Sustainable Recovery of Platinum Group Metals from Spent Automotive Three-Way Catalysts through a Biogenic Thiosulfate-Copper-Ammonia System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plackett–Burman Experimental Design
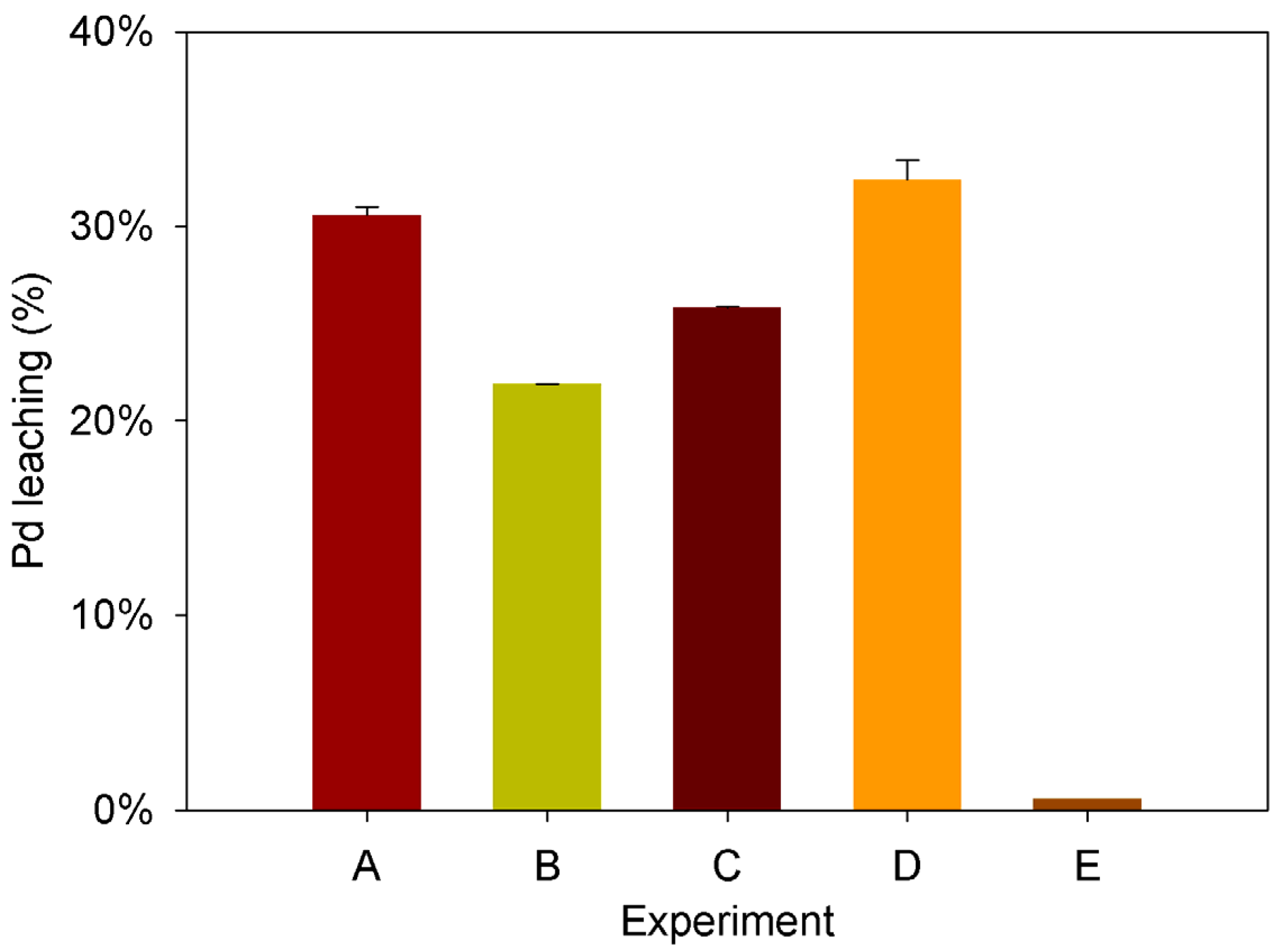
2.2. Effect of the Main Operational Variables
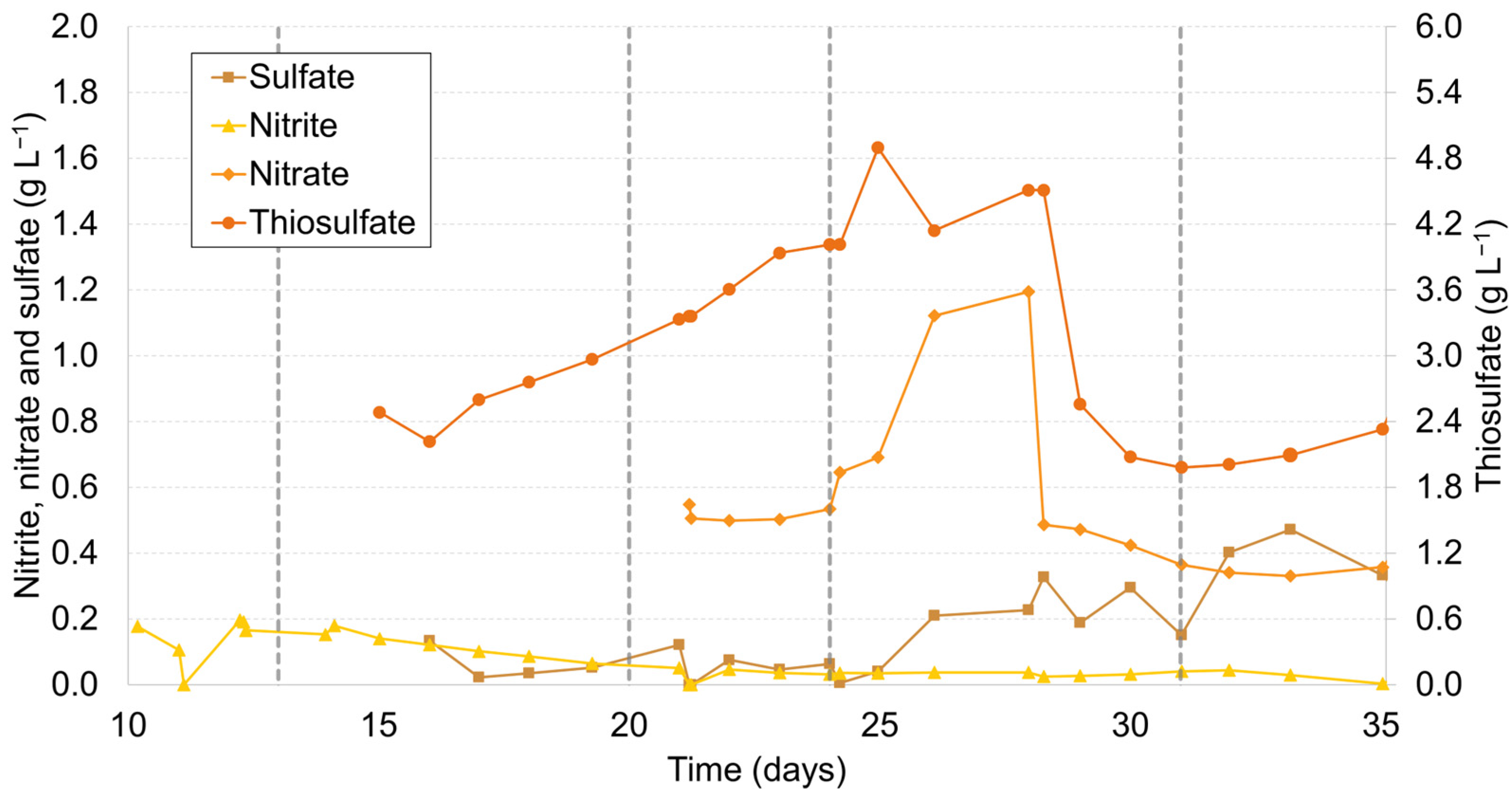
2.3. Biogenic Thiosulfate Production
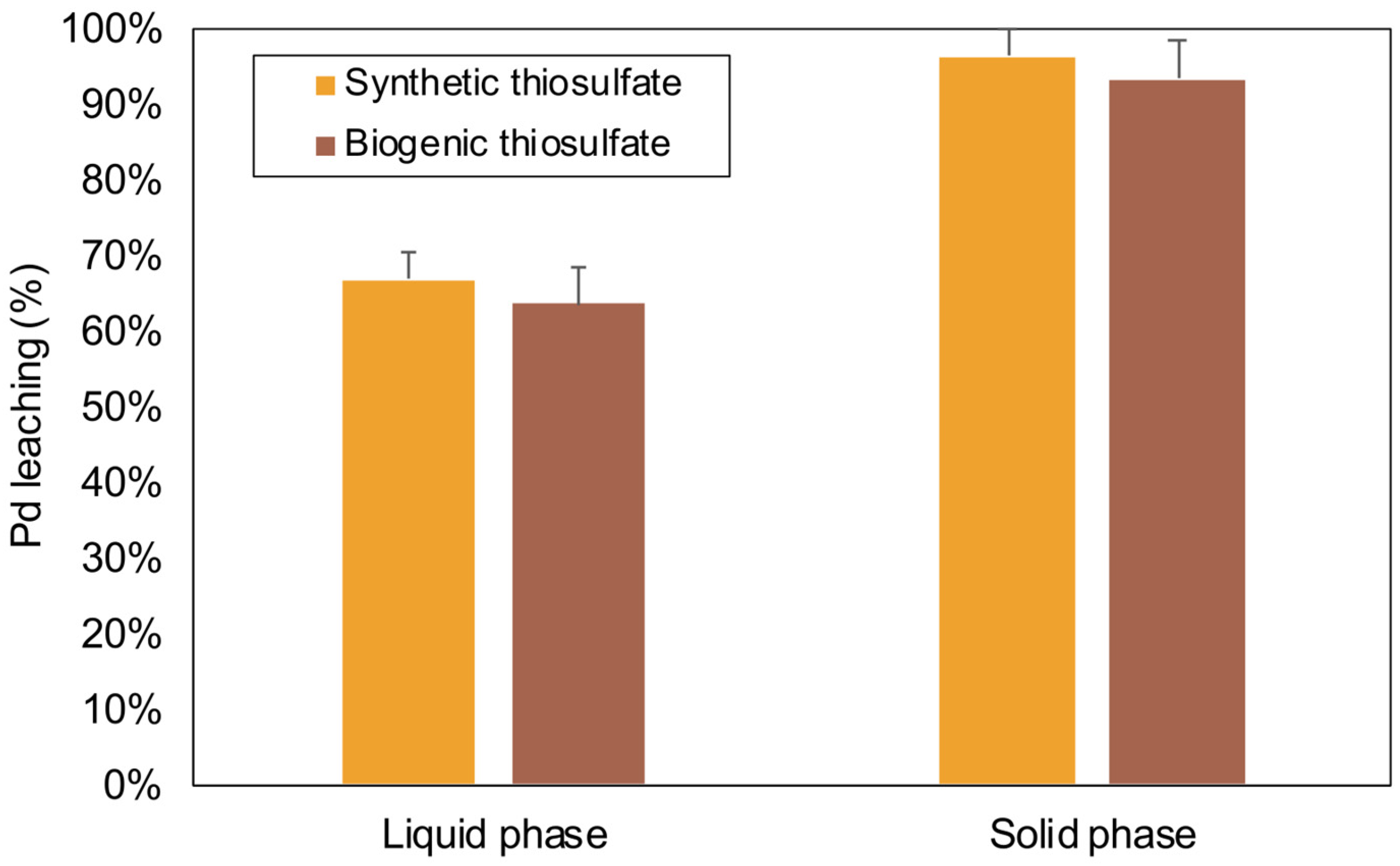
2.4. Comparative Analysis of PGM Recovery Using Lower Pulp Density with Biogenic and Synthetic Thiosulfate
3. Materials and Methods
3.1. Synthetic Catalyst (Pd/Al2O3)
3.2. Screening through a Plackett–Burman Design
3.3. Effect of the Main Operational Variables
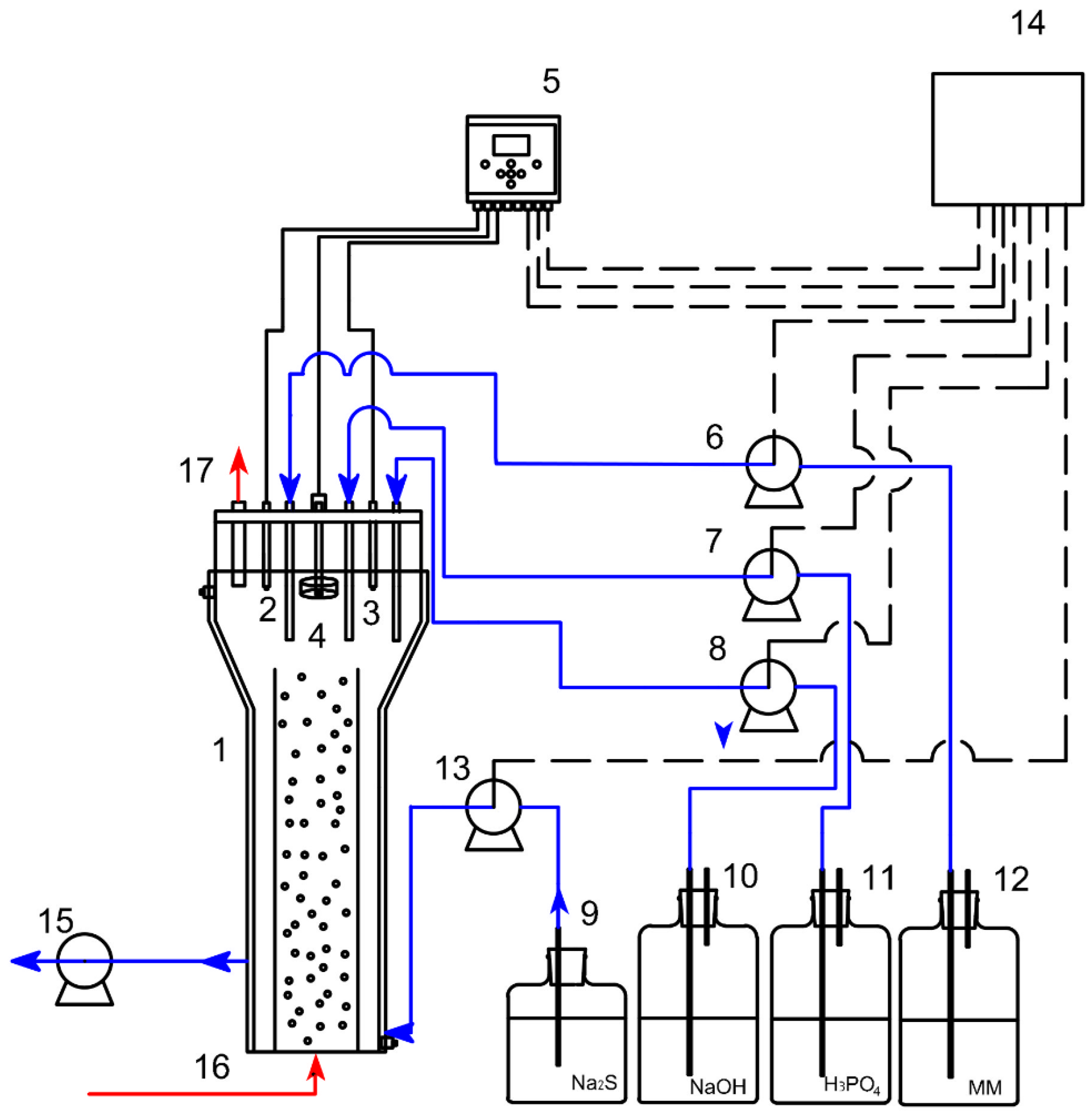
3.4. Biogenic Thiosulfate Production
3.5. Effect of Lower Pulp Density and Biogenic Thiosulfate
3.6. Analytical Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, J.; Ghahreman, A. Platinum Group Metals Recycling from Spent Automotive Catalysts: Metallurgical Extraction and Recovery Technologies. Sep. Purif. Technol. 2023, 311, 123357. [Google Scholar] [CrossRef]
- Paiva, A.P. Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points. Metals 2017, 7, 505. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of Platinum Group Metals from Spent Catalysts: A Review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Bloxham, L.; Brown, S.; Cole, L.; Cowley, A.; Fujita, M.; Girardot, N.; Jiang, J.; Raithatha, R.; Ryan, M. PGM Market Report. 2022. Available online: https://matthey.com/pgm-market-report-2022 (accessed on 11 December 2023).
- European Commission. Tackling the Challenges in Commodity Markets and on Raw Materials; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Fajar, A.T.N.; Hanada, T.; Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Separation of Platinum Group Metals via Sequential Transport through Polymer Inclusion Membranes Containing an Ionic Liquid Carrier. ACS Sustain. Chem. Eng. 2020, 8, 11283–11291. [Google Scholar] [CrossRef]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Bloxham, L.; Brown, S.; Cole, L.; Cowley, A.; Fujita, M.; Girardot, N.; Jiang, J.; Raithatha, R.; Ryan, M. PGM Market Report: February 2021. 2021. Available online: https://matthey.com/news/2021/pgm-market-report-may-2021-out-now (accessed on 11 December 2023).
- Thormann, L.; Buchspies, B.; Mbohwa, C.; Kaltschmitt, M. PGE Production in Southern Africa, Part I: Production and Market Trends. Minerals 2017, 7, 224. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Saternus, M. Removal of Platinum Group Metals from the Used Auto Catalytic Converter. Metalurgija 2009, 48, 133–136. [Google Scholar]
