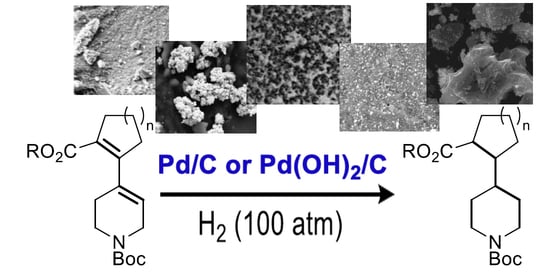
Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Pd/C Composites
2.2. Synthesis and Characterization of Pearlman’s Catalysts
2.3. Characteristics of the Porous Structure of the Composites
2.4. Catalytic Performance of the Composites in the Hydrogenation of Quinoline and Dienes
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schneider, C.; Leischner, T.; Ryabchuk, P.; Jackstell, R.; Junge, K.; Beller, M. Development of Bulk Organic Chemical Processes—History, Status, and Opportunities for Academic Research. CCS Chem. 2021, 3, 512–530. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective Hydrogenation over Supported Metal Catalysts: From Nanoparticles to Single Atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef]
- Stoffels, M.A.; Klauck, F.J.R.; Hamadi, T.; Glorius, F.; Leker, J. Technology Trends of Catalysts in Hydrogenation Reactions: A Patent Landscape Analysis. Adv. Synth. Catal. 2020, 362, 1258–1274. [Google Scholar] [CrossRef] [Green Version]
- Endean, R.T.; Rasu, L.; Bergens, S.H. Enantioselective Hydrogenations of Esters with Dynamic Kinetic Resolution. ACS Catal. 2019, 9, 6111–6117. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Zang, W.; Li, G.; Wang, L.; Zhang, X. Catalytic hydrogenation by noble-metal nanocrystals with well-defined facets: A review. Catal. Sci. Technol. 2015, 5, 2532–2553. [Google Scholar] [CrossRef]
- Louis, C.; Delannoy, L. Chapter One—Selective hydrogenation of polyunsaturated hydrocarbons and unsaturated aldehydes over bimetallic catalysts. Adv. Catal. 2019, 64, 1–88. [Google Scholar] [CrossRef]
- Côté, C.R.; Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Comparing the Pyrophoricity of Palladium Catalysts for Heterogeneous Hydrogenation. Org. Process Res. Dev. 2018, 22, 1852–1855. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-Based Bimetallic Catalysis: From Model Surfaces to Supported Catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef]
- Wiesenfeldt, M.P.; Nairoukh, Z.; Dalton, T.; Glorius, F. Selective Arene Hydrogenation for Direct Access to Saturated Carbo- and Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 10460–10476. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, H.; Bruneau, C.; Fischmeister, C. Iridium-Catalyzed Hydrogenation and Dehydrogenation of N-Heterocycles in Water under Mild Conditions. ChemSusChem 2019, 12, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Surkus, A.-E.; He, L.; Pohl, M.-M.; Radnik, J.; Topf, C.; Junge, K.; Beller, M. Selective Catalytic Hydrogenation of Heteroarenes with N-Graphene-Modified Cobalt Nanoparticles (Co3O4–Co/NGr@α-Al2O3). J. Am. Chem. Soc. 2015, 137, 11718–11724. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Renzetti, A.R.; Turbanti, L.; Di Bugno, C.; Fornai, F.; Vaglini, F.; Maggio, R.; Corsini, G.U. Stereoselective inhibition of muscarinic receptor subtypes by the eight stereoisomers related to rociverine. Eur. J. Pharmacol. 1995, 290, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Subissi, A.; Maggi, C.A.; Meli, A. Rociverine Citrate: A New Spasmolytic Agent, Potentially Useful in the Treatment of Urinary Bladder Hyperreflexia. Jpn. J. Pharmacol. 1986, 42, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Jadhav, A.; Jangid, P.; Patil, R.; Shelar, A.; Karuppayil, S.M. The human muscarinic acetylcholine receptor antagonist, Dicyclomine targets signal transduction genes and inhibits the virulence factors in the human pathogen, Candida albicans. J. Antibiot. 2018, 71, 456–466. [Google Scholar] [CrossRef]
