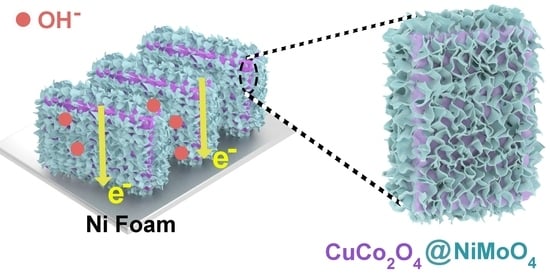
NiMoO4 Nanosheets Embedded in Microflake-Assembled CuCo2O4 Island-like Structure on Ni Foam for High-Performance Asymmetrical Solid-State Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Electrochemical Measurements
3. Experimental
3.1. Synthesis of CuCo2O4 Island-like Structure
3.2. Synthesis of CuCo2O4/NiMoO4 Heterostructures on Ni Foam
3.3. Materials Characterization
3.4. Electrochemical Measurements
3.5. Assemble of Asymmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Olabi, A.G.; Abbas, Q.; al Makky, A.; Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational design of electrode materials for advanced supercapacitors: From lab research to commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Dahiya, Y.; Hariram, M.; Kumar, M.; Jain, A.; Sarkar, D. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coord. Chem. Rev. 2022, 451, 214265. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhavar, A.A.; Kadam, R.A.; Velhal, N.B.; Nakate, U.T.; Teli, A.M.; Shin, J.C.; Nguyen, L.N.; Shin, D.K.; Kaushik, N.K. Hydrothermal synthesis of manganese oxide (Mn3O4) with granule-like morphology for supercapacitor application. Ceram. Int. 2022, 48, 29429–29437. [Google Scholar] [CrossRef]
- Asl, M.S.; Hadi, R.; Salehghadimi, L.; Tabrizi, A.G.; Farhoudian, S.; Babapoor, A.; Pahlevani, M. Flexible all-solid-state supercapacitors with high capacitance, long cycle life, and wide operational potential window: Recent progress and future perspectives. J. Energy Storage 2022, 50, 104223. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Yamani, Z.H. Recent progress in carbonaceous and redox-active nanoarchitectures for hybrid supercapacitors: Performance evaluation, challenges, and future prospects. Chem. Rec. 2022, 22, e202200018. [Google Scholar] [CrossRef]
- Hu, R.; Jiao, L.; Liang, H.; Feng, Z.; Gao, B.; Wang, X.; Song, X.; Liu, L.; Tan, Z. Engineering interfacial built-in electric field in polymetallic phosphide heterostructures for superior supercapacitors and electrocatalytic hydrogen evolution. Small 2023, 2023, 2304132. [Google Scholar] [CrossRef]
- Deshagani, S.; Maity, D.; Das, A.; Deepa, M. NiMoO4@NiMnCo2O4 heterostructure: A poly(3,4 propylenedioxythiophene) composite-based supercapacitor powers an electrochromic device. ACS Appl. Mater. Interfaces 2021, 13, 34518. [Google Scholar] [CrossRef]
- Wang, X.; Deng, C.; Hong, X.; Dong, W.; Liang, B. Controllable synthesis of NiCo2O4, NiCo2O4/graphene composite and their electrochemical application in supercapacitors. J. Energy Storage 2022, 55, 105837. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Zhang, Y.; Sun, J.; Zheng, J.; Wei, C.; Weimin, D.U.; Liu, L.; Cheng, C. Designed synthesis of yolk-shelled NiCo2O4/MnCo2O4 hollow sphere with boosted performance for supercapacitors. Appl. Surf. Sci. 2023, 611, 155758. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Lee, S.H.; Ryu, K.S. Porous thin layered nanosheets assembled ZnCo2O4 grown on Ni-foam as an efficient electrode material for hybrid supercapacitor applications. Int. J. Hydrog. Energy 2017, 42, 3122–3129. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Gao, H.; Shen, H.; Wu, H.; Xia, X.; Wu, X.; Lei, W.; Yang, J.; Hao, Q. Controllable synthesis of ZnCo2O4@NiCo2O4 heterostructures on Ni foam for hybrid supercapacitors with superior performance. J. Alloys Compd. 2022, 891, 162053. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Bao, E.; Du, S.; Xu, C.; Zhu, J.; Mao, H.; Chen, H. Assembly of high-performance hybrid supercapacitors using FeCo2O4 microflowers as battery-type cathode materials. Ceram. Int. 2023, 49, 10411–10419. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Dubai, D.P.; Ji, S.-H.; Kim, D.-H. Highly efficient and stable negative electrode for asymmetric supercapacitors based on graphene/FeCo2O4 nanocomposite hybrid material. Electrochim. Acta 2019, 295, 195–203. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, F.; Zhang, Z.; Zhuang, Q.; Liu, Q.; Yu, H.; Hu, M. In-situ growth of MnCo2O4 hollow spheres on nickel foam as pseudocapacitive electrodes for supercapacitors. J. Colloid Interface Sci. 2021, 587, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Naresh, B.; Kuchi, C.; Rajasekhar, D.; Reddy, P.S. Solvothermal synthesis of MnCo2O4 microspheres for high-performance electrochemical supercapacitors. Colloid Surf. A 2022, 640, 128443. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Zhang, W.-C.; Wu, X. Flexible CuCo2O4@Ni-Co-S hybrids as electrode materials for high-performance energy storage devices. Chin. Chem. Lett. 2023, 34, 107593. [Google Scholar] [CrossRef]
- Chen, S.; Cui, S.; Chandrasekaran, S.; Ke, C.; Li, Z.; Chen, P.; Zhang, C.; Jiang, Y. Growth of CuCo2O4@MnMoO4 core/shell nanosheet arrays for high energy density asymmetric supercapacitors. Electrochim. Acta 2020, 341, 135893. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Q.; Lu, L.; Zou, Y.; Xu, F.; Sun, L.; Cai, D.; Xiang, C. Hollow core-shell CuCo2O4@MoNi-layered double hydroxides as an electrode material for supercapacitors. J. Energy Storage 2023, 61, 106691. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Y.; Ren, F.; Tan, S.; Wang, Z. Three-dimensional hierarchical core-shell CuCo2O4@Co(OH)2 nanoflakes as high-performance electrode materials for flexible supercapacitors. J. Colloid Interface Sci. 2021, 586, 797–806. [Google Scholar] [CrossRef]
- Murugan, E.; Govindaraju, S.; Santhoshkumar, S. Hydrothermal synthesis, characterization and electrochemical behavior of NiMoO4 nanoflower and NiMoO4/rGO nanocomposite for high-performance supercapacitors. Electrochim. Acta 2021, 392, 138973. [Google Scholar] [CrossRef]
- Feng, X.; Ning, J.; Wang, D.; Zhang, J.; Xia, M.; Wang, Y.; Hao, Y. Heterostructure arrays of NiMoO4 nanoflakes on N-doping of graphene for high-performance asymmetric supercapacitors. J. Alloys Compd. 2020, 816, 152625. [Google Scholar] [CrossRef]
- Huang, B.; Yao, D.; Yuan, J.; Tao, Y.; Yin, Y.; He, G.; Chen, H. Hydrangea-like NiMoO4-Ag/rGO as battery-type electrode for hybrid supercapacitors with superior stability. J. Colloid Interface Sci. 2022, 606, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, C.; Gui, Y.; Blackwood, D.J. Rational design of self-supported Ni3S2 Nanosheets array for advanced asymmetric supercapacitor with a superior energy density. ACS Appl. Mater. Interfaces 2017, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Z.; Zhou, C.; Zhang, H. Tuning the morphology and size of NiMoO4 nanosheets anchored on NiCo2O4 nanowires: The optimized core-shell hybrid for high energy density asymmetric supercapacitors. Appl. Surf. Sci. 2021, 541, 148458. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Akilan, R.; Sakthivel, P.; Asaithambi, S.; Shankar, R.; Yuvakkumar, R.; Hayakawa, Y.; Ravi, G. Synthesis of self-assembled micro/nano structured manganese carbonate for high performance, long lifespan asymmetric supercapacitors and investigation of atomic-level intercalation properties of OH-ions via first principle calculation. J. Energy Storage 2020, 27, 101138. [Google Scholar] [CrossRef]
- Hong, J.; Lee, Y.W.; Ahn, D.; Pak, S.; Lee, J.; Jang, A.R.; Lee, S.; Hou, B.; Cho, Y.; Morris, S.M.; et al. Highly stable 3D porous heterostructures with hierarchically-coordinated octahedral transition metals for enhanced performance supercapacitors. Nano Energy 2017, 39, 337–345. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, B.; Huang, C.; Li, Y.; Wang, J.; Tang, S.; Deng, M.; Du, Y. Heterostructural three dimensional reduced graphene oxide/CoMn2O4 nanosheets toward a wide-potential window for high performance supercapacitors. ACS Appl. Energy Mater. 2019, 2, 5219–5230. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, S.S.; Wu, Y.J.; Zheng, Z.M.; Hong, K.Q.; Li, B.S.; Sun, Y.M. Polyhedron-core/double-shell CuO@C@MnO2 decorated nickel foam for high performance all-solid-state supercapacitors. Electrochim. Acta 2017, 246, 1065–1074. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Wang, G.; Hao, C.; Huang, C.; Jiang, C. Facile construction of a MgCo2O4@NiMoO4/NF core-shell nanocomposite for high-performance asymmetric supercapacitors. J. Mater. Chem. C. 2019, 7, 13267–13278. [Google Scholar] [CrossRef]
