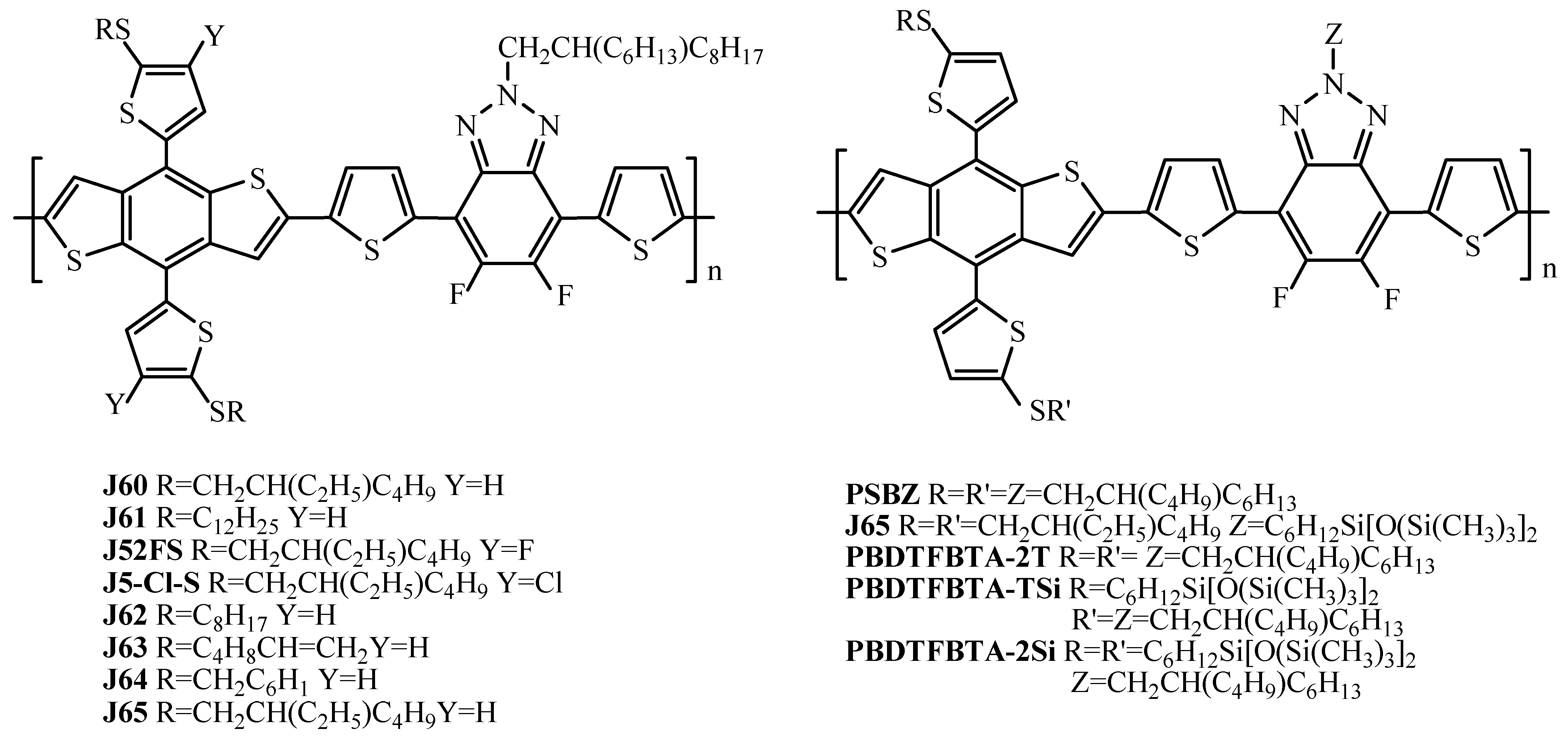
| J65 | ITIC | 4.92 (6.91) c | 4.58 ± 0.32 (6.01 ± 0.36)) | [61] |
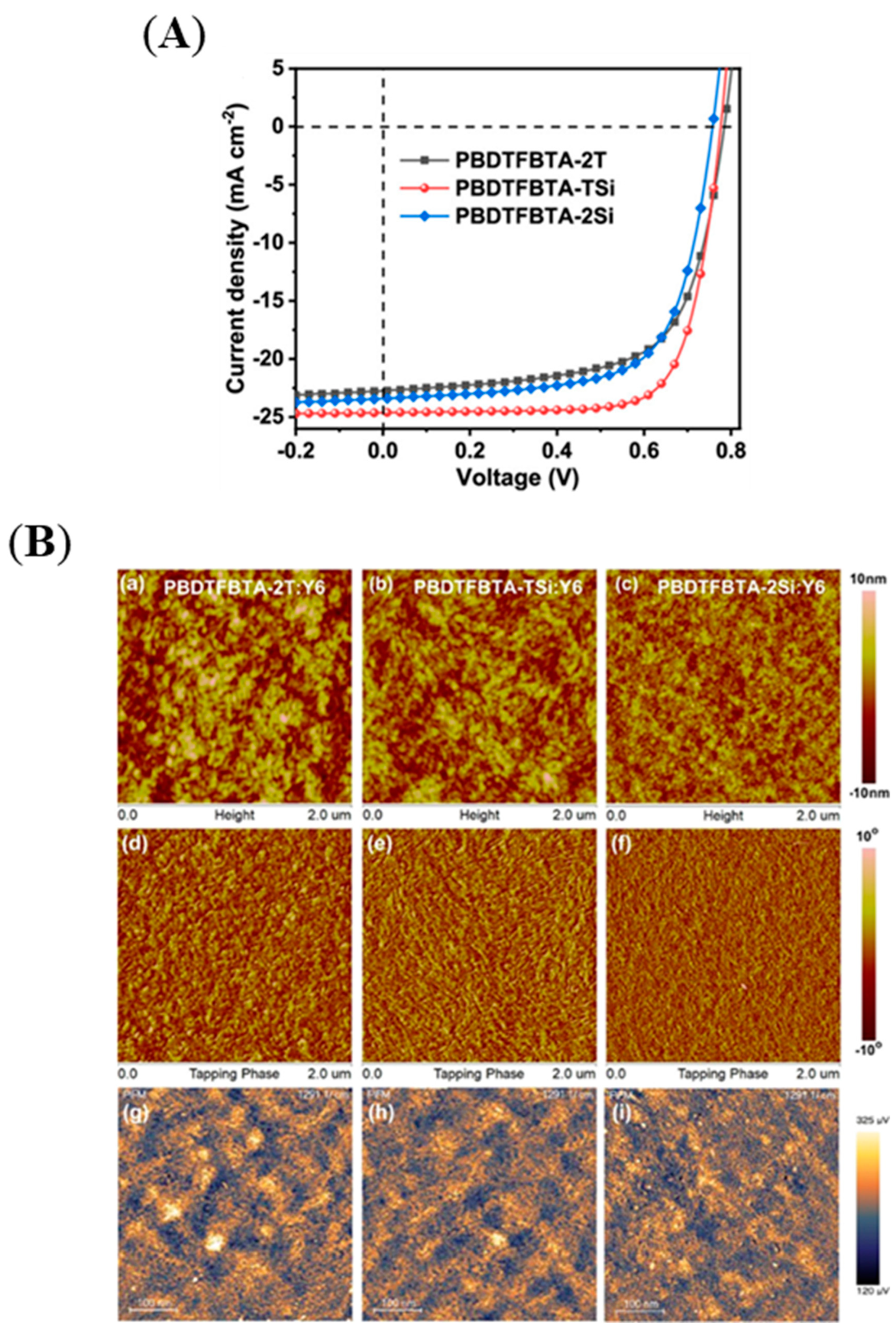
| PBDTFBTA-2T | Y6 | 11.76 | 11.37 ± 0.35 | [78] |
| PBDTFBTA-TSi | Y6 | 14.18 | 13.76 ± 0.40 | [78] |
| PBDTFBTA-2Si | Y6 | 11.92 | 11.45 ± 0.39 | [78] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
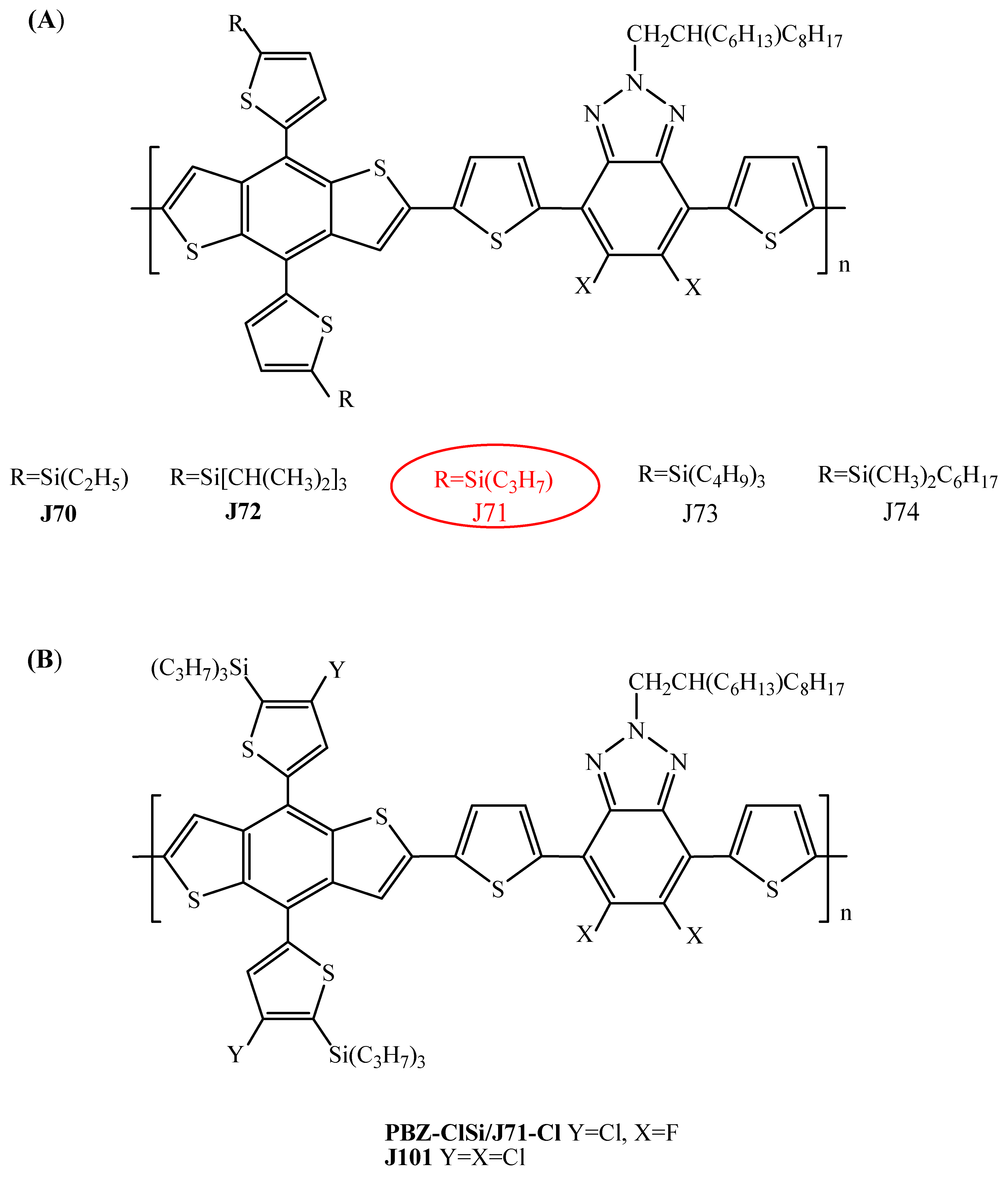
| J71 | ITIC | 9.03 (11.41) c | 8.94 ± 0.24 (11.2 ± 0.29) | [82] |
| J71 | m-ITIC | 12.05 | 11.77 ± 0.15 | [83] |
| J70 | m-ITIC | 11.62 | 11.40 ± 0.18 | [83] |
| J72 | m-ITIC | 10.23 | 9.95 ± 0.22 | [83] |
| J73 | m-ITIC | 10.71 | 10.43 ± 0.16 | [83] |
| J74 | m-ITIC | 9.63 | 9.47 ± 0.12 | [83] |
| J71 | ITCPTC | 6.38 (7.13) c | 5.88 ± 0.52 (6.61 ± 0.67) c | [84] |
| J71 | MeIC | 6.64 (8.83) c | 6.22 ± 0.47 (8.38 ± 0.56) | [84] |
| J71 | ITC6-IC | 11.32 | 10.89 | [85] |
| J71 | IDIC | 11.75 | 11.23 | [85] |
| J71 | Me-IC | 10.57 | 10.37 | [85] |
| J71 | ITCPTC | 10.46 | 10.03 | [85] |
| J71 | ITIC | 10.95 | 10.55 | [85] |
| PBZ-ClSi (J71-Cl) | IT-4F | 12.8 b | 12.5 | [43] |
| J71 | IT-4F | 7.79 (8.16) d | [30] | |
| J71-Cl (PBZ-ClSi) | IT-4F | 9.47 (11.10) e | [30] | |