- Dom, R.; Lommel, R.; Baro, F. A quantitative study of neuroleptic-induced extrapyramidal symptoms and their response o dexetimide, a potent and long-acting antiparkinsonian agent. Acta Psychiatr. Scand. 1971, 47, 399–410. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, I.; Soudijn, W. The metabolism and excretion of benzetimide hydrochloride (R 4929) by rats. Life Sci. 1968, 7, 225–229. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, I. Differential anticholinergic activity, metabolism and excretion of the optical isomers of 14C-benzetimide hydrochloride. Life Sci. 1969, 8, 517–523. [Google Scholar] [CrossRef]
- DeForrest, J.M.; Waldron, T.L.; Harvey, C.; Scalese, B.; Rubin, B.; Powell, J.R.; Petrillo, E.W.; Cushman, D.W. Fosinopril, a Phosphinic Acid Inhibitor of Angiotensin I Converting Enzyme: In Vitro and Preclinical In Vivo Pharmacology. J. Cardiovasc. Pharmacol. 1989, 14, 730–736. [Google Scholar] [CrossRef]
- Murdoch, D.; McTavish, D. Fosinopril. Drugs 1992, 43, 123–140. [Google Scholar] [CrossRef]
- Pilote, L.; Abrahamowicz, M.; Eisenberg, M.; Humphries, K.; Behlouli, H.; Tu, J.V. Effect of different angiotensin-converting-enzyme inhibitors on mortality among elderly patients with congestive heart failure. Can. Med. Assoc. J. 2008, 178, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungler, A.; Hegedus, L.; Fodor, K.; Farkas, G.; Furcht, A.; Karancsi, Z.P. PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Miyazaki, M.; Furukawa, S.; Komatsu, T. Regio- and Chemoselective Hydrogenation of Dienes to Monoenes Governed by a Well-Structured Bimetallic Surface. J. Am. Chem. Soc. 2017, 139, 18231–18239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Shon, Y.-S. Mechanistic interpretation of selective catalytic hydrogenation and isomerization of alkenes and dienes by ligand deactivated Pd nanoparticles. Nanoscale 2015, 7, 17786–17790. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Sun, J.; Kang, D.; Zhu, Q.; Yang, Y. Mechanistic insights into complete hydrogenation of 1,3-butadiene over Pt/SiO2: Effect of Pt dispersion and kinetic analysis. Catal. Sci. Technol. 2017, 7, 2717–2728. [Google Scholar] [CrossRef]
- Feller, D.; Craig, N.C. High Level ab Initio Energies and Structures for the Rotamers of 1,3-Butadiene. J. Phys. Chem. A 2009, 113, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Papa, V.; Cao, Y.; Spannenberg, A.; Junge, K.; Beller, M. Development of a practical non-noble metal catalyst for hydrogenation of N-heteroarenes. Nat. Catal. 2020, 3, 135–142. [Google Scholar] [CrossRef]
