Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022)
Abstract
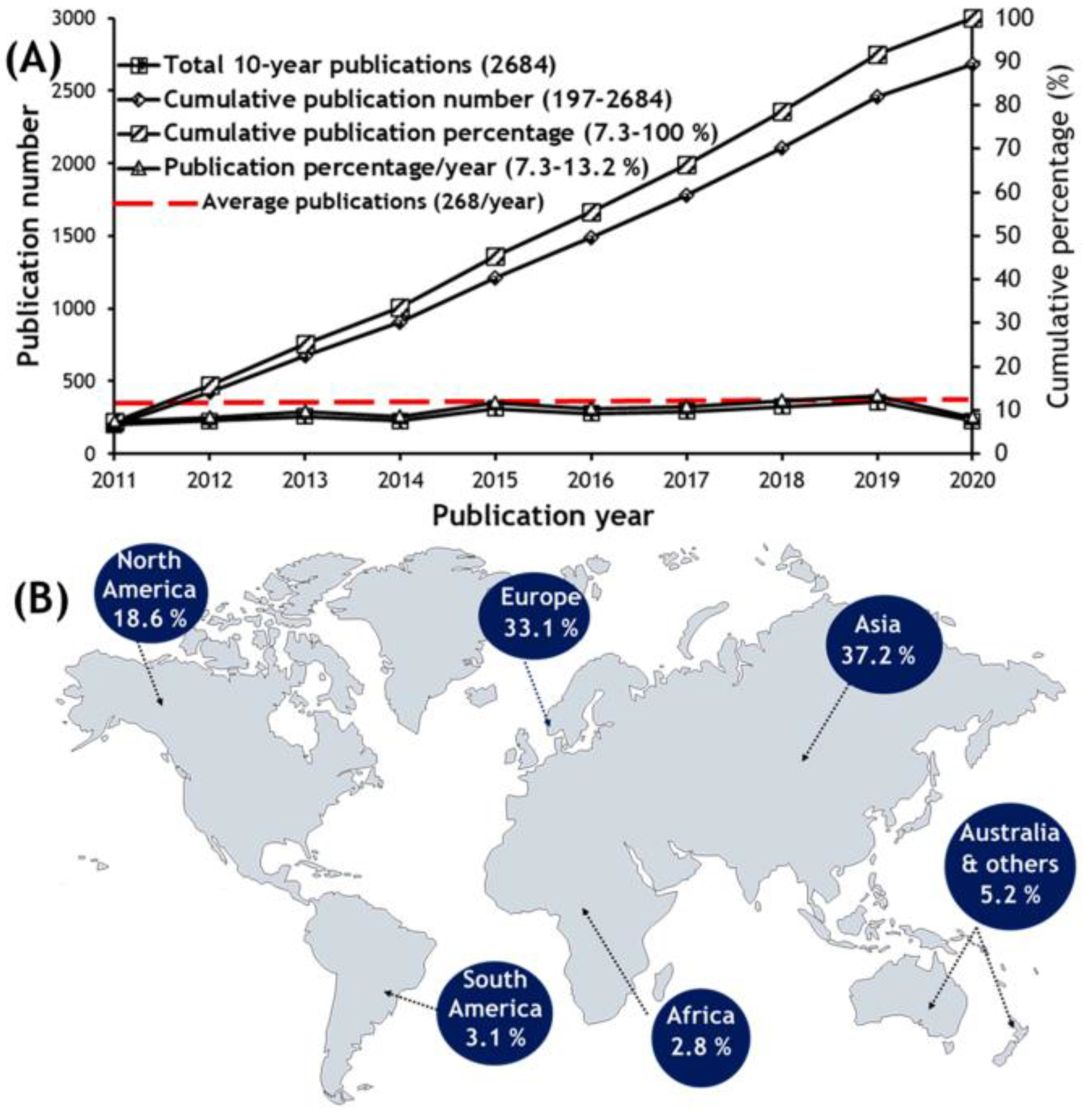
:1. Introduction
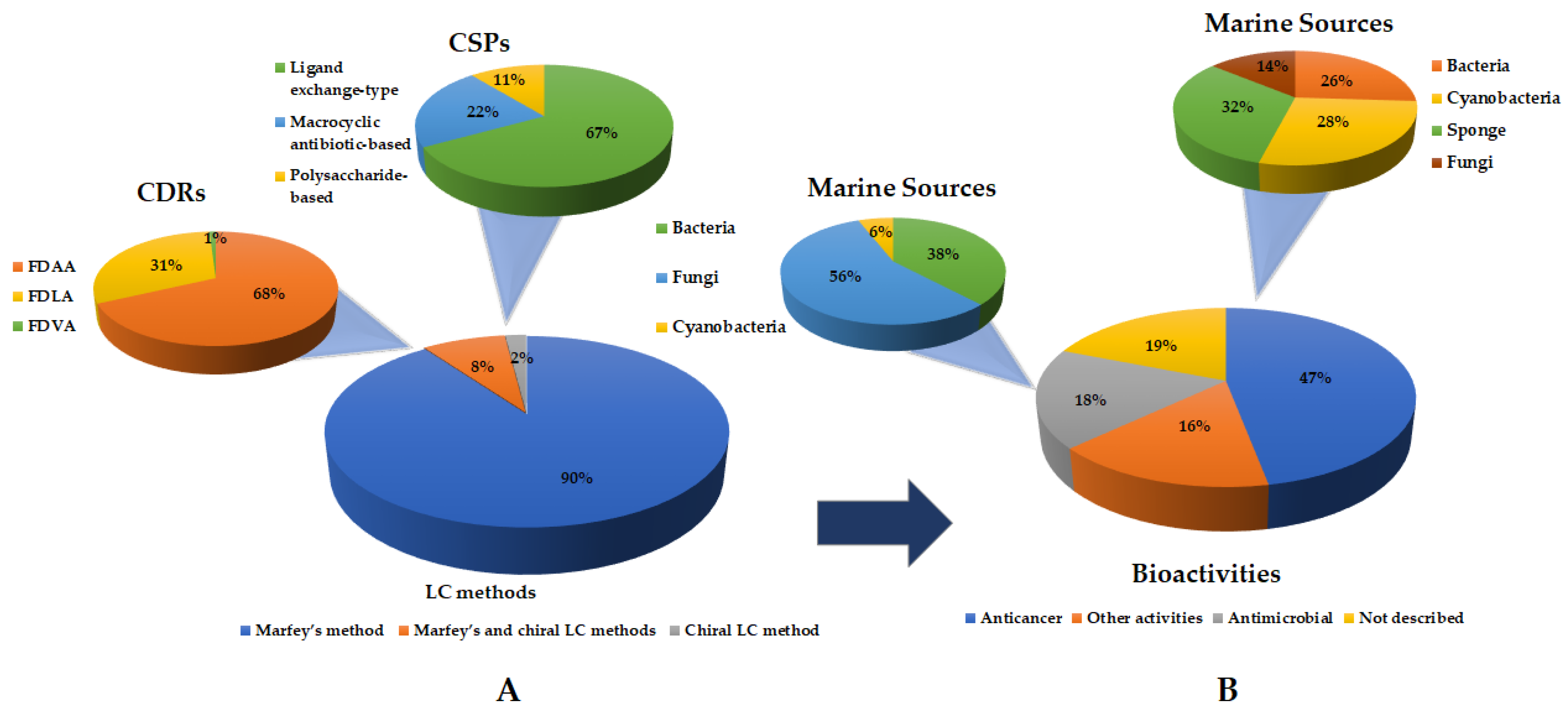
2. Liquid Chromatography Methods for Determination of Absolute Configurations of Peptides
3. New Marine-Derived Cyclopeptides Reported from January 2018 to November 2022
3.1. Anticancer Activity
3.2. Antimicrobial Activity
3.3. Other Activities
3.4. No Demonstrated Biological Activity
3.5. Final Remarks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, R.; Chhabra, P.; Goyal, A.P.; Srivastava, S. Predictive modelling to assess groundwater pollution and integration with water quality index. Int. J. Eng. Adv. Technol. 2019, 8, 1076–1084. [Google Scholar]
- Xing, L.; Wang, Z.; Hao, Y.; Zhang, W. Marine Products As a Promising Resource of Bioactive Peptides: Update of Extraction Strategies and Their Physiological Regulatory Effects. J. Agric. Food Chem. 2022, 70, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Mandhare, A.; Bagalkote, V. Marine natural products as source of new drugs: An updated patent review (July 2018–July 2021). Expert Opin. Ther. Pat. 2022, 32, 317–363. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.d.; Carneiro, J.A.; Costa, R.A.d.; Alencar, S.A.d.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci. 2021, 889, 667764. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Degen, D.; Jang, K.H.; Ebright, R.H.; Fenical, W. Correction to: Salinamide F, new depsipeptide antibiotic and inhibitor of bacterial RNA polymerase from a marine-derived Streptomyces sp. (The Journal of Antibiotics, (2015), 68, 3, (206-209), 10.1038/ja.2014.122). J. Antibiot. 2022, 75, 415. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; De Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Corrigendum to ‘Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp.’ [Tetrahedron Lett. 56(28) (2015) 4215–4219] (S0040403915008679) (10.1016/j.tetlet.2015.05.058)). Tetrahedron Lett. 2015, 56, 5482. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- O’connor, J.; Garcia-Vaquero, M.; Meaney, S.; Tiwari, B.K. Bioactive Peptides from Algae: Traditional and Novel Generation Strategies, Structure-Function Relationships, and Bioinformatics as Predictive Tools for Bioactivity. Mar. Drugs 2022, 20, 317. [Google Scholar] [CrossRef]
- Pesic, A.; Baumann, H.I.; Kleinschmidt, K.; Ensle, P.; Wiese, J.; Süssmuth, R.D.; Imhoff, J.F. Champacyclin, a new cyclic octapeptide from Streptomyces strain C42 isolated from the Baltic Sea. Mar. Drugs 2013, 11, 4834–4857. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, Q.; Liu, X.; Chen, Y.; Zhang, Y.; Sun, A.; Zhang, W.; Zhang, J.; Ju, J. Cyclic hexapeptides from the deep South China sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic gram-positive bacteria. J. Nat. Prod. 2014, 77, 1937–1941. [Google Scholar] [CrossRef]
- Sun, P.; Maloney, K.N.; Nam, S.J.; Haste, N.M.; Raju, R.; Aalbersberg, W.; Jensen, P.R.; Nizet, V.; Hensler, M.E.; Fenical, W. Fijimycins A-C, three antibacterial etamycin-class depsipeptides from a marine-derived Streptomyces sp. Bioorg. Med. Chem. 2011, 19, 6557–6562. [Google Scholar] [CrossRef] [Green Version]
- Linington, R.G.; González, J.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401. [Google Scholar] [CrossRef]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya mojuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- De Sá, J.D.M.; Kumla, D.; Dethoup, T.; Kijjoa, A. Bioactive Compounds from Terrestrial and Marine-Derived Fungi of the Genus Neosartorya†. Molecules 2022, 27, 2351. [Google Scholar] [CrossRef]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Zhang, P.; Li, X.M.; Li, C.S.; Cui, C.M.; Wang, B.G. Cyclohexadepsipeptides of the isaridin class from the marine-derived fungus Beauveria felina EN-135. J. Nat. Prod. 2014, 77, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Hou, Y.; Vazquez-Rivera, E.; Braun, D.; Bugni, T.S. Peptidolipins B-F, antibacterial lipopeptides from an ascidian-derived Nocardia sp. J. Nat. Prod. 2012, 75, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Amelia, T.S.M.; Suaberon, F.A.C.; Vad, J.; Fahmi, A.D.M.; Saludes, J.P.; Bhubalan, K. Recent Advances of Marine Sponge-Associated Microorganisms as a Source of Commercially Viable Natural Products. Mar. Biotechnol. 2022, 24, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.M.M.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef] [Green Version]
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinto, M.M.M.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Sekeroglu, N.; et al. New cyclotetrapeptides and a new diketopiperzine derivative from the marine sponge-associated fungus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.M.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Ghanbari, R. Review on the Bioactive Peptides from Marine Sources: Indication for Health Effects. Int. J. Pept. Res. Ther. 2019, 25, 1187–1199. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Rangel, M.; José Correia de Santana, C.; Pinheiro, A.; Dos Anjos, L.; Barth, T.; Rodrigues Pires Júnior, O.; Fontes, W.; Castro, M.S. Marine depsipeptides as promising pharmacotherapeutic agents. Curr.t Protein Pep. Sci. 2017, 18, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Curr. Protein Pept. Sci. 2017, 90, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds From Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- McIntosh, M.; Cruz, L.J.; Hunkapiller, M.W.; Gray, W.R.; Olivera, B.M. Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch. Biochem. Biophys. 1982, 218, 329–334. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyi, L.; Tomer, K.B.; Bontems, R.J. The Isolation and Structure of a Remarkable Marine Animal Antineoplastic Constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 468. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, V.; Nicholson, G.J.; Ries, J.; Recktenwald, J.r.; Schefer, A.B.; Shawky, R.M.; Schröder, J.; Wohlleben, W.; Pelzer, S. A polyketide synthase in glycopeptide biosynthesis: The biosynthesis of the non-proteinogenic amino acid (S)-3, 5-dihydroxyphenylglycine. J. Biol. Chem. 2001, 276, 38370–38377. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.P.; Nogawa, T.; Futamura, Y.; Shimizu, T.; Hashizume, D.; Takahashi, S.; Jang, J.H.; Ahn, J.S.; Osada, H. Octaminomycins A and B, cyclic octadepsipeptides active against Plasmodium falciparum. J. Nat. Prod. 2017, 80, 134–140. [Google Scholar] [CrossRef]
- Fukuhara, K.; Takada, K.; Okada, S.; Matsunaga, S. Nazumazoles A-C, cyclic pentapeptides dimerized through a disulfide bond from the marine sponge Theonella swinhoei. Org. Lett. 2015, 17, 2646–2648. [Google Scholar] [CrossRef]
