Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results and Discussion
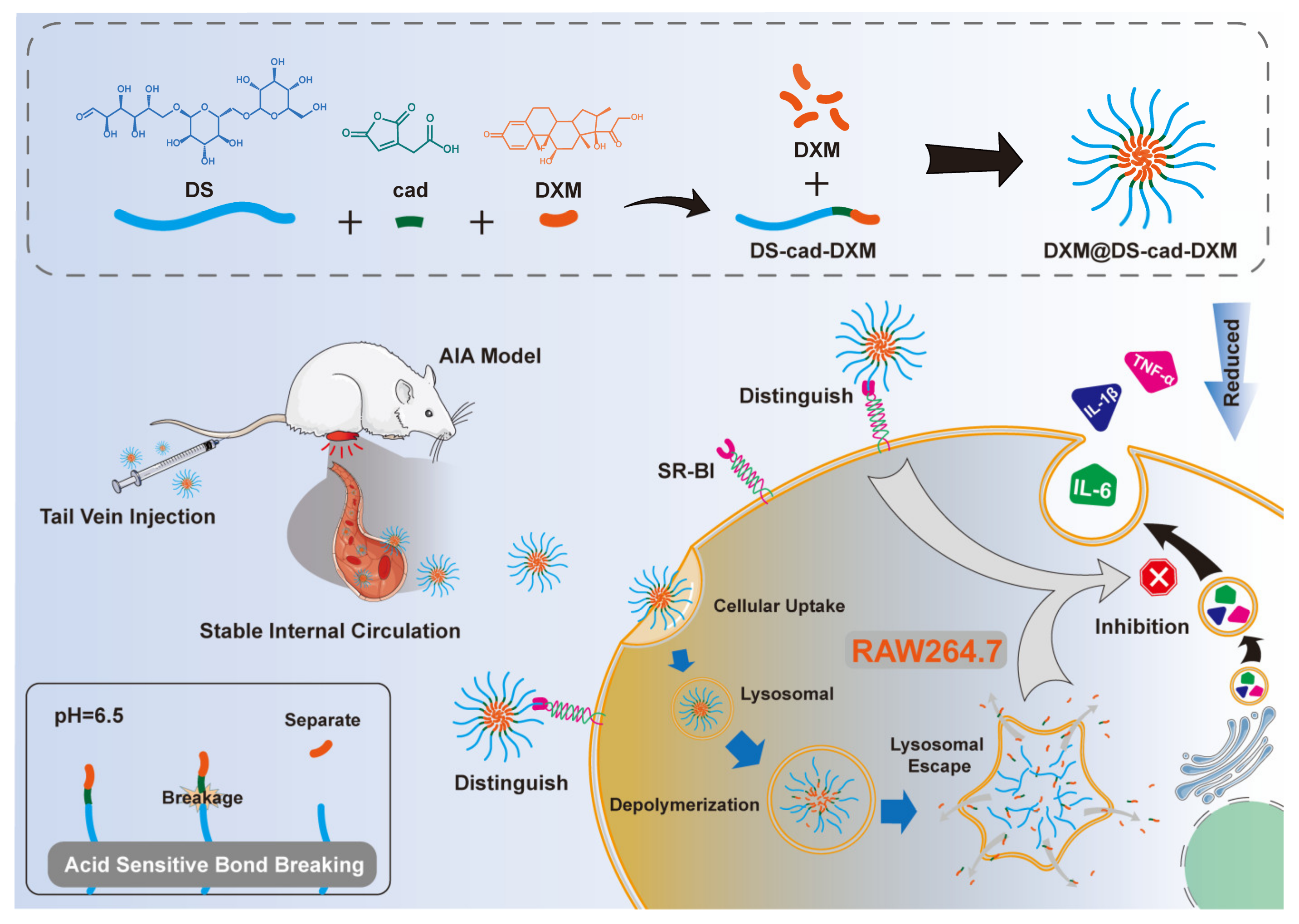
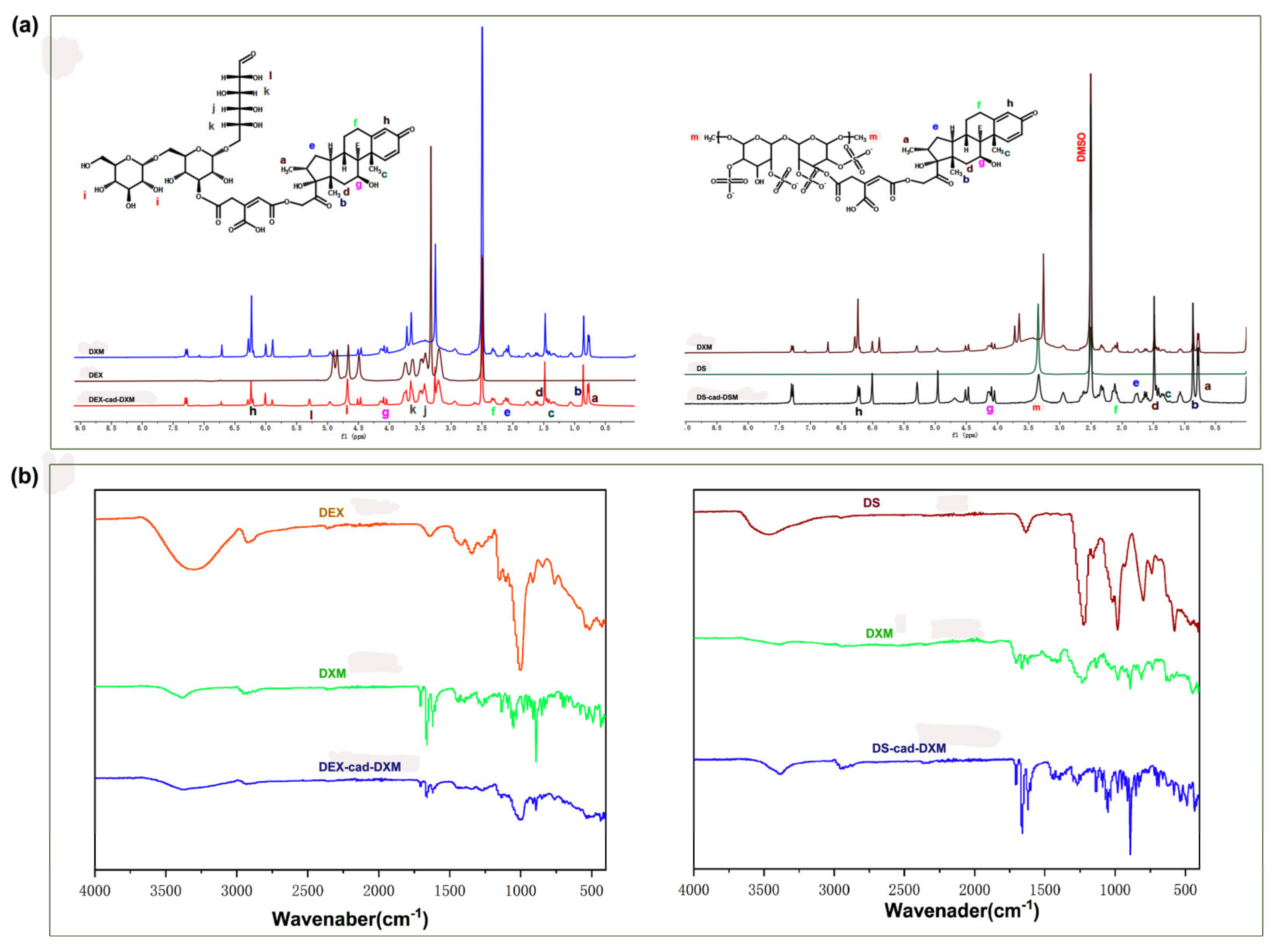
2.1. Characterizations of the Conjugates
2.2. Preparation and Characterization of Micelles
2.3. Release Profiles and Targeting Ability Analysis
2.4. Anti-Inflammatory Activity
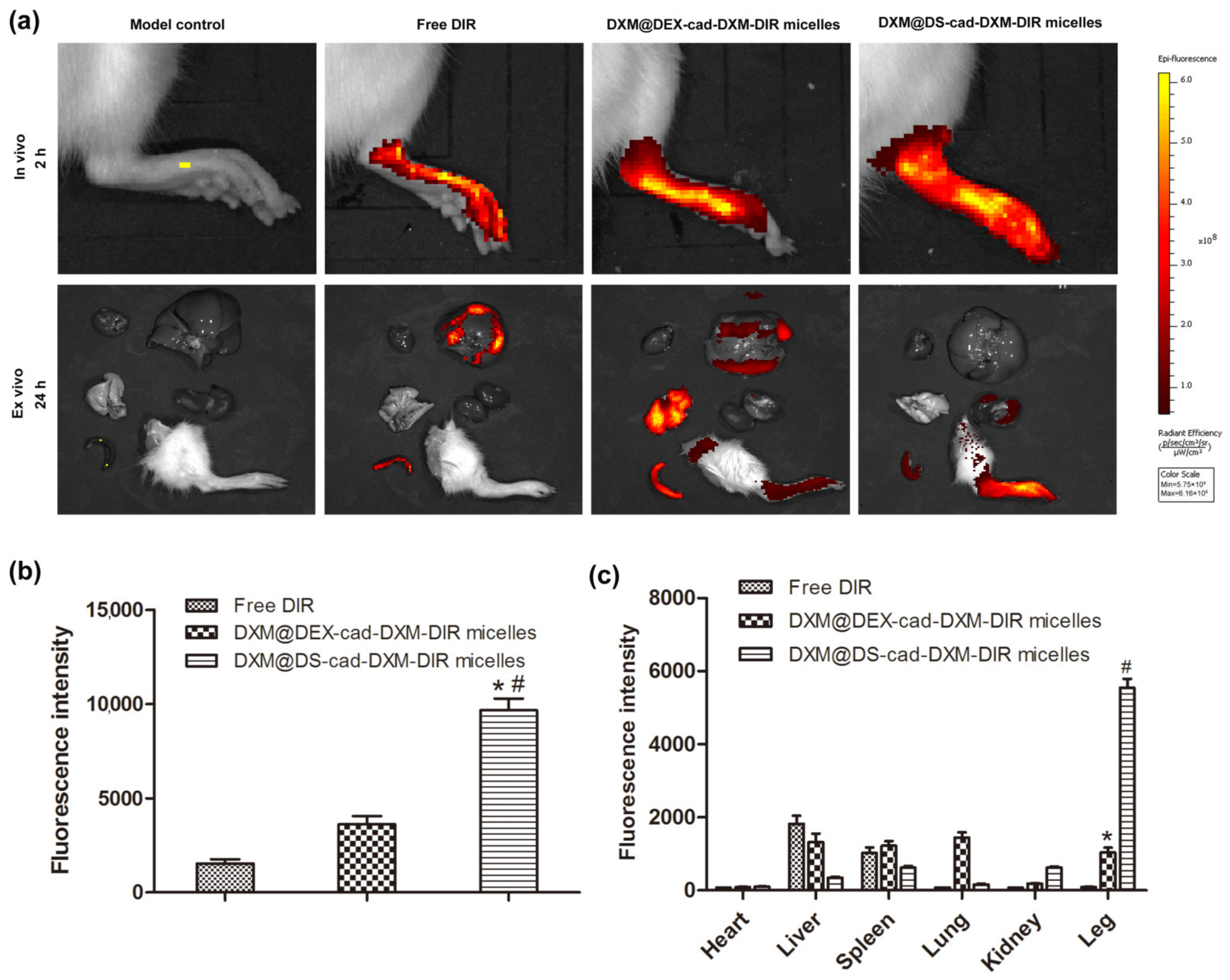
2.5. Selective Biodistribution In Vivo
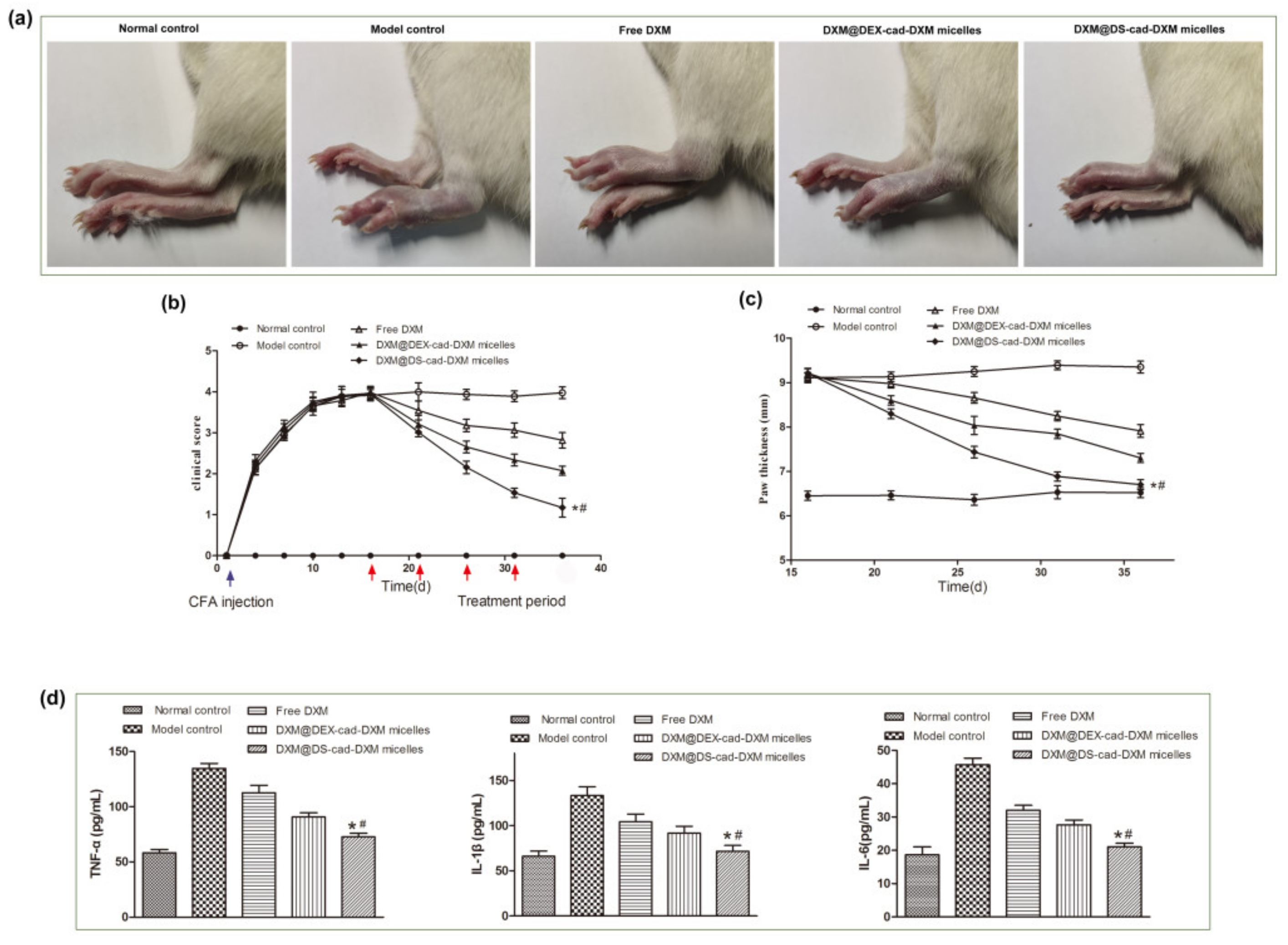
2.6. Therapeutic Efficacy of AIA Models
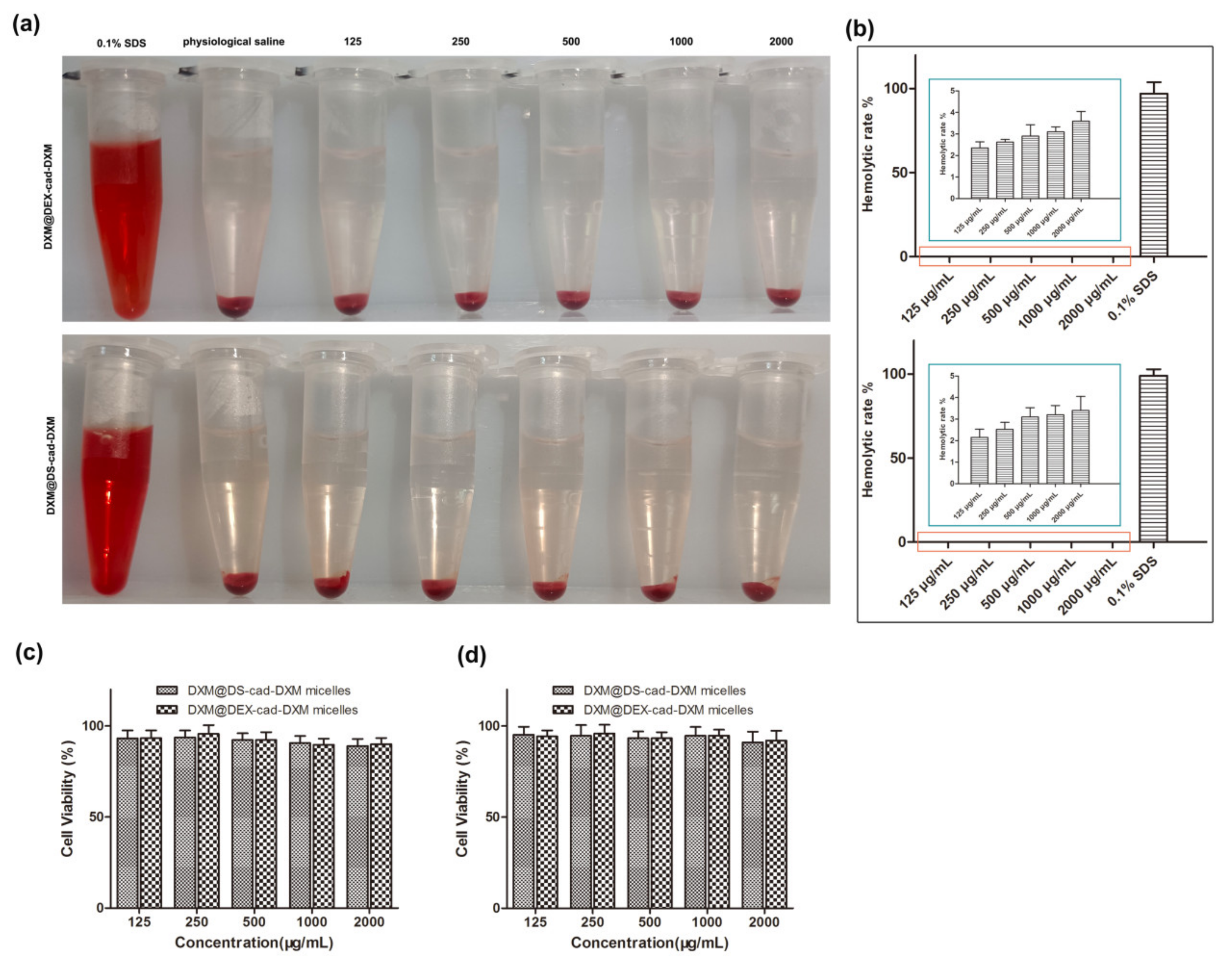
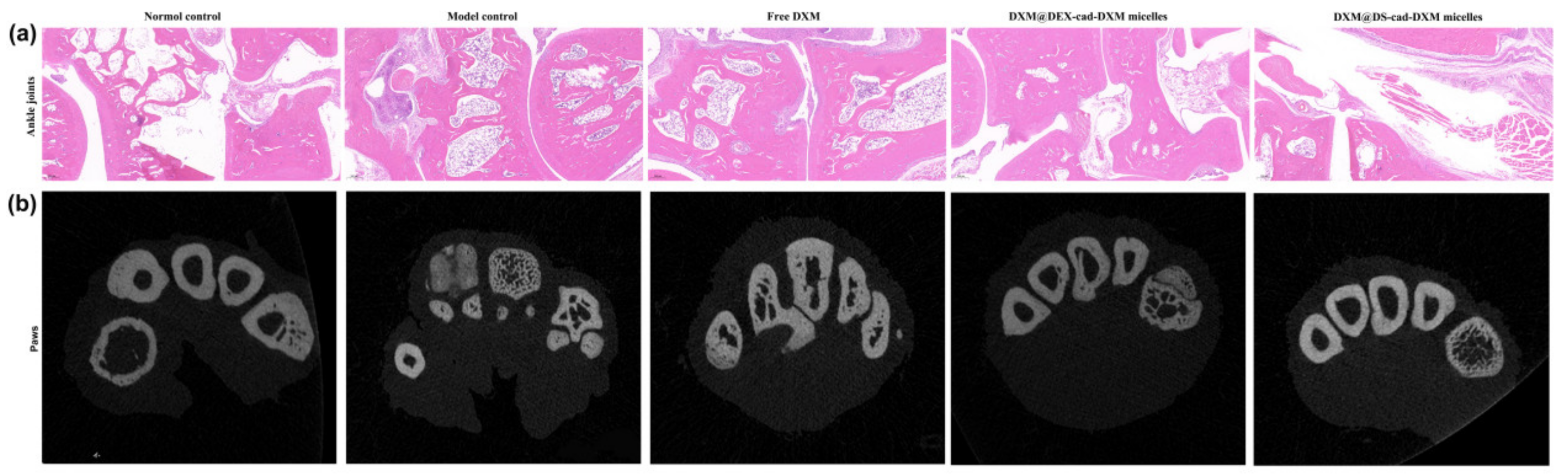
2.7. Safety Evaluation In Vivo
3. Materials and Methods
3.1. Materials
3.2. Cell Culture and Animals
3.3. Syntheses of DS-cad-DXM Conjugate
3.4. Preparation and Characterization of DS-cad-DXM Conjugate Micelles (DXM@DS-cad-DXM)
3.5. Release Profiles of DXM from DXM@DS-cad-DXM
3.6. Biocompatibility Evaluation of DXM@DS-cad-DXM
3.7. Targeting Ability of DXM@DS-cad-DXM
3.8. Anti-Inflammatory Efficacy of DXM@DS-cad-DXM
3.9. Biodistribution of DXM@DS-cad-DXM
3.10. In Vivo Therapeutic Efficacy of DXM@DS-cad-DXM
3.11. Safety Evaluation DXM@DS-cad-DXM
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Anzaghe, M.; Schulke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [PubMed] [Green Version]
