Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations
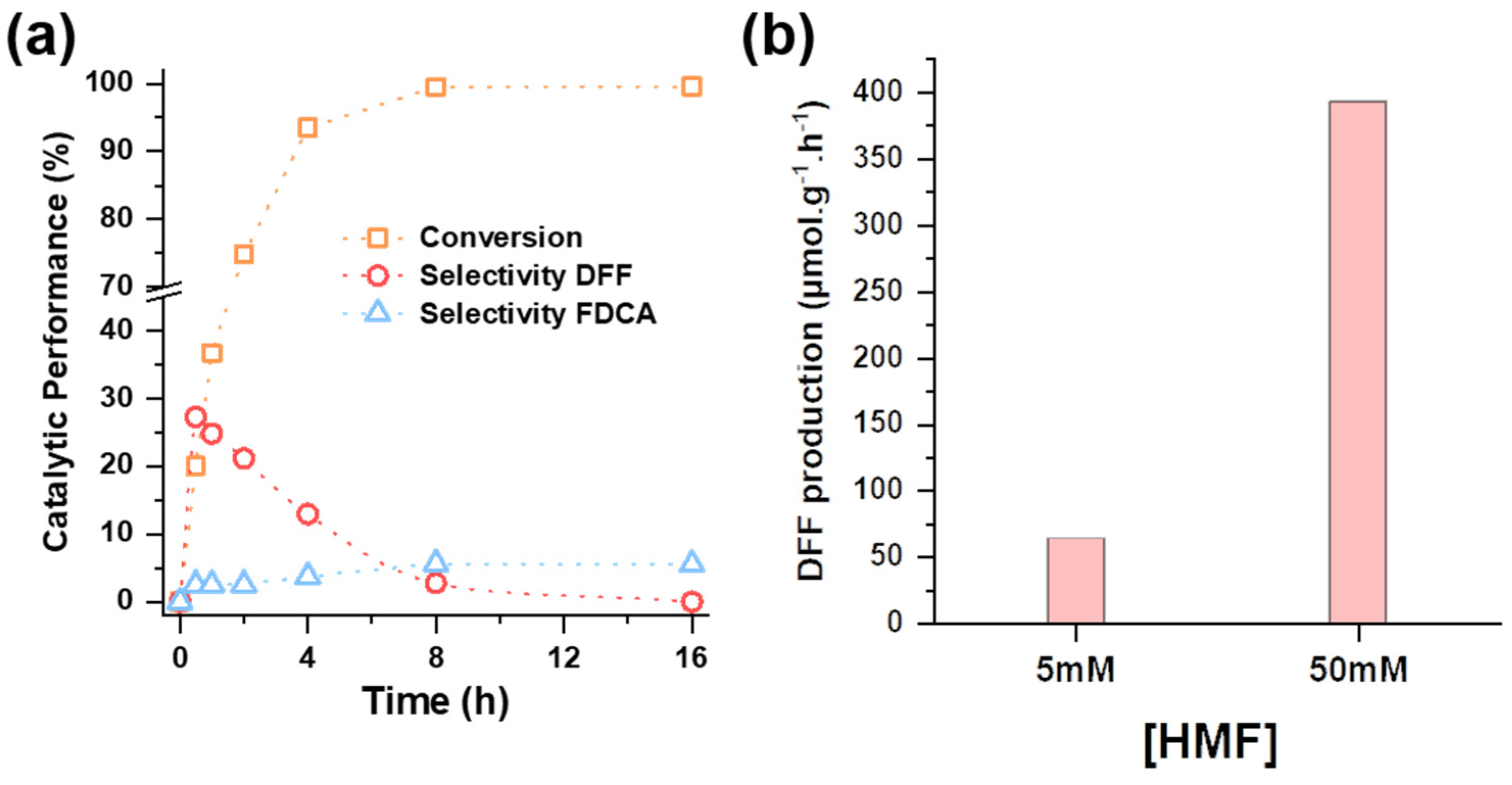
2.2. Photocatalytic Oxidation of HMF
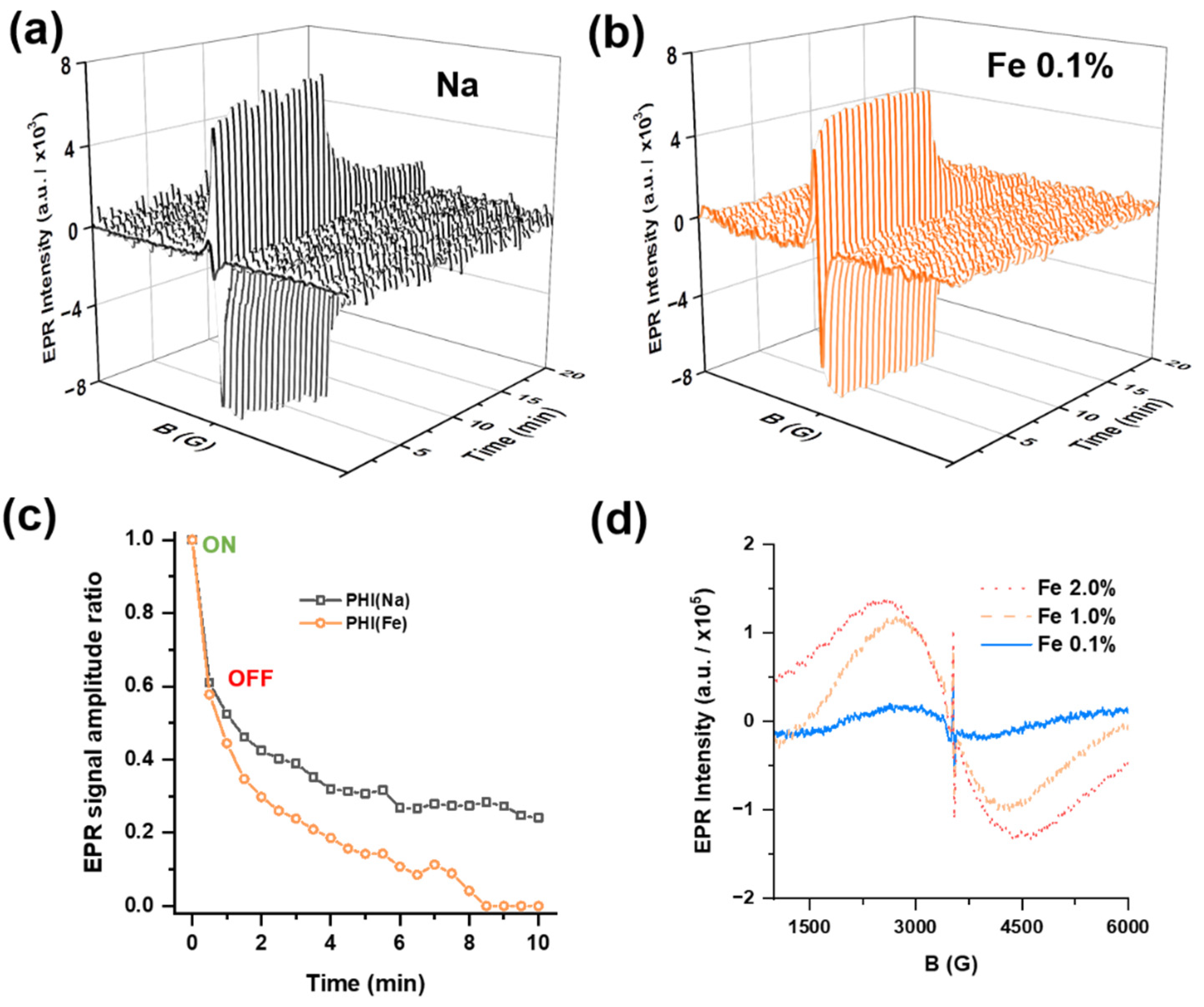
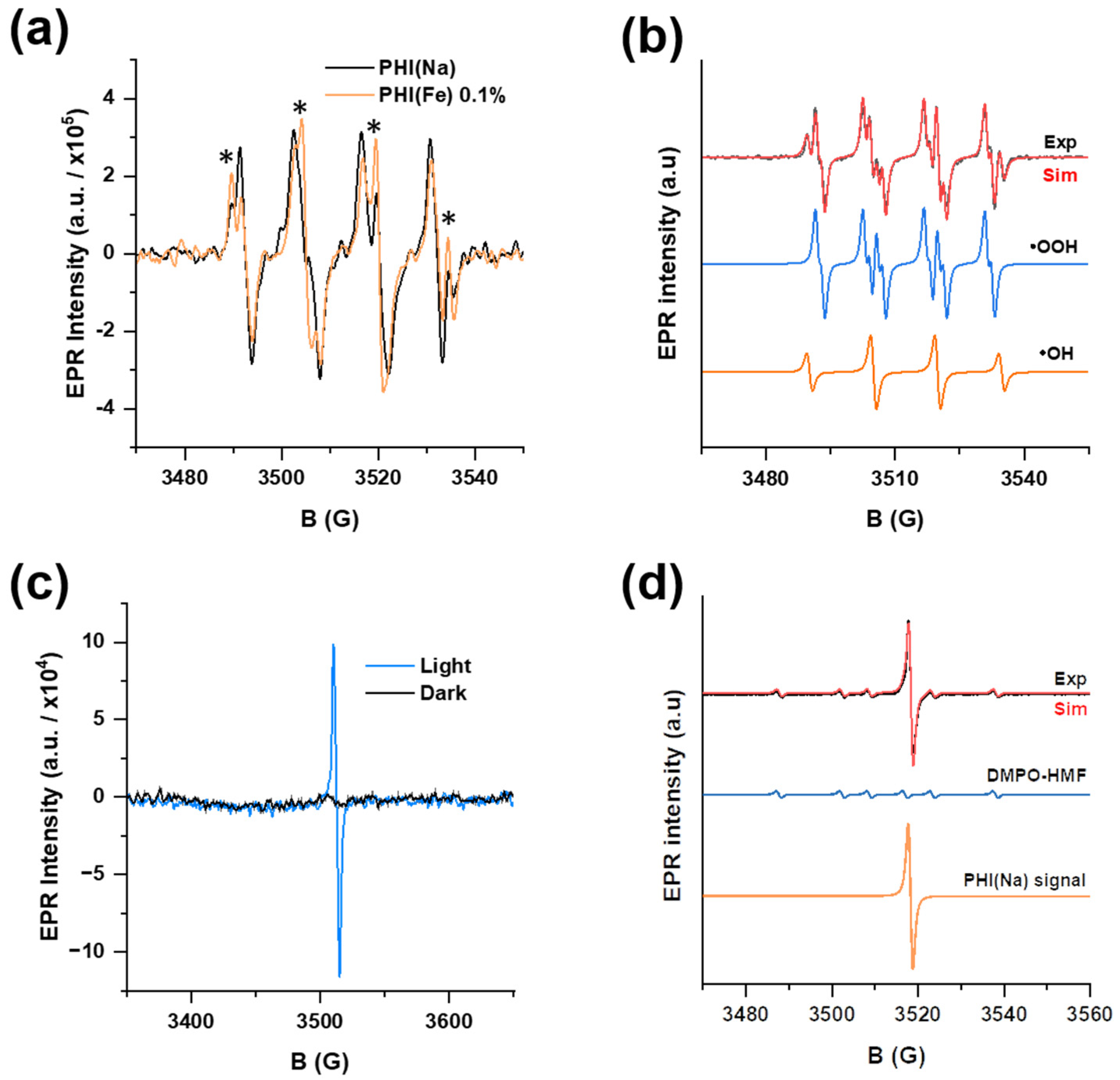
2.3. Insights into Surface Structure and Mechanism
3. Materials and Methods
3.1. Poly(heptazine imide)-Based Material Syntheses
3.1.1. PHI(Na) Synthesis
3.1.2. PHI(TM) Photocatalysts
3.2. Ex and In Situ Characterizations
3.3. Photocatalytic Tests and Reaction Medium Analysis
3.4. Hydrogen Peroxide Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filho, J.B.; Rios, R.D.F.; Bruziquesi, C.G.O.; Ferreira, D.C.; Victória, H.F.V.; Krambrock, K.; Pereira, M.C.; Oliveira, L.C.A. A promising approach to transform levulinic acid into γ-valerolactone using niobic acid photocatalyst and the accumulated electron transfer technique. Appl. Catal. B 2021, 285, 119814. [Google Scholar] [CrossRef]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Photocatalytic conversion of biomass into valuable products: A meaningful approach? Green. Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Oliveira, L.; Pereira, M.; Pacheli Heitman, A.; Filho, J.; Oliveira, C.; Ziolek, M. Niobium: The Focus on Catalytic Application in the Conversion of Biomass and Biomass Derivatives. Molecules 2023, 28, 1527. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Chhabra, T.; Krishnan, V.; Nagaraja, C.M. Visible-Light-Driven Selective Oxidation of Biomass-Derived HMF to DFF Coupled with H Generation by Noble Metal-Free ZnCdS/MnO Heterostructures. ACS Appl. Energy Mater. 2020, 3, 7138–7148. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I; US Department of Energy: Washington, DC, USA, 2004; p. 76. [Google Scholar]
- Hsiao, Y.W.; Zong, X.; Zhou, J.H.; Zheng, W.Q.; Vlachos, D.G. Selective hydrodeoxygenation of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) over carbon supported copper catalysts using isopropyl alcohol as a hydrogen donor. Appl. Catal. B 2022, 317, 121790. [Google Scholar] [CrossRef]
