The Expanded Vermiculite Was Quickly Prepared by the Catalytic Action of Manganese Dioxide on Hydrogen Peroxide and Its Adsorption Properties to Cd
Abstract
:1. Introduction
2. Results and Discussion
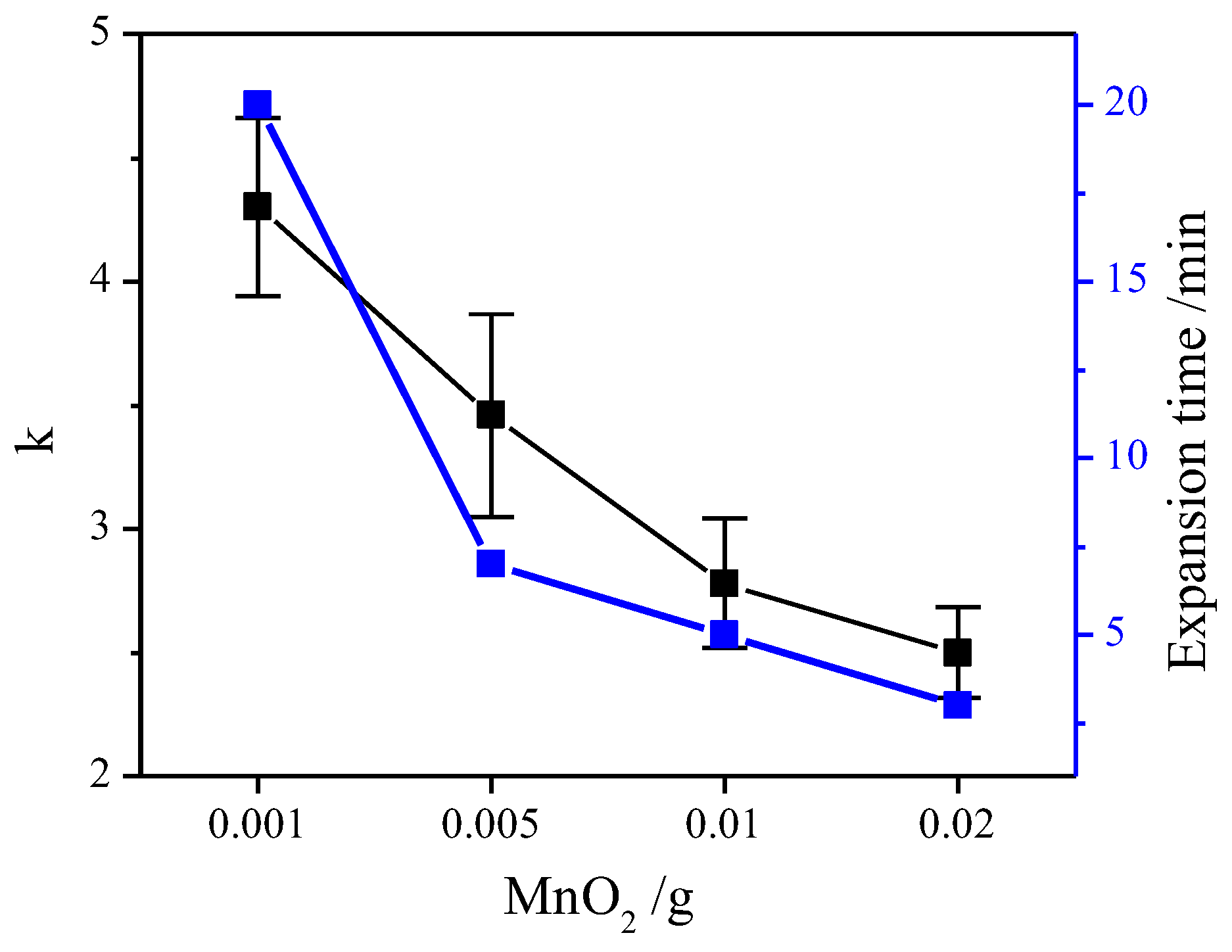
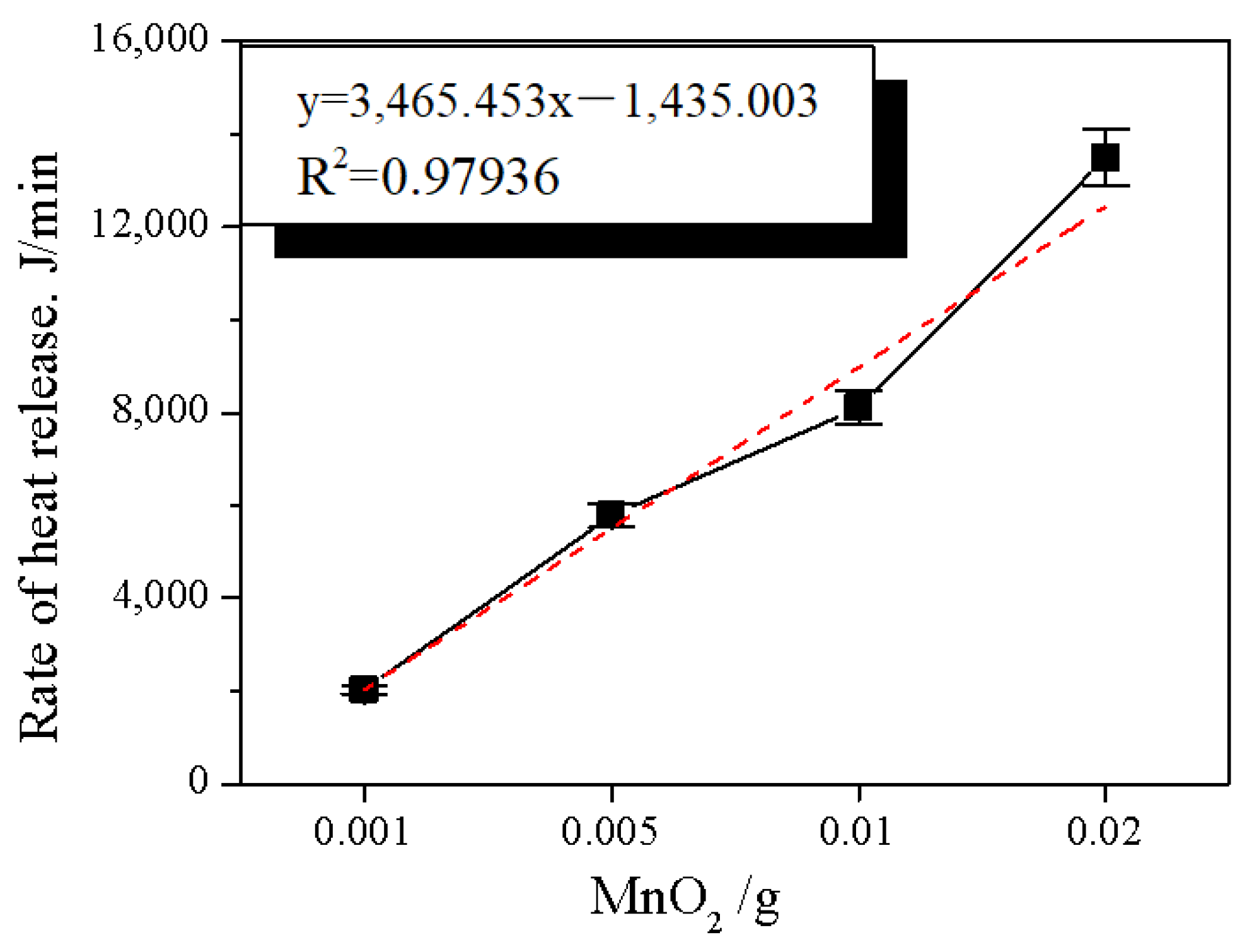
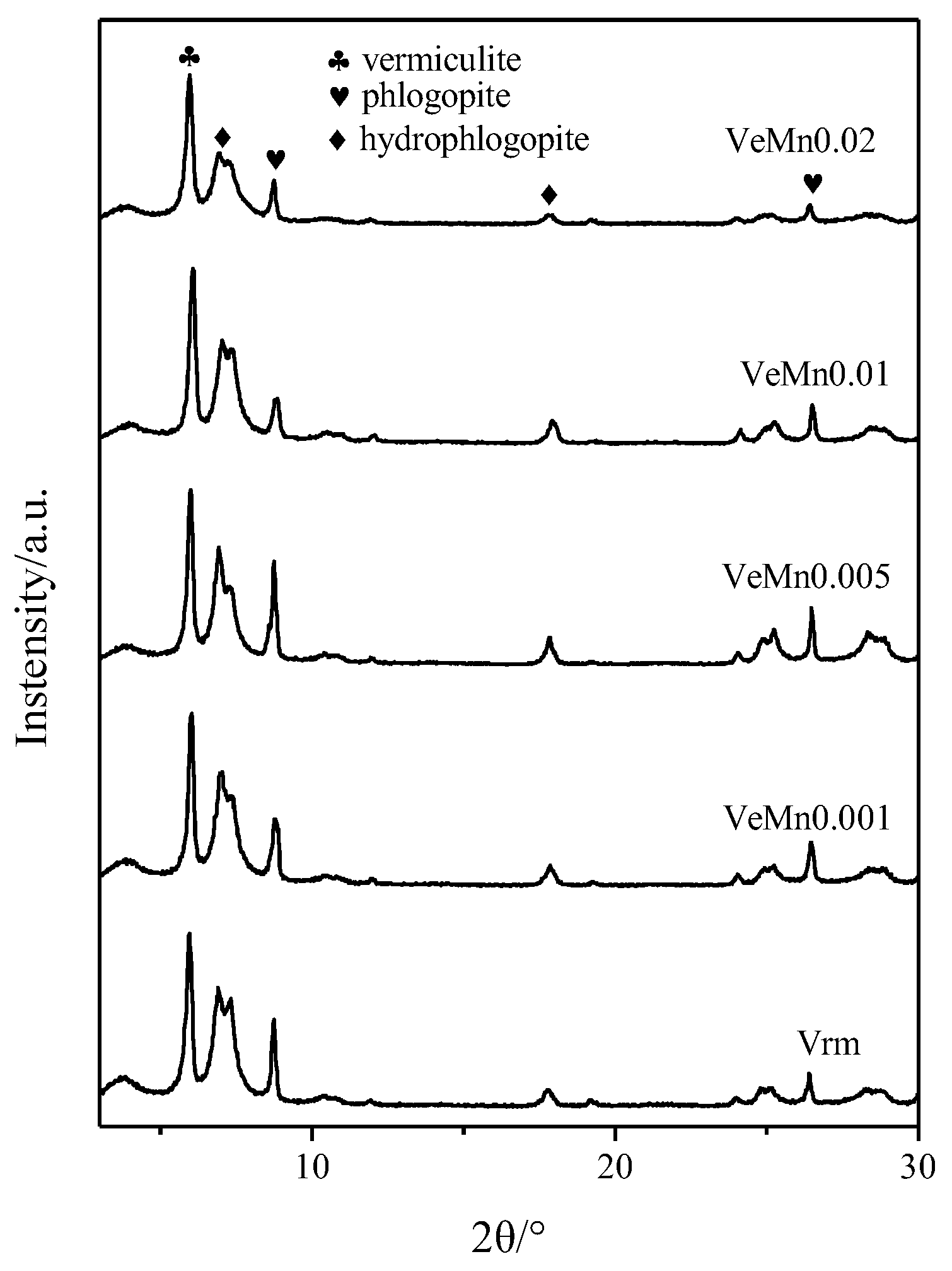
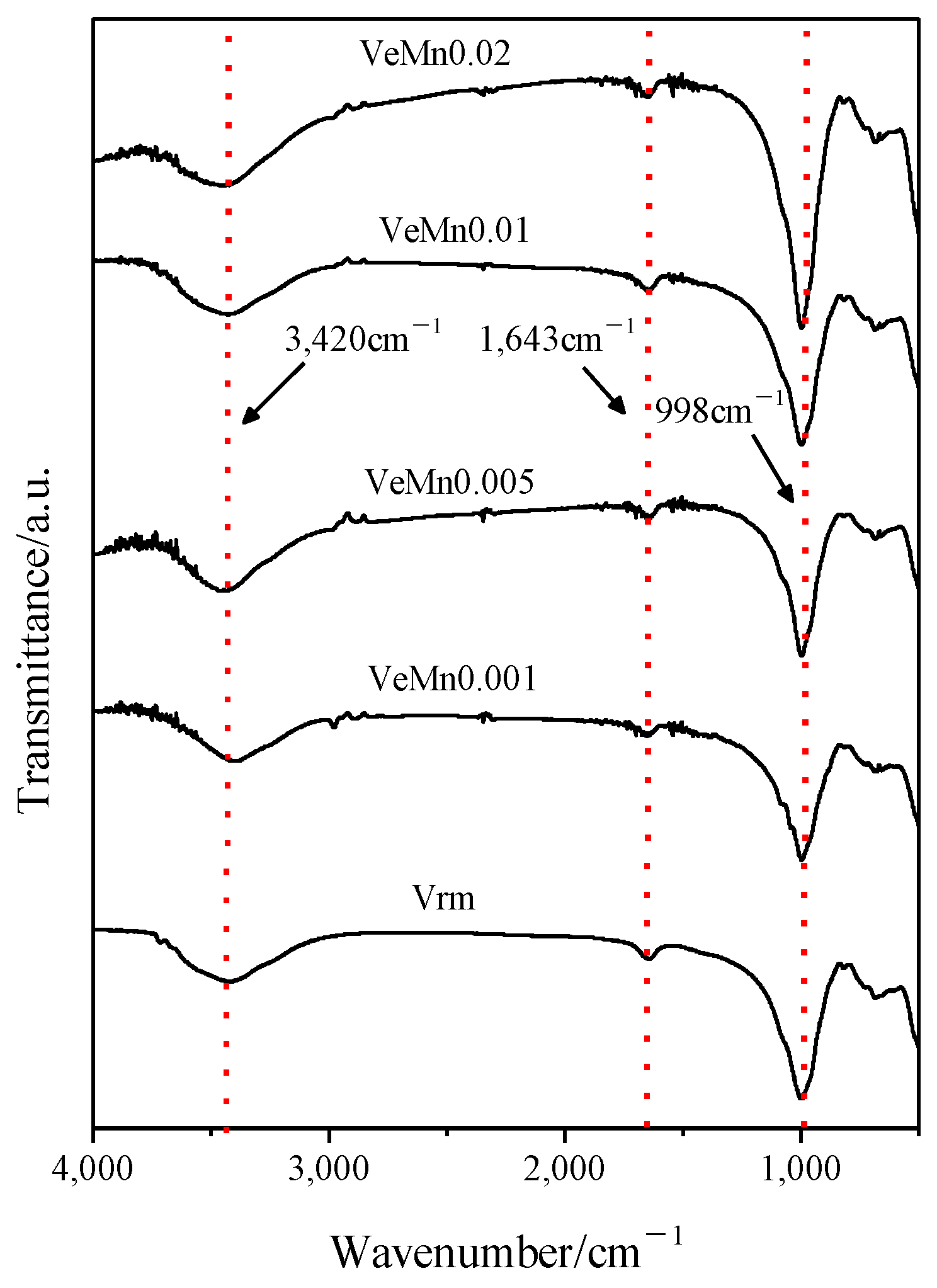
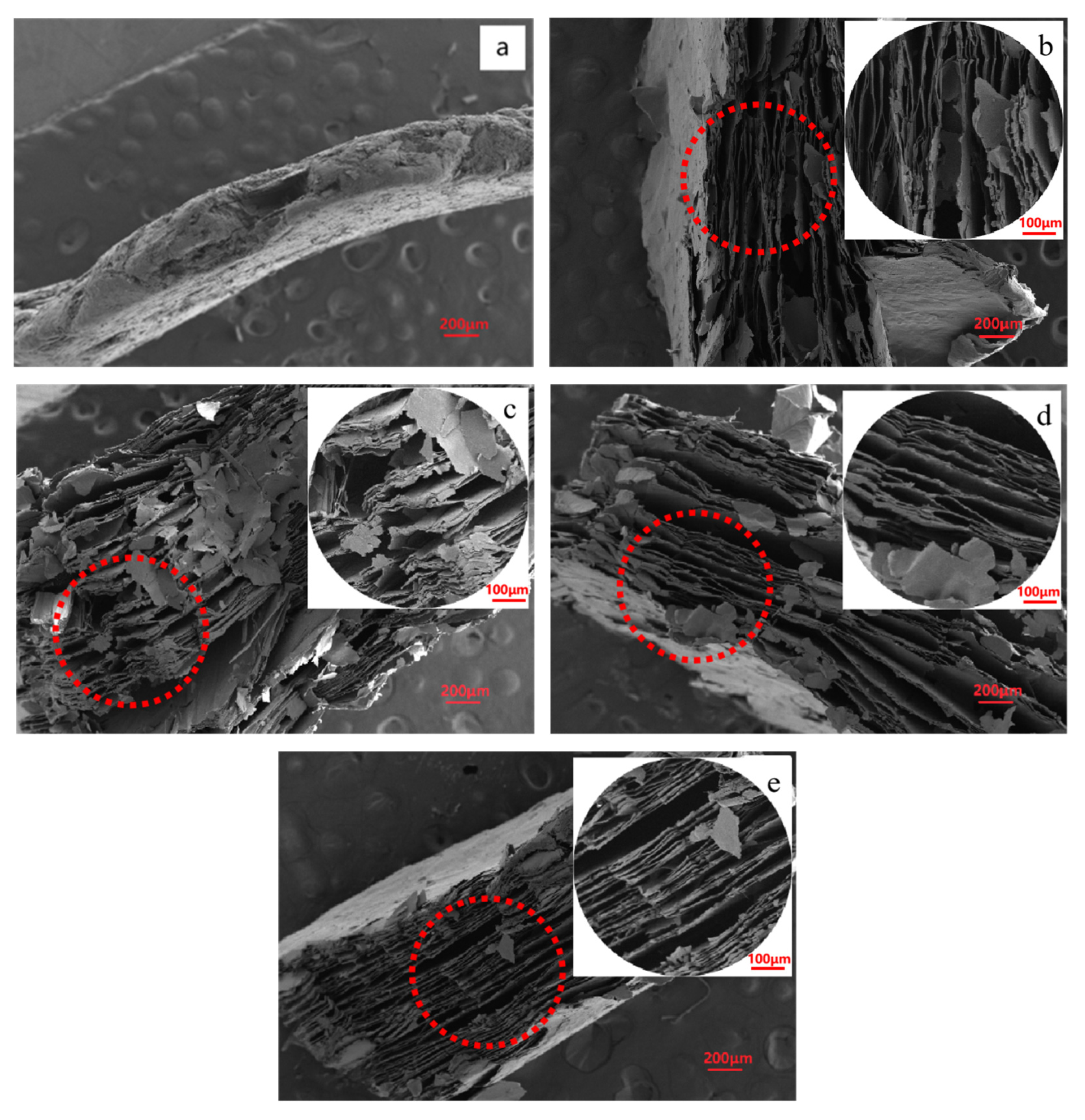
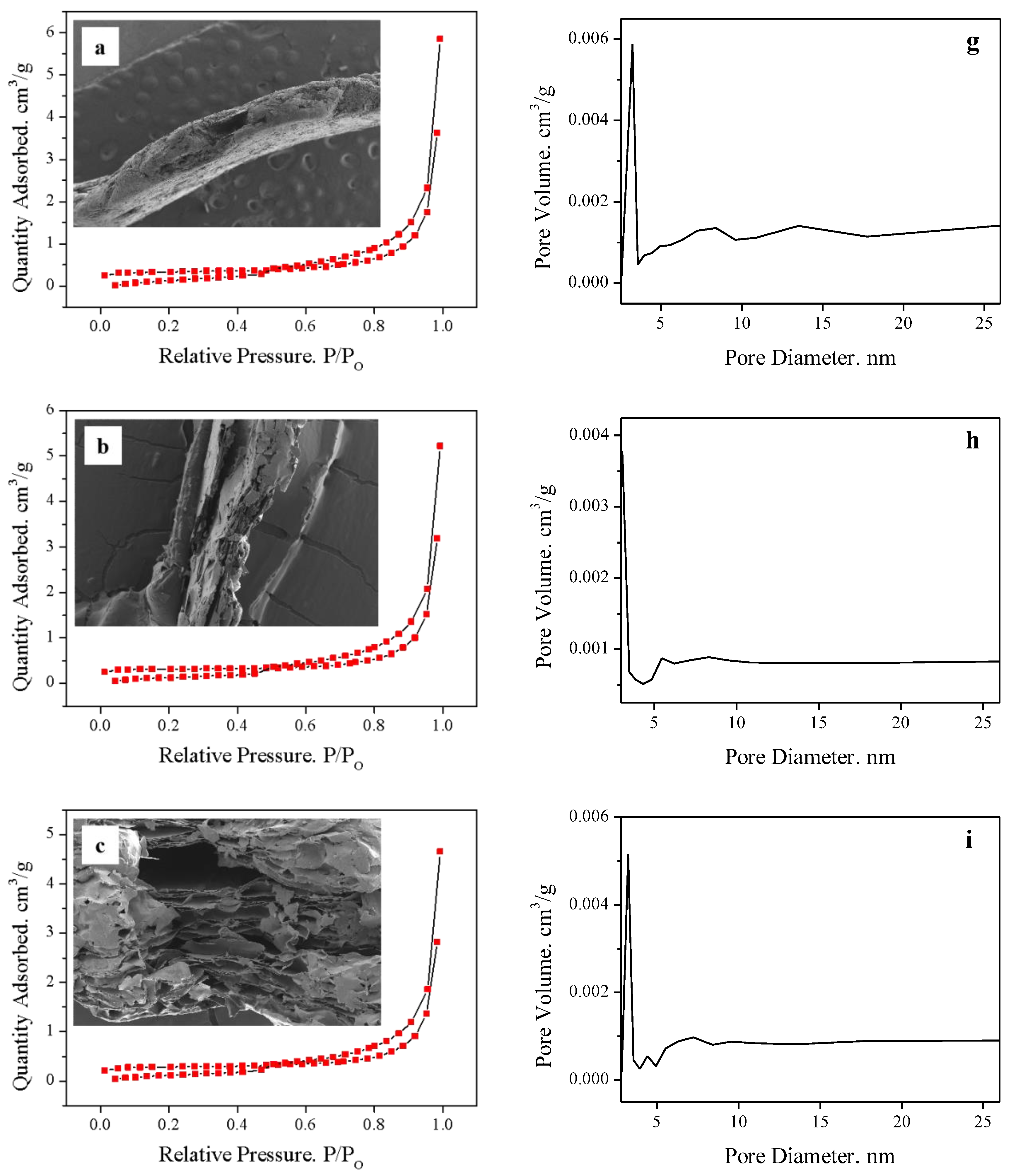
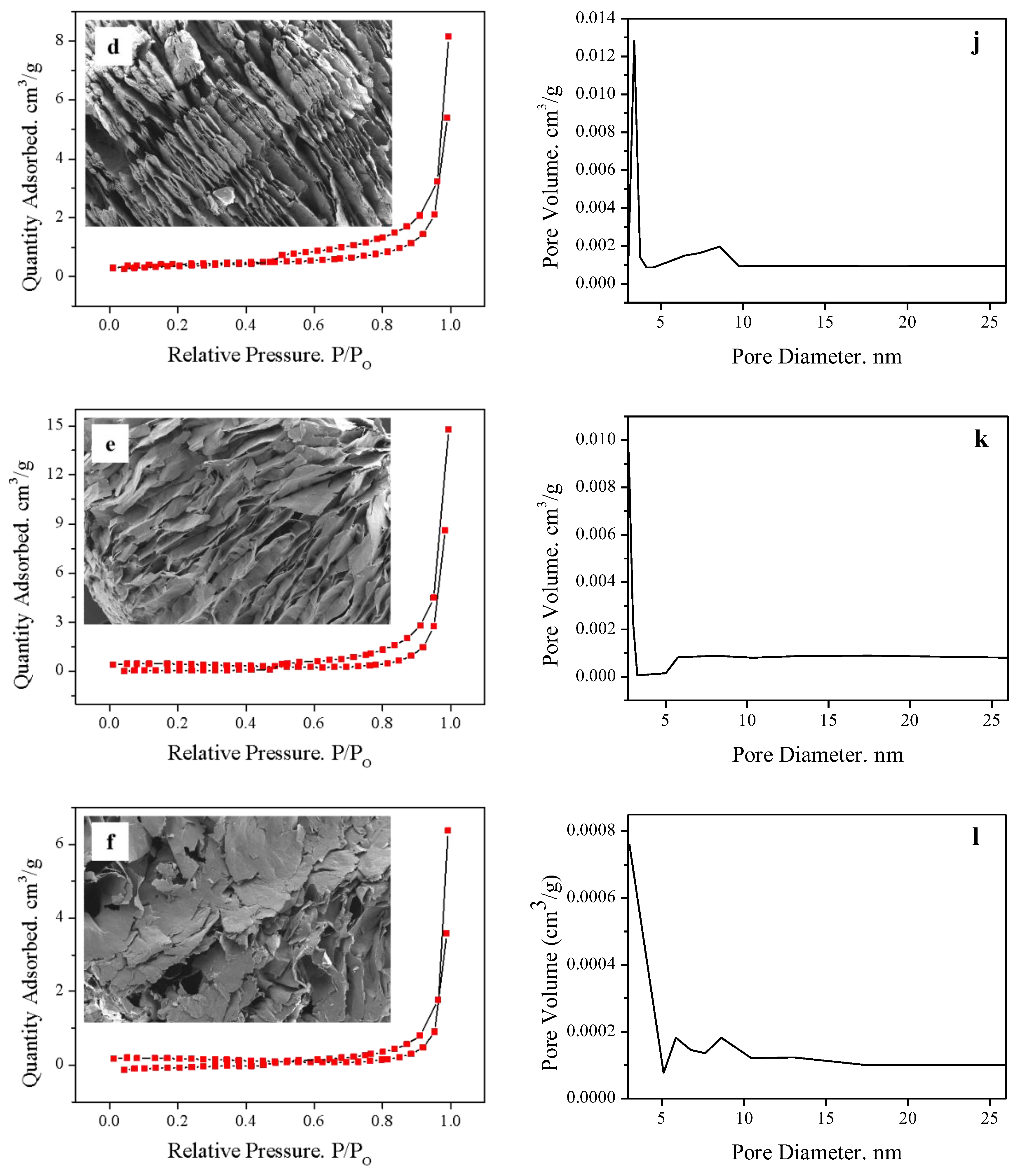
2.1. Effect of Manganese Dioxide on Expansion Properties
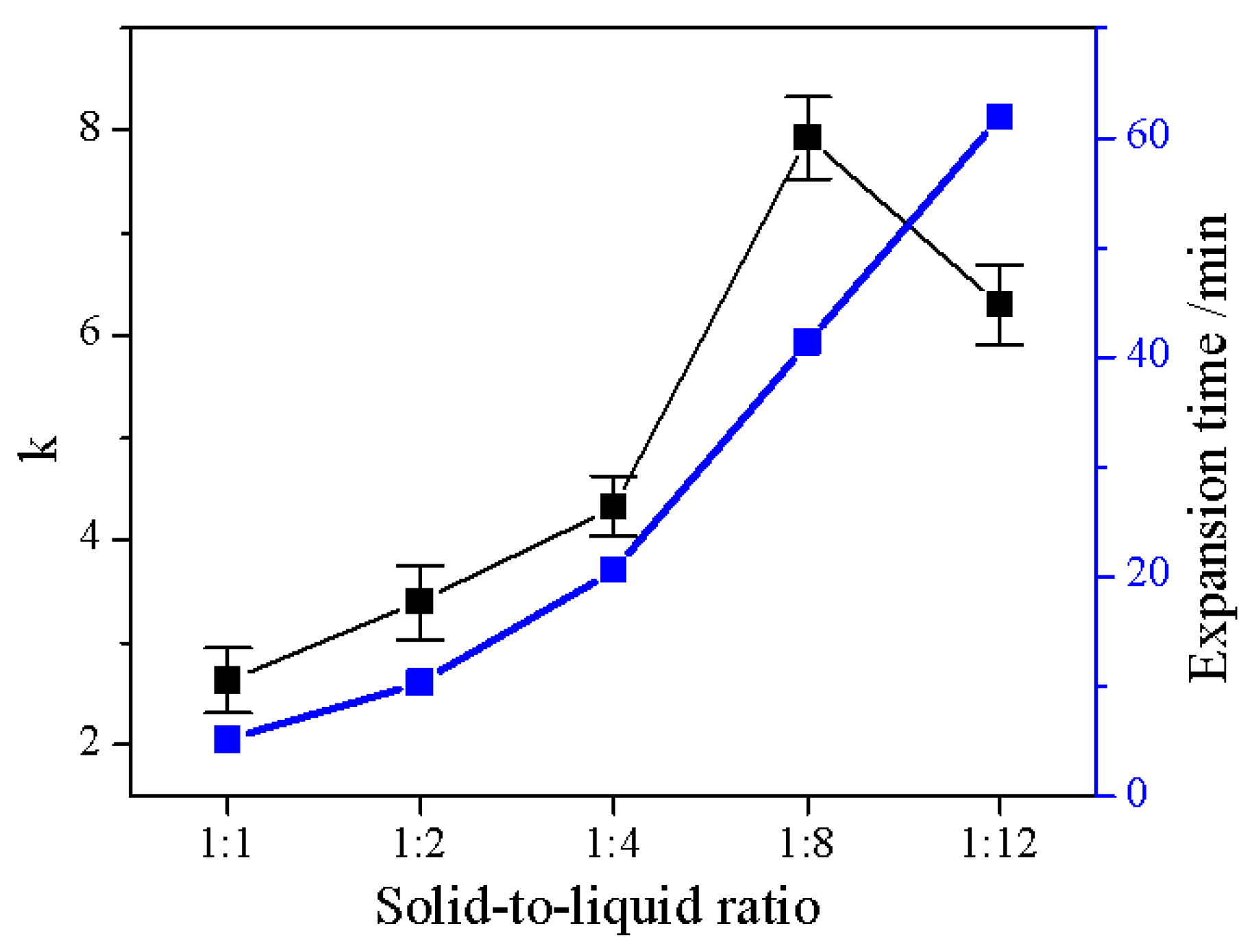
2.2. Effect of Solid–Liquid Ratio
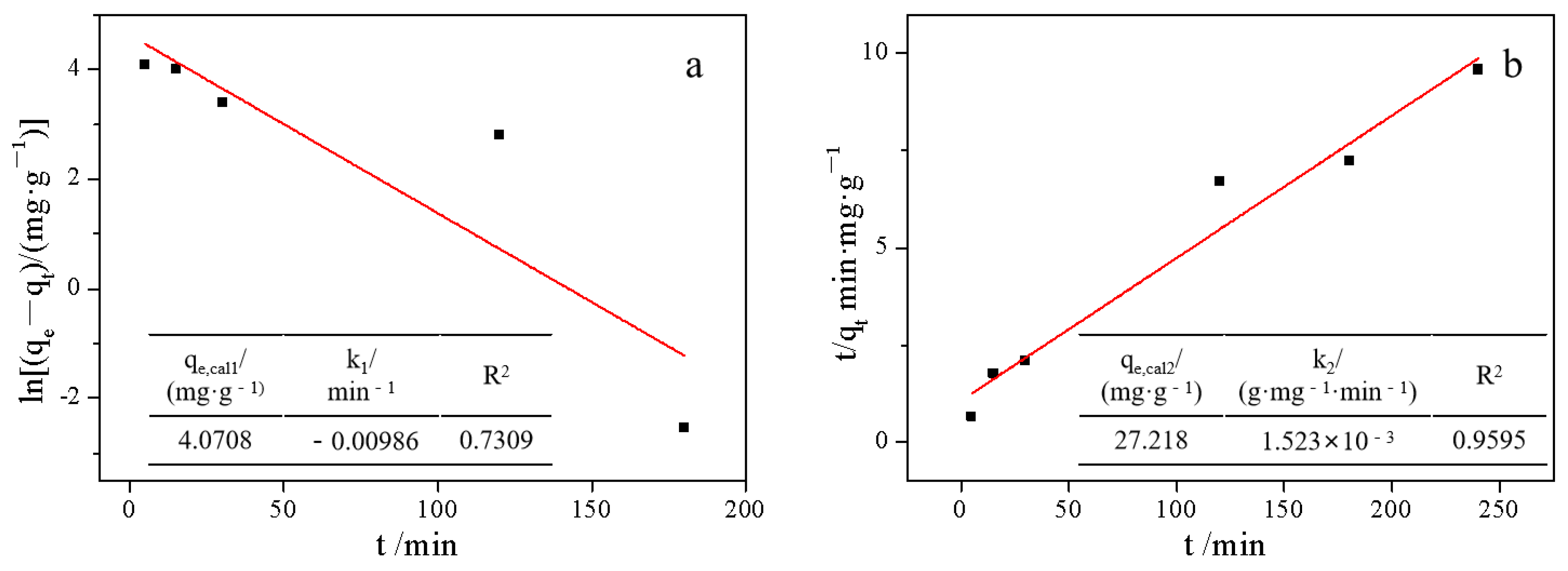
2.3. Adsorption Experiment
3. Materials and Methods
3.1. Materials
3.2. Preparation of Samples
3.3. Cd adsorption Experiment
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brião, G.D.V.; da Silva, M.G.; Vieira, M.G.A. Expanded vermiculite as an alternative adsorbent for the dysprosium recovery. J. Taiwan Inst. Chem. Eng. 2021, 127, 228–235. [Google Scholar] [CrossRef]
- Feng, J.; Liu, M.; Mo, W.; Su, X. Heating temperature effect on the hygroscopicity of expanded vermiculite. Ceram. Int. 2021, 47, 25373–25380. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Ai, Z.; Bai, H.; Zhang, T.; Song, S. Preparation and application of expanded and exfoliated vermiculite: A critical review. Chem. Phys. 2021, 550, 111313. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, T.; Kong, X.; Liu, Y.; Xu, J.; Zhang, B.; Chen, H.; Chen, Z. Synthesis and characterization of nano-layered Mg-vermiculite by hydrothermal method. Mater. Lett. 2019, 238, 175–178. [Google Scholar]
- Janica, I.; Del Buffa, S.; Mikołajczak, A.; Eredia, M.; Pakulski, D.; Ciesielski, A.; Samorì, P. Thermal insulation with 2D materials: Liquid phase exfoliated vermiculite functional nanosheets. Nanoscale 2018, 10, 23182–23190. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Zang, L.; Luo, J.; Guo, J.; Liu, H.; Ru, J. Preparation and characterization of poly(ethylene glycol)/organo-vermiculite nanocomposite polymer electrolytes. Polym. Bull. 2010, 65, 669–680. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Giacomino, A.; Aceto, M.; Mentasti, E. Adsorption of heavy metals on vermiculite: Influence of pH and organic ligands. J. Colloid Interface Sci. 2006, 299, 537–546. [Google Scholar] [CrossRef]
- Brião, G.D.V.; Franco, D.S.P.; da Silva, F.V.; da Silva, M.G.C.; Vieira, M.G.A. Critical rare earth metal adsorption onto expanded vermiculite: Accurate modeling through response surface methodology and machine learning techniques. Sustain. Chem. Pharm. 2023, 31. [Google Scholar] [CrossRef]
- Briao, G.V.; Meuris, G.C.D.S. Dysprosium adsorption on expanded vermiculite: Kinetics, selectivity and desorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127616. [Google Scholar] [CrossRef]
- Han, L.; Wang, T.; Gong, J.; Li, X.; Ji, Y.; Wang, S. Multi-hydroxyl containing organo-vermiculites for enhanced adsorption of coexisting methyl blue and Pb(II) and their adsorption mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 650, 129542. [Google Scholar] [CrossRef]
- Sis, H.; Uysal, T. Removal of heavy metal ions from aqueous medium using Kuluncak (Malatya) vermiculites and effect of precipitation on removal. Appl. Clay Sci. 2014, 95, 1–8. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Exfoliation of vermiculites with chemical treatment using hydrogen peroxide and thermal treatment using microwaves. Appl. Clay Sci. 2013, 87, 219–227. [Google Scholar] [CrossRef]