- Luo, S.; Krunic, A.; Kang, H.S.; Chen, W.L.; Woodard, J.L.; Fuchs, J.R.; Swanson, S.M.; Orjala, J. Trichormamides A and B with antiproliferative activity from the cultured freshwater cyanobacterium Trichormus sp. UIC 10339. J. Nat. Prod. 2014, 77, 1871–1880. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; de Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Sieber, S.A.; Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. [Google Scholar] [CrossRef]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- van der Pijl, P.C.; Kies, A.K.; Ten Have, G.A.; Duchateau, G.S.; Deutz, N.E. Pharmacokinetics of proline-rich tripeptides in the pig. Peptides 2008, 29, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Stokvis, E.; Jimeno, J.M.; López-Lázaro, L.; Schellens, J.H.; Beijnen, J.H. Chemical and enzymatic stability of a cyclic depsipeptide, the novel, marine-derived, anti-cancer agent kahalalide F. Anti-Cancer Drugs 2001, 12, 575–582. [Google Scholar] [CrossRef]
- Sera, Y.; Adachi, K.; Nishida, F.; Shizuri, Y. Isolation and evaluation of nonsiderophore cyclic peptides from marine sponges. Biochem. Biophys. Res. Commun. 2001, 283, 976–981. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.-Y.; Shao, C.-L.; Wang, C.-Y. Metabolites from marine invertebrates and their symbiotic microorganisms: Molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef] [Green Version]
- Vining, O.B.; Medina, R.A.; Mitchell, E.A.; Videau, P.; Li, D.; Serrill, J.D.; Kelly, J.X.; Gerwick, W.H.; Proteau, P.J.; Ishmael, J.E. Depsipeptide companeramides from a panamanian marine cyanobacterium associated with the coibamide producer. J. Nat. Prod. 2015, 78, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.Y.G.; Ong, J.F.M.; Goh, H.C.; Coffill, C.R.; Tan, L.T. Benderamide A, a Cyclic Depsipeptide from a Singapore Collection of Marine Cyanobacterium cf. Lyngbya sp. Mar. Drugs 2018, 16, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, N.; Kawabata, K.; Adachi, K.; Katsuta, A.; Shizuri, Y. Unnarmicins A and C, new antibacterial depsipeptides produced by marine bacterium Photobacterium sp. MBIC06485. J. Antibiot. 2008, 61, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-K.; Song, J.-w.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, S.; Kumaravel, K.; Rameshkumar, G.; Ajithkumar, T. Antimicrobial peptides from the marine fishes. Res. J. Immunol. 2010, 3, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Partridge, A.W.; Kaan, H.Y.K.; Juang, Y.-C.; Sadruddin, A.; Lim, S.; Brown, C.J.; Ng, S.; Thean, D.; Ferrer, F.; Johannes, C. Incorporation of putative helix-breaking amino acids in the design of novel stapled peptides: Exploring biophysical and cellular permeability properties. Molecules 2019, 24, 2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine antimicrobial peptides: Nature provides templates for the design of novel compounds against pathogenic bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Fenner, A.M. Drug discovery from marine microbes. Microb. Ecol. 2013, 65, 800–806. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Harrington, C.; Dobson, A.D.; O’Gara, F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs 2014, 12, 3516–3559. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, A.; Gaifem, J.; Ramos, V.; Glukhov, E.; Dorrestein, P.C.; Gerwick, W.H.; Vasconcelos, V.M.; Mendes, M.V.; Tamagnini, P. Bioprospecting Portuguese Atlantic coast cyanobacteria for bioactive secondary metabolites reveals untapped chemodiversity. Algal Res. 2015, 9, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Desriac, F.; Jégou, C.; Balnois, E.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Antimicrobial peptides from marine proteobacteria. Mar. Drugs 2013, 11, 3632–3660. [Google Scholar] [CrossRef] [Green Version]
- Fisch, K.M. Biosynthesis of natural products by microbial iterative hybrid PKS–NRPS. RSC advances 2013, 3, 18228–18247. [Google Scholar] [CrossRef] [Green Version]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Fang, W.Y.; Dahiya, R.; Qin, H.L.; Mourya, R.; Maharaj, S. Natural proline-rich cyclopolypeptides from marine organisms: Chemistry, synthetic methodologies and biological status. Mar. Drugs 2016, 14, 194. [Google Scholar] [CrossRef] [Green Version]
- Evidente, A. Bioactive Lipodepsipeptides Produced by Bacteria and Fungi †. Int. J. Mol. Sci. 2022, 23, 12342. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.M.; Walsh, C.T.; Burkart, M.D. Biomimetic synthesis and optimization of cyclic peptide antibiotics. Nature 2002, 418, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Z.; Ding, K.; Roller, P.P. Recent progress of synthetic studies to peptide and peptidomimetic cyclization. Curr. Org. Chem. 2008, 12, 1502–1542. [Google Scholar] [CrossRef]
- Lambert, J.N.; Mitchell, J.P.; Roberts, K.D. The synthesis of cyclic peptides. J. Chem. Soc. Perkin Trans. 1 2001, 5, 471–484. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Shepherd, N.E.; Xu, W.; Lucke, A.J.; Stoermer, M.J.; Fairlie, D.P. Orally absorbed cyclic peptides. Chem. Rev. 2017, 117, 8094–8128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, Y.; Shioiri, T. Recent progress of the synthetic studies of biologically active marine cyclic peptides and depsipeptides. Chem. Rev. 2005, 105, 4441–4482. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Al Musaimi, O.; De La Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef]
- Gentilucci, L.; de Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J. Acetylation of peptides inhibits their degradation by rumen micro-organisms. Br. J. Nutr. 1992, 68, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239. [Google Scholar] [PubMed]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Trier, S.; Linderoth, L.; Bjerregaard, S.; Strauss, H.M.; Rahbek, U.L.; Andresen, T.L. Acylation of salmon calcitonin modulates in vitro intestinal peptide flux through membrane permeability enhancement. Eur. J. Pharm. Biopharm. 2015, 96, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.Y.; Wang, P. Determination of the absolute configuration of natural products. Chin. J. Nat. Med. 2013, 11, 193–198. [Google Scholar] [CrossRef]
- Zhu, T.H.; Ma, H.G.; Wang, C.; Zhao, C.Y.; Mei, X.G.; Zhu, W.M. Determination methods for absolute configuration of natural products. J. Int. Pharm. Res. 2015, 42, 773–785. [Google Scholar] [CrossRef]
- Harada, N. Determination of absolute configurations by X-ray crystallography and 1H NMR anisotropy. Chirality 2008, 20, 691–723. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef]
- Freedman, T.B.; Cao, X.; Dukor, R.; Nafie, L.A. Absolute Configuration Determination of Chiral Molecules in the Solution State Using Vibrational Circular Dichroism. Chirality 2003, 15, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Nathan, P.; Gordillo-Román, B. Vibrational circular dichroism absolute configuration determination of natural products. Prog. Chem. Org. Nat. Prod. 2015, 100, 311–452. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.M.; Da Silva Bolzani, V. Determination of the absolute configuration of natural product molecules using vibrational circular dichroism. Stud. Nat. Prod. Chem. 2014, 41, 383–417. [Google Scholar] [CrossRef]
