Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review
Abstract
:1. Introduction
2. Biologically Active Copper, Zinc, and Nickel Coordination Compounds
2.1. Copper Coordination Compounds
2.1.1. Antitumor Properties of Copper Coordination Compounds
2.1.2. Antibacterial and Antifungicidal Properties of Copper Coordination Compounds
2.2. Zinc Coordination Compounds
2.3. Nickel Coordination Compounds
2.4. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands
2.5. Dicarboxylic Acids
- The chelating mode fac-SO2, where the acid acts as a tridentate ligand, exemplified by octahedral complexes of Cu(II), Ni(II), and Zn(II), specifically [Cu(tda)(bipy)(H2O)], fac-O2 + S(apical) (Scheme 5A);
- fac-SO2 (tridentate) in chelation mode + μ-bridging carboxyl group, the first type of this mode is μ-η1 in [Cu(tda)(phen)]2·H2tda (fac-O2 + S(apical) + O-monoatomic carboxylate bridging ligand), the second type is anti,syn-μ-η1:η1, where the O,O-diatomic carboxylate bridging group in [(phen)2Cu(μ-tda)Cu(phen)](NO3)2·5H2O (fac-SO + O(apical)) (Scheme 5B), or the third type is fac-SO + O(apical) pentadentate (double anti,syn-μ-η1:η1);
3. Copper, Zinc, and Nickel Dicarboxylate Compounds
3.1. Copper
3.1.1. Copper with 2,2′-Thiodiacetic Acid and N Donor Ligands
3.1.2. Copper with 3,3′-Thiodipropionic Acid and N Donor Ligands
3.1.3. Copper with 3,3′-Dithiodipropionic Acid and N Donor Ligands
3.1.4. Copper with Fumaric Acid and N Donor Ligands
3.2. Zinc
3.2.1. Zinc with 2,2′-Thiodiacetic Acid and N Donor Ligands
3.2.2. Zinc with 3,3′-Thiodipropionic Acid and N Donor Ligands
3.2.3. Zinc with 3,3′-Dithiodipropionic Acid and N Donor Ligands
3.2.4. Zinc with Fumaric Acid and N Donor Ligands
3.3. Nickel
3.3.1. Nickel with 2,2′-Thiodiacetic Acid and N Donor Ligands
3.3.2. Nickel with 3,3′-Thiodipropionic Acid/3,3′-Dithiodipropionic Acids and N Donor Ligands
3.3.3. Nickel with Fumaric Acid and N Donor Ligands
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bakalbassis, E.G.; Bozopoulos, A.P.; Mrozinski, J.; Rentzeperis, P.J.; Tsipis, C.A. Crystal-structure, magnetic-properties, and orbital interactions of the (mu-terephthalato)(ethylenediamine)diaquocopper(II) zigzag chain. Inorg. Chem. 1988, 27, 529–532. [Google Scholar] [CrossRef]
- Mrozinski, J. New trends of molecular magnetism. Coord. Chem. Rev. 2005, 249, 2534–2548. [Google Scholar] [CrossRef]
- Habib, H.A.; Sanchiz, J.; Janiak, C. Magnetic and luminescence properties of Cu(II), Cu(II)(4)O-4 core, and Cd(II) mixed-ligand metal-organic frameworks constructed from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzene-1,3,5-tricarboxylate. Inorg. Chim. Acta 2009, 362, 2452–2460. [Google Scholar] [CrossRef]
- Grirrane, A.; Pastor, A.; Galindo, A.; Ienco, A.; Mealli, C.; Rosa, P. First example of a tetra-carboxylate bridged dimanganese species. Chem. Commun. 2003, 512–513. [Google Scholar] [CrossRef]
- Grirrane, A.; Pastor, A.; Alvarez, E.; Mealli, C.; Ienco, A.; Masi, D.; Galindo, A. Thiodiacetate cobalt(II) complexes: Synthesis, structure and properties. Inorg. Chem. Commun. 2005, 8, 463–466. [Google Scholar] [CrossRef]
- Kopel, P.; Sindelar, Z.; Klicka, R. Complexes of iron(III) salen and saloph Schiff bases with bridging dicarboxylic and tricarboxylic acids. Transit. Met. Chem. 1998, 23, 139–142. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Marek, J.; Mrozinski, J. Syntheses and study on nickel(II) complexes with thiodiglycolic acid and nitrogen-donor ligands. X-ray structures of Ni(bpy)(tdga)(H2O)center dot 4H(2)O and (en)Ni(mu-tdga)(2)NI(en) center dot 4H(2)O (tdgaH(2)= thiodiglycolic acid). Polyhedron 2004, 23, 1573–1578. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Marek, J.; Korabik, M.; Mrozinski, J. Syntheses and properties of binuclear copper(II) mixed-ligand complexes involving thiodiglycolic acid. The crystal structures of (phen)(2)Cu(mu-tdga)Cu(phen) (NO3)(2)center dot 5H(2)O and (H2O)(pmdien)Cu(mu-tdga)Cu(pmdien)(H2O) (ClO4)(2). Polyhedron 2003, 22, 411–418. [Google Scholar] [CrossRef]