- Yan-Zhao, D.U.; Chao-Yang, C.; Wei, F.Y. A New Method Study on Preparation of Vermiculite with High Expansion Rate. Conserv. Util. Miner. Resour. 2008, 5, 13–17. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Feng, J.; Mo, W.; Su, X.; Fu, L. A novel quantitative analysis method of microwave energy absorption during vermiculite expansion process. Thermochim. Acta 2020, 691, 178718. [Google Scholar] [CrossRef]
- Yanyan, X. Preparation and Photocatalytic Properties of TiO2/Thin-Layer Phlogopite-Vermiculite Nanocomposite; Southwest University of Science and Technology: Mianyang, China, 2020. [Google Scholar]
- Kehal, M.; Reinert, L.; Duclaux, L. Characterization and boron adsorption capacity of vermiculite modified by thermal shock or H2O2 reaction and/or sonication. Appl. Clay Sci. 2010, 48, 561–568. [Google Scholar] [CrossRef]
- Obut, A.; Girgin, I. Hydrogen peroxide exfoliation of vermiculite and phlogopite. Miner. Eng. 2002, 15, 683–687. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.; Luo, L.; Peng, T.; Tian, J. Preparation of high diameter-thickness ratio thin-layer phlogopite-vermiculite nano-functional material by liquid phase exfoliation. Appl. Clay Sci. 2020, 191, 105612. [Google Scholar] [CrossRef]
- Baldikova, E.; Pospiskova, K.; Safarikova, M.; Safarik, I. Non-woven fabric supported manganese dioxide microparticles as a low-cost, easily recoverable catalyst for hydrogen peroxide decomposition. Mater. Chem. Phys. 2018, 203, 280–283. [Google Scholar] [CrossRef]