- Otón, T.; Carmona, L. The epidemiology of established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101477. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Kumar, A.; Pawar, S.V. A Review on Rheumatoid Arthritis Interventions and Current Developments. Curr. Drug Targets 2021, 22, 463–483. [Google Scholar] [CrossRef]
- Hannemann, N.; Apparailly, F.; Courties, G. New insights into macrophage heterogeneity in rheumatoid arthritis. Jt. Bone Spine 2021, 88, 105091. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A. Insights of rheumatoid arthritis risk factors and associations. J. Transl. Autoimmun. 2019, 2, 100012. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Liu, Q.; Zhou, M.; Wang, K.; Wang, Y.; Nie, J.; Gui, S.; Peng, D.; He, Z.; et al. Emerging nanotherapeutics alleviating rheumatoid arthritis by readjusting the seeds and soils. J. Control. Release 2022, 345, 851–879. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef]
- Lorscheider, M.; Tsapis, N.; Ur-Rehman, M.; Gaudin, F.; Stolfa, I.; Abreu, S.; Mura, S.; Chaminade, P.; Espeli, M.; Fattal, E. Dexamethasone palmitate nanoparticles: An efficient treatment for rheumatoid arthritis. J. Control. Release 2019, 296, 179–189. [Google Scholar] [CrossRef]