- Alavijeh, R.K.; Beheshti, S.; Akhbari, K.; Morsali, A. Investigation of reasons for metal-organic framework’s antibacterial activities. Polyhedron 2018, 156, 257–278. [Google Scholar] [CrossRef]
- Soltani, S.; Akhbari, K.; Phuruangrat, A. Investigation of effective factors on antibacterial activity of Pillared-Layered MOFs. J. Mol. Struct. 2021, 1225, 8. [Google Scholar] [CrossRef]
- Kalati, M.; Akhbari, K. Optimizing the metal ion release and antibacterial activity of ZnO@ZIF-8 by modulating its synthesis method. New J. Chem. 2021, 45, 22924–22931. [Google Scholar] [CrossRef]
- Nakhaei, M.; Akhbari, K.; Kalati, M.; Phuruangrat, A. Antibacterial activity of three zinc-terephthalate MOFs and its relation to their structural features. Inorg. Chim. Acta 2021, 522, 9. [Google Scholar] [CrossRef]
- Soltani, S.; Akhbari, K.; Phuruangrat, A. Incorporation of silver nanoparticles on Cu-BTC metal-organic framework under the influence of reaction conditions and investigation of their antibacterial activity. Appl. Organomet. Chem. 2022, 36, 10. [Google Scholar] [CrossRef]
- Soltani, S.; Akhbari, K. Cu-BTC metal-organic framework as a biocompatible nanoporous carrier for chlorhexidine antibacterial agent. J. Biol. Inorg. Chem. 2022, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kruszynski, R.; Swiatkowski, M. The structure of coordination precursors as an effective tool for governing of size and morphology of ZnS and ZnO nanoparticles. J. Saudi Chem. Soc. 2018, 22, 816–825. [Google Scholar] [CrossRef]
- Swiatkowski, M.; Kruszynski, R. Structurally diverse coordination compounds of zinc as effective precursors of zinc oxide nanoparticles with various morphologies. Appl. Organomet. Chem. 2019, 33, 18. [Google Scholar] [CrossRef]
- Stepankova, H.; Swiatkowski, M.; Kruszynski, R.; Svec, P.; Michalkova, H.; Smolikova, V.; Ridoskova, A.; Splichal, Z.; Michalek, P.; Richtera, L.; et al. The Anti-Proliferative Activity of Coordination Compound-Based ZnO Nanoparticles as a Promising Agent Against Triple Negative Breast Cancer Cells. Int. J. Nanomed. 2021, 16, 4431–4449. [Google Scholar] [CrossRef]
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem. Rev. 2020, 412, 13. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef]
- Ban, I.; Stergar, J.; Maver, U. NiCu magnetic nanoparticles: Review of synthesis methods, surface functionalization approaches, and biomedical applications. Nanotechnol. Rev. 2018, 7, 187–207. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef]
- Tardito, S.; Marchio, L. Copper Compounds in Anticancer Strategies. Curr. Med. Chem. 2009, 16, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; Gabe, E.J.; Glusker, J.P.; Minkin, J.A.; Patterso, A.L. Crystal structures of compounds with antitumor activity. 2-keto-3-ethoxybutyraldehyde bis(thiosemicarbazone) and its cupric complex. J. Am. Chem. Soc. 1966, 88, 1845–1846. [Google Scholar] [CrossRef]
- Garcia-Tojal, J.; Garcia-Orad, A.; Serra, J.L.; Pizarro, J.L.; Lezama, L.; Arriortua, M.I.; Rojo, T. Synthesis and spectroscopic properties of copper(II) complexes derived from thiophene-2-carbaldehyde thiosemicarbazone. Structure and biological activity of Cu(C6H6N3S2)(2). J. Inorg. Biochem. 1999, 75, 45–54. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Siddikuzzaman; Palanimuthu, D.; Somasundaram, K.; Samuelson, A.G. Biotin Decorated Gold Nanoparticles for Targeted Delivery of a Smart-Linked Anticancer Active Copper Complex: In Vitro and In Vivo Studies. Bioconjugate Chem. 2016, 27, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, C.Y.; Zou, G.L.; Tao, D.D.; Gong, J.P. G(1)-phase specific apoptosis in liver carcinoma cell line induced by copper-1,10-phenanthroline. Int. J. Biochem. Cell Biol. 2002, 34, 678–684. [Google Scholar] [CrossRef]