- Xu, B.; Chen, Y.; Zhou, Y.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y.; Jiang, T. A Review of Recovery of Palladium from the Spent Automobile Catalysts. Metals 2022, 12, 533. [Google Scholar] [CrossRef]
- Compagnone, M.; González-Cortés, J.J.; del Pilar Yeste, M.; Cantero, D.; Ramírez, M. Bioleaching of the α-Alumina Layer of Spent Three-Way Catalysts as a Pretreatment for the Recovery of Platinum Group Metals. J. Environ. Manag. 2023, 345, 118825. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Efficient Extraction of Critical Elements from End-of-Life Automotive Catalytic Converters via Alkaline Pretreatment Followed by Leaching with a Complexing Agent. J. Clean. Prod. 2022, 344, 131064. [Google Scholar] [CrossRef]
- Paiva, A.P.; Piedras, F.V.; Rodrigues, P.G.; Nogueira, C.A. Hydrometallurgical Recovery of Platinum-Group Metals from Spent Auto-Catalysts—Focus on Leaching and Solvent Extraction. Sep. Purif. Technol. 2022, 286, 120474. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. An Innovative Hybrid Hydrometallurgical Approach for Precious Metals Recovery from Secondary Resources. J. Environ. Manag. 2022, 307, 114567. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of Gold and Silver from a Complex Sulfide Concentrate in Copper-Tartrate-Thiosulfate Solutions. Metals 2022, 12, 1152. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Yin, W.; Jiang, T.; Li, G. Thiosulfate Leaching of Au, Ag and Pd from a High Sn, Pb and Sb Bearing Decopperized Anode Slime. Hydrometallurgy 2016, 164, 278–287. [Google Scholar] [CrossRef]
- Karim, S.; Ting, Y.P. Recycling Pathways for Platinum Group Metals from Spent Automotive Catalyst: A Review on Conventional Approaches and Bio-Processes. Resour. Conserv. Recycl. 2021, 170, 105588. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and Recovery of Gold Using Ammoniacal Thiosulfate Leach Liquors (a Review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Aylmore, M.G. Thiosulfate as an Alternative Lixiviant to Cyanide for Gold Ores. In Gold Ore Processing; Adams, Mike, D., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2016; pp. 485–523. ISBN 9780444636584. [Google Scholar] [CrossRef]
- Huy Do, M.; Tien Nguyen, G.; Dong Thach, U.; Lee, Y.; Huu Bui, T. Advances in Hydrometallurgical Approaches for Gold Recovery from E-Waste: A Comprehensive Review and Perspectives. Miner. Eng. 2023, 191, 107977. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of Platinum Group Metals from Spent Automotive Catalysts: A Review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- González-Cortés, J.J.; Quijano, G.; Ramírez, M.; Cantero, D. Methane Concentration and Bacterial Communities’ Dynamics during the Anoxic Desulfurization of Landfill Biogas under Diverse Nitrate Sources and Hydraulic Residence Times. J. Environ. Chem. Eng. 2023, 11, 109285. [Google Scholar] [CrossRef]