- Zhao, S.D.; Shen, L.; Luo, D.Q.; Zhu, H.J. Progression of absolute configuration determination in natural product chemistry using optical rotation (dispersion), matrix determinant and electronic circular dichroism methods. Curr. Org. Chem. 2011, 15, 1843–1862. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Ferreira, D.; Ding, Y. Determination of absolute configuration of natural products: Theoretical calculation of electronic circular dichroism as a tool. Curr. Org. Chem. 2010, 14, 1678–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Tan, R.X. Theoretical calculation of electronic circular dichroism: A promising tool for the determination of absolute configuration of natural products. J. Int. Pharm. Res. 2015, 42, 734–737. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Chisholm, C.D. Assignment of absolute configuration using chiral reagents and NMR spectroscopy. Chirality 2011, 23, 190–214. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suryaprakash, N. Some new protocols for the assignment of absolute configuration by NMR spectroscopy using chiral solvating agents and CDAs. Tetrahedron Asymmetry 2017, 28, 1220–1232. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar] [CrossRef]
- Takiguchi, S.; Hirota-Takahata, Y.; Nishi, T. Application of the advanced Marfey’s method for the determination of the absolute configuration of ogipeptins. Tetrahedron Lett. 2022, 96, 153760. [Google Scholar] [CrossRef]
- Vijayasarathy, S.; Prasad, P.; Fremlin, L.J.; Ratnayake, R.; Salim, A.A.; Khalil, Z.; Capon, R.J. C3 and 2D C3 Marfey’s Methods for Amino Acid Analysis in Natural Products. J. Nat. Prod. 2016, 79, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Ikai, Y.; Mayumi, T.; Oka, H.; Suzuki, M.; Harada, K.I. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Elucidation of limitations of Marfey’s method and of its separation mechanism. Anal. Chem. 1997, 69, 3346–3352. [Google Scholar] [CrossRef]
- Fujii, K.; Harada, K.I. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; She, Z.; Lin, Y.; Vrijmoed, L.L.P.; Lin, W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel). J. Nat. Prod. 2007, 70, 1696–1699. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Matsubara, T.; Shimada, S.; Sato, T.; Suenaga, K. Mebamamides A and B, Cyclic Lipopeptides Isolated from the Green Alga Derbesia marina. J. Nat. Prod. 2015, 78, 901–908. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Shin, H.J. Gageostatins A-C, antimicrobial linear lipopeptides from a marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M. Marine natural peptides: Determination of absolute configuration using liquid chromatography methods and evaluation of bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.S.; Mehjabin, J.J.; Anas, A.R.J.; Hayasaka, M.; Onoki, R.; Wang, J.; Umezawa, T.; Washio, K.; Morikawa, M.; Okino, T. Nostosin G and Spiroidesin B from the Cyanobacterium Dolichospermum sp. NIES-1697. J. Nat. Prod. 2022, 85, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Ban, Y.H.; Shin, J.; Park, I.W.; Yoon, S.; Ko, K.; Shin, J.; Nam, S.J.; Winter, J.M.; Kim, Y.; et al. Azetidine-Bearing Non-Ribosomal Peptides, Bonnevillamides D and E, Isolated from a Carrion Beetle-Associated Actinomycete. J. Org. Chem. 2021, 86, 11149–11159. [Google Scholar] [CrossRef]
- Ekanayake, D.I.; Perlatti, B.; Swenson, D.C.; Põldmaa, K.; Bills, G.F.; Gloer, J.B. Broomeanamides: Cyclic Octapeptides from an Isolate of the Fungicolous Ascomycete Sphaerostilbella broomeanafrom India. J. Nat. Prod. 2021, 84, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pastor, I.; González-Menéndez, V.; Annang, F.; Toro, C.; Mackenzie, T.A.; Bosch-Navarrete, C.; Genilloud, O.; Reyes, F. Pipecolisporin, a novel cyclic peptide with antimalarial and antitrypanosome activities from a wheat endophytic Nigrospora oryzae. Pharmaceuticals 2021, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Significance of Chromatographic Techniques in Pharmaceutical Analysis. Processes 2022, 10, 172. [Google Scholar] [CrossRef]
- Bhushan, R.; Brückner, H. Use of Marfey’s reagent and analogs for chiral amino acid analysis: Assessment and applications to natural products and biological systems. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3148–3161. [Google Scholar] [CrossRef]
- Péter, A.; Olajos, E.; Casimir, R.; Tourwé, D.; Broxterman, Q.B.; Kaptein, B.; Armstrong, D.W. High-performance liquid chromatographic separation of the enantiomers of unusual α-amino acid analogues. J. Chromatogr. A 2000, 871, 105–113. [Google Scholar] [CrossRef]
- Moon, K.; Lim, C.; Kim, S.; Oh, D.C. Facile determination of the absolute configurations of α-hydroxy acids by chiral derivatization coupled with liquid chromatography-mass spectrometry analysis. J. Chromatogr. A 2013, 1272, 141–144. [Google Scholar] [CrossRef]
- Bhushan, R.; Brückner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef]
- Bhushan, R.; Kumar, R. Reversed-phase high performance liquid chromatographic separation of diastereomers of β-amino alcohols and microwave assisted synthesis of Marfey’s reagent, its chiral variants and diastereomers. J. Chromatogr. A 2009, 1216, 2592–2596. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Martens, J.; Bhushan, R. Assessment and application of Marfey’s reagent and analogs in enantioseparation: A decade’s perspective. Biomed. Chromatogr. 2021, 35, 4990. [Google Scholar] [CrossRef] [PubMed]
- Hess, S. A universal HPLC-MS method to determine the stereochemistry of common and unusual amino acids. Methods Mol. Biol. 2012, 828, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Dolowy, M.; Pyka, A. Application of TLC, HPLC and GC methods to the study of amino acid and peptide enantiomers: A review. Biomed. Chromatogr. 2014, 28, 84–101. [Google Scholar] [CrossRef]
- Harada, K.i.; Fujii, K.; Mayumi, T.; Hibino, Y.; Suzuki, M.; Ikai, Y.; Oka, H. A method usingL/CMS for determination of absolute configuration of constituent amino acids in peptide–advanced Marfey’s method. Tetrahedron Lett. 1995, 36, 1515–1518. [Google Scholar] [CrossRef]
- Harada, K.I.; Fujii, K.; Hayashi, K.; Suzuki, M.; Ikai, Y.; Oka, H. Application of D,L-FDLA derivatization to determination of absolute configuration of constituent amino acids in peptide by advanced Marfey’s method. Tetrahedron Lett. 1996, 37, 3001–3004. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.M.; Yuan, L.M. Recent progress in the development of chiral stationary phases for high-performance liquid chromatography. J. Sep. Sci. 2022, 45, 51–77. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Ribeiro, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography based on chitin- and chitosan-derived marine polysaccharides. Symmetry 2017, 9, 190. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography: Recent developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.; Lima, R.; Pinto, M.M.M.; Tiritan, M.E. Chromatographic supports for enantioselective liquid chromatography: Evolution and innovative trends. J. Chromatogr. A 2022, 1684, 463555. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Teixeira, J.; Pinto, M.M.M.; Tiritan, M.E. Strategies for preparation of chiral stationary phases: Progress on coating and immobilization methods. Molecules 2021, 26, 5477. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Pataj, Z.; Aranyi, A.; Péter, A. Macrocyclic antibiotic selectors in direct HPLC enantioseparations. Sep. Purif. Rev. 2012, 41, 207–249. [Google Scholar] [CrossRef]