- Valdez-Camacho, J.R.; Ramirez-Solis, A.; Escalante, J.; Ruiz-Azuara, L.; Ho, M. Theoretical determination of half-wave potentials for phenanthroline-, bipyridine-, acetylacetonate-, and glycinate-containing copper (II) complexes. J. Mol. Model. 2020, 26, 13. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Yasui, H. Zinc Complexes Developed as Metallopharmaceutics for Treating Diabetes Mellitus based on the Bio-Medicinal Inorganic Chemistry. Curr. Top. Med. Chem. 2012, 12, 210–218. [Google Scholar] [CrossRef]
- Sakurai, H.; Yoshikawa, Y.; Yasui, H. Current state for the development of metallopharmaceutics and anti-diabetic metal complexes. Chem. Soc. Rev. 2008, 37, 2383–2392. [Google Scholar] [CrossRef]
- Roguin, L.P.; Chiarante, N.; Vior, M.C.G.; Marino, J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 2019, 114, 14. [Google Scholar] [CrossRef]
- Kuzyniak, W.; Ermilov, E.A.; Atilla, D.; Gurek, A.G.; Nitzsche, B.; Derkow, K.; Hoffmann, B.; Steinemann, G.; Ahsen, V.; Hopfner, M. Tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine for photodynamic cancer therapy. Photodiagnosis Photodyn. Ther. 2016, 13, 148–157. [Google Scholar] [CrossRef]
- Drewry, J.A.; Gunning, P.T. Recent advances in biosensory and medicinal therapeutic applications of zinc(II) and copper(II) coordination complexes. Coord. Chem. Rev. 2011, 255, 459–472. [Google Scholar] [CrossRef]
- Afrasiabi, Z.; Sinn, E.; Lin, W.S.; Ma, Y.F.; Campana, C.; Padhye, S. Nickel (II) complexes of naphthaquinone thiosemicarbazone and semicarbazone: Synthesis, structure, spectroscopy, and biological activity. J. Inorg. Biochem. 2005, 99, 1526–1531. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Rizzarelli, E.; Brescianipahor, N.; Nardin, G. Properties and x-ray crystal-structures of copper(ii) mixed complexes with thiodiacetate and 2,2′-bipyridyl or 2,2′-6′,2′′-terpyridyl. J. Chem. Soc.-Dalton Trans. 1982, 681–685. [Google Scholar] [CrossRef]
- Pavlova, A.; Cernak, J.; Harms, K. catena-Poly diaqua(di-2-pyridylamine-kappa N-2,N ′)nickel(II)-mu-fumarato-kappa O-2(1):O-4 tetrahydrate. Acta Crystallogr. Sect. E.-Struct Rep. Online 2010, 66, m501–m502. [Google Scholar] [CrossRef]
- Bora, S.J.; Das, B.K. Synthesis, structure and properties of a fumarate bridged Ni(II) coordination polymer. J. Mol. Struct. 2011, 999, 83–88. [Google Scholar] [CrossRef]
- Bienko, A.; Kopel, P.; Kizek, R.; Kruszynski, R.; Bienko, D.; Titis, J.; Boca, R. Synthesis, crystal structure and magnetic properties of trithiocyanurate or thiodiacetate polynuclear Ni(II) and Co(II) complexes. Inorg. Chim. Acta 2014, 416, 147–156. [Google Scholar] [CrossRef]
- Yu, J. catena-Poly di-mu(2)-hydroxido-bis (di-2-pyridylamine)nickel(II)-mu-fumarato dihydrate. Acta Crystallogr. Sect. E.-Struct Rep. Online 2009, 65, m535–m536. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, K.N.; Terzis, A.; Perlepes, S.P.; Raptopoulou, C.P. Synthetic, structural and spectroscopic studies of complexes derived from the copper(II) perchlorate/fumaric acid/N,N ′-chelates tertiary reaction systems. Polyhedron 2010, 29, 46–53. [Google Scholar] [CrossRef]
- Chawla, S.K.; Arora, M.; Nattinen, K.; Rissanen, K.; Yakhmi, J.V. Syntheses and crystal structures of three novel Cu(II) coordination polymers of different dimensionality constructed from Cu(II) carboxylates (carboxylate=malonate (mal), 2 acetate (ac), fumarate (fum)) and conformationally flexible 1,4-bis(imidazole-1-yl-methylene)benzene(IX). Polyhedron 2004, 23, 3007–3019. [Google Scholar] [CrossRef]