- Xiang-Xi, L.; Cui Qing, L.; Ru Jun, W.; Si Ning, C. Analysis of Thermal Stability of 30% Hydrogen Peroxide. Chem. Adhes. 2019, 41, 240–243. [Google Scholar]
- Pingxiao, W. Existing status of interlayer water in vermiculite mineral material. J. Chin. Ceram. Soc. 2005, 33, 209–214. [Google Scholar]
- Suquet, H.; Franck, R.; Lambert, J.-F.; Elsass, F.; Marcilly, C.; Chevalier, S. Catalytic properties of two pre-cracking matrices: A leached vermiculite and a Al-pillared saponite. Appl. Clay Sci. 1994, 8, 349–364. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef] [PubMed]
- de Farias, A.B.V.; da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Development of novel composite adsorbents based on biopolymers/vermiculite using the ionic imprinting technique for cerium biosorption. J. Environ. Chem. Eng. 2022, 10, 108730. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, Z.; Fan, X.; Jing, R.; Yang, J.; Shao, Y.; Liu, X.; Wu, M.; Zhang, Q.; Liu, A. Removal of heavy metals from soil by vermiculite supported layered double hydroxides with three-dimensional hierarchical structure. Chem. Eng. J. 2020, 390, 124554. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Tsurtsumia, A.; Witkowski, S.; Freitas, O.; Figueiredo, S.; Cybińska, J.; Stawiński, W. Vermiculite as a potential functional additive for water treatment bioreactors inhibiting toxic action of heavy metal cations upsetting the microbial balance. J. Hazard. Mater. 2022, 433, 128812. [Google Scholar] [CrossRef]
- Yan Yan, X.; Hong Juan, S.; Tong Jiang, P.; Li Ming, L.; Jing Fei, T.; Ya Ting, Q. High Expansion Rate Expanded Vermiculite: Preparation by Chemical-Microwave Method and the Adsorption Mechanism of Methylene Blue. Chin. J. Inorg. Chem. 2020, 36, 113–122. [Google Scholar]
- Jauhar, S.; Singhal, S.; Dhiman, M. Manganese substituted cobalt ferrites as efficient catalysts for H2O2 assisted degradation of cationic and anionic dyes: Their synthesis and characterization. Appl. Catal. A Gen. 2014, 486, 210–218. [Google Scholar] [CrossRef]
- Gómez-Largo, P.M.; Miranda, C.D.; Villagrán-Olivares, A.C.; López, C.A.; Barbero, B.P. Study of cobalt-iron mixed oxides and catalytic behavior for decomposition of hydrogen peroxide. Mol. Catal. 2022, 530, 112639. [Google Scholar] [CrossRef]