- Janakiraman, K.; Krishnaswami, V.; Rajendran, V.; Natesan, S.; Kandasamy, R. Novel nano therapeutic materials for the effective treatment of rheumatoid arthritis-recent insights. Mater. Today Commun. 2018, 17, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ismail, M.; Shan, Q.; Zhao, J.; Zhu, Y.; Zhang, L.; Du, Y.; Ling, L. ROS-mediated liposomal dexamethasone: A new FA-targeted nanoformulation to combat rheumatoid arthritis via inhibiting iRhom2/TNF-alpha/BAFF pathways. Nanoscale 2021, 13, 20170–20185. [Google Scholar] [CrossRef] [PubMed]
- Heo, R.; Gil You, D.; Um, W.; Choi, K.Y.; Jeon, S.; Park, J.-S.; Choi, Y.; Kwon, S.; Kim, K.; Kwon, I.C.; et al. Dextran sulfate nanoparticles as a theranostic nanomedicine for rheumatoid arthritis. Biomaterials 2017, 131, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, H.; Guo, C.; Chen, Q.; Su, Y.; Guo, H.; Hou, X.; Zhao, F.; Fan, H.; Xu, H.; et al. Dextran sulfate-based MMP-2 enzyme-sensitive SR-A receptor targeting nanomicelles for the treatment of rheumatoid arthritis. Drug Deliv. 2022, 29, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, J.; Feng, X.; Chang, F.; Wang, Y.; Gao, Z.; Zhuang, X.; Chen, X. Scavenger Receptor-Mediated Targeted Treatment of Collagen-Induced Arthritis by Dextran Sulfate-Methotrexate Prodrug. Theranostics 2017, 7, 97–105. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, J.; Zhang, F.; Liao, T.; Na, R.; Yuan, X.; He, G.; Ye, W. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv. 2022, 29, 2269–2282. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Xu, X.; Su, M.; Xi, M.; Yin, Z. Dextran sulfate-modified pH-sensitive layered double hydroxide nanocomposites for treatment of rheumatoid arthritis. Drug Deliv. Transl. Res. 2021, 11, 1096–1106. [Google Scholar]
- Yan, F.; Li, H.; Zhong, Z.; Zhou, M.; Lin, Y.; Tang, C.; Li, C. Co-Delivery of Prednisolone and Curcumin in Human Serum Albumin Nanoparticles for Effective Treatment of Rheumatoid Arthritis. Int. J. Nanomed. 2019, 14, 9113–9125. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.; Zhang, Y.; Dusad, A.; Ren, K.; Purdue, P.E.; Goldring, S.R.; Wang, D. The Evaluation of the Therapeutic Efficacy and Side Effects of a Macromolecular Dexamethasone Prodrug in the Collagen-Induced Arthritis Mouse Model. Pharm. Res. 2016, 33, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.; Zhang, P.; Deng, C.; Xiao, Y.; Gong, T.; Zhang, Z. An effective and safe treatment strategy for rheumatoid arthritis based on human serum albumin and Kolliphor((R)) HS 15. Nanomedicine 2019, 14, 2169–2187. [Google Scholar]