- Mautner, F.A.; Vicente, R.; Louka, F.R.Y.; Massoud, S.S. Dinuclear fumarato-and terephthalato-bridged copper(II) complexes: Structural characterization and magnetic properties. Inorg. Chim. Acta 2008, 361, 1339–1348. [Google Scholar] [CrossRef]
- Arici, M.; Yesilel, O.Z.; Keskin, S.; Sahin, O.; Buyukgungor, O. The synthesis, characterization, and theoretical hydrogen gas adsorption properties of copper(II)-3,3 ′-thiodipropionate complexes with imidazole derivatives. J. Coord. Chem. 2013, 66, 4093–4106. [Google Scholar] [CrossRef]
- Alarcon-Payer, C.; Pivetta, T.; Choquesillo-Lazarte, D.; Gonzalez-Perez, J.M.; Crisponi, G.; Castineiras, A.; Niclos-Gutierrez, J. Thiodiacetato-copper(II) chelates with or without N-heterocyclic donor ligands: Molecular and/or crystal structures of Cu(tda) (n), Cu(tda)(Him)(2)(H2O) and Cu(tda)(5Mphen) center dot 2H(2)O (Him = imidazole, 5Mphen=5-methyl-1,10-phenanthroline). Inorg. Chim. Acta 2005, 358, 1918–1926. [Google Scholar] [CrossRef]
- Nath, J.K.; Mondal, A.; Powell, A.K.; Baruah, J.B. Structures, Magnetic Properties, and Photoluminescence of Dicarboxylate Coordination Polymers of Mn, Co, Ni, Cu Having N-(4-Pyridylmethyl)-1,8-naphthalimide. Cryst. Growth Des. 2014, 14, 4735–4748. [Google Scholar] [CrossRef]
- Tellez-Lopez, A.; Jaramillo-Garcia, J.; Martinez-Dominguez, R.; Morales-Luckie, R.A.; Camacho-Lopez, M.A.; Escudero, R.; Sanchez-Mendieta, V. M(II)(H2O)(2) (5,5 ′-dimethy-2,2 ′-bipyridine)(fumarato) M = Co and Zn complexes bearing a unique distorted trigonal-prismatic geometry and displaying 2D supramolecular structures. Polyhedron 2015, 100, 373–381. [Google Scholar] [CrossRef]
- Mori, W.; Takamizawa, S.; Kato, C.N.; Ohmura, T.; Sato, T. Molecular-level design of efficient microporous materials containing metal carboxylates: Inclusion complex formation with organic polymer, gas-occlusion properties, and catalytic activities for hydrogenation of olefins. Microporous Mesoporous Mat. 2004, 73, 31–46. [Google Scholar] [CrossRef]
- Farnum, G.A.; Martin, D.P.; Sposato, L.K.; Supkowski, R.M.; LaDuca, R.L. Zinc maleate and fumarate coordination polymers containing hydrogen-bonding capable organodiimines featuring ligand dependent in situ cis-trans isomerization. Inorg. Chim. Acta 2010, 363, 250–256. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Ghoshal, D.; Zangrando, E.; Mallah, T.; Chaudhuri, N.R. Use of different unsaturated dicarboxylates toward the design of new 3D and 2D networks of copper(II). Eur. J. Inorg. Chem. 2004, 4675–4680. [Google Scholar] [CrossRef]
- Buchtelova, H.; Skubalova, Z.; Strmiska, V.; Michalek, P.; Kociova, S.; Smerkova, K.; Kruszynski, R.; Bienko, A.; Kaj, M.; Lewinska, A.; et al. Synthesis and structural characterization of antimicrobial binuclear copper (II) coordination compounds bridged by hydroxy-and/or thiodipropionic acid. J. Inorg. Biochem. 2019, 191, 8–20. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, G.J.; Liu, B.X. Aqua(2,2′-diamino-4,4′-bi-1,3-thiazole-kappa 2 N 3,N 3′)(thiodiacetato-kappa 3 O,S,O′)nickel(II) monohydrate. Acta Crystallogr. Sect. E.-Struct Rep. Online 2011, 67, m681. [Google Scholar] [CrossRef] [PubMed]
- Baggio, R.; Perec, M.; Garland, M.T. Aqua(2,2′-bipyriayl-N,N′)(thiodiacetato-O,O′,S)zinc(II) tetrahydrate. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1996, 52, 2457–2460. [Google Scholar] [CrossRef]
- He, X.; Lu, C.Z. Hydrothermal synthesis of two mixed-valence copper complexes with mixed ligands. Z. Anorg. Allg. Chem. 2004, 630, 756–759. [Google Scholar] [CrossRef]