| J101 | IT-4F | 8.07 (11.30) e | [30] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PDTTz-N | ITIC | 6.61 | [92] | |
| PBDTTz-BP | ITIC | 8.03 | [92] | |
| PBDTTz-BP | ITIC | 9.20 | 8.97 | [47] |
| PBDTTz-SBP | ITIC | 12.09 | 11.89 | [47] |
| P2 | ITIC | 5.91 (6.59) c | [94] | |
| PBZ1 | ITIC | 4.3 (5.8) c | 4.1 (5.7) c | [95] |
| PBZ-m-CF3 | ITIC | 7.9 (10.4) c | 7.8 (10.3) c | [95] |
| PBTA-PS | ITIC | 11.85 | 11.52 | [98] |
| PBTA-PSF | ITIC | 13.91 | 13.60 | [98] |
| PBTZa | ITIC-4Cl | 6.02 (8.50) c | 5.96 (8.31) c | [99] |
| PBTZb | ITIC-4Cl | 12.09 (14.53) c | 11.81 (14.34) c | [99] |
| T1 | ITIC | 11.83 | 11.50 ±0.33 | [93] |
| DZ1 | MeIC | 8.26 | 7.94 ± 0.19 | [97] |
| DZ2 | MeIC | 10.21 | 9.98 ± 0.20 | [97] |
| DZ3 | MeIC | 5.97 | 5.77 ± 0.20 | [97] |
| PBDTPSi-FTAZ | IDIC | 5.78 (7.07) c | 5.62 ± 0.16 (6.91 ± 0.16) c | [100] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PE51 | Y6 | 13.34 | 13.17 ± 0.12 | [101] |
| PE52 | Y6 | 14.61 | 14.33 ± 0.28 | [101] |
| PE53 | Y6 | 13.72 | 13.52 ± 0.15 | [101] |
| PTBFBz | ITIC | 6.54 (8.33) c | 6.42 (8.12) c | [102] |
| PTSDO-Bz | ITIC | 8.66 | [103] | |
| PTDO-Bz | ITIC | 6.59 | [103] | |
| PPEH-Bz | ITIC | 8.23 | [103] | |