- Zhan, Z.Y.; Chen, S.; Xie, J.; Yang, Y.; Xiong, J. Growth of highly mesoporous CuCo2O4 nanoflakes@Ni(OH)2 nanosheets as advanced electrodes for high-performance hybrid supercapacitors. J. Alloys Compd. 2017, 722, 928–937. [Google Scholar] [CrossRef]
- Hao, C.; Guo, Y.N.; Xian, S.L.; Zheng, W.H.; Gao, H.W.; Wang, X.H. Fabrication of flower-shaped CuCo2O4@MgMoO4 nanocomposite for high-performance supercapacitors. J. Energy Storage 2021, 41, 102972. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Zhang, X.; Yu, D.; Ji, Y.; Sun, Q.; Wang, Y.; Liu, X. Facile synthesis of hierarchical CoMoO4@NiMoO4 core–shell nanosheet arrays on nickel foam as an advanced electrode for asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 18578–18584. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.S.; Hui, K.N.; Yun, J.M.; Kim, K.H. Vertically stacked bilayer CuCo2O4/MnCo2O4 heterostructures on functionalized graphite paper for high-performance electrochemical capacitors. J. Mater. Chem. A 2016, 4, 8061–8071. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; Lou, X.W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Lu, S.; Yang, W.; Zhou, M.; Qiu, L.; Tao, B.; Zhao, Q.; Wang, X.; Zhang, L.; Xie, Q.; Ruan, Y. Nitrogen-and oxygen-doped carbon with abundant micropores derived from biomass waste for all-solid-state flexible supercapacitors. J. Colloid Interface Sci. 2022, 610, 1088–1099. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.; Yu, X.; Li, Q.; Wang, T. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Liu, T.; Chai, H.; Jia, D.Z.; Su, Y.; Wang, T.; Zhou, W.Y. Three-dimensional Co3O4@NiMoO4 core/shell nanowire arrays on Ni Foam for electrochemical energy storage. Electrochim. Acta 2015, 180, 998–1006. [Google Scholar] [CrossRef]
- Budhiraju, V.S.; Kumar, R.; Sharma, A.; Sivakumar, S. Structurally stable hollow mesoporous graphitized carbon nanofibers embedded with NiMoO4 nanoparticles for high performance asymmetric supercapacitors. Electrochim. Acta 2017, 238, 337–348. [Google Scholar] [CrossRef]
- Wan, L.; Liu, J.; Li, X.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Fabrication of core-shell NiMoO4@MoS2 nanorods for high-performance asymmetric hybrid supercapacitors. Int. J. Hydrogen Energy 2020, 45, 4521–4533. [Google Scholar] [CrossRef]









| Electrode Materials | Max Capacitance | Min Capacitance | Rate Capability | Retention (Cycles) |
|---|---|---|---|---|
| NiMoO4 nanosheet arrays | 1220 (1 A/g) | 490 (10 A/g) | 40.2% | 69.7% (3000) |
| CuCo2O4 nanosheet-assembled island-like | 797 (1 A/g) | 366 (10 A/g) | 45.9% | 91.0% (5000) |
| CuCo2O4/NiMoO4 hierarchically structure | 2350 (1 A/g) | 1235 (10 A/g) | 52.5% | 91.5% (5000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Chen, L.; Li, L. NiMoO4 Nanosheets Embedded in Microflake-Assembled CuCo2O4 Island-like Structure on Ni Foam for High-Performance Asymmetrical Solid-State Supercapacitors. Molecules 2023, 28, 6840. https://doi.org/10.3390/molecules28196840
Li G, Chen L, Li L. NiMoO4 Nanosheets Embedded in Microflake-Assembled CuCo2O4 Island-like Structure on Ni Foam for High-Performance Asymmetrical Solid-State Supercapacitors. Molecules. 2023; 28(19):6840. https://doi.org/10.3390/molecules28196840
Chicago/Turabian StyleLi, Gaofeng, Lingling Chen, and Longfei Li. 2023. "NiMoO4 Nanosheets Embedded in Microflake-Assembled CuCo2O4 Island-like Structure on Ni Foam for High-Performance Asymmetrical Solid-State Supercapacitors" Molecules 28, no. 19: 6840. https://doi.org/10.3390/molecules28196840
APA StyleLi, G., Chen, L., & Li, L. (2023). NiMoO4 Nanosheets Embedded in Microflake-Assembled CuCo2O4 Island-like Structure on Ni Foam for High-Performance Asymmetrical Solid-State Supercapacitors. Molecules, 28(19), 6840. https://doi.org/10.3390/molecules28196840