- Sareen, N.; Singh, S.; Bhattacharya, S. A Cu(II) mediated new desulfurization pathway involving elimination of ethylene sulfide. Dalton Trans. 2014, 43, 4635–4638. [Google Scholar] [CrossRef]
- Wen, G.L.; Wang, Y.Y.; Zhang, W.H.; Ren, C.; Liu, R.T.; Shi, Q.Z. Self-assembled coordination polymers of V-shaped bis(pyridyl)thiadiazole dependent upon the spacer length and flexibility of aliphatic dicarboxylate ligands. Crystengcomm 2010, 12, 1238–1251. [Google Scholar] [CrossRef]
- Ren, B.D.; Zhao, Y.J. catena-Poly (di-2-pyridylamine-kappa N-2(2),N-2 ′)copper(II)-mu-3,3 ′-dithiodipropionato-kappa O,O ′:kappa O ″. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2006, 62, M170–M171. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, X.; Shi, Q.Z.; Gao, Y.C. A novel binuclear copper(II) complex with fumarate and 1,10-phenanthroline. Transit. Met. Chem. 2002, 27, 481–484. [Google Scholar] [CrossRef]
- Cheng, H.J.; Shen, Y.L.; Zhang, S.Y.; Ji, H.W.; Yin, W.Y.; Li, K.; Yuan, R.X. Three Coordination Polymers Constructed with Zinc(II), 3,3-Thiodipropionic Acid, and Bipyridyl Ligands: Syntheses, Crystal Structures and Luminescent Properties. Z. Anorg. Allg. Chem. 2015, 641, 1575–1580. [Google Scholar] [CrossRef]
- Yang, P.P.; Li, B.; Wang, Y.H.; Gu, W.; Liu, X. Synthesis, structure, and luminescence properties of zinc(II) and cadmium(II) complexes containing the flexible ligand of 3,3 ′-thiodipropionic acid. Z. Anorg. Allg. Chem. 2008, 634, 1221–1224. [Google Scholar] [CrossRef]
- Grirrane, A.; Pastor, A.; Alvarez, E.; Mealli, C.; Ienco, A.; Galindo, A. Novel results on thiodiacetate zinc(II) complexes: Synthesis and structure of Zn(tda)(phen) (2) center dot 5H(2)O. Inorg. Chem. Commun. 2006, 9, 160–163. [Google Scholar] [CrossRef]
- Das, M.; Biswas, A.; Kundu, B.K.; Charmier, M.A.J.; Mukherjee, A.; Mobin, S.M.; Udayabhanu, G.; Mukhopadhyay, S. Enhanced pseudo-halide promoted corrosion inhibition by biologically active zinc(II) Schiff base complexes. Chem. Eng. J. 2019, 357, 447–457. [Google Scholar] [CrossRef]
- Nie, F.M.; Chen, J.; Lu, F. Synthesis, crystal structures and magnetic studies of terephthalato-and fumarato-bridged dinickel(II) complexes with tripodal poly-benzimidazole ligand. Inorg. Chim. Acta 2011, 365, 190–195. [Google Scholar] [CrossRef]
- Kansiz, S.; Dege, N.; Kalibabchuk, V.A. Synthesis, crystal structure and Hirshfeld surface analysis of a 1D coordination polymer catenapoly diaquabis(nicotinamide-kappa N-1)nickel(II)-mu-fumarato-kappa O-2(1):O-4. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2018, 74, 1263–1266. [Google Scholar] [CrossRef]
- Lin, J.G.; Wu, P.H.; Kang, L.; Lu, C.S.; Meng, Q.J. Synthesis and characterization of a three dimensional zinc(II) metal-organic framework constructed from flexible 1,2,3,4-tetra-(4-pyridyl)-butane ligand. Solid State Sci. 2011, 13, 1538–1541. [Google Scholar] [CrossRef]
- Xu, J.Y.; Hurtado, E.J.; Lobkovsky, E.B.; Chen, B.L. Poly (mu(2)-trans-di-4-pyridylethylene-kappa N-2:N ′)(mu(2)-fumarato-kappa O-2: O ′)zinc(II). Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2007, 63, m2205. [Google Scholar] [CrossRef]
- Tseng, T.W.; Luo, T.T.; Wu, J.Y.; Tsai, C.C.; Huang, C.Y.; Chiang, M.H.; Lu, K.L. Adaptation of guest molecules: A simple system that amplifies the gentle perturbation of host lattices from nickel(II) to cobalt(II). Inorg. Chim. Acta 2016, 445, 96–102. [Google Scholar] [CrossRef]
- Uhrinova, A.; Kuchar, J.; Cernak, J. The chain structure of Ni(C4H2O4)(C12H8N2)(H2O) (n) with different types of fumarate bridging. Acta Crystallogr. Sect. E.-Struct Rep. Online 2012, 68, m92–m93. [Google Scholar] [CrossRef]