- Asaula, V.M.; Buryanov, V.V.; Solod, B.Y.; Tryus, D.M.; Pariiska, O.O.; Kotenko, I.E.; Volovenko, Y.M.; Volochnyuk, D.M.; Ryabukhin, S.V.; Kolotilov, S.V. Catalytic Hydrogenation of Substituted Quinolines on Co–Graphene Composites. Eur. J. Org. Chem. 2021, 2021, 6616–6625. [Google Scholar] [CrossRef]
- Asaula, V.M.; Lytvynenko, A.S.; Mishura, A.M.; Kurmach, M.M.; Buryanov, V.V.; Gavrilenko, K.S.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. In-situ formation of NixB/MIL-101(Cr) and Pd/MIL-101(Cr) composites for catalytic hydrogenation of quinoline. Inorg. Chem. Commun. 2020, 121, 108203. [Google Scholar] [CrossRef]
- Asaula, V.M.; Shvets, O.V.; Pariiska, O.O.; Bur’yanov, V.V.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Composites Based on Nanodispersed Nickel, Graphene-Like Carbon, and Aerosil for Catalytic Hydrogenation of Furfural and Quinoline. Theor. Experim. Chem. 2020, 56, 261–267. [Google Scholar] [CrossRef]
- Mozingo, R. Palladium Catalysts. Org. Synth. 1946, 26, 77. [Google Scholar] [CrossRef]
- Cho, H.-B.; Park, J.-H.; Hong, B.-E.; Park, Y.-H. Effect of Catalyst Preparation on the Selective Hydrogenation of Biphenol over Pd/C Catalysts. Bull. Korean Chem. Soc. 2008, 29, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Simakova, O.A.; Simonov, P.A.; Romanenko, A.V.; Simakov, I.L. Preparation of Pd/C catalysts via deposition of palladium hydroxide onto Sibunit carbon and their application to partial hydrogenation of rapeseed oil. React. Kin. Catal. Lett. 2008, 95, 3–12. [Google Scholar] [CrossRef]
- Toebes, M.L.; van Dillen, J.A.; de Jong, K.P. Synthesis of supported palladium catalysts. J. Mol. Cat. A Chem. 2001, 173, 75–98. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Li, J.; Tian, Z.; Yu, F. Highly-Dispersed Ni-NiO Nanoparticles Anchored on an SiO2 Support for an Enhanced CO Methanation Performance. Catalysts 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Hull, A.W. X-ray crystal analysis of 13 common metals. Phys. Rev. 1921, 17, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Gorban, O.; Danilenko, I.; Nosolev, I.; Abdullayev, E.; Islamov, A.; Gavrilenko, K.; Doroshkevich, A.; Shvets, O.; Kolotilov, S. Impact of chemical and physical modification of zirconia on structure, surface state, and catalytic activity in oxidation of α-tetralol. J. Nanopart. Res. 2022, 24, 197. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Yu, T.; Camargo, P.H.C.; Xia, Y. Nucleation and growth mechanisms for Pd-Pt bimetallic nanodendrites and their electrocatalytic properties. Nano Res. 2010, 3, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Simonov, P.A.; Troitskii, S.Y.; Likholobov, V.A. Preparation of the Pd/C Catalysts: A Molecular-Level Study of Active Site Formation. Kinet. Catal. 2000, 41, 255–269. [Google Scholar] [CrossRef]
- Albers, P.W.; Möbus, K.; Wieland, S.D.; Parker, S.F. The fine structure of Pearlman’s catalyst. Phys. Chem. Chem. Phys. 2015, 17, 5274–5278. [Google Scholar] [CrossRef] [Green Version]
- Pearlman, W.M. Noble metal hydroxides on carbon nonpyrophoric dry catalysts. Tetrahedron Lett. 1967, 8, 1663–1664. [Google Scholar] [CrossRef]