- Gabriel, J.B.; Oliveira, V.; Souza, T.E.; Padula, I.; Oliveira, L.C.A.; Gurgel, L.V.A.; Baeta, B.E.L.; Silva, A.C. New Approach to Dehydration of Xylose to 2-Furfuraldehyde Using a Mesoporous Niobium-Based Catalyst. ACS Omega 2020, 5, 21392–21400. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, X.; Zeng, X.H.; Sun, Y.; Ke, X.X.; Li, T.Y.; Zhang, J.R.; Lin, L. Catalyst design strategy toward the efficient heterogeneously-catalyzed selective oxidation of 5-hydroxymethylfurfural. Green Energy Environ. 2022, 7, 900–932. [Google Scholar] [CrossRef]
- Zhang, W.J.; Qian, H.L.; Hou, Q.D.; Ju, M.T. The functional and synergetic optimization of the thermal-catalytic system for the selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran: A review. Green Chem. 2023, 25, 893–914. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Gomes, G.H.M.; Silva, I.F.; Rios, R.D.F.; Victoria, H.F.V.; Krambrock, K.; Pereira, M.C.; Oliveira, L.C.A. Photocatalytic reduction of levulinic acid using thermally modified niobic acid. Chem. Eng. J. 2022, 450, 137935. [Google Scholar] [CrossRef]
- Dai, Y.; Xiong, Y. Control of selectivity in organic synthesis via heterogeneous photocatalysis under visible light. Nano Res. Energy 2022, 1, e9120006. [Google Scholar] [CrossRef]
- Schlomberg, H.; Kroger, J.; Savasci, G.; Terban, M.W.; Bette, S.; Moudrakovski, I.; Duppel, V.; Podjaski, F.; Siegel, R.; Senker, J.; et al. Structural Insights into Poly(Heptazine Imides): A Light-Storing Carbon Nitride Material for Dark Photocatalysis. Chem. Mater. 2019, 31, 7478–7486. [Google Scholar] [CrossRef] [PubMed]
- Markushyna, Y.; Lamagni, P.; Teutloff, C.; Catalano, J.; Lock, N.; Zhang, G.G.; Antonietti, M.; Savateev, A. Green radicals of potassium poly(heptazine imide) using light and benzylamine. J. Mater. Chem. A 2019, 7, 24771–24775. [Google Scholar] [CrossRef]