- Ma, J.F.; Yang, J.; Liu, J.F. A nickel(II) fumarate complex with o-phenanthroline. Acta Crystallogr. Sect. E.-Struct Rep. Online 2003, 59, M900–M902. [Google Scholar] [CrossRef]
- Pan, T.T.; Su, J.R.; Xu, D.J. Tris(1H-imidazole-kappa N-3)(thiodiacetato-kappa O-3,S,O ′)-nickel(II) monohydrate. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2005, 61, M1576–M1578. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Safari, N.; Amani, V.; Notash, B.; Raei, F.; Eftekhar, F. Mononuclear and Dinuclear Copper(II) Complexes Containing N, O and S Donor Ligands: Synthesis, Characterization, Crystal Structure Determination and Antimicrobial Activity of Cu(phen)(tda) center dot 2H(2)O and (phen)(2)Cu(mu-tda)Cu(phen) (ClO4)(2 center dot)1.5H(2)O. Iran J. Chem. Chem. Eng.-Int. Engl. Ed. 2014, 33, 1–13. [Google Scholar]
- Neuman, N.I.; Perec, M.; Gonzalez, P.J.; Passeggi, M.C.G.; Rizzi, A.C.; Brondino, C.D. Single Crystal EPR Study of the Dinuclear Cu(II) Complex Cu(tda)(phen) (2)center dot H(2)tda (tda = Thiodiacetate, phen = Phenanthroline): Influence of Weak Interdimeric Magnetic Interactions. J. Phys. Chem. A 2010, 114, 13069–13075. [Google Scholar] [CrossRef] [PubMed]
- Khullar, S.; Mandal, S.K. Effect of Spacer Atoms in the Dicarboxylate Linkers on the Formation of Coordination Architectures-Molecular Rectangles vs 1D Coordination Polymers: Synthesis, Crystal Structures, Vapor/Gas Adsorption Studies, and Magnetic Properties. Cryst. Growth Des. 2014, 14, 6433–6444. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M.; Monika; Ansari, A.; Ashafaq, M. Exploring catecholase activity in dinuclear Mn(ii) and Cu(ii) complexes: An experimental and theoretical approach. New J. Chem. 2020, 44, 7998–8009. [Google Scholar] [CrossRef]
- Banu, K.S.; Chattopadhyay, T.; Banerjee, A.; Mukherjee, M.; Bhattacharya, S.; Patra, G.K.; Zangrando, E.; Das, D. Mono-and dinuclear manganese(III) complexes showing efficient catechol oxidase activity: Syntheses, characterization and spectroscopic studies. Dalton Trans. 2009, 8755–8764. [Google Scholar] [CrossRef]
- Arici, M.; Yesilel, O.Z.; Sahin, O.; Tas, M. Dinuclear and polynuclear copper(II) complexes with 3,3 ′-thiodipropionate and unprecedented coordination mode. Polyhedron 2014, 71, 62–68. [Google Scholar] [CrossRef]
- Arici, M.; Yesilel, O.Z.; Tas, M. Naked-eye detection and thermochromic properties of Cu(II)-3,3 ′-thiodipropionate complexes with benzimidazole. Dalton Trans. 2015, 44, 1627–1635. [Google Scholar] [CrossRef]
- Tella, A.C.; Owalude, S.O.; Adimula, V.O.; Oladipo, A.C.; Olayemi, V.T.; Ismail, B.; Mumtaz, A.; Rehman, A.U.; Khan, A.M.; Clayton, H.S.; et al. Synthesis, Structure, and Properties of a Dinuclear Cu(II) Coordination Polymer Based on Quinoxaline and 3,3-Thiodipropionic Acid Ligands. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3089–3100. [Google Scholar] [CrossRef]
- Lahiri, D.; Bhowmick, T.; Pathak, S.; Shameema, O.; Patra, A.K.; Ramakumar, S.; Chakravarty, A.R. Anaerobic Photocleavage of DNA in Red Light by Dicopper(II) Complexes of 3,3 ′-Dithiodipropionic Acid. Inorg. Chem. 2009, 48, 339–349. [Google Scholar] [CrossRef]
- Loubalová, I.; Zahradníková, E.; Masaryk, L.; Nemec, I.; Hochvaldová, L.; Panáček, A.; Kvítek, L.; Večeřová, R.; Świątkowski, M.; Kopel, P. Antibacterial study on nickel and copper dicarboxylate complexes. Inorg. Chim. Acta 2023, 545, 121273. [Google Scholar] [CrossRef]
- Young, S.W.; Woodburn, K.W.; Wright, M.; Mody, T.D.; Fan, Q.; Sessler, J.L.; Dow, W.C.; Miller, R.A. Lutetium texaphyrin (PCI-0123): A near-infrared, water-soluble photosensitizer. Photochem. Photobiol. 1996, 63, 892–897. [Google Scholar] [CrossRef]