- Berkecz, R.; Németi, G.; Péter, A.; Ilisz, I. Liquid chromatographic enantioseparations utilizing chiral stationary phases based on crown ethers and cyclofructans. Molecules 2021, 26, 4648. [Google Scholar] [CrossRef]
- Carenzi, G.; Sacchi, S.; Abbondi, M.; Pollegioni, L. Direct chromatographic methods for enantioresolution of amino acids: Recent developments. Amino Acids 2020, 52, 849–862. [Google Scholar] [CrossRef]
- Ianni, F.; Pucciarini, L.; Carotti, A.; Natalini, S.; Raskildina, G.Z.; Sardella, R.; Natalini, B. Last ten years (2008–2018) of chiral ligand-exchange chromatography in HPLC: An updated review. J. Sep. Sci. 2019, 42, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M. Chiral Pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemicl Technology; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Kanki, D.; Nakamukai, S.; Ogura, Y.; Takikawa, H.; Ise, Y.; Morii, Y.; Yamawaki, N.; Takatani, T.; Arakawa, O.; Okada, S.; et al. Homophymamide A, Heterodetic Cyclic Tetrapeptide from a Homophymia sp. Marine Sponge: A Cautionary Note on Configurational Assignment of Peptides That Contain a Ureido Linkage. J. Nat. Prod. 2021, 84, 1848–1853. [Google Scholar] [CrossRef]
- Martin, M.J.; Rodriguez-Acebes, R.; Garcia-Ramos, Y.; Martinez, V.; Murcia, C.; Digon, I.; Marco, I.; Pelay-Gimeno, M.; Fernández, R.; Reyes, F.; et al. Stellatolides, a new cyclodepsipeptide family from the sponge Ecionemia acervus: Isolation, solid-phase total synthesis, and full structural assignment of stellatolide a. J. Am. Chem. Soc. 2014, 136, 6754–6762. [Google Scholar] [CrossRef]
- Coello, L.; Reyes, F.; Martín, M.J.; Cuevas, C.; Fernández, R. Isolation and structures of pipecolidepsins A and B, cytotoxic cyclic depsipeptides from the madagascan sponge Homophymia lamellosa. J. Nat. Prod. 2014, 77, 298–303. [Google Scholar] [CrossRef]
- Gao, J.; Caballero-George, C.; Wang, B.; Rao, K.V.; Shilabin, A.G.; Hamann, M.T. 5-OHKF and NorKA, Depsipeptides from a hawaiian collection of Bryopsis pennata: Binding properties for NorKA to the human neuropeptide Y Y1 receptor. J. Nat. Prod. 2009, 72, 2172–2176. [Google Scholar] [CrossRef] [Green Version]
- Urda, C.; Pérez, M.; Rodríguez, J.; Jiménez, C.; Cuevas, C.; Fernández, R. Pembamide, a N-methylated linear peptide from a sponge Cribrochalina sp. Tetrahedron Lett. 2016, 57, 3239–3242. [Google Scholar] [CrossRef]
- Junk, L.; Kazmaier, U. Total Synthesis and Configurational Revision of Mozamide A, a Hydroxy-Brunsvicamide. J. Org. Chem. 2019, 84, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Junk, L.; Kazmaier, U. Total Synthesis of Keramamides A and L from a Common Precursor by Late-Stage Indole Synthesis and Configurational Revision. Angew. Chem. Int. Ed. 2018, 57, 11432–11435. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef]
- Zhou, H.; He, Y.; Tian, Y.; Cong, B.; Yang, H. Bacilohydrin A, a new cytotoxic cyclic lipopeptide of surfactins class produced by Bacillus sp. sy27f from the indian ocean hydrothermal vent. Nat. Pro. Comm. 2019, 14, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Han, J.; Jang, S.C.; An, J.S.; Kang, I.; Kwon, Y.; Nam, S.J.; Shim, S.H.; Cho, J.C.; Lee, S.K.; et al. Epoxinnamide: An Epoxy Cinnamoyl-Containing Nonribosomal Peptide from an Intertidal Mudflat-Derived Streptomyces sp. Mar. Drugs 2022, 20, 455. [Google Scholar] [CrossRef]
- Wu, C.; Tang, J.; Limlingan Malit, J.J.; Wang, R.; Sung, H.H.Y.; Williams, I.D.; Qian, P.Y. Bathiapeptides: Polythiazole-Containing Peptides from a Marine Biofilm-Derived Bacillus sp. J. Nat. Prod. 2022, 85, 1751–1762. [Google Scholar] [CrossRef]
- Shin, D.; Byun, W.S.; Moon, K.; Kwon, Y.; Bae, M.; Um, S.; Lee, S.K.; Oh, D.C. Coculture of marine Streptomyces sp. with Bacillus sp. produces a new piperazic acid-bearing cyclic peptide. Front. Chem. 2018, 6, 498. [Google Scholar] [CrossRef] [Green Version]
- Le, T.C.; Pulat, S.; Lee, J.; Kim, G.J.; Kim, H.; Lee, E.Y.; Hillman, P.F.; Choi, H.; Yang, I.; Oh, D.C.; et al. Marine Depsipeptide Nobilamide i Inhibits Cancer Cell Motility and Tumorigenicity via Suppressing Epithelial-Mesenchymal Transition and MMP2/9 Expression. ACS Omega 2022, 7, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Attia, E.Z.; Hajjar, D.; Anany, M.A.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Wajant, H.; Gulder, T.A.M.; Abdelmohsen, U.R. New cytotoxic cyclic peptide from the marine sponge-associated Nocardiopsis sp. Ur67. Mar. Drugs 2018, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Phyo, M.Y.; Ding, C.Y.G.; Goh, H.C.; Goh, J.X.; Ong, J.F.M.; Chan, S.H.; Yung, P.Y.M.; Candra, H.; Tan, L.T. Trikoramide A, a Prenylated Cyanobactin from the Marine Cyanobacterium Symploca hydnoides. J. Nat. Prod. 2019, 82, 3482–3488. [Google Scholar] [CrossRef] [PubMed]
- Phyo, M.Y.; Goh, T.M.B.; Goh, J.X.; Tan, L.T. Trikoramides b–d, bioactive cyanobactins from the marine cyanobacterium Symploca hydnoides. Mar. Drugs 2021, 19, 548. [Google Scholar] [CrossRef]
- Phyo, M.Y.; Goh, J.X.; Tan, L.T. Triproamide and Pemukainalides, Cyclic Depsipeptides from the Marine Cyanobacterium Symploca hydnoides. J. Nat. Prod. 2022, 85, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J.; Sikandar, A.; Naman, C.B.; Glukhov, E.; Luo, D.; Duggan, B.M.; et al. Tutuilamides A-C: Vinyl-Chloride-Containing Cyclodepsipeptides from Marine Cyanobacteria with Potent Elastase Inhibitory Properties. ACS Chem. Biol. 2020, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Matthew, S.; Chen, Q.Y.; Paul, V.J.; Luesch, H. Discovery of new A- and B-type laxaphycins with synergistic anticancer activity. Bioorg. Med. Chem. 2018, 26, 2310–2319. [Google Scholar] [CrossRef]
- Long, J.; Chen, Y.; Chen, W.; Wang, J.; Zhou, X.; Yang, B.; Liu, Y. Cyclic peptides from the soft coral-derived fungus Aspergillus sclerotiorum scsio 41031. Mar. Drugs 2021, 19, 701. [Google Scholar] [CrossRef]
- Jiao, F.R.; Zhu, H.R.; Gu, B.B.; Wu, Y.; Sun, F.; Wang, S.P.; Zhang, A.; Jiao, W.H.; Xu, S.H.; Lin, H.W. Asperflomide and asperflosamide, new N-methylated cyclopeptides from the marine sponge-derived fungus Aspergillus flocculosus 16D-1. Tetrahedron 2022, 109, 132579. [Google Scholar] [CrossRef]