- De la Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, J.; Fatieiev, Y.; Almalik, A.; Khashab, N. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Kang, X.; Ren, S.; Ouyang, X.; Chang, M. Construction of a Nano-Controlled Release Methotrexate Delivery System for the Treatment of Rheumatoid Arthritis by Local Percutaneous Administration. Nanomaterials 2021, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, F.; Wang, H.; Wu, X.; Eltahan, A.S.; Stanford, S.; Bottini, N.; Xiao, H.; Bottini, M.; Guo, W.; et al. Secreted Protein Acidic and Rich in Cysteine Mediated Biomimetic Delivery of Methotrexate by Albumin-Based Nanomedicines for Rheumatoid Arthritis Therapy. ACS Nano 2019, 13, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, W.; Chen, X.; Xu, X.; Zhang, X.; Zhang, X. pH and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102008. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Zhang, C.; Zhi, X.; Cheng, J.; de la Fuente, J.; Song, J.; Cui, D. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018, 68, 308–319. [Google Scholar] [CrossRef]
- Liu, L.; Guo, W.; Liang, X.-J. Move to Nano-Arthrology: Targeted Stimuli-Responsive Nanomedicines Combat Adaptive Treatment Tolerance (ATT) of Rheumatoid Arthritis. Biotechnol. J. 2019, 14, e1800024. [Google Scholar] [CrossRef] [Green Version]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6–Induced Osteoclasts in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1145–1154. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, K.; Yang, Y.; Wang, F.; Li, W. Glaucocalyxin A suppresses inflammatory responses and induces apoptosis in TNF-a-induced human rheumatoid arthritis via modulation of the STAT3 pathway. Chem. Interact. 2021, 341, 109451. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, D.; Wang, R.; Wu, M.; Zhu, L.; Peng, W.; Tu, H.; Deng, X.; Zhu, H.; Zhang, Z.; et al. Targeted therapy of rheumatoid arthritis via macrophage repolarization. Drug Deliv. 2021, 28, 2447–2459. [Google Scholar] [CrossRef]
- Yuan, F.; Quan, L.-D.; Cui, L.; Goldring, S.R.; Wang, D. Development of macromolecular prodrug for rheumatoid arthritis. Adv. Drug Deliv. Rev. 2012, 64, 1205–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, P.; Bian, S.; Cheng, Y.; Dong, S.; Liu, J.; Liu, W.; Xiao, C. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. Int. J. Biol. Macromol. 