- Almenglo, F.; González-Cortés, J.J.; Ramírez, M.; Cantero, D. Recent Advances in Biological Technologies for Anoxic Biogas Desulfurization. Chemosphere 2023, 321, 138084. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, P.L.F.; Sorokin, D.Y.; Buisman, C.J.N.; Janssen, A.J.H. The Effect of PH on Thiosulfate Formation in a Biotechnological Process for the Removal of Hydrogen Sulfide from Gas Streams. Environ. Sci. Technol. 2008, 42, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Kuenen, J.G.; Muyzer, G. The Microbial Sulfur Cycle at Extremely Haloalkaline Conditions of Soda Lakes. Front. Microbiol. 2011, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Pourhossein, F.; Mousavi, S.M. Improvement of Gold Bioleaching Extraction from Waste Telecommunication Printed Circuit Boards Using Biogenic Thiosulfate by Acidithiobacillus thiooxidans. J. Hazard. Mater. 2023, 450, 131073. [Google Scholar] [CrossRef] [PubMed]
- Pourhossein, F.; Mousavi, S.M. A Novel Rapid and Selective Microbially Thiosulfate Bioleaching of Precious Metals from Discarded Telecommunication Printed Circuited Boards (TPCBs). Resour. Conserv. Recycl. 2022, 187, 106599. [Google Scholar] [CrossRef]
- McNeice, J.; Mahandra, H.; Ghahreman, A. Biogenic Production of Thiosulfate from Organic and Inorganic Sulfur Substrates for Application to Gold Leaching. Sustainability 2022, 14, 16666. [Google Scholar] [CrossRef]
- González-Cortés, J.J.; Torres-Herrera, S.; Almenglo, F.; Ramírez, M.; Cantero, D. Anoxic Biogas Biodesulfurization Promoting Elemental Sulfur Production in a Continuous Stirred Tank Bioreactor. J. Hazard. Mater. 2021, 401, 123785. [Google Scholar] [CrossRef]
- Lawson, J. Regression Analysis of Experiments with Complex Confounding Patterns Guided by the Alias Matrix. Comput. Stat. Data Anal. 2002, 39, 227–241. [Google Scholar] [CrossRef]
- Senanayake, G.; Senaputra, A.; Nicol, M.J. Effect of Thiosulfate, Sulfide, Copper(II), Cobalt(II)/(III) and Iron Oxides on the Ammoniacal Carbonate Leaching of Nickel and Ferronickel in the Caron Process. Hydrometallurgy 2010, 105, 60–68. [Google Scholar] [CrossRef]