- Xing, L.; Yang, Q.; Zhu, C.; Bai, Y.; Tang, Y.; Rueping, M.; Cai, Y. Poly(heptazine imide) ligand exchange enables remarkable low catalyst loadings in heterogeneous metallaphotocatalysis. Nat. Commun. 2023, 14, 1501. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.R.; Gil, J.C.; Tarakina, N.V.; Silva, G.; Filho, J.B.G.; Krambrock, K.; Antonietti, M.; Ribeiro, C.; Teixeira, I.F. Selective methane photooxidation into methanol under mild conditions promoted by highly dispersed Cu atoms on crystalline carbon nitrides. Chem. Commun. 2022, 58, 7419–7422. [Google Scholar] [CrossRef]
- da Silva, M.A.R.; Silva, I.F.; Xue, Q.; Lo, B.T.W.; Tarakina, N.V.; Nunes, B.N.; Adler, P.; Sahoo, S.K.; Bahnemann, D.W.; López-Salas, N.; et al. Sustainable oxidation catalysis supported by light: Fe-poly (heptazine imide) as a heterogeneous single-atom photocatalyst. App. Catal. B 2022, 304, 120965. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Cai, Q.G.; Li, Y.; Wang, S.F.; Sun, Y.X.; Zhou, Q.Y.; Zhao, D.Y. Single-Atom Mo Anchored on a Poly(heptazine imide) Nanosheet as a Novel Electrocatalyst Showing Excellent Behavior toward Nitrogen Reduction Reaction. J. Phys. Chem. C 2022, 126, 7859–7869. [Google Scholar] [CrossRef]
- Rocha, G.F.S.R.; da Silva, M.A.R.; Rogolino, A.; Diab, G.A.A.; Noleto, L.F.G.; Antonietti, M.; Teixeira, I.F. Carbon nitride based materials: More than just a support for single-atom catalysis. Chem. Soc. Rev. 2023, 52, 4878–4932. [Google Scholar] [CrossRef]
- Gentile, G.; Marchi, M.; Melchionna, M.; Fornasiero, P.; Prato, M.; Filippini, G. Use of Carbon Nitrides as Photoactive Supports in Single-Atom Heterogeneous Catalysis for Synthetic Purposes. Eur. J Org. Chem. 2022, 2022, e202200944. [Google Scholar] [CrossRef]