- Yu, X.; Wei, C.; Wu, H. Effect of molecular structure on the adsorption behavior of cationic dyes onto natural vermiculite. Sep. Purif. Technol. 2015, 156, 489–495. [Google Scholar] [CrossRef]
- Jie, M.; Yuling, M.; Qian, X.; Wang, Z.; Chen, Y. Phosphorus adsorption onto ferrihydrite and predicting colloidal phosphorus transport. Chin. J. Eco-Agric. 2021, 29, 85–93. [Google Scholar]
- Peng, T.; Wan, P.; Pan, Z.; Zhang, J. A study of the interstratified structure of phlogopite and vermiculite from Weili mine, Xinjiang. Acta Petrol. Mineral. 1996, 15, 9. [Google Scholar]
- Committee, C.N.S. JC/T 441-2009 Expanded Vermiculite; Chinese Building Material Press: Beijing, China, 2009. [Google Scholar]

| Component | SiO2 | MgO | Al2O3 | Fe2O3 | K2O | Na2O | TiO2 | CaO | F | BaO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 38.32 | 20.18 | 12.34 | 7.36 | 4.86 | 1.50 | 1.39 | 1.26 | 0.16 | 0.10 | 12.53 |
| *LOI (loss on ignition) | |||||||||||
| No. | Vrm: Hydrogen Peroxide (g:mL) | Manganese Dioxide Addition (g) |
|---|---|---|
| VeMn0.001 | 1∶4 | 0.001 |
| VeMn0.005 | 1∶4 | 0.005 |
| VeMn0.01 | 1∶4 | 0.01 |
| VeMn0.02 | 1∶4 | 0.02 |
| VeH1 | 1∶1 | 0.001 |
| VeH2 | 1∶2 | 0.001 |
| VeH4 | 1∶4 | 0.001 |
| VeH8 | 1∶8 | 0.001 |