- Senanayake, G. Gold Leaching by Thiosulphate Solutions: A Critical Review on Copper(II)-Thiosulphate-Oxygen Interactions. Miner. Eng. 2005, 18, 995–1009. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W.; Xu, B.; Li, Q.; Jiang, T. Study on Oxygen Pressure Thiosulfate Leaching of Gold without the Catalysis of Copper and Ammonia. Hydrometallurgy 2019, 187, 71–80. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G.; Nicol, M.J. A Study of the Gold Colloid Dissolution Kinetics in Oxygenated Ammoniacal Thiosulfate Solutions. Hydrometallurgy 2004, 74, 243–257. [Google Scholar] [CrossRef]
- Xia, C.; Yen, W.T.; Deschenes, G. Improvement of Thiosulfate Stability in Gold Leaching. Miner. Metall. Process. 2003, 20, 68–72. [Google Scholar] [CrossRef]
- Junior, F.; Pedrosa, B.; Mano, E.S.; Neto, D. Using Thiosulphate as Gold Leaching Agent in a Brazilian Carbonaceous Ore: Batch Tests Analysis. In Proceedings of the 12th International Conference on Process Hydrometallurgy, Santiago, Chile, 28–30 October 2020. [Google Scholar]
- Navarro, P.; Vargas, C.; Villarroel, A.; Alguacil, F.J. On the Use of Ammoniacal/Ammonium Thiosulphate for Gold Extraction from a Concentrate. Hydrometallurgy 2002, 65, 37–42. [Google Scholar] [CrossRef]
- Azaroual, M.; Romand, B.; Freyssinet, P.; Disnar, J.; Azaroual, M.; Romand, B.; Freyssinet, P.; Solubility, J.D. Solubility of Platinum in Aqueous Solutions at 25 °C and PHs 4 to 10 under Oxidizing Conditions. Geochim. Cosmochim. Acta 2001, 65, 4453–4466. [Google Scholar] [CrossRef]
- Chen, Y.; Shang, H.; Ren, Y.; Yue, Y.; Li, H.; Bian, Z. Systematic Assessment of Precious Metal Recovery to Improve Environmental and Resource Protection. ACS EST Eng. 2022, 2, 1039–1052. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y. A Systematic and Comparative Study of Copper, Nickel and Cobalt-Ammonia Catalyzed Thiosulfate Processes for Eco-Friendly and Efficient Gold Extraction from an Oxide Gold Concentrate. Sep. Purif. Technol. 2021, 272, 118929. [Google Scholar] [CrossRef]
- Gui, Q.; Khan, M.I.; Wang, S.; Zhang, L. The Ultrasound Leaching Kinetics of Gold in the Thiosulfate Leaching Process Catalysed by Cobalt Ammonia. Hydrometallurgy 2020, 196, 105426. [Google Scholar] [CrossRef]
- Wan, R.Y.; LeVier, K.M. Solution Chemistry Factors for Gold Thiosulfate Heap Leaching. Int. J. Miner. Process. 2003, 72, 311–322. [Google Scholar] [CrossRef]
- Pietrelli, L.; Fontana, D. Automotive Spent Catalysts Treatment and Platinum Recovery. Int. J. Environ. Waste Manag. 2013, 11, 222–232. [Google Scholar] [CrossRef]
- Chen, J.; Huang, K. A New Technique for Extraction of Platinum Group Metals by Pressure Cyanidation. Hydrometallurgy 2006, 82, 164–171. [Google Scholar] [CrossRef]