- Tian, T.; Takada, K.; Ise, Y.; Ohtsuka, S.; Okada, S.; Matsunaga, S. Microsclerodermins N and O, cytotoxic cyclic peptides containing a p-ethoxyphenyl moiety from a deep-sea marine sponge Pachastrella sp. Tetrahedron 2020, 76, 130997. [Google Scholar] [CrossRef]
- Hasin, O.; Shoham, S.; Kashman, Y.; Ilan, M.; Carmeli, S. Theonellamides j and k and 5-cis-apoa-theopalauamide, bicyclic glycopeptides of the red sea sponge Theonella swinhoei. Mar. Drugs 2022, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, L.; Chen, H.F.; Jiao, W.H.; Sun, F.; Liu, L.Y.; Zhu, H.R.; Wang, S.P.; Lin, H.W. Fuscasins A-D, Cycloheptapeptides from the Marine Sponge Phakellia fusca. J. Nat. Prod. 2019, 82, 970–979. [Google Scholar] [CrossRef]
- Ortiz-Celiseo, A.; Valerio-Alfaro, G.; Sosa-Rueda, J.; López-Fentanes, F.C.; Domínguez-Melendez, V.; Cen-Pacheco, F. Ectyoplasin, a novel cytotoxic cyclic peptide from Ectyoplasia ferox sponge. Nat. Prod. Res. 2022, 36, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liao, H.; Liu, L.Y.; Sun, F.; Chen, H.F.; Jiao, W.H.; Zhu, H.R.; Yang, F.; Huang, G.; Zeng, D.Q.; et al. Phakefustatins A-C: Kynurenine-bearing cycloheptapeptides as RXRα modulators from the marine sponge Phakellia fusca. Org. Lett. 2020, 22, 6703–6708. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Kanki, D.; Watanabe, R.; Matsushima, R.; Ise, Y.; Yokose, H.; Morii, Y.; Yamawaki, N.; Ninomiya, A.; Okada, S.; et al. Aciculitin D, a cytotoxic heterodetic cyclic peptide from a Poecillastra sp. marine sponge. Tetrahedron 2022, 119, 132859. [Google Scholar] [CrossRef]
- Wiese, J.; Abdelmohsen, U.R.; Motiei, A.; Humeida, U.H.; Imhoff, J.F. Bacicyclin, a new antibacterial cyclic hexapeptide from Bacillus sp. strain BC028 isolated from Mytilus edulis. Bioorg. Med. Chem. Lett. 2018, 28, 558–561. [Google Scholar] [CrossRef]
- Cui, J.; Kim, E.; Moon, D.H.; Kim, T.H.; Kang, I.; Lim, Y.; Shin, D.; Hwang, S.; Du, Y.E.; Song, M.C.; et al. Taeanamides A and B, Nonribosomal Lipo-Decapeptides Isolated from an Intertidal-Mudflat-Derived Streptomyces sp. Mar. Drugs 2022, 20, 400. [Google Scholar] [CrossRef]
- Kozuma, S.; Hirota-Takahata, Y.; Fukuda, D.; Kuraya, N.; Nakajima, M.; Ando, O. Identification and biological activity of ogipeptins, novel LPS inhibitors produced by marine bacterium. J. Antibiot. 2017, 70, 79–83. [Google Scholar] [CrossRef]
- Yao, F.H.; Liang, X.; Cheng, X.; Ling, J.; Dong, J.D.; Qi, S.H. Antifungal peptides from the marine gorgonian-associated fungus Aspergillus sp. SCSIO41501. Phytochemistry 2021, 192, 112967. [Google Scholar] [CrossRef]
- Karim, F.; Liang, X.; Qi, S.H. Bioassay-guided isolation of antifungal cyclopeptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. Phytochem. Lett. 2022, 48, 68–71. [Google Scholar] [CrossRef]
- Luo, M.; Zang, R.; Wang, X.; Chen, Z.; Song, X.; Ju, J.; Huang, H. Natural Hydroxamate-Containing Siderophore Acremonpeptides A-D and an Aluminum Complex of Acremonpeptide D from the Marine-Derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Iwasaki, A.; Kurisawa, N.; Suzuki, R.; Jeelani, G.; Matsubara, T.; Sato, T.; Nozaki, T.; Suenaga, K. Motobamide, an Antitrypanosomal Cyclic Peptide from a Leptolyngbya sp. Marine Cyanobacterium. J. Nat. Prod. 2021, 84, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, B.; Yang, L.; Yang, F.; Lin, H. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.Z.; Liu, J.T.; Hu, Q.; He, R.J.; Guan, X.Q.; Ge, G.B.; Han, H.; Yang, F.; Lin, H.W. Pancreatic Lipase Inhibitory Cyclohexapeptides from the Marine Sponge-Derived Fungus Aspergillus sp. 151304. J. Nat. Prod. 2020, 83, 2287–2293. [Google Scholar] [CrossRef]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Sano, T.; Hitomi, Y.; Ohno, O.; Suenaga, K. Croissamide, a proline-rich cyclic peptide with an N-prenylated tryptophan from a marine cyanobacterium Symploca sp. Tetrahedron Lett. 2018, 59, 3806–3809. [Google Scholar] [CrossRef]
- Seo, J.; Shin, Y.H.; Jo, S.J.; Du, Y.E.; Um, S.; Kim, Y.R.; Moon, K. Cystargamides C and D, New Cyclic Lipopeptides From a Tidal Mudflat-Derived Streptomyces sp. JMS132. Front. Microbiol. 2022, 13, 904954. [Google Scholar] [CrossRef]
- Hou, X.M.; Li, Y.Y.; Shi, Y.W.; Fang, Y.W.; Chao, R.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Integrating Molecular Networking and 1H NMR to Target the Isolation of Chrysogeamides from a Library of Marine-Derived Penicillium Fungi. J. Org. Chem. 2019, 84, 1228–1237. [Google Scholar] [CrossRef]
- Grunwald, A.L.; Cartmell, C.; Kerr, R.G. Auyuittuqamides A-D, Cyclic Decapeptides from Sesquicillium microsporum RKAG 186 Isolated from Frobisher Bay Sediment. J. Nat. Prod. 2021, 84, 56–60. [Google Scholar] [CrossRef]
- Fernández, R.; Bayu, A.; Hadi, T.A.; Bueno, S.; Pérez, M.; Cuevas, C.; Putra, M.Y. Unique polyhalogenated peptides from the marine sponge Ircinia sp. Mar. Drugs 2020, 18, 396. [Google Scholar] [CrossRef]
- Li, W.; Jiao, F.W.; Wang, J.Q.; Shi, J.; Wang, T.T.; Khan, S.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Unguisin G, a new kynurenine-containing cyclic heptapeptide from the sponge-associated fungus Aspergillus candidus NF2412. Tetrahedron Lett. 2020, 61, 152322. [Google Scholar] [CrossRef]
- Chao, R.; Hou, X.M.; Xu, W.F.; Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Targeted Isolation of Asperheptatides from a Coral-Derived Fungus Using LC-MS/MS-Based Molecular Networking and Antitubercular Activities of Modified Cinnamate Derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.B.; Zhang, Y.; Leao, T.; Glukhov, E.; Pierce, M.L.; Zhang, C.; Kim, H.; Mao, H.H.; Fang, F.; et al. Pagoamide A, a Cyclic Depsipeptide Isolated from a Cultured Marine Chlorophyte, Derbesia sp., Using MS/MS-Based Molecular Networking. J. Nat. Prod. 2020, 83, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Scopularides revisited: Molecular networking guided exploration of lipodepsipeptides in Australian marine fish gastrointestinal tract-derived fungi. Mar. Drugs 2019, 17, 475. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, C.L.; Zhang, B.; Guo, W.J.; Zhu, J.; Chang, C.Y.; Zhao, E.J.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Genome mining and biosynthesis of kitacinnamycins as a STING activator. Chem. Sci. 2019, 10, 4839–4846. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Yu, J.; Wang, Y.; Connolly, J.A.; Liu, Y.; Zhang, Y.; Yu, L.; Cen, S.; Goss, R.J.M.; Gan, M. Zelkovamycins B-E, Cyclic Octapeptides Containing Rare Amino Acid Residues from an Endophytic Kitasatospora sp. Org. Lett. 2020, 22, 9346–9350. [Google Scholar] [CrossRef]