- Jia, T.; Meng, D.; Duan, R.; Ji, H.; Sheng, H.; Chen, C.; Li, J.; Song, W.; Zhao, J. Single-Atom Nickel on Carbon Nitride Photocatalyst Achieves Semihydrogenation of Alkynes with Water Protons via Monovalent Nickel. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216511. [Google Scholar] [CrossRef]
- Stephanopoulos, F.; Commu, N. Single atom catalysts push the boundaries of heterogeneous catalysis. Nat. Commun. 2021, 12, 5884. [Google Scholar]
- Colombari, F.M.; da Silva, M.A.R.; Homsi, M.S.; de Souza, B.R.L.; Araujo, M.; Francisco, J.L.; da Silva, G.; Silva, I.F.; de Moura, A.F.; Teixeira, I.F. Graphitic carbon nitrides as platforms for single-atom photocatalysis. Faraday Discuss 2021, 227, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, H.; Wu, Y. Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite. Molecules 2022, 27, 8537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, D.; Zhang, Y.; Wu, Y. Photocatalytic selective oxidation of HMF to DFF over Bi2WO6/mpg–C3N4 composite under visible light. Appl. Organomet. Chem. 2021, 35, e6404. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Dong, Y.; Zhu, Y.; Li, J. Highly Selective Photocatalytic Oxidation Biomass Valorization Over Nb2O5/g-C3N4 Heterojunction. Adv. Energy Sustain. Res. 2022, 3, 2200116. [Google Scholar] [CrossRef]
- Qian, H.L.; Hou, Q.D.; Zhang, W.J.; Nie, Y.F.; Lai, R.T.; Ren, H.R.; Yu, G.J.; Bai, X.Y.; Wang, H.Z.; Ju, M.T. Construction of electron transport channels and oxygen adsorption sites to modulate reactive oxygen species for photocatalytic selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Appl. Catal. B 2022, 319, 121907. [Google Scholar] [CrossRef]