| PPFOEH-Bz | ITIC | 8.16 | [103] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PBDFT-Bz | ITIC | 9.26 | 9.08 | [105] |
| PBDFT-Bz | m-ITIC | 9.84 | 9.62 | [105] |
| PBDFF-Bz | ITIC | 9.46 | 9.28 | [105] |
| PBDFF-Bz | m-ITIC | 10.28 | 10.02 | [105] |
| PBDFS-fBz | ITIC | 9.00 | [26] | |
| PBDFT-FFBz | m-ITIC | 7.57 | 7.32 ± 0.20 | [106] |
| PBDFF-FFBz | m-ITIC | 8.79 | 8.32 ± 0.32 | [106] |
| J81 | ITIC | 10.6 | [107] | |
| J81 | m-ITIC | 11.5 | [107] | |
| L2 (F11/P-ClT) | TTPT-T-4F | 14.0 | 13.68 ± 0.21 | [67] |
| F10 (P-FT) | m-ITIC | 10.5 | [62] | |
| F11 (L2/PClT) | m-ITIC | 11.37 | [62] | |
| P-FT (F10) | m-ITIC | 11.43 c | 10.87 ± 0.23 a | [108] |
| P-FT (F10) | Y6 | 10.61 c | 10.03 ± 0.48 a | [108] |
| P-ClT (L2/F11) | m-ITIC | 11.61 c | 11.04 ± 0.55 a | [108] |
| P-ClT (L2/F11) | Y6 | 11.03 c | 10.64 ± 0.15 a | [108] |
| P-P | m-ITIC | 8.28 c | 7.32 ± 0.59 a | [108] |
| P-P | Y6 | 9.49 c | 8.11 ± 0.11 a | [108] |
| P-4FP | m-ITIC | 8.86 c | 8.70 ± 0.26 a | [108] |
| P-4FP | Y6 | 13.34 c | 12.71 ± 0.51 a | [108] |
| PBDFTz-SBP | ITIC | 12.42 | 12.24 ± 0.22 | [109] |
| PBDFP-Bz | ITIC | 11.10 d | 10.69 ± 0.27 | [110] |
| PBDFP-Bz | IT-M | 12.93 e | 12.19 ± 0.38 | [110] |
| PBDFP-Bz | Y6 | 14.62 | 13.49 ± 0.62 | [111] |
| PBDFP-dT-Bz | Y6 | 16.03 | [112] | |
