Multiplexing Quadrupole and Ion Trap Operation Modes on a “Brick” Miniature Mass Spectrometer
Abstract
:1. Introduction
2. Results and Discussions
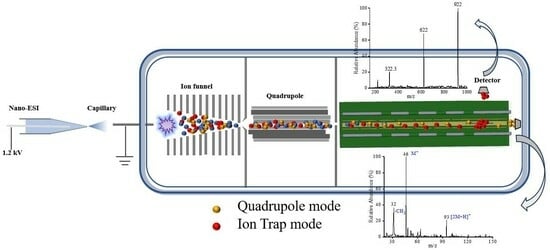
2.1. Design of a PCB-Based Multi-Electrode Quadrupole–Ion Trap Mass Analyzer
2.2. Multiplexing Quadrupole and Ion Trap Operation Modes
2.3. Characterization of the Quadrupole Operation Mode
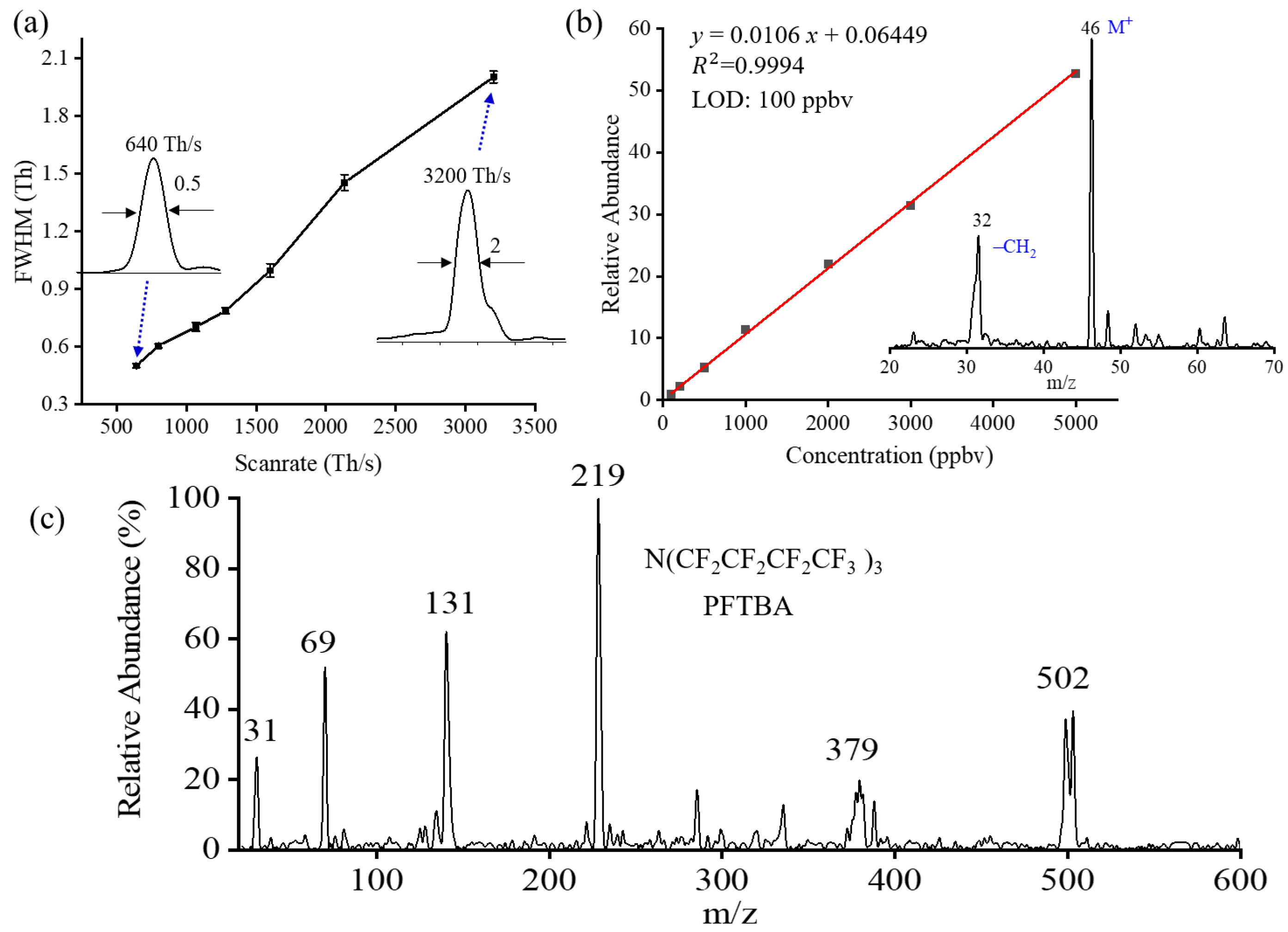
2.3.1. Mass Resolution
2.3.2. Sensitivity
2.3.3. Mass Range
2.4. Characterization of the Ion Trap Operation Mode
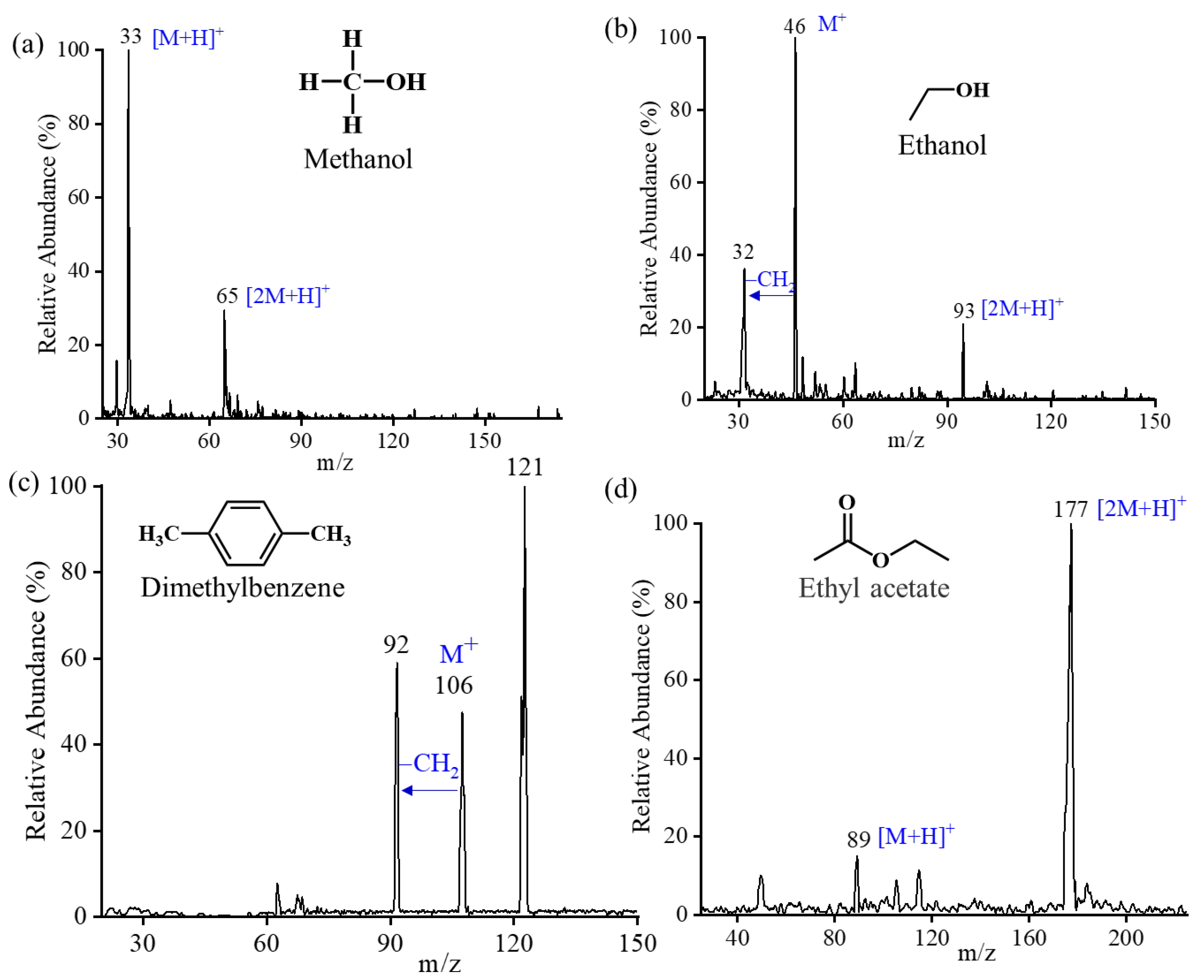
2.4.1. Mass Resolution
2.4.2. Mass Range
2.4.3. Sensitivity
3. Instrumental Section
3.1. Chemical Samples
3.2. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, Z.; Cooks, R.G. Miniature Mass Spectrometers. Annu. Rev. Anal. Chem. 2009, 2, 187–214. [Google Scholar] [CrossRef]
- Giannoukos, S.; Brkic, B.; Taylor, S.; France, N. Membrane inlet mass spectrometry for homeland security and forensic applications. J. Am. Soc. Mass Spectrom. 2015, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Su, Y.; Wang, H.Y.; Guo, Y.L. Gas chromatographic-ion trap mass spectrometric analysis of volatile organic compounds by ion-molecule reactions using the electron-deficient reagent ion CCl3(+). J. Am. Soc. Mass Spectrom. 2011, 22, 1839–1850. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Multi-residue quantification of veterinary drugs in milk with a novel extraction and cleanup technique: Salting out supported liquid extraction (SOSLE). Anal. Chim. Acta 2014, 820, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Peng, Y.; Chen, F.; Zhao, L.; Li, X.; Qin, J.; Li, Q.; Wang, B.; Pan, B.; et al. A liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based assay to profile 20 plasma steroids in endocrine disorders. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Feng, Y.; Wei, Y.; Wang, Y.; Xu, W. Development of a miniature mass spectrometer with continuous atmospheric pressure interface. Analyst 2015, 140, 3406–3414. [Google Scholar] [CrossRef]
- Zhai, Y.; Jiang, T.; Huang, G.; Wei, Y.; Xu, W. An aerodynamic assisted miniature mass spectrometer for enhanced volatile sample analysis. Analyst 2016, 141, 5404–5411. [Google Scholar] [CrossRef]
- Gao, L.; Cooks, R.G.; Ouyang, Z. Breaking the pumping speed barrier in mass spectrometry: Discontinuous atmospheric pressure interface. Anal. Chem. 2008, 80, 4026–4032. [Google Scholar] [CrossRef]