- Shams, K.; Beiggy, M.R.; Shirazi, A.G. Platinum Recovery from a Spent Industrial Dehydrogenation Catalyst Using Cyanide Leaching Followed by Ion Exchange. Appl. Catal. A Gen. 2004, 258, 227–234. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. A Review on Management of Waste Three-Way Catalysts and Strategies for Recovery of Platinum Group Metals from Them. J. Environ. Manag. 2022, 305, 114383. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Eksteen, J.J.; Aldrich, C.; Dyer, L. Direct Leach Approaches to Platinum Group Metal (PGM) Ores and Concentrates: A Review. Miner. Eng. 2015, 78, 93–113. [Google Scholar] [CrossRef]
- Li, N.; Liu, T.; Xiao, S.; Yin, W.; Zhang, L.; Chen, J.; Wang, Y.; Zhou, X.; Zhang, Y. Thiosulfate Enhanced Cu(II)-Catalyzed Fenton-like Reaction at Neutral Condition: Critical Role of Sulfidation in Copper Cycle and Cu(III) Production. J. Hazard. Mater. 2023, 445, 130536. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, G.; Zou, M.; Shi, C. Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals 2019, 9, 1178. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. Silver Leaching with the Thiosulfate-Nitrite-Sulfite-Copper Alternative System. Hydrometallurgy 2015, 152, 120–128. [Google Scholar] [CrossRef]
- Torres, R.; Lapidus, G.T. Platinum, Palladium and Gold Leaching from Magnetite Ore, with Concentrated Chloride Solutions and Ozone. Hydrometallurgy 2016, 166, 185–194. [Google Scholar] [CrossRef]
- Bax, A.; Dunn, G.M.; Lewins, J.D. Recovery of Platinum Group Metals. U.S. Patent No. 7544231, 9 June 2009. [Google Scholar]
- Ilyas, S.; Kim, H. Recovery of Platinum-Group Metals from an Unconventional Source of Catalytic Converter Using Pressure Cyanide Leaching and Ionic Liquid Extraction. JOM 2022, 74, 1020–1026. [Google Scholar] [CrossRef]
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A Conceptual Flowsheet for Heap Leaching of Platinum Group Metals (PGMs) from a Low-Grade Ore Concentrate. Hydrometallurgy 2012, 111–112, 129–135. [Google Scholar] [CrossRef]
- Fotoohi, B.; Mercier, L. Recovery of Precious Metals from Ammoniacal Thiosulfate Solutions by Hybrid Mesoporous Silica: 2—A Prospect of PGM Adsorption. Sep. Purif. Technol. 2015, 149, 82–91. [Google Scholar] [CrossRef]
- Fotoohi, B.; Mercier, L. Recovery of Precious Metals from Ammoniacal Thiosulfate Solutions by Hybrid Mesoporous Silica: 3—Effect of Contaminants. Sep. Purif. Technol. 2015, 139, 14–24. [Google Scholar] [CrossRef]