2020, 158, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Saravanakumar, G.; Son, S.; Han, H.; Heo, R.; Kim, K.; Kwon, I.; Lee, J.; Park, J. Dextran sulfate-coated superparamagnetic iron oxide nanoparticles as a contrast agent for atherosclerosis imaging. Carbohyd. Polym. 2014, 101, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Qin, X.; Fan, D.; Feng, C.; Wang, Q.; Fang, J. Dual-Stimuli Responsive Polymeric Micelles for the Effective Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 21076–21086. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Wang, T.; Yuan, Y.; Yang, Y.; Luo, X.; Hu, S.; Ding, J.; Zhou, W. Rational design of metal-organic frameworks to deliver methotrexate for targeted rheumatoid arthritis therapy. J. Control. Release 2021, 330, 119–131. [Google Scholar] [CrossRef]
- Gou, K.-J.; Zeng, R.; Ren, X.-D.; Dou, Q.-L.; Yang, Q.-B.; Dong, Y.; Qu, Y. Anti-rheumatoid arthritis effects in adjuvant-induced arthritis in rats and molecular docking studies of Polygonum orientale L. extracts. Immunol. Lett. 2018, 201, 59–69. [Google Scholar] [CrossRef]
- Meng, M.; Yue, Z.; Chang, L.; Liu, Y.; Hu, J.; Song, Z.; Tang, Z.; Zhou, R.; Wang, C. Anti-Rheumatoid Arthritic Effects of Paris Saponin VII in Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and Adjuvant-Induced Arthritis in Rats. Front. Pharmacol. 2021, 12, 683698. [Google Scholar] [CrossRef]