- Xu, W.; Charipar, N.; Kirleis, M.A.; Xia, Y.; Ouyang, Z. Study of Discontinuous Atmospheric Pressure Interfaces for Mass Spectrometry Instrumentation Development. Anal. Chem. 2010, 82, 6584–6592. [Google Scholar] [CrossRef]
- Li, L.; Chen, T.C.; Ren, Y.; Hendricks, P.I.; Cooks, R.G.; Ouyang, Z. Mini 12, miniature mass spectrometer for clinical and other applications--introduction and characterization. Anal. Chem. 2014, 86, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.S.; Shelley, J.T.; Cooks, R.G. Handheld low-temperature plasma probe for portable “point-and-shoot” ambient ionization mass spectrometry. Anal. Chem. 2013, 85, 6545–6552. [Google Scholar] [CrossRef]
- Wells, J.M.; Badman, E.R.; Cooks, R.G. A quadrupole ion trap with cylindrical geometry operated in the mass-selective instability mode. Anal. Chem. 1998, 70, 438–444. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Senko, M.W.; Syka, J.E. A two-dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002, 13, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.W. A new linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 2002, 16, 512–526. [Google Scholar] [CrossRef]
- Ouyang, Z.; Wu, G.; Song, Y.; Li, H.; Plass, W.R.; Cooks, R.G. Rectilinear ion trap: Concepts, calculations, and analytical performance of a new mass analyzer. Anal. Chem. 2004, 76, 4595–4605. [Google Scholar] [CrossRef]
- March, R.E. Quadrupole ion traps. Mass Spectrom. Rev. 2009, 28, 961–989. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; van Amerom, F.H.; Short, R.T. A novel planar ion funnel design for miniature ion optics. Rev. Sci. Instrum. 2014, 85, 105101. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, X.; Xu, H.; Zheng, Y.; Yuan, T.; Xu, W. Mini Mass Spectrometer Integrated with a Miniature Ion Funnel. Anal. Chem. 2017, 89, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, T.; Xu, Q.; Zhai, Y.; Li, D.; Xu, W. Pseudo-Multiple Reaction Monitoring (Pseudo-MRM) Mode on the “Brick” Mass Spectrometer, Using the Grid-SWIFT Waveform. Anal. Chem. 2019, 91, 13838–13846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Gao, Z.; Xiang, Y.; Jiang, T.; Xu, W. Parallel Pseudo-MRM on the “brick” miniature mass spectrometer for high throughput multi-target screening. Talanta 2023, 252, 123866. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, F.; Ding, C.F. Dipolar direct current driven collision-induced dissociation in a digital ceramic-based rectilinear ion trap mass spectrometer. Anal. Chem. 2013, 85, 1271–1275. [Google Scholar] [CrossRef]