- Portmann, C.; Sieber, S.; Wirthensohn, S.; Blom, J.F.; Da Silva, L.; Baudat, E.; Kaiser, M.; Brun, R.; Gademann, K. Balgacyclamides, antiplasmodial heterocyclic peptides from Microcystis aeruguinosa EAWAG 251. J. Nat. Prod. 2014, 77, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012, 2012, 179782. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Zhang, J.; He, S.; Yan, X. New peptides isolated from marine cyanobacteria, an overview over the past decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J. Natural products from marine fungi. Mar. Drugs 2020, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, X.; He, Y.; Lin, X.; Zhou, X.; Liu, Y.; Yang, B. Secondary Metabolites from Coral-Associated Fungi: Source, Chemistry and Bioactivities. J. Fungi 2022, 8, 1043. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, A.; Chugh, A.; Kumar, S.; Kumar, P. Tankyrase inhibitors: Emerging and promising therapeutics for cancer treatment. Med. Chem. Res. 2021, 30, 50–73. [Google Scholar] [CrossRef]
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 81–196. [Google Scholar] [CrossRef] [PubMed]
- Andavan, G.S.B.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Liu, W.; Su, Y.; Wei, Z.; Liu, J.; Kolluri, S.K.; Wu, H.; Cao, Y.; Chen, J.; Wu, Y.; et al. NSAID Sulindac and Its Analog Bind RXRα and Inhibit RXRα-Dependent AKT Signaling. Cancer Cell 2010, 17, 560–573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Li, H.; Zhang, S.; Zhang, Y.; Wang, N.; Zhou, H.; He, H.; Hu, G.; Zhang, T.C.; Ma, W. RXRα provokes tumor suppression through p53/p21/p16 and PI3K-AKT signaling pathways during stem cell differentiation and in cancer cells article. Cell Death Dis. 2018, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Roy, A. Antimicrobial Peptides as Potential Therapeutic Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 555–577. [Google Scholar] [CrossRef]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine Cyclic Peptides: Antimicrobial Activity and Synthetic Strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Hirota-Takahata, Y.; Kozuma, S.; Kuraya, N.; Fukuda, D.; Nakajima, M.; Takatsu, T.; Ando, O. Ogipeptins, novel inhibitors of LPS: Physicochemical properties and structural elucidation. J. Antibiot. 2017, 70, 84–89. [Google Scholar] [CrossRef]
- Batista, A.N.L.; dos Santos, F.M.; Batista, J.M.; Cass, Q.B. Enantiomeric mixtures in natural product chemistry: Separation and absolute configuration assignment. Molecules 2018, 23, 492. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Shin, D.; Kim, T.H.; An, J.S.; Jo, S.I.; Jang, J.; Hong, S.; Shin, J.; Oh, D.C. Structural Revision of Lydiamycin A by Reinvestigation of the Stereochemistry. Org. Lett. 2020, 22, 3855–3859. [Google Scholar] [CrossRef]
- Tarazona, G.; Fernández, R.; Cruz, P.G.; Pérez, M.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Combining JBCA and Marfey’s methodology to determine the absolute configuration of threonines: The case of gunungamide A, a new cyclic depsipeptide containing chloropyrrole from the sponge Discodermia sp. Org. Chem. Front. 2019, 6, 15–21. [Google Scholar] [CrossRef]
- Dewapriya, P.; Khalil, Z.G.; Prasad, P.; Salim, A.A.; Cruz-Morales, P.; Marcellin, E.; Capon, R.J. Talaropeptides A-D: Structure and Biosynthesis of Extensively N-methylated Linear Peptides From an Australian Marine Tunicate-Derived Talaromyces sp. Front. Chem. 2018, 6, 394. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Genta-Jouve, G. Asperopiperazines A and B: Antimicrobial and Cytotoxic Dipeptides from a Tunicate-Derived Fungus Aspergillus sp. DY001. Mar. Drugs 2022, 20, 451. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Ni, J.; Wang, G.; Li, F.; Li, Q.; Chen, S.; Shu, J.; Gan, M. Acremopeptaibols A-F, 16-Residue Peptaibols from the Sponge-Derived Acremonium sp. IMB18-086 Cultivated with Heat-Killed Pseudomonas aeruginosa. J. Nat. Prod. 2021, 84, 2990–3000. [Google Scholar] [CrossRef]
- Routhu, S.R.; Ragi, N.C.; Yedla, P.; Shaik, A.B.; Venkataraman, G.; Cheemalamarri, C.; Chityala, G.K.; Amanchy, R.; Sripadi, P.; Kamal, A. Identification, characterization and evaluation of novel antifungal cyclic peptides from Neobacillus drentensis. Bioorg. Chem. 2021, 115, 105180. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hong, X.; Yang, J.; Qin, J.J.; Zhang, B.; Lin, J.; Shao, Z.; Wang, W. Structure elucidation of a novel cyclic tripeptide from the marine-derived fungus Aspergillus ochraceopetaliformis DSW-2. Nat. Prod. Res. 2022, 36, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, P.; Ren, B.; Song, Z.; Zhu, G.; He, W.; Zhang, J.; Oyeleye, A.; Dai, H.; Zhang, L.; et al. Antibacterial polyene-polyol macrolides and cyclic peptides from the marine-derived Streptomyces sp. MS110128. Appl. Microbiol. Biotechnol. 2021, 105, 4975–4986. [Google Scholar] [CrossRef] [PubMed]















| Marine Peptide | Type of Cyclopeptide | Marine Source | Chromatographic Method | Biological Activities | Ref. |
|---|---|---|---|---|---|
| Bacilohydrin A (1) | Cyclic lipoheptapeptide | Bacillus sp. SY27F from the Indian ocean Hydrothermal Vent | Modified Marfey’s method combined with UPLC-ESIMS: CDR: l-FDAA; Column: C18 (1.7 µm, 2.1 × 100 mm); MP: 0.05% NH4OH: CH3CN:0.01% HCO2H in elution gradient; Flow rate: 0.3 mL/min; Detection: ESIMS | Significant cytotoxicity against DU-145, MCF-7, and HepG2 cancer cell lines. | [148] |
| Epoxinnamide (2) | Cyclic decapeptide | Intertidal Mudflat-Derived Streptomyces sp. OID44 | Advanced Marfey’s method combined with LC-MS: CDR: l-FDAA and D-FDAA; Column: C18 (5 µm, 4.6 × 100 mm): MP: CH3CN:H2O: 0.1% HCO2H in elution gradient; Flow rate: 0.7 mL/min; Detection: UV at 340 nm and MS | Induced QR activity in murine Hepa-1c1c7 cells. Antiangiogenesis activity in HUVECs. | [149] |
| Bathiapeptides A1 (3), A2 (4) and B-E (5–8) | Cyclic hexapeptides | Marine biofilm-derived Bacillus sp. B19-2 | Advanced Marfey’s method combined with UPLC-MS: CDR: l-FDLA or d-FDLA; Column: C18; MP: CH3CN:0.05% TFA in elution gradient; Detection: MS | Cytotoxicity against Hep G2, HeLa, MCF-7, and MGC-803 cell lines. | [150] |
| Dentigerumycin E (9) | Cyclic hexapeptide | Coculture of marine Streptomyces and Bacillus strains isolated together from an intertidal mudflat | Advanced Marfey’s method combined with LC-MS: CDR: l-FDAA and d-FDAA; Column: Phenomenex Luna C18(2), (5 µm 4.6 × 100 mm); MP: CH3CN:H2O:0.1% HCO2H in elution gradient; Flow rate: 0.7 mL/min; Detection: UV at 340 nm and MS | Moderate cytotoxicity against A549, HCT-116, MDA-MB-231, and SK-HEP-1 cancer cell lines. Inhibition of MDA-MB-231 cell migration and cell invasion inhibition. | [151] |
| Nobilamide I (10) | Cyclic depsiheptapeptide | Marine-derived bacterium Saccharomonospora sp., strain CNQ-490 | C3 Marfey’s analysis combined with LC-ESI-MS: CDR: l-FDAA; Column: Agilent Zorbax SB-C3 (5 μm, 4.6 × 150 mm); MP: H2O:MeOH:0.02% HCO2H in elution gradient; Flow rate: 1 mL/min; Detection: UV at 340 nm and ESI-MS | Inhibition of A549, AGS, and Caco2 cancer cell lines motility and tumorigenicity via suppressing EMT effectors and MMP2/9 expression. | [152] |
| Nocardiotide A (11) | Cyclic hexapeptide | Nocardiopsis sp. UR67 strain associated with the marine sponge Callyspongia sp. | Marfey’s method combined with LC: CDR: l-FDAA; Column: Gemini-NX RP-C18; MP: H2O:CH3CN in elution gradient; Flow rate: 1 mL/min; Detection: UV at 340 nm | Cytotoxicity towards MM.1S, HeLa, and CT26 cell lines. | [153] |