- Ye, W.-L.; Zhao, Y.-P.; Cheng, Y.; Liu, D.-Z.; Cui, H.; Liu, M.; Zhang, B.-L.; Mei, Q.-B.; Zhou, S.-Y. Bone metastasis target redox-responsive micell for the treatment of lung cancer bone metastasis and anti-bone resorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 380–391. [Google Scholar] [CrossRef]
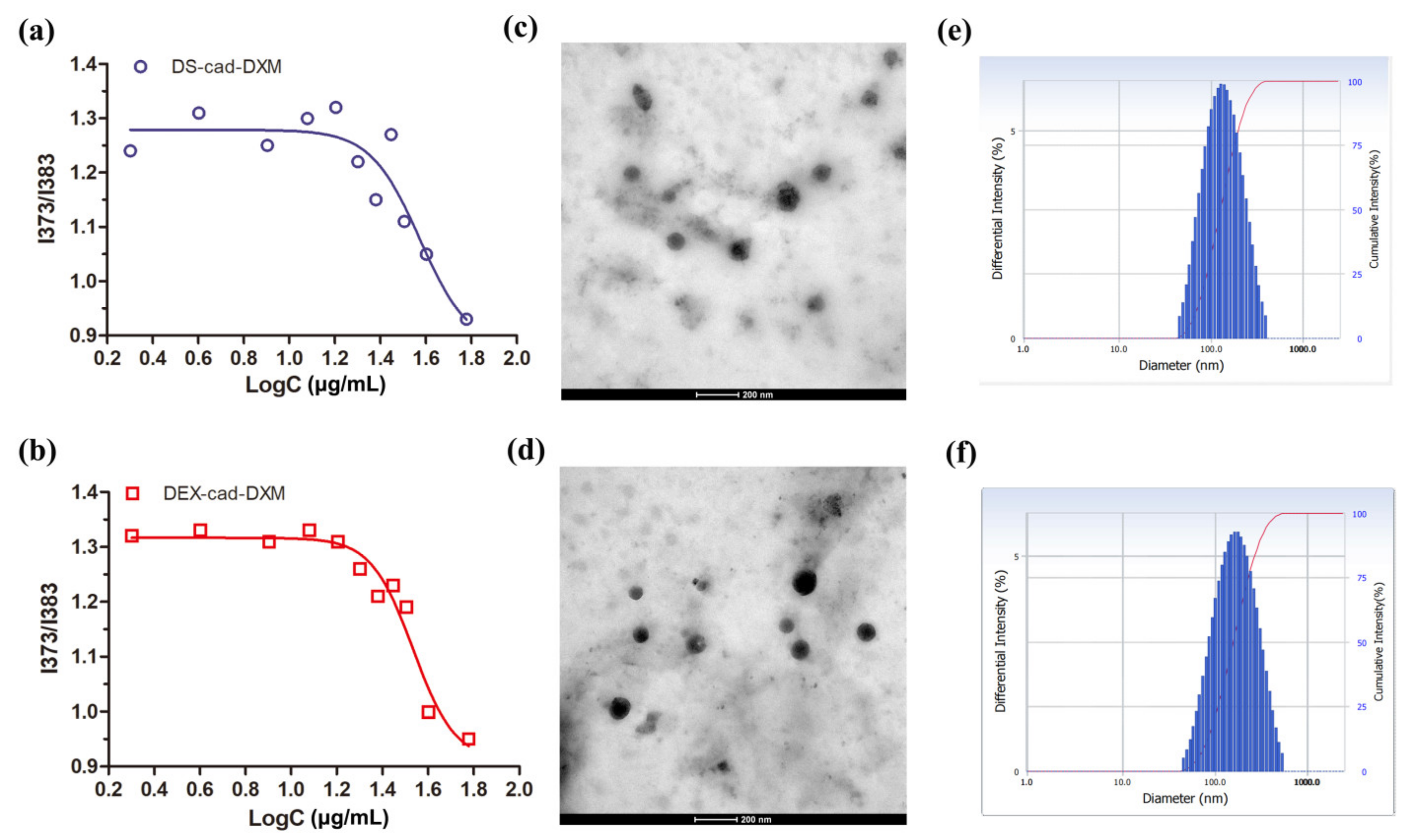



| DXM-Loaded Micelles | Size (nm) | Zeta (mV) | PDI | DL (%) |
|---|---|---|---|---|
| DXM@DEX-cad-DXM | 147 ± 12.1 | −17.4 ± 1.8 | 0.209 ± 0.5 | 24.3 ± 3.7 |
| DXM@DS-cad-DXM | 136 ± 11.3 | −19.9 ± 2.1 | 0.214 ± 0.7 | 25.4 ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Na, R.; Zhao, N.; Yuan, X.; Fu, L.; Jing, J.; Qian, A.; Ye, W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules 2023, 28, 591. https://doi.org/10.3390/molecules28020591
Han J, Na R, Zhao N, Yuan X, Fu L, Jing J, Qian A, Ye W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules. 2023; 28(2):591. https://doi.org/10.3390/molecules28020591
Chicago/Turabian StyleHan, Jiangfan, Ren Na, Ningning Zhao, Xiaofeng Yuan, Linke Fu, Jianmei Jing, Airong Qian, and Weiliang Ye. 2023. "Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis" Molecules 28, no. 2: 591. https://doi.org/10.3390/molecules28020591