- Bao, H.X.; Li, Z.R.; Song, Z.B.; Wang, A.J.; Zhang, X.N.; Qian, Z.M.; Sun, Y.L.; Cheng, H.Y. Mitigating Nitrite Accumulation during S0-Based Autotrophic Denitrification: Balancing Nitrate-Nitrite Reduction Rate with Thiosulfate as External Electron Donor. Environ. Res. 2022, 204, 112016. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhou, J.; Zhang, Z.; Liu, R.; Wang, Q. Biological Nitrogen Removal through Nitritation Coupled with Thiosulfate-Driven Denitritation. Sci. Rep. 2016, 6, 27502. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Van Den Bosch, P.L.F.; Abbas, B.; Janssen, A.J.H.; Muyzer, G. Microbiological Analysis of the Population of Extremely Haloalkaliphilic Sulfur-Oxidizing Bacteria Dominating in Lab-Scale Sulfide-Removing Bioreactors. Appl. Microbiol. Biotechnol. 2008, 80, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.A.B.; Bijmans, M.F.M.; Stams, A.J.M.; Plugge, C.M. Thiosulfate Conversion to Sulfide by a Haloalkaliphilic Microbial Community in a Bioreactor Fed with H2 Gas. Environ. Sci. Technol. 2017, 51, 914–923. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, M.; Jing, R.; Bai, L.; Zhou, B.; Zhao, M.; Pei, X.; Wei, L.; Chen, G.H. Thiosulfate as the Electron Acceptor in Sulfur Bioconversion-Associated Process (SBAP) for Sewage Treatment. Water Res. 2019, 163, 114850. [Google Scholar] [CrossRef]
- Van Den Bosch, P.L.F. Biological Sulfide Oxidation by Natron-Alkaliphilic Bacteria: Application in Gas Desulfurization. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2008. ISBN 978-3-540-44855-6. [Google Scholar]
- Kleinjan, W.E.; de Keizer, A.; Janssen, A.J.H. Biologically Produced Sulfur. In Elemental Sulfur and Sulfur-Rich Compounds I; Steudel, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 167–188. ISBN 978-3-540-44855-6. [Google Scholar]
- Hedderich, R.; Klimmek, O.; Kröger, A.; Dirmeier, R.; Keller, M.; Stetter, K.O. Anaerobic Respiration with Elemental Sulfur and with Disulfides. FEMS Microbiol. Rev. 1998, 22, 353–381. [Google Scholar] [CrossRef]
- Chen, K.Y.; Morris, J.C. Kinetics of Oxidation of Aqueous Sulfide by O2. Environ. Sci. Technol. 1972, 6, 529–537. [Google Scholar] [CrossRef]
- Xolo, L.; Moleko-Boyce, P.; Makelane, H.; Faleni, N.; Tshentu, Z.R. Status of Recovery of Strategic Metals from Spent Secondary Products. Minerals 2021, 11, 673. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Riaño, S.; Aktan, E.; Deferm, C.; Fransaer, J.; Binnemans, K. Solvometallurgical Recovery of Platinum Group Metals from Spent Automotive Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 337–350. [Google Scholar] [CrossRef]