| PBDFP-dF-Bz | Y6 | 15.59 | [112] | |
| PBDFP-TF-Bz | Y6 | 17.01 | [112] | |
| PBDFP-TF-Bz | Y6:PCBO12 | 18.1 | [112] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PE1 | BTA3 | 8.43 | 8.36 ± 0.09 | [118] |
| PE2 | BTA3 | 5.83 | 5.75 ± 0.05 | [118] |
| PE2 | Y6 | 13.50 | 13.05 ± 0.26 | [77] |
| PE4 (PE5) | Y6 | 14.02 | 13.90 ± 0.08 | [29] |
| PE5 (PE4) | Y6 | 14.48 | 14.25 ± 0.21 | [57] |
| PE40 | Y6 | 7.07 | 6.29 ± 0.86 | [119] |
| PE42 | Y6 | 10.11 | 9.93 ± 0.14 | [119] |
| PE44 | Y6 | 13.62 | 12.81 ± 0.50 | [119] |
| PE45 | Y5 | 13.76 | 13.40 ± 0.26 | [120] |
| PE46 | Y5 | 13.55 | 13.32 ± 0.19 | [120] |
| PE47 | Y5 | 6.51 | 6.14 ± 0.18 | [120] |
| PE45 | Y6 | 10.30 | 10.16 ± 0.12 | [120] |
| PE46 | Y6 | 14.25 | 13.72 ± 0.38 | [120] |
| PE47 | Y6 | 15.58 | 15.16 ± 0.38 | [120] |
| PCN1 | BTA3 | 12.07 | 11.63 ± 0.44 | [58] |
| PCN2 | BTA3 | 15.2 | 14.83 ± 0.37 | [58] |
| Polymer Donor | Non-Fullerene Acceptor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PTzBI-DT | ITIC | 8.11 (9.43) c | 7.99 (9.32) c | [121] |
| PTzBI | ITIC | 8.64 (10.23) d | 8.60 (10.13) d | [121] |
| PTzBI | N2200 | 9.16 e | 9.06 ± 0.14 e | [122] |
| PTzBI | N2200 | 8.21 f | 8.05 ± 0.15 f | [123] |