| VeH12 | 1∶12 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, H.; Peng, T.; Gao, T.; Ding, W.; Hui, T.; Jiang, L. The Expanded Vermiculite Was Quickly Prepared by the Catalytic Action of Manganese Dioxide on Hydrogen Peroxide and Its Adsorption Properties to Cd. Molecules 2023, 28, 817. https://doi.org/10.3390/molecules28020817
Chen Y, Sun H, Peng T, Gao T, Ding W, Hui T, Jiang L. The Expanded Vermiculite Was Quickly Prepared by the Catalytic Action of Manganese Dioxide on Hydrogen Peroxide and Its Adsorption Properties to Cd. Molecules. 2023; 28(2):817. https://doi.org/10.3390/molecules28020817
Chicago/Turabian StyleChen, Yunzhu, Hongjuan Sun, Tongjiang Peng, Tongxi Gao, Wenjin Ding, Tao Hui, and Lei Jiang. 2023. "The Expanded Vermiculite Was Quickly Prepared by the Catalytic Action of Manganese Dioxide on Hydrogen Peroxide and Its Adsorption Properties to Cd" Molecules 28, no. 2: 817. https://doi.org/10.3390/molecules28020817
APA StyleChen, Y., Sun, H., Peng, T., Gao, T., Ding, W., Hui, T., & Jiang, L. (2023). The Expanded Vermiculite Was Quickly Prepared by the Catalytic Action of Manganese Dioxide on Hydrogen Peroxide and Its Adsorption Properties to Cd. Molecules, 28(2), 817. https://doi.org/10.3390/molecules28020817