- Wang, X.-X.; Meng, S.; Zhang, S.; Zheng, X.; Chen, S. 2D/2D MXene/g-C3N4 for photocatalytic selective oxidation of 5-hydroxymethylfurfural into 2,5-formylfuran. Catal. Commun. 2020, 147, 106152. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green. Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Savateev, A.; Tarakina, N.V.; Strauss, V.; Hussain, T.; Ten Brummelhuis, K.; Sanchez Vadillo, J.M.; Markushyna, Y.; Mazzanti, S.; Tyutyunnik, A.P.; Walczak, R.; et al. Potassium Poly(Heptazine Imide): Transition Metal-Free Solid-State Triplet Sensitizer in Cascade Energy Transfer and [3+2]-cycloadditions. Angew. Chem. Int. Ed. Engl. 2020, 59, 15061–15068. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric Determination of Hydrogen-Peroxide Using Potassium Titanium(Iv) Oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Li, C.; Hofmeister, E.; Krivtsov, I.; Mitoraj, D.; Adler, C.; Beranek, R.; Dietzek, B. Photodriven Charge Accumulation and Carrier Dynamics in a Water-Soluble Carbon Nitride Photocatalyst. ChemSusChem 2021, 14, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.L.; Gelman, D.; Vaccaro, L. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Wang, W.H.; Niu, M.G.; Hou, Y.C.; Wu, W.Z.; Liu, Z.Y.; Liu, Q.Y.; Ren, S.H.; Marsh, K.N. Catalytic conversion of biomass-derived carbohydrates to formic acid using molecular oxygen. Green Chem. 2014, 16, 2614–2618. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO(2) Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Mazúr, M.; García-López, E.I.; Marcì, G.; Lušpai, K.; Brezová, V. EPR investigations of polymeric and H2O2-modified C3N4-based photocatalysts. J. Photoch. Photobio. A 2019, 375, 100–113. [Google Scholar] [CrossRef]
- Fujihara, K.; Izumi, S.; Ohno, T.; Matsumura, M. Time-resolved photoluminescence of particulate TiO2 photocatalysts suspended in aqueous solutions. J. Photoch. Photobio. A 2000, 132, 99–104. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Bruziquesi, C.G.O.; Rios, R.D.F.; Castro, A.A.; Victória, H.F.V.; Krambrock, K.; Mansur, A.A.P.; Mansur, H.S.; Siniterra, R.D.; Ramalho, T.C.; et al. Selective visible-light-driven toxicity breakdown of nerve agent simulant methyl paraoxon over a photoactive nanofabric. Appl. Catal. B 2021, 285, 119774. [Google Scholar] [CrossRef]