| PTzBI | Y6 | 9.24 | 9.06 ± 0.18 | [124] |
| PTzBI-Si | N2200 | 8.3 e (10.1) g | 8.2 ± 0.1 e (9.9± 0.2) g | [126] |
| PTzBI-Si | N2200 | 10.1 e | 9.9 ± 0.2 e | [127] |
| PTzBI-Si | N2200 | 7.6 g | 7.4 ± 0.2 g | [127] |
| PTzBI-Si | N2200 | 11.0 h | 10.8 ± 0.2 h | [127] |
| PTzBI-Si | PNDICI | 5.4 i (3.0) j | 5.2 ± 0.2 i (2.8 ± 0.1) j | [128] |
| P2F-Si | PNDICI | 4.6 i (4.2) j | 4.4 ± 0.1 i (4.1 ± 0.1) j | [128] |
| P2F-EHP | BTPT-4F | 1.09 c | 1.06 ± 0.06 c | [129] |
| P2F-EHP | BTPTT-4F (Y6) | 16.02 d | 15.75 ± 0.25 d | [129] |
| P2F-EHP | Y6 | 15.65 | 15.50 ± 0.15 | [130] |
| P2F-EHP | Y6+PC61BM | 16.18 | 16.07 ± 0.13 | [130] |
| P2F-EHP | BTA3 | 4.62 | 4.45 ± 0.02 | [131] |
| P2F-EHP | BTA3-F | 8.38 | 8.25 ± 0.02 | [131] |
| PTzBI-2Fp | ITIC-4F+5% N2200 | 13.0 | 12.9 ± 0.1 | [132] |
| PTzBIoF | PS1 | 13.8 g | 13.5 | [125] |
| PM6-TzBI-10 | L8-BO | 18.36 | [135] | |
| PHT-EHp | Y6 | 7.7 d | 7.63 ± 0.2 | [133] |
| PFT-EHp | Y6 | 14.16 | 14.02 ± 0.13 | [130] |
| PFT-EHp | Y6 | 15.4 d | 15.0 ± 0.2 | [133] |
| PCT-EHp (PTzBI-Cl) | Y6 | 15.06 | 14.96 ± 0.06 | [130] |
| PCT-EHp (PTzBI-Cl) | Y6 | 15.8 d | 15.6 ± 0.1 | [133] |
| PCT-EHp (PTzBI-Cl) | Y6DT | 16.4 d | 16.2 ± 0.2 | [133] |
| PTzBI-Cl (PCT-EHp) | Y6 | 10.35 | 9.99 ± 0.36 | [124] |