| Trikoramide A (12) | Cyclic decapeptide | Marine cyanobacterium Symploca hydnoides | Marfey’s method combined with LC-MS: CDR: l-FDVA; Column: Phenomenex Kinetex C18 (2.6 μm, 4.6 × 100 mm); MP: H2O:CH3CN:0.1% HCO2H in gradient elution; Flow rate: 0.2 mL/min; Detection: MS | Cytotoxicity against MOLT-4 and AML2 cancer cell lines. | [154] |
| Trikoramides B–D (13–15) | Cyclic decapeptides | Marine cyanobacterium Symploca hydnoides | Marfey’s method combined with LC: CDR: l-FDAA; Column: Phenomenex Kinetex C18 (2.6 µm, 4.6 × 250 mm,); MP: CH3CN: 0.05 M TFA (40:60); Flow rate: 0.5 mL/min; Detection: UV at 340 nm | Cytotoxicity against MOLT-4 cell line. QSI activity for 15 against PAO1 lasB-gpf and rhlA-gfp bioreporter strains. | [155] |
| Triproamide (16) and pemukainalides A (17) and B (18) | Cyclic depsiocta and hexapeptides | Marine cyanobacterium Symploca hydnoides | Marfey’s method combined with HR-LCMS: CDR: l-FDAA; Column; Phenomenex Kinetex C18 (2.6 μm, 4.6 × 250 mm); MP: CH3CN:0.05 M TFA (40:60); Flow rate: 1.0 mL/min flow rate; Detection: MS Chiral LC: Column: Phenomenex Chirex 3126 (d)-penicillamine (4.6 × 50 mm); CSP: ligand exchange-based; MP: 1 mM CuSO4:IPA (85:15); Flow rate: 1.0 mL/min; Detection: UV at 254 nm | 17 exhibited cytotoxicity against the MOLT-4 cell line. | [156] |
| Tutuilamides A–C (19–21) | Cyclic hexadepsipeptides | Marine cyanobacteria Schizothrix sp. (19–20) and Coleofasciculus sp. (21) | Marfey’s method combined with LC-MS: CDR: l-FDAA or d-FDAA; Column: Phenomenex Kinetex C18 (5 μm, 4.6 × 100 mm); MP: H2O: CH3CN:0.1% HCO2H in elution gradient; Flow rate: 0.6 mL/min; Detection: UV at 220, 254 and 280 nm, and HRESIMS | Cytotoxicity in H-460 cell line. Potent elastase inhibitory activity. | [157] |
| Laxaphycins B4 (22) and A2 (23) | Cyclic lipoundeca-peptides | Marine cyanobacterium Hormothamnion enteromorphoides | Chiral LC-MS: Column: Chirobiotic TAG (4.6 × 250 mm); CSP: Macrocyclic antibiotic-based; MP: MeOH:10 mM NH4OAc (40:60, pH 5.12) or (90:10, pH 6.0); Flow rate: 0.5 mL/min; Detection: ESIMS Advanced Marfey’s method combined with LC-MS: CDR: l-FDLA or dl-FDLA; Column: Phenomenex Kinetex C18 (2.6 μm, 2.1 × 100 mm) or Alltech Alltima C18 (5 μm, 4.6 × 250 mm), MP: H2O: CH3CN:0.1% HCO2H or MeOH:0.1% HCO2H, in elution gradient; Flow rate: 0.2 or 1.0 mL/min; Detection: MS | Antiproliferative effects of 22 against HCT-116 cell line, while 23 exhibited weak activity. | [158] |
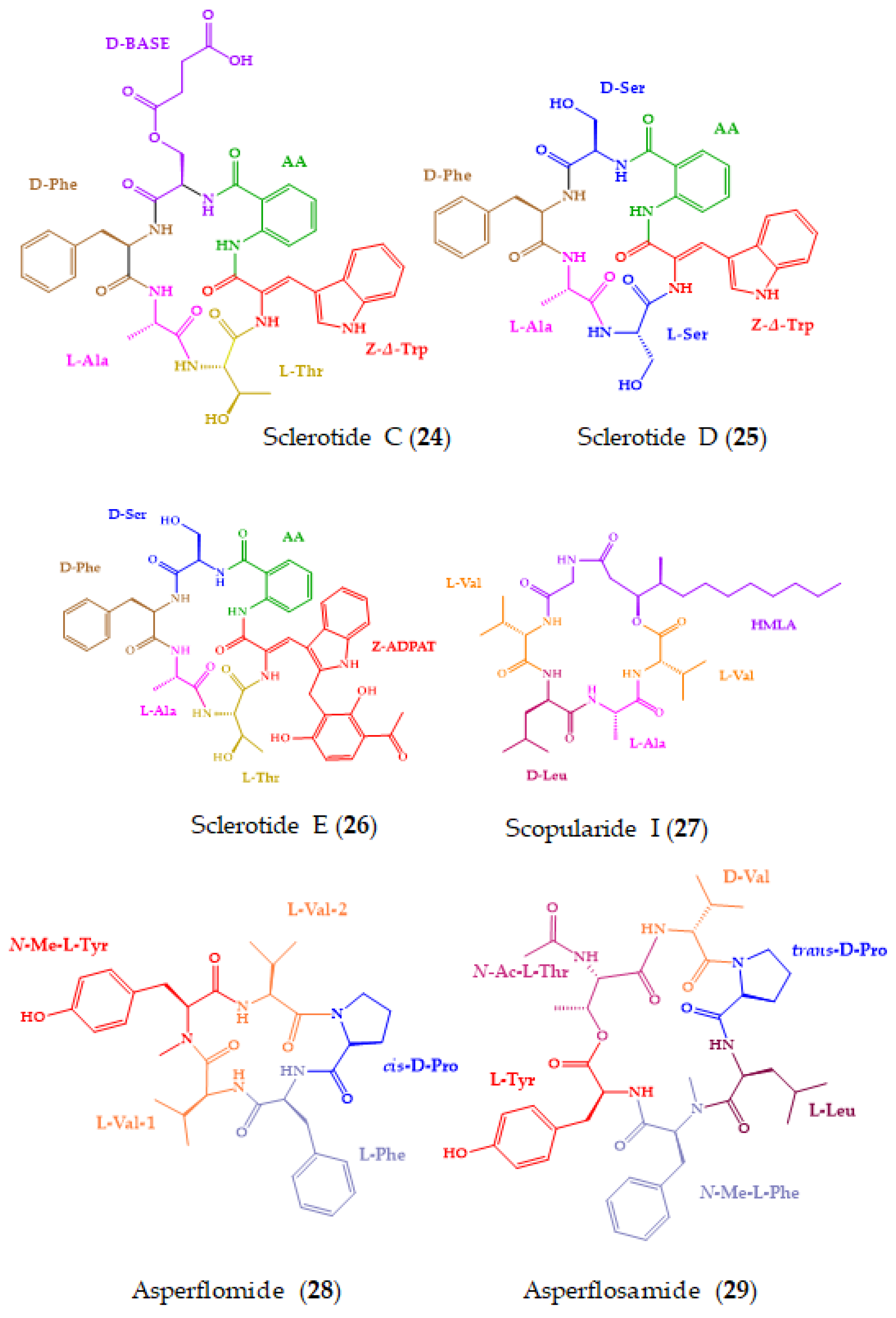
| Sclerotides C–E (24–26) and scopularide I (27) | Cyclic hexapeptides (24–26) and lipodepsipeptide (27) | Soft coral-derived fungus Aspergillus sclerotiorum SCSIO 41031 | Marfey’s method combined with LC-ESIMS/MS: CDR: l-FDAA; Column: YMC-Pack ODS-A (5 µm 4.6 × 250 mm); Mobile phase: CH3CN:H2O:0.03% TFA in elution gradient; Flow rate: 1 mL/min; Detection: UV at 340 nm and MS | 27 showed cytotoxicity against HONE-EBV cancer cell line and AChE inhibitory activity. | [159] |
| Asperflomide (28) and asperflosamide (29) | Cyclic pentapeptide (28) and depsihexapeptide (29) | Marine sponge-derived fungus Aspergillus flocculosus 16D-1 | Marfey’s analysis combined with UPLC-HRMS: CDR: d-/l-FDLA; Column: Acquity UPLC HSS T3 (1.8 µm; 2.1 × 100 mm); MP: CH3CN:H2O in elution gradient; Flow rate: 0.4 mL/min; Detection: HRMS | Weak tankyrase1/2 inhibitory activity. | [160] |
| Microsclerodermins N (30) and O (31) | Cyclic hexapeptides | Deep-sea marine sponge Pachastrella sp. | Marfey’s analysis combined with LC-MS: CDR: d-FDAA or l-FDAA; Column: COSMOSIL 2.5 π NAP (2.1 mm); MP: CH3CN:0.45% CH3CO2H in elution gradient; Flow rate of 0.5 mL/min; Detection: MS | Cytotoxic against HeLa cells. | [161] |
| Theonellamides J (32) and K (33), and 5-cis-Apoa-theopalauamide (34) | Bicyclic glycoundecapeptides | Red sea sponge Theonella swinhoei | Advanced Marfey’s method combined with UPLC-MS: CDR: l,d-FDAA; Column: C18 (1.7 µm, 2.1 × 100 mm); MP: (A) H2O:CH3CN: 0.1% HCO2H in elution gradient; Flow rate: 0.5 mL/min; Detection: UV at 340 nm and ESIMS Marfey’s method combined with HPLC: CDR: FDAA; Column: LiChroCART RP-18 (5 µm, 4.6 × 250 mm); MP: aq. TFA buffer (pH 3):CH3CN in elution gradient; Flow rate: 1 mL/min; Detection: UV at 340 nm | Significant cytotoxicity against the HTC-116 cell line. | [162] |
| Fuscasins A–D (35–38) | Cyclic heptapeptides | Marine sponge Phakellia fusca | Advanced Marfey’s method combined with UPLC-HRMS: CDR: l-FDLA; Column: Acquity UPLC HSS T3 (2.1 × 100 mm, 1.8 μm); MP: CH3CN:H2O: 0.1% HCO2H in gradient elution; Flow rate: 0.4 mL/min; Detection: HRMS | 35 displayed growth-inhibitory activity against HepG2 cells. | [163] |
| Ectyoplasin (39) | Cyclic heptapeptide | Marine sponge Ectyoplasia ferox | Marfey’s method combined with LC: CDR: l-FDLA; Column: BridgeVR C-18 (5 µm, 4.6 × 100 mm); MP: CH3CN:H2O:0.05% HCO2H in elution gradient; Flow rate: 0.8 mL/min; Detection: 340 nm | Cytotoxicity against DU-145, Jurkat, MM144, HeLa and CADO-ES1 cancer cell lines. Apoptotic cell death of DU-145 cell line. | [164] |
| Phakefutatins A–C (40–42) | Cyclic heptapeptides | Marine sponge Phakellia fusca | Advanced Marfey’s method combined with UPLC-HRMS: CDR: l-FDLA and d-FDLA; Column: Acquity UPLC HSS T3 (2.1 × 100 mm, 1.8 μm); Flow rate: 0.4 mL/min; Detection: MS | 40 is a RXRα modulator to inhibit cancer cell growth. | [165] |
| Aciculitin D (43) | Cyclic lipopeptide | Marine sponge Poecillastra sp. collected in the deep-sea | Marfey’s method combined with LC-MS: CDR: d- or l-FDAA; Column: C18; MP: CH3CN:0.45% CH3CO2H in gradient elution; Detection: MS Chiral LC: Column: CHIRALCEL OJ-RH; CSP: polysaccharide-based; MP: CH3CN:0.45% CH3CO2H in gradient elution | Cytotoxicity against HeLa and HCT-116 cells. | [166] |
| Bacicyclin (44) | Cyclic hexapeptide | Marine Bacillus sp. strain associated with Mytilus edulis | Marfey’s analysis combined with LC: CDR: l-FDAA; Column: Gemini-NX RP-C18; MP: H2O:CH3CN in elution gradient; Flow rate: 1 mL/min; Detection: UV at 340 nm | Antibacterial activity against the E. faecalis JH212 and S. aureus NCTC 8325. | [167] |
| Taeanamide A (45) | Cyclic lipo-decapeptide | Intertidal-mudflat-derived Streptomyces sp. AMD43 | Advanced Marfey’s method combined with LC-MS: CDR: FDAA; Column: Phenomenex Luna C18 (2) (5 µm, 4.6 × 100 mm); MP: H2O:CH3CN:0.1% HCO2H in gradient elution; Flow rate: 0.7 mL/min; Detection: UV at 340 nm and MS | Anti-tuberculosis activity. | [168] |
| Ogipeptins A–D (46–49) | Cyclic heptapeptides | Marine bacterium Pseudoalteromonas sp. SANK 71,903 by Daiichi Sankyo | Advanced Marfey’s method combined with LC-MS: CDR: FDLA; Column: C30 (5 µm, 4.6 × 50 mm); MP: H2O:CH3CN:0.1% HCO2H in gradient elution; Flow rate: 2.5 mL/min; Detection: UV at 254 nm and MS | Antimicrobial activity against E. coli. 46–49 blocked LPS binding to CD14. | [101,169] |