- Moore, W.J.; Pauling, L. The crystal structures of the tetragonal monoxides of lead, tin, palladium, and platinum. J. Am. Chem. Soc. 1941, 63, 1392–1394. [Google Scholar] [CrossRef]
- Fotouhi-Far, F.; Bashiri, H.; Hamadanian, M.; Keshavarz, M.H. Increment of activity of Pd(OH)2/C catalyst in order to improve the yield of high performance 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (HNIW). Inorg. Nanomet. Chem. 2017, 47, 1489–1494. [Google Scholar] [CrossRef]
- Noack, K.; Zbinden, H.; Schlögl, R. Identification of the state of palladium in various hydrogenation catalysts by XPS. Catal. Lett. 1990, 4, 145–156. [Google Scholar] [CrossRef]
- Dubois, V.; Desmecht, D.; Rkiouak, L.; Jacquet, A.-S.; Hoshinoo, T.; Nakagawa, K.; Hermans, S. Protection of industrially-relevant Pd/C catalysts for cyclohexene hydrogenation: Effect of a siliceous coating on the thermal treatment of covered catalysts. React. Kin. Mech. Catal. 2019, 126, 399–415. [Google Scholar] [CrossRef]
- Lamme, W.S.; van der Heijden, O.; Krans, N.A.; Nöllen, E.; Mager, N.; Hermans, S.; Zečević, J.; de Jong, K.P. Origin and prevention of broad particle size distributions in carbon supported palladium catalysts prepared by liquid-phase reduction. J. Catal. 2019, 375, 448–455. [Google Scholar] [CrossRef]
- Crawford, C.J.; Qiao, Y.; Liu, Y.; Huang, D.; Yan, W.; Seeberger, P.H.; Oscarson, S.; Chen, S. Defining the Qualities of High-Quality Palladium on Carbon Catalysts for Hydrogenolysis. Org. Process Res. Dev. 2021, 25, 1573–1578. [Google Scholar] [CrossRef]
- Crawford, C.J.; Qiao, Y.; Liu, Y.; Huang, D.; Yan, W.; Oscarson, S.; Chen, S. Variation in the Structure and Activity of Commercial Palladium on Carbon Catalysts. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Burmeister, R.; Despeyroux, B.; Deller, K.; Seibold, K.; Albers, P. On the XPS-Surface Characterization of Activated Carbons resp. Pd/C Catalysts and a Correlation to the Catalytic Activity. In Heterogeneous Catalysis and Fine Chemicals III; Barbier, J., Barrault, J., Bouchoule, C., Duprez, D., Montassier, C., Guisnet, M., Pérot, G., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Oh, S.-H.; Hoflund, G.B. Low-temperature catalytic carbon monoxide oxidation over hydrous and anhydrous palladium oxide powders. J. Catal. 2007, 245, 35–44. [Google Scholar] [CrossRef]
- Brunner, E. Solubility of hydrogen in 10 organic solvents at 298.15, 323.15, and 373.15 K. Chem. Eng. Data 1985, 30, 269–273. [Google Scholar] [CrossRef]
- Liu, Q.; Takemura, F.; Yabe, A. Solubility of Hydrogen in Liquid Methanol and Methyl Formate at 20 °C to 140 °C. J. Chem. Eng. Data 1996, 41, 1141–1143. [Google Scholar] [CrossRef]
- Teschner, D.; Révay, Z.; Borsodi, J.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Milroy, D.; Jackson, S.D.; Torres, D.; Sautet, P. Understanding Palladium Hydrogenation Catalysts: When the Nature of the Reactive Molecule Controls the Nature of the Catalyst Active Phase. Angew. Chem. Int. Ed. 2008, 47, 9274–9278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals; Elsevier: Oxford, UK, 2003. [Google Scholar]










| Sample | SBET a, m2/g | VT, cm3/g | Vmicro, cm3/g | Dmicro, nm |