| PTzBI-dF | Y6 | 9.13 | 8.77 ± 0.36 | [124] |
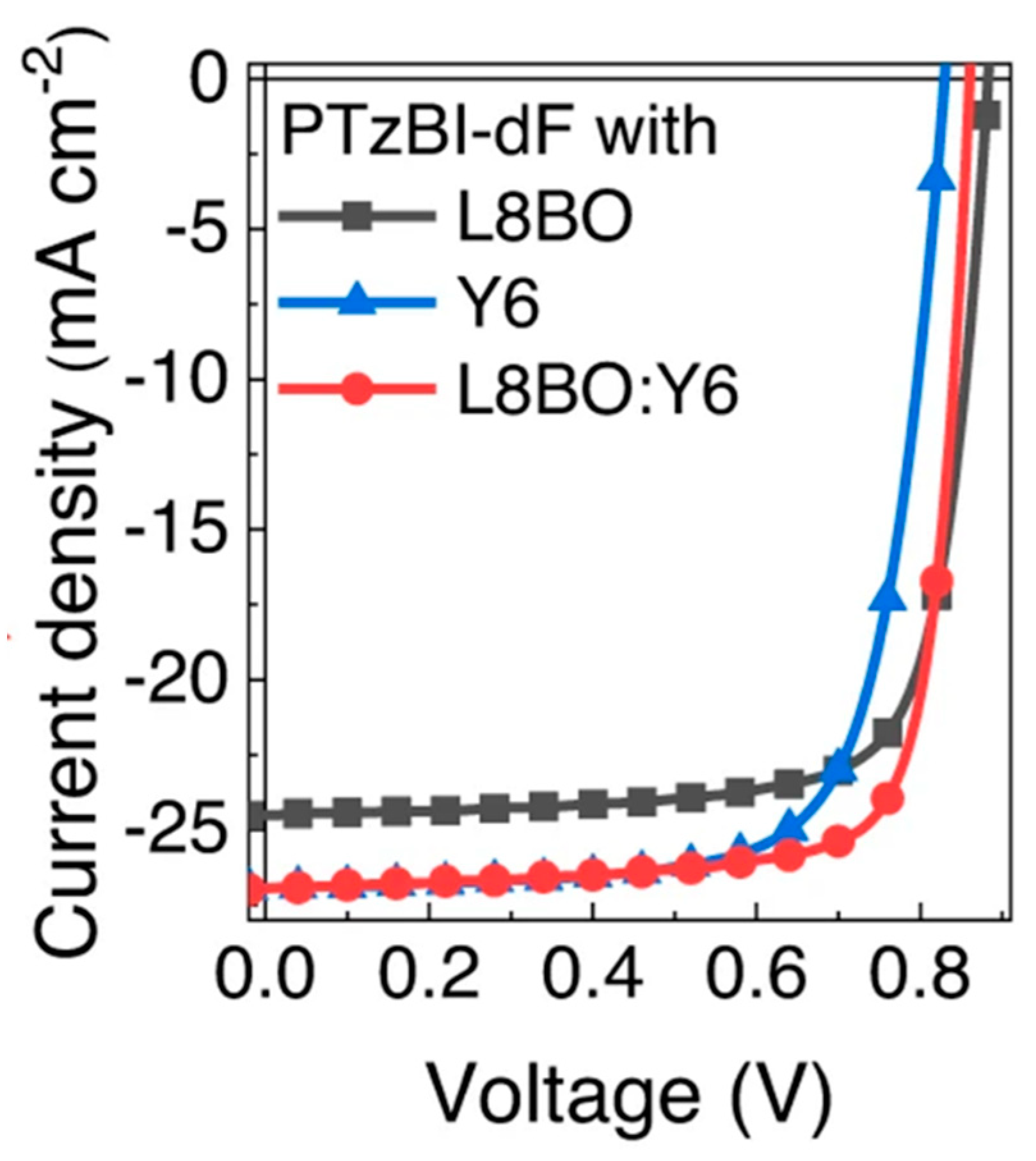
| PTzBI-dF | L8-BO | 16.74 k | 16.49 ± 0.25 | [134] |
| PTzBI-dF | Y6 | 16.23 k | 16.03 ± 0.20 | [134] |
| PTzBI-dF | L8-BO+Y6 | 18.26 | 17.95 ± 0.31 | [134] |
| P1 | ITIC | 7.14 l | 7.02 ± 0.08 | [136] |
| P2 | ITIC | 4.17 l | 3.96 ± 0.14 | [136] |
| P3 | ITIC | 3.66 l | 3.60 ± 0.07 | [136] |
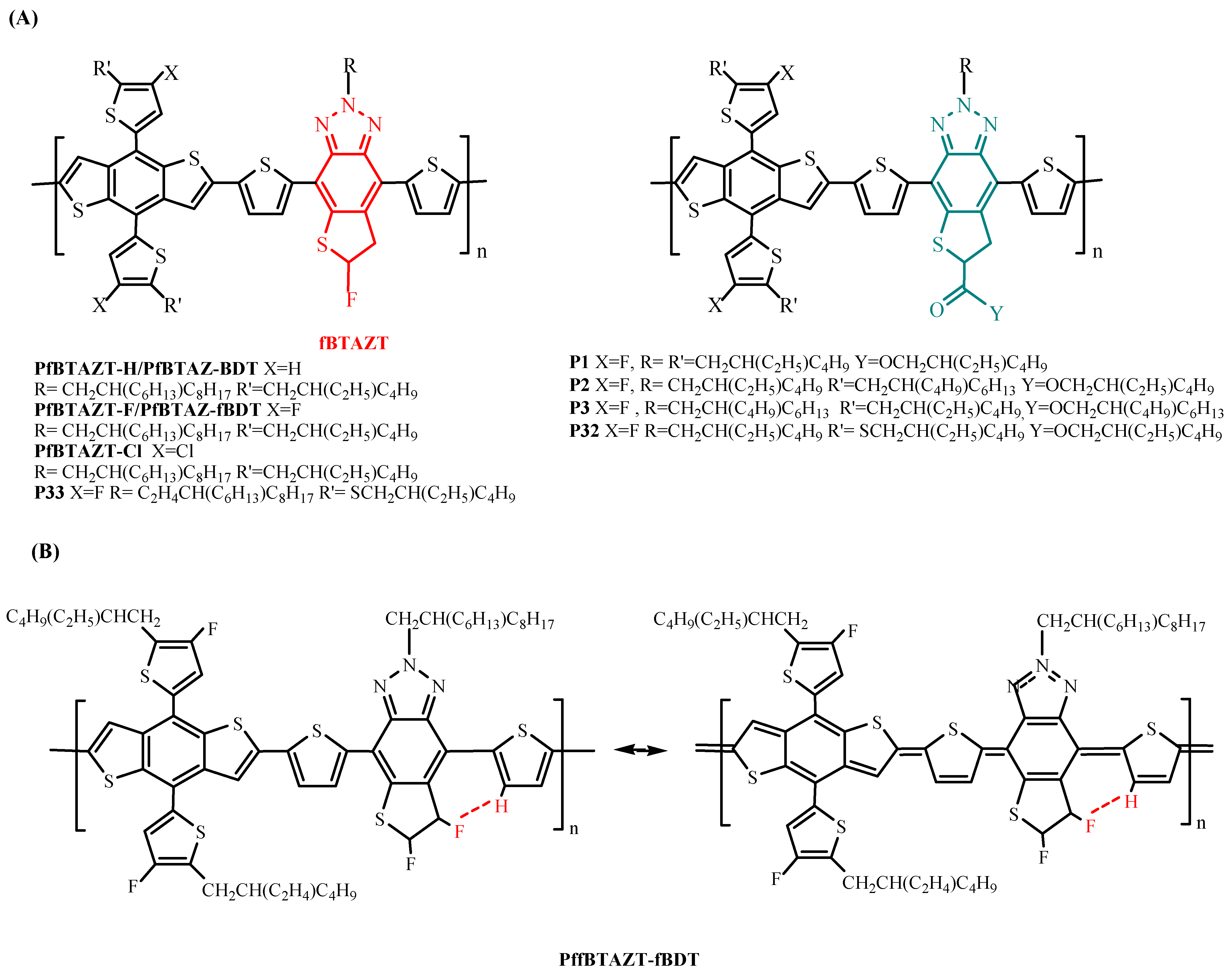
| PfBTAZT-BDT (PfBTAZT-H) | ITIC | 6.04 | 5.71 | [137] |
| PfBTAZT-fBDT (PfBTAZT-F) | ITIC | 6.59 d | 6.05 d | [137] |
| PfBTAZT-H (PfBTAZT-BDT) | BTA3 | 6.65 | 6.53 ± 0.16 | [138] |
| PfBTAZT-F (PfBTAZT-fBDT) | BTA3 | 7.69 | 7.53 ± 0.09 | [138] |
| PfBTAZT-Cl | BTA3 | 8.00 | 7.95 ± 0.06 | [138] |
| P32 | IHIC | 8.59 m | [139] | |
| P33 | IHIC | 6.63 m | [139] | |
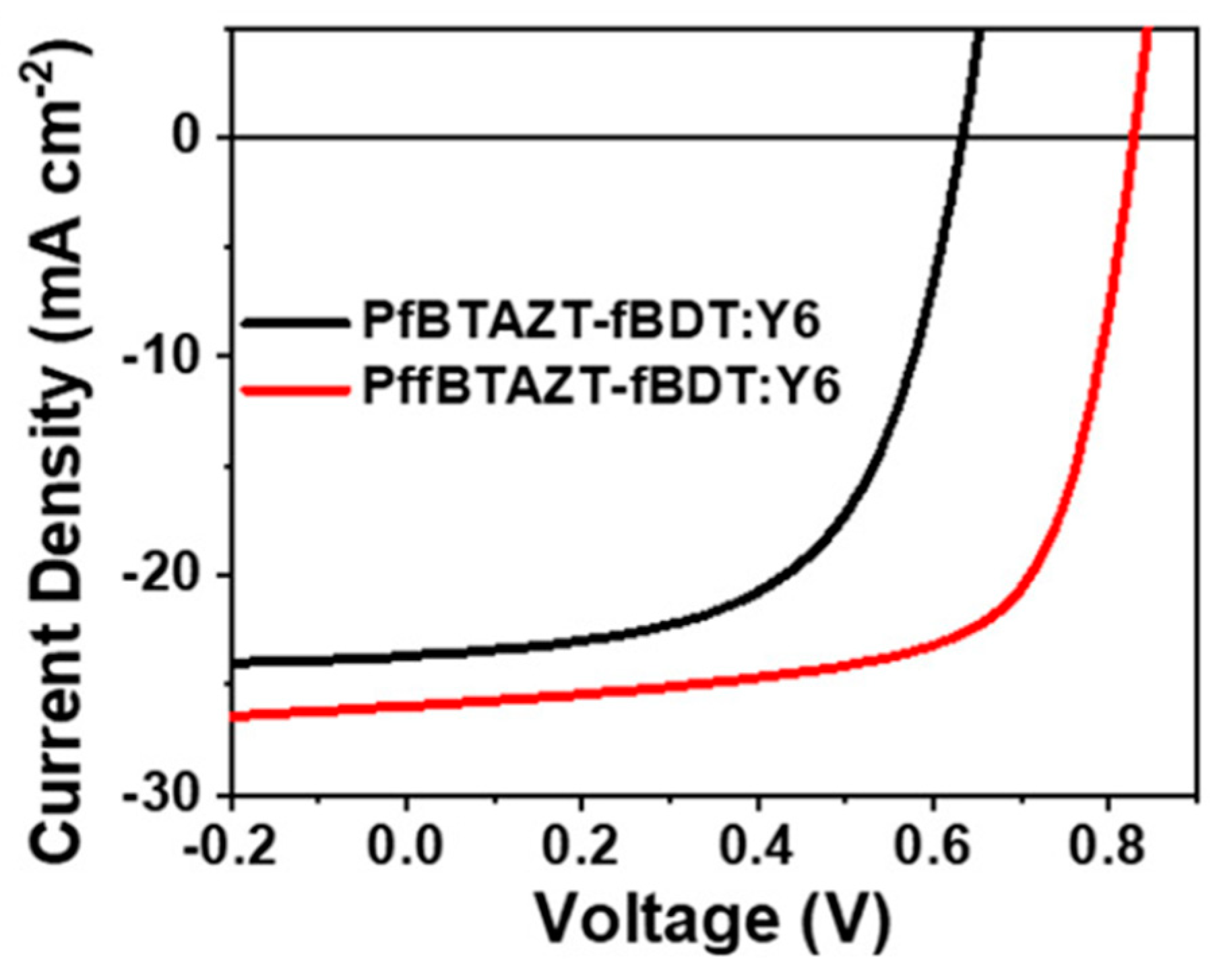
| PfBTAZT-fBDT (PfBTAZT-F) | Y6 | 8.77 | 8.50 ± 0.14 | [140] |
| PffBTAZT-fBDT | Y6 | 14.53 | 14.29 ± 0.16 | [140] |
| PY1 | IT-M | 12.49 | 12.20 ± 0.30 | [19] |
| PY39 | Y18 | 12.45 | 12.26 ± 0.19 | [20] |
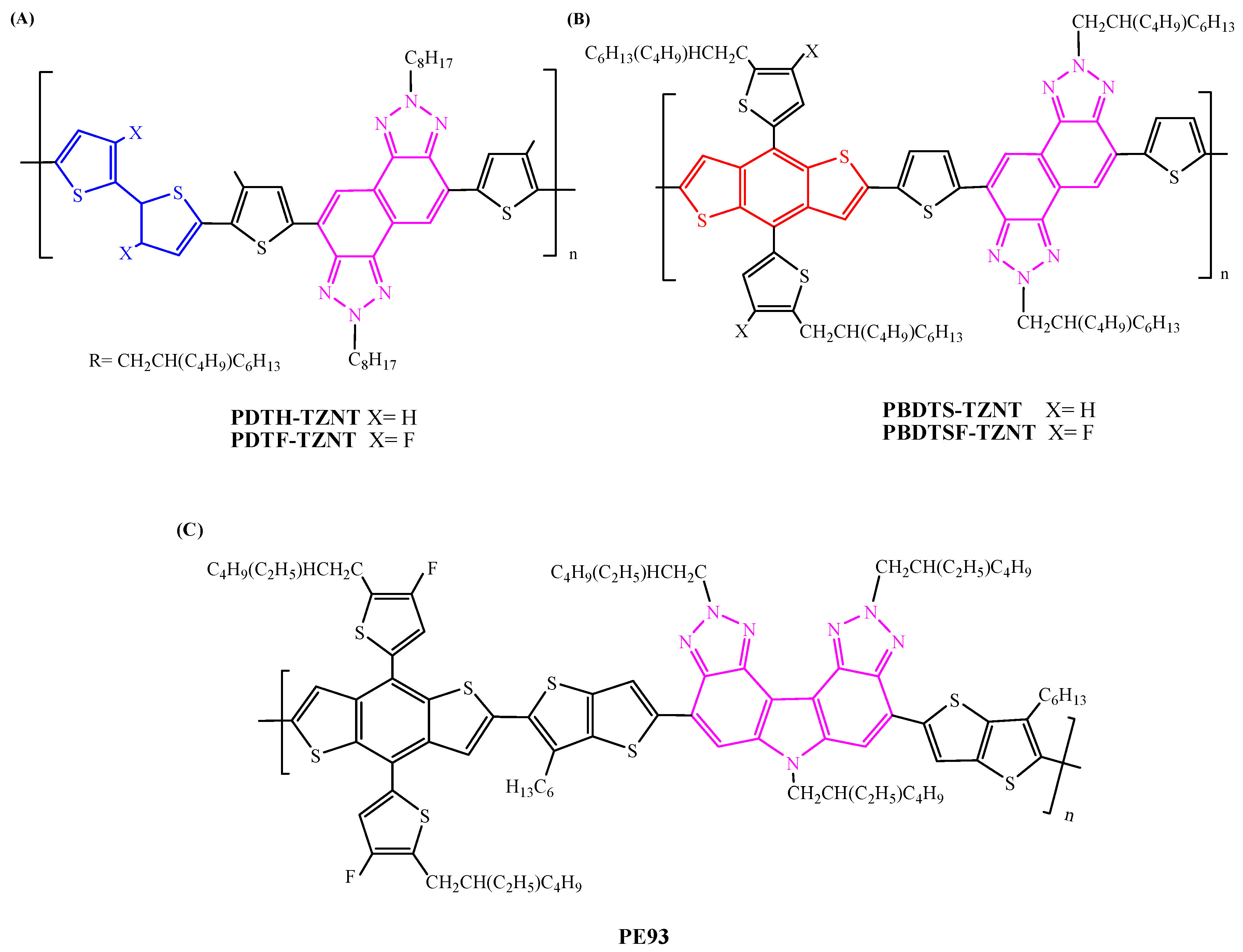
| PDTH-TZNT | IT-M | 4.42 | 4.05 ± 0.37 | [141] |
| PDTF-TZNT | IT-M | 10.05 (11.48) n | 9.74 ± 0.31 (11.16 ± 0.32) n | [141] |