- Ding, L.; Sudakov, M.; Brancia, F.L.; Giles, R.; Kumashiro, S. A digital ion trap mass spectrometer coupled with atmospheric pressure ion sources. J. Mass Spectrom. 2004, 39, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Sudakov, M.; Kumashiro, S. A simulation study of the digital ion trap mass spectrometer. Int. J. Mass Spectrom. 2002, 221, 117–138. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, H.; Tang, Y.; Zhai, Y.; Xu, W.; Xu, H.; Zhao, X.; Li, D.; Xu, W. A “Brick Mass Spectrometer” Driven by a Sinusoidal Frequency Scanning Technique. Anal. Chem. 2017, 89, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Yang, B.; Li, X.; Xu, W. Development of a handheld mass spectrometer driven by the sinusoidal frequency scanning technique. Int. J. Mass Spectrom. 2022, 481, 116915. [Google Scholar] [CrossRef]
- Ekroos, K.; Chernushevich, I.V.; Simons, K.; Shevchenko, A. Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Anal. Chem. 2002, 74, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Hong, J.; Zhang, M.; Jiang, T.; Xu, W. Development of a High-Field “Brick” Mass Spectrometer with Extended Mass Range and Capable of Characterizing Native Proteins. Anal. Chem. 2023, 95, 13503–13508. [Google Scholar] [CrossRef] [PubMed]
- Hopfgartner, G.; Husser, C.; Zell, M. Rapid screening and characterization of drug metabolites using a new quadrupole-linear ion trap mass spectrometer. J. Mass Spectrom. 2003, 38, 138–150. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Wen, C.; Jiang, T.; Tian, W.; Xie, X.; Cui, X.; Lu, R.; Feng, J.; Jin, A.; et al. Metabolome analysis of genus Forsythia related constituents in Forsythia suspensa leaves and fruits using UPLC-ESI-QQQ-MS/MS technique. PLoS ONE 2022, 17, e0269915. [Google Scholar] [CrossRef]
- Feng, S.-X.; Zhang, H.-J.; Zhao, D.; Li, R.-R.; Du, X.-H.; Wang, P.-Y.; Shu, S.-N.; Qu, L.-B.; Li, J.-S. Chemical profiling and quantification of Tanreqing injection, a systematic quality control strategy equipped with UPLC-Q-Orbitrap fusion MS. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 255–270. [Google Scholar] [CrossRef]