- Kang, Y.F.; Liu, Q.; Yin, W.T.; Zhang, W.T.; Liu, P. 3D Ni(II)/Cu(II) Supermolecular Frameworks Based on Pyridylamine and Fumarate Co-ligands Containing a Trinodal (4,5,6)-Connected Network and a (H2O)16 Water Cluster. Chin. J. Chem. 2013, 31, 256–262. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Lin, J.L.; Ying, E.B. New mixed ligand copper(II) complexes: Syntheses and crystal structures of Cu(Imid)(2)(H2O)L with imid = imidazole, L = succinic and fumaric anions. Z. Anorg. Allg. Chem. 2003, 629, 673–676. [Google Scholar] [CrossRef]
- Paul, A.; Figuerola, A.; Bertolasi, V.; Manna, S.C. DNA/protein binding and magnetic properties of a 1D Cu(II) complex containing fumarate and tridentate Schiff base ligands. Polyhedron 2016, 119, 460–470. [Google Scholar] [CrossRef]
- Che, G.B.; Liu, C.B.; Xu, Z.L. Poly pyrazino 2,3-f 1,10 phenanthroline copper(II)-mu(4)-fumarato-mu (2)-fumarato. Acta Crystallogr. Sect. E.-Struct Rep. Online 2006, 62, M1948–M1949. [Google Scholar] [CrossRef]
- Dong, G.Y.; Cui, G.H.; Lin, J. catena-poly aqua(4,4 ′-dimethyl-2,2 ′-bipyridine)-copper(II)-mu-fumarato-kappa O-2,O ′:kappa O-2 ″ O ‴-aqua-(4,4 ′-dimethyl-2,2 ′-bipyridine)copper(II)-mu-fumarato-kappa O :kappa O ′. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2006, 62, M628–M630. [Google Scholar] [CrossRef]
- Dalai, S.; Mukherjee, P.S.; Rogez, G.; Mallah, T.; Drew, M.G.B.; Chaudhuri, N.R. Synthesis, crystal structures, and magnetic properties of two new 1D copper(II) coordination polymers containing fumarate(-2) and chelating N,N ′-donor as ligands. Eur. J. Inorg. Chem. 2002, 2002, 3292–3297. [Google Scholar] [CrossRef]
- He, T.; Yue, K.F.; Zhao, Y.X.; Chen, S.P.; Zhou, C.S.; Yan, N. Crystal structures and thermodynamics/kinetics of Zn(II) coordination polymers with helical chains. J. Solid State Chem. 2016, 239, 113–120. [Google Scholar] [CrossRef]
- Tella, A.C.; Oladipo, A.C.; Adimula, V.O.; Ameen, O.A.; Bourne, S.A.; Ogunlaja, A.S. Synthesis and crystal structures of a copper(ii) dinuclear complex and zinc(ii) coordination polymers as materials for efficient oxidative desulfurization of dibenzothiophene. New J. Chem. 2019, 43, 14343–14354. [Google Scholar] [CrossRef]
- Grirrane, A.; Alvarez, E.; Pastor, A.; Galindo, A. Thiodipropionate Zn-II Complexes: Synthesis, DFT Studies, and X-ray Structure of {Zn(phen)(H2O)}(2)(mu-tdp)(2) center dot 3H(2)O. Z. Anorg. Allg. Chem. 2010, 636, 2409–2412. [Google Scholar] [CrossRef]
- Xing, G.E.; Liu, Q.F.; Zhang, Y.; Zhang, S.F.; Dong, Y.L. Microporous Zinc(II) Metal-Organic Framework with 6-Connected pcu Topology: Synthesis, Structure, and Gas Adsorption Properties. Z. Anorg. Allg. Chem. 2015, 641, 1556–1559. [Google Scholar] [CrossRef]
- Gong, W.; Niu, H.L.; Zhang, J.; Song, J.M.; Mao, C.J.; Zhang, S.Y. Synthesis, structure and properties of three isostructure polymer networks based on mixed ligands. Inorg. Chim. Acta 2014, 418, 93–98. [Google Scholar] [CrossRef]
- Abdolalian, P.; Morsali, A.; Bruno, G. Sonochemical synthesis and characterization of microrod to nanoparticle of new mixed-ligand zinc(II) fumarate metal-organic polymer. Ultrason. Sonochem. 2017, 37, 654–659. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Saha, D.; Maity, D.K.; Dey, R.; Ghoshal, D. Syntheses, X-ray structures, gas adsorption and luminescent properties of three coordination polymers of Zn(II) dicarboxylates mixed with a linear, neutral, and rigid N,N ′-donor ligand. Crystengcomm 2014, 16, 4783–4795. [Google Scholar] [CrossRef]
- Burrows, A.D.; Harrington, R.W.; Mahon, M.F.; Price, C.E. The influence of hydrogen bonding on the structure of zinc co-ordination polymers. J. Chem. Soc.-Dalton Trans. 2000, 3845–3854. [Google Scholar] [CrossRef]
- Burke, N.J.; Burrows, A.D.; Donovan, A.S.; Harrington, R.W.; Mahon, M.F.; Price, C.E. Zinc dicarboxylate polymers and dimers: Thiourea substitution as a tool in supramolecular synthesis. Dalton Trans. 2003, 3840–3849. [Google Scholar] [CrossRef]