- Trinh, H.B.; Lee, J.C.; Srivastava, R.R.; Kim, S.; Ilyas, S. Eco-Threat Minimization in HCl Leaching of Pgms from Spent Automobile Catalysts by Formic Acid Prereduction. ACS Sustain. Chem. Eng. 2017, 5, 7302–7309. [Google Scholar] [CrossRef]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A Review of Recent Studies into Hydrometallurgical Methods for Recovering PGMs from Used Catalytic Converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Lanaridi, O.; Platzer, S.; Nischkauer, W.; Betanzos, J.H.; Iturbe, A.U.; Del Rio Gaztelurrutia, C.; Sanchez-Cupido, L.; Siriwardana, A.; Schnürch, M.; Limbeck, A.; et al. Benign Recovery of Platinum Group Metals from Spent Automotive Catalysts Using Choline-Based Deep Eutectic Solvents. Green Chem. Lett. Rev. 2022, 15, 404–414. [Google Scholar] [CrossRef]
- Canda, L.; Heput, T.; Ardelean, E. Methods for Recovering Precious Metals from Industrial Waste. IOP Conf. Ser. Mater. Sci. Eng. 2016, 106, 012020. [Google Scholar] [CrossRef]
- Grad, O.; Ciopec, M.; Negrea, A.; Duțeanu, N.; Vlase, G.; Negrea, P.; Dumitrescu, C.; Vlase, T.; Vodă, R. Precious Metals Recovery from Aqueous Solutions Using a New Adsorbent Material. Sci. Rep. 2021, 11, 2016. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Bello, O.S.; Agboola, O.S.; Adeeyo, R.O.; Oyetade, J.A.; Alabi, M.A.; Edokpayi, J.N.; Makungo, R. Recovery of Precious Metals from Processed Wastewater: Conventional Techniques Nexus Advanced and Pragmatic Alternatives. Water Reuse 2023, 13, 134–161. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, M.; Wang, S.; Dai, L.; Zhang, L.; Wang, C. Selective Recovery of Gold Ions in Aqueous Solutions by a Novel Trithiocyanuric-Zr Based MOFs Adsorbent. J. Mol. Liq. 2020, 298, 112090. [Google Scholar] [CrossRef]
- Fernández, M.; Ramírez, M.; Gómez, J.M.; Cantero, D. Biogas Biodesulfurization in an Anoxic Biotrickling Filter Packed with Open-Pore Polyurethane Foam. J. Hazard. Mater. 2014, 264, 529–535. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Waste Water, 20th ed.; APHA: Washington, DC, USA, 1999. [Google Scholar]
- Rowley, K.; Swift, E.H. Goulometric Titration of Thiosulfate with Iodine: Application to the Determination of Oxidizing Agents. Anal. Chem. 1954, 26, 373–375. [Google Scholar] [CrossRef]
| Run No. | Na2S2O3 (M) | CuSO4 (M) | (NH4)2SO4 (M) | Na2SO3 (M) | pH | T (°C) | Airflow Rate (vvm) | Pd Leaching (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.2 (+) | 0.06 (+) | 1.5 (+) | 0.0 (−) | 12.0 (+) | 60.0 (+) | 0 (−) | 2.0 |
| 2 | 0.6 (−) | 0.06 (+) | 1.5 (+) | 0.0 (−) | 12.0 (+) | 25.0 (−) | 0 (−) | 1.1 |
| 3 | 1.2 (+) | 0.03 (−) | 0.5 (−) | 0.0 (−) | 12.0 (+) | 60.0 (+) | 2 (+) | 1.0 |
| 4 | 0.6 (−) | 0.03 (−) | 0.5 (−) | 0.1 (+) | 12.0 (+) | 60.0 (+) | 0 (−) | 1.4 |
| 5 | 0.6 (−) | 0.06 (+) | 0.5 (−) | 0.0 (−) | 8.0 (−) | 60.0 (+) | 2 (+) | 0.1 |