| PDTS-TZNT | ITIC | 10.45 | 10.13 ± 0.32 | [142] |
| PDTS-TZNT | IT-4F | 11.31 | 11.00 ± 0.31 | [142] |
| PDTSF-TZNT | ITIC | 12.16 | 11.81 ± 0.35 | [142] |
| PDTSF-TZNT | IT-4F | 13.25 | 12.92 ± 0.33 | [142] |
| PE93 | Y6 | 9.32 | 9.28 ± 0.09 | [143] |
| PE93 | BTA75 | 10.11 | 10.05 ± 0.11 | [143] |
| PE93 | BTA76 | 12.16 | 11.98 ± 0.18 | [143] |
| BzT-Acceptor | Polymer Donor | PCEmax a (%) | PCEavg b (%) | References |
|---|---|---|---|---|
| PS1 | PTzBI-of | 13.8 c | 13.8 c | [125] |
| PS1 | PTzBI-of | 12.1 d | 12.1 d | [125] |
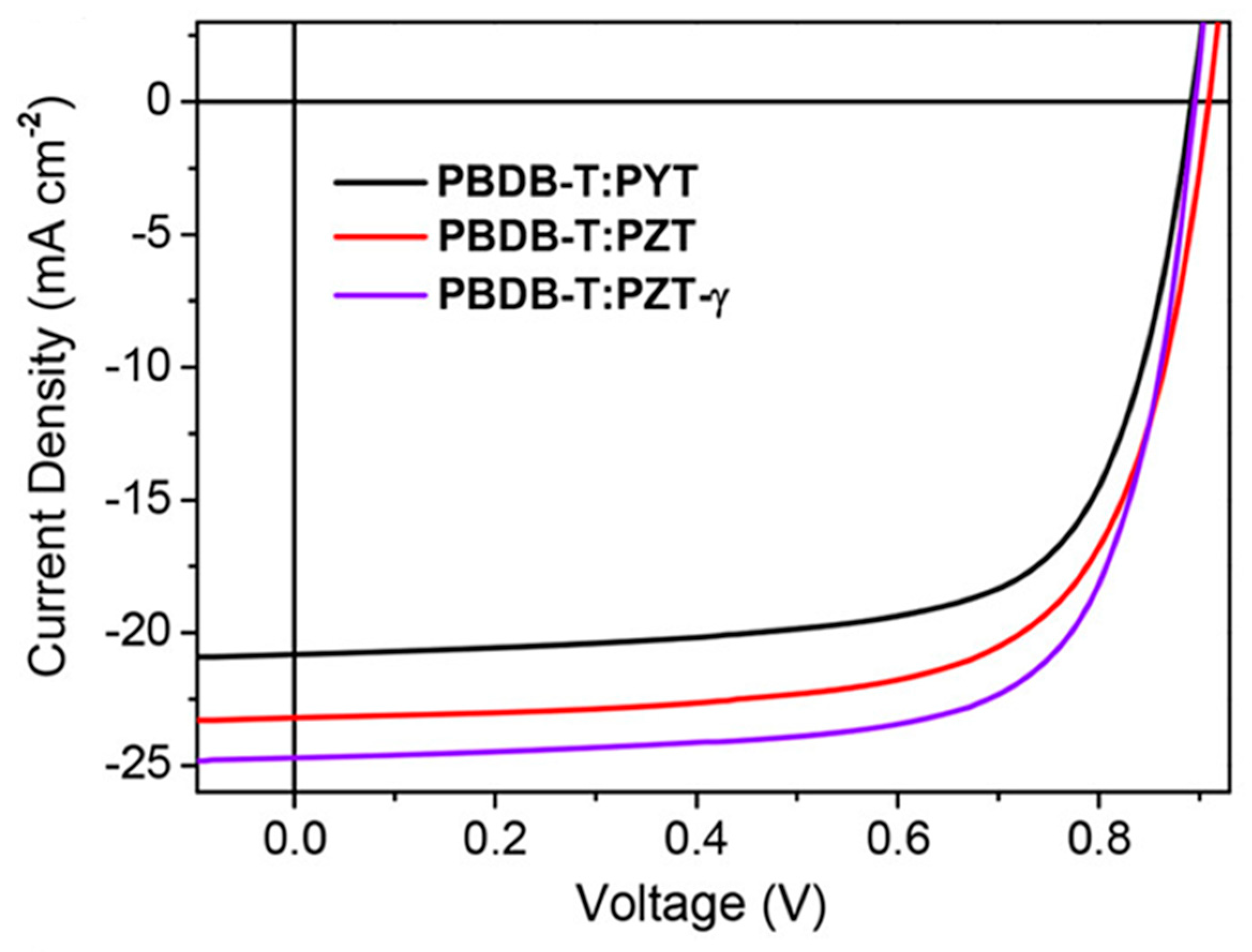
| PYT | PBDB-T | 12.9 | 12.7 ± 0.2 | [144] |
| PZT (PZT-C1) | PBDB-T | 14.5 | 14.2 ± 0.3 | [144] |
| PZT-γ (PTz-C11) | PBDB-T | 15.8 | 15.6 ± 0.2 | [144] |
| Graded index(GI)-PZT | PBDB-T | 25.1 | 25.1 | [150] |
| PYV-Tz | PBDB-T | 13.02 | 12.78 ± 0.24 | [152] |
| PYBzT | J52 | 11.22 | 10.67 ± 0.28 | [153] |
| PYBzDT | J52 | 8.93 | 8.69 ± 0.26 | [153] |
| PZT-C12 | PBDB-T | 13.1 | 12.8 ± 0.3 | [148] |
| PZT-C8 | PBDB-T | 13.8 | 13.5 ± 0.3 | [148] |
| PZT-C1 (PZT) | PBDB-T | 14.9 | 14.5 ± 0.4 | [148] |
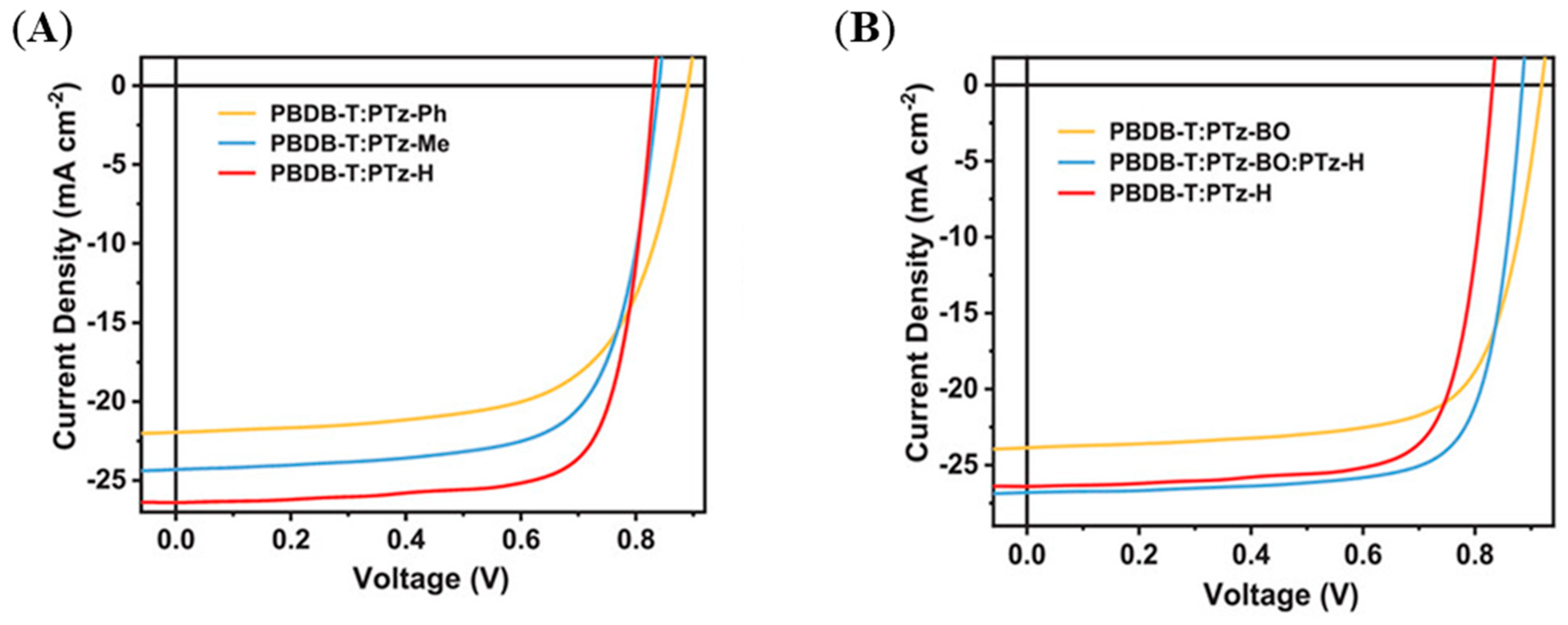
| PTz-BO | PBDB-T | 15.76 | 15.56 ± 0.19 | [149] |