|---|---|---|---|---|
| Charcoal (carrier) | 825 | 0.395 | 0.302 | 0.569 |
| Pd/C-1 | 580 | 0.313 | 0.243 | 0.566 |
| Pd/C-2 | 635 | 0.344 | 0.264 | 0.560 |
| Pd/C-3 | 690 | 0.331 | 0.249 | 0.570 |
| Pd(OH)2/C-2 | 623 | 0.290 | 0.226 | 0.554 |
| Catalyst | Yield a, % |
|---|---|
| Pd/C-1 | 42 |
| Pd/C-2 | 28 |
| Pd/C-3 | 10 |
| Pd/C-4 | 9 |
| Pd(OH)2/C-1 | 61 |
| Pd(OH)2/C-2 | 67 |
| Pd(OH)2/C-3 | 55 |
| No. | Diene | Palladium Catalysts | Pd Loading, mol. % | Solvent | Yield of 3 or 4 (%) a |
|---|---|---|---|---|---|
| 1 | 1 | 5% Pd/C (commercial) | 10 | hexanes | 3.5 |
| 2 | 1 | 5% Pd/C (commercial) | 10 | THF | 3.5 |
| 3 | 1 | 5% Pd/C (commercial) | 10 | MeOH | 21 |
| 4 | 1 | 10% Pd/C-1 | 10 | MeOH | 35 |
| 5 | 1 | 10% Pd/C-2 | 10 | MeOH | 35 |
| 6 | 1 | 10% Pd/C-3 | 10 | MeOH | 0, N/D b (12% partially reduced products) |
| 7 | 1 | 20% Pd/C-4 | 10 | MeOH | 0, N/D b (18% partially reduced products) |
| 8 | 1 | 20% Pd(OH)2/C-1 | 10 | MeOH | 33 |
| 9 | 1 | 20% Pd(OH)2/C-2 | 10 | MeOH | 35 |
| 10 | 1 | 20% Pd(OH)2/C-3 | 10 | MeOH | 27 |
| 11 | 1 | 20% Pd(OH)2/C (commercial) | 10 | MeOH | 20 |
| 12 | 2 | 5% Pd/C (commercial) | 10 | hexanes | 0, N/D b |
| 13 | 2 | 5% Pd/C (commercial) | 10 | THF | 1.3 |
| 14 | 2 | 5% Pd/C (commercial) | 10 | MeOH | 56 |
| 15 | 2 | 10% Pd/C-1 | 10 | MeOH | 92 |
| 16 | 2 | 10% Pd/C-2 | 10 | MeOH | 89 |
| 17 | 2 | 10% Pd/C-3 | 10 | MeOH | 24 (59% partially reduced products) |
| 18 | 2 | 10% Pd/C-4 | 10 | MeOH | 26 (58% partially reduced products) |
| 19 | 2 | 20% Pd(OH)2/C-1 | 10 | MeOH | 66 (19% partially reduced products) |
| 20 | 2 | 20% Pd(OH)2/C-2 | 10 | MeOH | 64 (12% partially reduced products) |
| 21 | 2 | 20% Pd(OH)2/C-3 | 10 | MeOH | 55 (15% partially reduced products) |
| 22 | 2 | 20% Pd(OH)2/C (commercial) | 10 | MeOH | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subotin, V.V.; Vashchenko, B.V.; Asaula, V.M.; Verner, E.V.; Ivanytsya, M.O.; Shvets, O.; Ostapchuk, E.N.; Grygorenko, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; et al. Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold. Molecules 2023, 28, 1201. https://doi.org/10.3390/molecules28031201
Subotin VV, Vashchenko BV, Asaula VM, Verner EV, Ivanytsya MO, Shvets O, Ostapchuk EN, Grygorenko OO, Ryabukhin SV, Volochnyuk DM, et al. Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold. Molecules. 2023; 28(3):1201. https://doi.org/10.3390/molecules28031201
Chicago/Turabian StyleSubotin, Vladyslav V., Bohdan V. Vashchenko, Vitalii M. Asaula, Eduard V. Verner, Mykyta O. Ivanytsya, Oleksiy Shvets, Eugeniy N. Ostapchuk, Oleksandr O. Grygorenko, Sergey V. Ryabukhin, Dmitriy M. Volochnyuk, and et al. 2023. "Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold" Molecules 28, no. 3: 1201. https://doi.org/10.3390/molecules28031201
APA StyleSubotin, V. V., Vashchenko, B. V., Asaula, V. M., Verner, E. V., Ivanytsya, M. O., Shvets, O., Ostapchuk, E. N., Grygorenko, O. O., Ryabukhin, S. V., Volochnyuk, D. M., & Kolotilov, S. V. (2023). Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold. Molecules, 28(3), 1201. https://doi.org/10.3390/molecules28031201