- March, R.E. An Introduction to Quadrupole Ion Trap Mass Spectrometry. J. Mass Spectrom. 1997, 32, 351–369. [Google Scholar] [CrossRef]
- Douglas, D.J. Linear quadrupoles in mass spectrometry. Mass Spectrom. Rev. 2009, 28, 937–960. [Google Scholar] [CrossRef]
- Barlow, S.E.; Tinkle, M.D. The ion cyclotron resonance frequency of short, single-species plasmas in Penning traps. J. Appl. Phys. 2006, 99, 013306. [Google Scholar] [CrossRef]
- Damale, R.D.; Dutta, A.; Shaikh, N.; Pardeshi, A.; Shinde, R.; Babu, K.D.; Gaikwad, N.N.; Banerjee, K. Multiresidue analysis of pesticides in four different pomegranate cultivars: Investigating matrix effect variability by GC-MS/MS and LC-MS/MS. Food Chem. 2023, 407, 135179. [Google Scholar] [CrossRef]
- Abdelraheem, E.; Wise, J.; Murphy, C.; Jiang, W. Triple-stage Quadrupole Mass Spectrometer to Determine Ubiquitously Present Per- and Polyfluorinated Alkyl Substances in Drinking Water at Part Per Trillion Levels Using Solid Phase Extraction Approach. Bull. Environ. Contam. Toxicol. 2022, 110, 32. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, H.; Muller, M.; Sinclair, A.J.; Wang, W.; Guo, X.; Vetter, W.; Wang, Y. Targeted quantitation of furan fatty acids in edible oils by gas chromatography/triple quadrupole tandem mass spectrometry (GC-TQ/MS). Food Chem. 2023, 404 Pt B, 134521. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Norskov, N.P. Comprehensive quantification of flavonoids and salicylic acid representative of Salix spp. using microLiquid Chromatography-Triple Quadrupole Mass Spectrometry: The importance of drying procedures and extraction solvent when performing classical solid-liquid extraction. J. Chromatogr. A 2023, 1705, 464139. [Google Scholar]
- Jiang, T.; Xu, Q.; Zhang, H.; Li, D.; Xu, W. Improving the Performances of a “Brick Mass Spectrometer” by Quadrupole Enhanced Dipolar Resonance Ejection from the Linear Ion Trap. Anal. Chem. 2018, 90, 11671–11679. [Google Scholar] [CrossRef]
- Meng, X.; Tang, C.; Zhang, C.; Li, D.; Xu, W.; Zhai, Y. A “Brick” Mass Spectrometer with Photoionization for Direct Analysis of Trace Volatile Compounds. J. Am. Soc. Mass Spectrom. 2020, 31, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cooks, R.G.; Ouyang, Z. Geometry optimization for the cylindrical ion trap: Field calculations, simulations and experiments. Int. J. Mass Spectrom. 2005, 241, 119–132. [Google Scholar] [CrossRef]