| PTz-C11 (PZT-γ) | PBDB-T | 15.59 | 15.36 ± 0.22 | [149] |
| PTz-BO:PTz-C11 | PBDB-T | 16.58 | 16.34 ± 0.22 | [149] |
| PY-FBTA | PM6 | 13.95 | 13.74 | [145] |
| PTz-H | PBDB-T | 16.53 | 16.28 ± 0.19 | [151] |
| PTz-Me | PBDB-T | 14.48 | 14.22 ± 0.22 | [151] |
| PTz-Ph | PBDB-T | 12.82 | 12.56 ± 0.25 | [151] |
| PTz-BO:PTz-H | PBDB-T | 18.16 | 17.86 ± 0.25 | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crociani, L. The Double-Cross of Benzotriazole-Based Polymers as Donors and Acceptors in Non-Fullerene Organic Solar Cells. Molecules 2024, 29, 3625. https://doi.org/10.3390/molecules29153625
Crociani L. The Double-Cross of Benzotriazole-Based Polymers as Donors and Acceptors in Non-Fullerene Organic Solar Cells. Molecules. 2024; 29(15):3625. https://doi.org/10.3390/molecules29153625
Chicago/Turabian StyleCrociani, Laura. 2024. "The Double-Cross of Benzotriazole-Based Polymers as Donors and Acceptors in Non-Fullerene Organic Solar Cells" Molecules 29, no. 15: 3625. https://doi.org/10.3390/molecules29153625
APA StyleCrociani, L. (2024). The Double-Cross of Benzotriazole-Based Polymers as Donors and Acceptors in Non-Fullerene Organic Solar Cells. Molecules, 29(15), 3625. https://doi.org/10.3390/molecules29153625