- Billany, M.R.; Khatib, K.; Gordon, M.; Sugden, J.K. Alcohols and ethanolamines as hydroxyl radical scavengers. Int. J. Pharm. 1996, 137, 143–147. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Bao, X.L.; Liu, M.; Wang, Z.Y.; Dai, D.J.; Wang, P.; Cheng, H.F.; Liu, Y.Y.; Zheng, Z.K.; Dai, Y.; Huang, B.B. Photocatalytic Selective Oxidation of HMF Coupled with H Evolution on Flexible Ultrathin g-CN Nanosheets with Enhanced N–H Interaction. ACS Catal. 2022, 12, 1919–1929. [Google Scholar] [CrossRef]
- Shan, T.; Luo, L.T.; Chen, T.R.; Deng, L.X.; Li, M.Q.; Yang, X.H.; Shen, L.J.; Yang, M.Q. Visible-light-driven anaerobic oxidative upgrading of biomass-derived HMF for co-production of DFF and H over a 1D CdZnS/NiSe Schottky junction. Green Chem. 2023, 25, 2745–2756. [Google Scholar] [CrossRef]
- Samuni, A.; Carmichael, A.J.; Russo, A.; Mitchell, J.B.; Riesz, P. On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc. Natl. Acad. Sci. USA 1986, 83, 7593–7597. [Google Scholar] [CrossRef]
- Reinke, L.A.; Kotake, Y.; Mccay, P.B.; Janzen, E.G. Spin-trapping studies of hepatic free radicals formed following the acute administration of ethanol to rats: In vivo detection of 1-hydroxyethyl radicals with PBN. Free Radic. Biol. Med. 1991, 11, 31–39. [Google Scholar] [CrossRef]
- Chen, J.; Draksharapu, A.; Angelone, D.; Unjaroen, D.; Padamati, S.K.; Hage, R.; Swart, M.; Duboc, C.; Browne, W.R. H2O2 Oxidation by Fe(III)-OOH Intermediates and Its Effect on Catalytic Efficiency. ACS Catal. 2018, 8, 9665–9674. [Google Scholar] [CrossRef]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reason. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Ye, L.; Yu, Z.; Liu, R.; Qiao, Y.; Sun, L.; Cui, J.; Lu, X. In situ fabrication Ti3C2Fx MXene/CdIn2S4 Schottky junction for photocatalytic oxidation of HMF to DFF under visible light. Appl. Catal. B Environ. 