- Konenkov, N.; Londry, F.; Ding, C.; Douglas, D.J. Linear quadrupoles with added hexapole fields. J. Am. Soc. Mass Spectrom. 2006, 17, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Sudakov, M.; Douglas, D.J. Linear quadrupoles with added octopole fields. Rapid Commun. Mass Spectrom. 2003, 17, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, W.; Jin, L.; Qian, B.; Ding, C.F. Measurement of the effective electric field radius on digital ion trap spectrometer. Analyst 2021, 146, 3810–3817. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Jiang, Y.; Xiong, X.; Deng, Y.; Fang, X.; Xu, W. The coupling effects of hexapole and octopole fields in quadrupole ion traps: A theoretical study. J. Mass Spectrom. 2013, 48, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, T.; Taylor, C.; Knowlton, M.; Williams, S.; Evans-Nguyen, T. Enabling Isotope Ratio Measurements on an Ion Trap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2020, 31, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; He, M.; Wang, Y.; Xiong, X.; Fang, X.; Xu, W. GPU Assisted Simulation Study of Ion-Ion Reactions within Quadrupole Ion Traps. J. Am. Soc. Mass Spectrom. 2015, 26, 1233–1241. [Google Scholar] [CrossRef]



| Concentration (ng/mL) | 100 | 200 | 500 | 1000 | 2000 | 3000 | 5000 |
|---|---|---|---|---|---|---|---|
| RSD (%) | 3.46 | 1.87 | 2.37 | 1.11 | 0.29 | 0.33 | 0.17 |
| Concentration (ng/mL) | 10 | 20 | 50 | 100 | 300 | 500 | 700 | 1000 |
|---|---|---|---|---|---|---|---|---|
| RSD (%) | 5.48 | 8.31 | 7.18 | 5.35 | 3.60 | 2.97 | 1.41 | 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Liu, S.; Jiang, T.; Xu, W. Multiplexing Quadrupole and Ion Trap Operation Modes on a “Brick” Miniature Mass Spectrometer. Molecules 2023, 28, 7640. https://doi.org/10.3390/molecules28227640
Feng C, Liu S, Jiang T, Xu W. Multiplexing Quadrupole and Ion Trap Operation Modes on a “Brick” Miniature Mass Spectrometer. Molecules. 2023; 28(22):7640. https://doi.org/10.3390/molecules28227640
Chicago/Turabian StyleFeng, Chaohong, Siyu Liu, Ting Jiang, and Wei Xu. 2023. "Multiplexing Quadrupole and Ion Trap Operation Modes on a “Brick” Miniature Mass Spectrometer" Molecules 28, no. 22: 7640. https://doi.org/10.3390/molecules28227640