| 6 | 1.2 (+) | 0.03 (−) | 1.5 (+) | 0.0 (−) | 8.0 (−) | 25.0 (−) | 2 (+) | 0.1 |
| 7 | 1.2 (+) | 0.03 (−) | 1.5 (+) | 0.1 (+) | 8.0 (−) | 60.0 (+) | 0 (−) | 26.0 |
| 8 | 1.2 (+) | 0.06 (+) | 0.5 (−) | 0.1 (+) | 12.0 (+) | 25.0 (−) | 2 (+) | 0.6 |
| 9 | 0.6 (−) | 0.03 (−) | 1.5 (+) | 0.1 (+) | 12.0 (+) | 25.0 (−) | 2 (+) | 1.0 |
| 10 | 1.2 (+) | 0.06 (+) | 0.5 (−) | 0.1 (+) | 8.0 (−) | 25.0 (−) | 0 (−) | 0.1 |
| 11 | 0.6 (−) | 0.06 (+) | 1.5 (+) | 0.1 (+) | 8.0 (−) | 60.0 (+) | 2 (+) | 0.1 |
| 12 | 0.6 (−) | 0.03 (−) | 0.5 (−) | 0.0 (−) | 8.0 (−) | 25.0 (−) | 0 (−) | 0.1 |
| Test | Na2S2O3 (M) | CuSO4 (M) | (NH4)2SO4 (M) | Na2SO3 (M) | pH |
|---|---|---|---|---|---|
| A | 2.4 | 0.030 | 1.5 | 0.1 | 8.0 |
| B | 1.2 | 0.015 | 1.5 | 0.1 | 8.0 |
| C | 1.2 | 0.030 | 3.0 | 0.1 | 8.0 |
| D | 1.2 | 0.030 | 1.5 | 0.2 | 8.0 |
| E | 1.2 | 0.030 | 1.5 | 0.1 | 6.0 |
| Stage | Time (days) | Action | pH | O2 (vvm) | Electron Acceptor Source | Operation Mode | HRT (Days) |
|---|---|---|---|---|---|---|---|
| I | 0–13 | Start-up | 8.5 | 0.033 | NO2− | Continuous | 3.5 |
| II | 13–20 | pH increase | 10 | 0.033 | NO2− | Continuous | 3.5 |
| III | 20–24 | Anoxic operation (nitrate) | 10 | 0.033 | NO3− | Continuous | 3.5 |
| IV | 24–31 | Batch mode | 10 | 0.033 | NO3− | Batch | - |
| V | 31–35 | Air supply cut | 10 | 0 | NO3− | Batch | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compagnone, M.; González-Cortés, J.J.; Pilar Yeste, M.; Cantero, D.; Ramírez, M. Sustainable Recovery of Platinum Group Metals from Spent Automotive Three-Way Catalysts through a Biogenic Thiosulfate-Copper-Ammonia System. Molecules 2023, 28, 8078. https://doi.org/10.3390/molecules28248078
Compagnone M, González-Cortés JJ, Pilar Yeste M, Cantero D, Ramírez M. Sustainable Recovery of Platinum Group Metals from Spent Automotive Three-Way Catalysts through a Biogenic Thiosulfate-Copper-Ammonia System. Molecules. 2023; 28(24):8078. https://doi.org/10.3390/molecules28248078
Chicago/Turabian StyleCompagnone, Mariacristina, José Joaquín González-Cortés, María Pilar Yeste, Domingo Cantero, and Martín Ramírez. 2023. "Sustainable Recovery of Platinum Group Metals from Spent Automotive Three-Way Catalysts through a Biogenic Thiosulfate-Copper-Ammonia System" Molecules 28, no. 24: 8078. https://doi.org/10.3390/molecules28248078
APA StyleCompagnone, M., González-Cortés, J. J., Pilar Yeste, M., Cantero, D., & Ramírez, M. (2023). Sustainable Recovery of Platinum Group Metals from Spent Automotive Three-Way Catalysts through a Biogenic Thiosulfate-Copper-Ammonia System. Molecules, 28(24), 8078. https://doi.org/10.3390/molecules28248078