| Maribasins C–E (50–52) | Cyclic lipopeptides | Marine gorgonian-associated fungus Aspergillus sp. SCSIO41501 | Marfey’s method combined with LC: CDR: FDAA; Column: YMC-Pack ODS-A (5 μm, 250 × 4.6 mm); MP: CH3CN:H2O: 0.03% TFA in gradient elution; Flow rate: 1 mL/min; Detection: UV at 340 nm | Antifungal activity against phytopathogenic fungi A. solani, P. oryzae, C. australiensis, C. gloeosporioiles, F. oxysporum. | [170] |
| Simplicilliumtides N (53) and O (54) | Cyclic hexapeptides | Deep-sea-derived fungal strain Simplicillium obclavatum EIODSF 020 | Marfey’s method combined with LC: CDR: FDAA; Column: YMC-Pack ODS-A (250 × 4.6 mm, S-5 mm, 12 nm); MP: CH3CN/H2O/TFA in gradient elution; Flow rate: 1 mL/min; Detection: UV at 340 nm | Antifungal activity against phytopathogenic fungi A. solani and C. asianum. | [171] |
| Acremonpeptides A–D (55–58) | Cyclic hexapeptides | Marine fungus Acremonium persicinum SCSIO 115 | Marfey’s method combined with LC: CDR: l-FDAA; Column: Prodigy ODS (2) (5 μm, 4.6 × 150 mm); MP: H2O:CH3CN:0.1% TFA in gradient elution; Flow rate: 1 mL/min; Detection: UV at 340 | Antiviral activity against H. simplex virus 1, for 55 and 56. | [172] |
| Motobamide (59) | Cyclic decapeptide | Marine cyanobacterium Leptolyngbya sp. | Chiral LC: Column: CHIRALPAK MA (+) (4.6 × 50 mm); CSP: ligand exchange-based; MP: 2 mM CuSO4, and CH3CN:2 mM CuSO4 (15:85); Flow rate: 1.0 mL/min; Detection: UV at 254 nm | Inhibition of the growth of bloodstream forms of T. brucei. | [173] |
| Violaceotide A (60), and sclerotiotide L (61) | Cyclic tetrapeptide (60) and lipotripeptide (61) | Marine sponge-derived fungus Aspergillus violaceofuscus | Marfey’s method combined with LC-MS: CDR: l-FDLA; Column: Waters XBridge C18 (5 µm, 4.6 × 250 mm); MP: H2O:CH3CN: 0.1% HCO2H in gradient elution; Flow rate: 1.0 mL/min; Detection: MS | Anti-inflammatory activity against IL-10 expression of the LPS-induced THP-1 cells. | [174] |
| Petrosamides A–C (62–64) | Cyclic hexapeptides | Sponge-derived Aspergillus sp. 151304 | Advanced Marfey’s method combined with UPLC-MS: CDR: l-FDLA; Column: Waters HSS T3 (1.8 μm, 2.1 × 100 mm); MP: CH3CN:H2O: 0.1% HCO2H in gradient elution; Flow rate: 0.4 mL/min); Detection: MS | Pancreatic lipase inhibitory activity. | [175] |
| Croissamide (65) | Cyclic decapeptide | Marine cyanobacterium Symploca sp. | Chiral LC: Column: CHIRALPAK MA(+) (4.6 × 50 mm); CSP: ligand exchange-based; Mobile phase: different proportions of CH3CN:2 mM CuSO4; Flow rate: 1.0 mL/min; Detection: UV at 254 nm | Inhibitory activity against NO production in LPS-stimulated RAW 264.3 cells. | [176] |
| Cystargamides C and D (66–67) | Cyclic lipohexadepsipeptides | Marine actinomycete strain Streptomyces sp. JMS132 | Advanced Marfey’s method combined with LC-MS: CDR: l-FDLA or d-FDLA; Column: Phenomenex C18 (5 µm, 4.6 × 100 mm); MP: CH3CN:H2O: 0.1% HCO2H in gradient elution; Flow rate: 0.4 mL/min; Detection: ESIMS | Antioxidant activity. 66 decreased DPPH free radicals. 67 decreased ABTS free radicals. | [177] |
| Chrysogeamides A–G (68–74) | Cyclic hexadepsipeptides | Coral-derived fungus Penicillium chrysogenum CHNSCLM-0003 | Marfey’s method combined with HPLC-DAD and UPLC-MS: CDR: l-FDAA; Column: YMC C18 (5 µm, 2.1 × 250 mm) or ACQUITY UPLC BEH C18 (1.7 µm, 2.1 × 50 mm); MP: H2O:CH3CN: 0.1% HCO2H in gradient elution; Flow rate: 0.5 or 1.0 mL/min; Detection: DAD and MS | Pro-angiogenic activity towards Tg(kdrl:EGFP) transgenic zebrafish line. | [178] |
| Auyuittuqamides A–D (75–78) | Cyclic decapeptides | Sesquicillium microsporum RKAG 186 | Marfey’s method combined with LC-HRMS: CDR: l-FDAA; Column: C18 (1.9 μm, 2.1 × 50 mm); MP: H2O:CH3CN:0.1% HCO2H in elution gradient; Flow rate: 0.4 mL/min; Detection: HRMS | Inactive against MCF-7 and HTB-26 cancer cell lines as well as against a human epithelial keratinocyte cell line. No antimicrobial activity was observed. | [179] |
| Haloirciniamide A (79) | Cyclic pentapeptide | Indonesian marine sponge of the genus Ircinia | Marfey’s method combined with LC/MS: CDR: l-FDAA; Column: Waters Symmetry (3.5 µm, 4.6 × 150 mm); MP: H2O:CH3CN: 0.04% HCO2H in gradient elution; Flow rate: 0.8 mL/min; Detection: LC/MS | Low cytotoxicity against A-549, HT-29, MDA-MB-231, and PSN-1 tumor cell lines. | [180] |
| Unguisin G (80) | Cyclic heptapeptide | Sponge-derived fungus Aspergillus candidus NF2412 | Advanced Marfey’s method combined with LC-HRESIMS: CDR: l- and d-FDAA; Column: Agilent Poroshell 120 EC-C18 (2.7 μm, 3.0 × 50 mm); MP: CH3OH:H2:O:0.1% TFA in gradient elution; Flow rate: 0.5 mL/min; Detection: HRESIMS | No antimicrobial activity against a series of pathogens. | [181] |
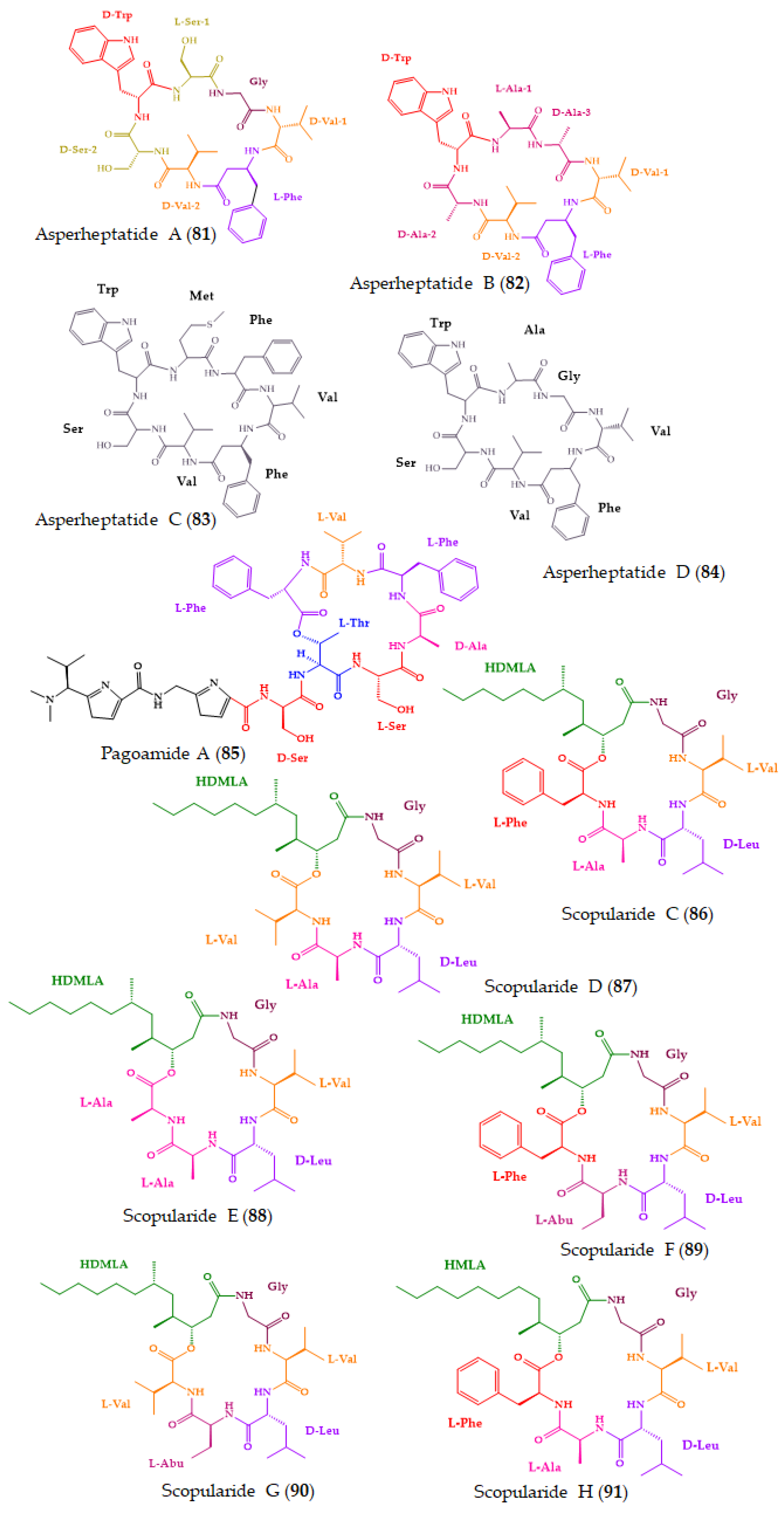
| Asperheptatides A–D (81–84) | Cyclic heptapeptides | Coral-derived fungus Aspergillus versicolor | Advanced Marfey’s method combined with LC: CDR: l-FDAA; Column: C18; MP: CH3CN:H2O in gradient elution; Flow rate: 1 mL/min; Detection: UV at 340 nm | No antitubercular activity against M. tuberculosis H37Ra. | [182] |
| Pagoamide A (85) | Cyclic depsiundecapeptide | Cultured Marine Chlorophyte, Derbesia sp. | Advanced Marfey’s method combined with LC-MS: CDR: d-FDAA; Column: YMC-Triart C18 (5 μm, 10 × 250 mm); Detection: MS Chiral LC: Column: Phenomenex Chirex 3126 (d)-penicillamine (5 μm, 4.6 × 250 mm); CSP: ligand-exchange based; MP: 2 M CuSO4; Flow rate: 2 mL/min; Detection: MS | No cytotoxicity against H-460 cancer cell line. | [183] |
| Scopularides C–G (86–90) and H (91) | Cyclic lipopentadepsipeptides | Marine sponge-derived fungus Beauveria sp. CMB-F585, and Scopulariopsis sp. CMB-F115 | C3 Marfey’s method combined with LC-DAD and MS: CDR: l and d-FDAA; Column: Agilent Zorbax SB-C3 (5 µm, 4.6 × 150 mm,); MP: H2O:MeOH: CH3CN: 0.1% HCO2H in gradient elution; Flow rate: 1.0 mL/min; Detection: DAD and ESIMS | No antimicrobial activity against a series of pathogens. No cytotoxicity against a panel of human carcinoma cell lines. | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.; Ribeiro, R.; Pinto, M.; Kijjoa, A. Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022). Molecules 2023, 28, 615. https://doi.org/10.3390/molecules28020615
Fernandes C, Ribeiro R, Pinto M, Kijjoa A. Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022). Molecules. 2023; 28(2):615. https://doi.org/10.3390/molecules28020615
Chicago/Turabian StyleFernandes, Carla, Ricardo Ribeiro, Madalena Pinto, and Anake Kijjoa. 2023. "Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022)" Molecules 28, no. 2: 615. https://doi.org/10.3390/molecules28020615
APA StyleFernandes, C., Ribeiro, R., Pinto, M., & Kijjoa, A. (2023). Absolute Stereochemistry Determination of Bioactive Marine-Derived Cyclopeptides by Liquid Chromatography Methods: An Update Review (2018–2022). Molecules, 28(2), 615. https://doi.org/10.3390/molecules28020615