2023, 330, 122635. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, Y.; Zhao, H.; Zhan, P.; Ren, C.; Su, C.; Ren, W.; Zhang, J.; Cai, D.; Qin, P. 2,5-Diformylfuran production by photocatalytic selective oxidation of 5-hydroxymethylfurfural in water using MoS2/CdIn2S4 flower-like heterojunctions. Chinese J. Chem. Eng. 2023, 54, 180–191. [Google Scholar] [CrossRef]
- Ayed, C.; Huang, W.; Kizilsavas, G.; Landfester, K.; Zhang, K.A.I. Photocatalytic Partial Oxidation of 5-Hydroxymethylfurfural (HMF) to 2,5-Diformylfuran (DFF) Over a Covalent Triazine Framework in Water. ChemPhotoChem. 2020, 4, 571–576. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on metal-free g-C3N4 under visible light irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Xue, J.; Huang, C.; Zong, Y.; Gu, J.; Wang, M.; Ma, S. Fe (III)-grafted Bi2MoO6 nanoplates for enhanced photocatalytic activities on tetracycline degradation and HMF oxidation. Appl. Organomet. Chem. 2019, 33, 1–10. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, J.B.G.; Silva, I.F.; Alafandi, M.; Rabeah, J. Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance. Molecules 2023, 28, 8077. https://doi.org/10.3390/molecules28248077
Filho JBG, Silva IF, Alafandi M, Rabeah J. Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance. Molecules. 2023; 28(24):8077. https://doi.org/10.3390/molecules28248077
Chicago/Turabian StyleFilho, José B. G., Ingrid F. Silva, Mamdouh Alafandi, and Jabor Rabeah. 2023. "Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance" Molecules 28, no. 24: 8077. https://doi.org/10.3390/molecules28248077
APA StyleFilho, J. B. G., Silva, I. F., Alafandi, M., & Rabeah, J. (2023). Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance. Molecules, 28(24), 8077. https://doi.org/10.3390/molecules28248077