- Che, G.B.; Liu, B. catena-Poly pyrazino 2,3-f 1,10 phenanthroline zinc(II)-mu(4)-fumara to-mu(2)-fumarato. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2006, 62, M2036–M2038. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jiang, Y.Y.; Yang, J.; Liu, Y.Y.; Ma, J.F. Syntheses, structures and photoluminescence of zinc(II) and silver(I) coordination polymers based on 1,1 ′-(1,4-butanediyl)bis(2-methylbenzimidazole) and different carboxylate ligands. Crystengcomm 2011, 13, 6118–6129. [Google Scholar] [CrossRef]
- Tao, J.; Tong, M.L.; Shi, J.X.; Chen, X.M.; Ng, S.W. Blue photoluminescent zinc coordination polymers with supertetranuclear cores. Chem. Commun. 2000, 2043–2044. [Google Scholar] [CrossRef]
- Uebler, J.W.; Wilson, J.A.; LaDuca, R.L. Donor disposition and aliphatic conformation effects on structure in luminescent zinc dicarboxylate coordination polymers with isomeric dipyridylamide coligands. Crystengcomm 2013, 15, 1586–1596. [Google Scholar] [CrossRef]
- Wang, B.C.; Chen, X.L.; Hu, H.M.; Yao, H.L.; Xue, G.L. Two novel Zn(II) coordination polymers based on trigonal ligand: 4 ′-(4-pyridyl)-3,2 ′:6 ′,3 ″-terpyridine. Inorg. Chem. Commun. 2009, 12, 856–859. [Google Scholar] [CrossRef]
- Zhao, R.L.; Yue, K.F.; Zhou, C.S.; Cheng, Q.D.M.; Shi, J.T.; Liu, Y.L.; Wang, Y.Y. A study of zinc(II) coordination polymers with identical meso-helix based on 1,4-bis(2-methyl-imidazol-1-yl)butane. Inorg. Chim. Acta 2013, 402, 25–32. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Lin, J.L.; Chen, B.Y. New catenary coordination polymers using fumarato ligand as bridging spacer: Crystal structures of Mn(phen)(2)(H2O)(2) L center dot 4H(2)O, Mn(phen)(H2O)(2)L and Zn(phen)L center dot H2L with-H2L fumaric acid. J. Mol. Struct. 2003, 646, 151–159. [Google Scholar] [CrossRef]
- Deng, H.X.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple Functional Groups of Varying Ratios in Metal-Organic Frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef]
- Konar, S.; Zangrando, E.; Drew, M.G.B.; Ribas, J.; Chaudhuri, N.R. Synthesis, structural analysis, and magnetic behaviour of three fumarate bridged coordination polymers: Five-fold interpenetrated diamond-like net of Ni-II, sheets of Ni-II and Co-II. Dalton Trans. 2004, 260–266. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, D.J. catena-poly diaquabis(1H-benzimidazole-kappa N-3)-nickel(II)-mu-fumarato-kappa O-2:O ′. Acta Crystallogr. Sect. E.-Crystallogr. Commun. 2004, 60, M1002–M1004. [Google Scholar] [CrossRef]
- Ohmura, T.; Mori, W.; Hasegawa, M.; Takei, T.; Ikeda, T.; Hasegawa, E. Crystal structures and magnetic and gas-occlusion properties of microporous materials containing infinite chains of mononuclear metal (Cu(II), Zn(II), and Ni(II)) dicarboxylates unit. Bull. Chem. Soc. Jpn. 2003, 76, 1387–1395. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, Q.; Chen, S.P.; Zhang, G.C.; Zhou, C.S.; Gao, S.L. Solid state synthesis, thermodynamics and catalytic combustion effect of a high energy nickel(II) coordination compound. J. Anal. Appl. Pyrolysis 2013, 99, 66–70. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Rizzato, S. New polymeric networks from the self-assembly of silver(I) salts and the flexible ligand 1,3-bis(4-pyridyl)propane (bpp). A systematic investigation of the effects of the counterions and a survey of the coordination polymers base on bpp. Crystengcomm 2002, 4, 121–129. [Google Scholar] [CrossRef]
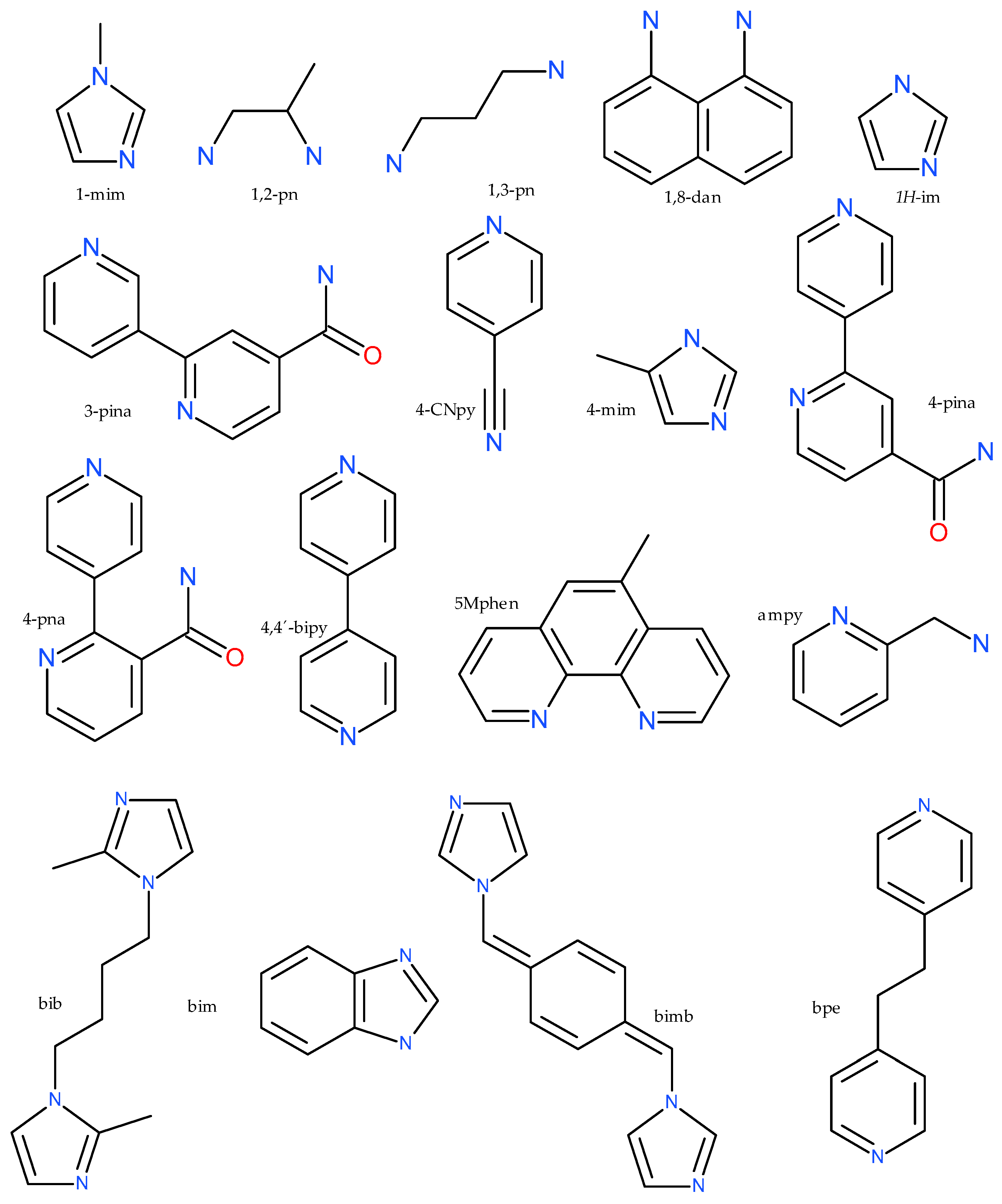
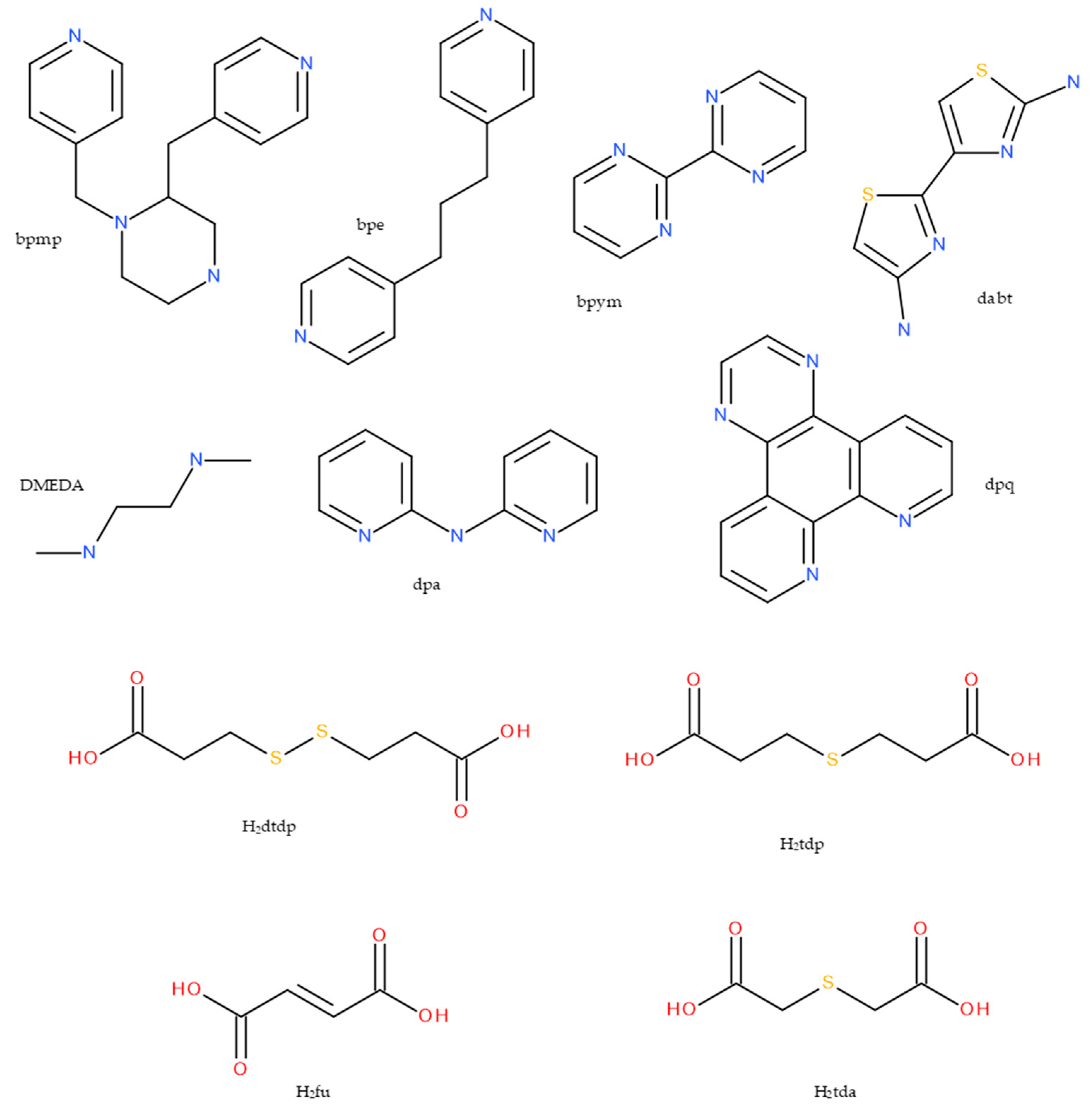
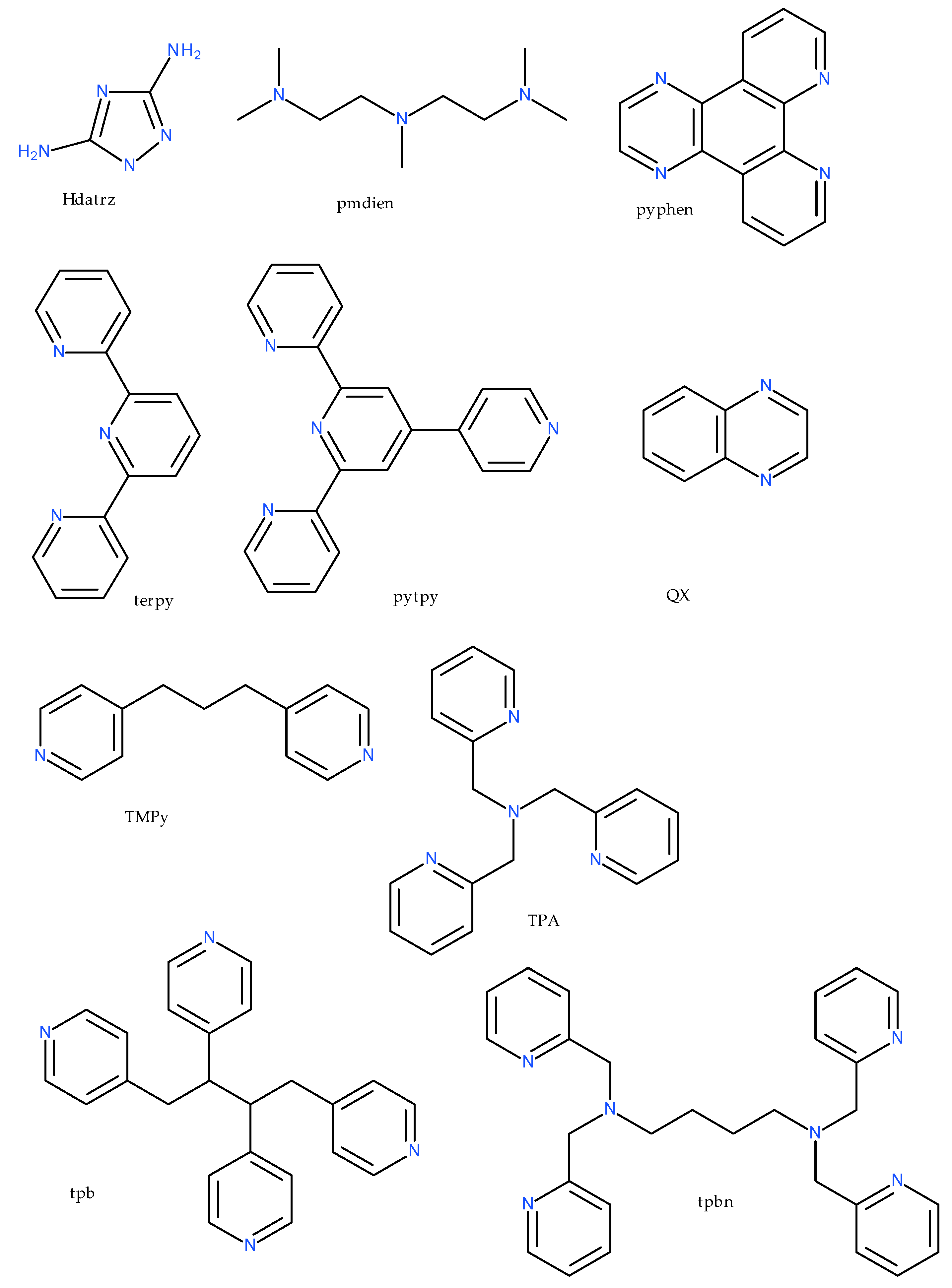


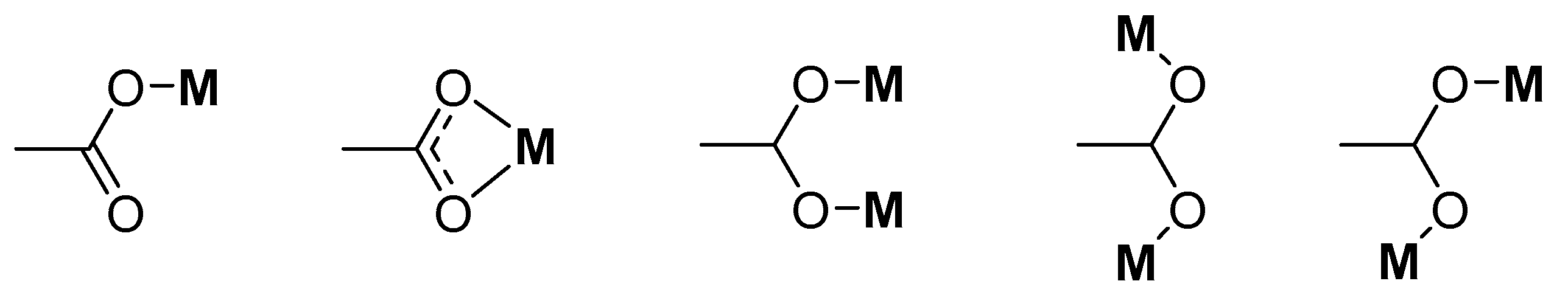
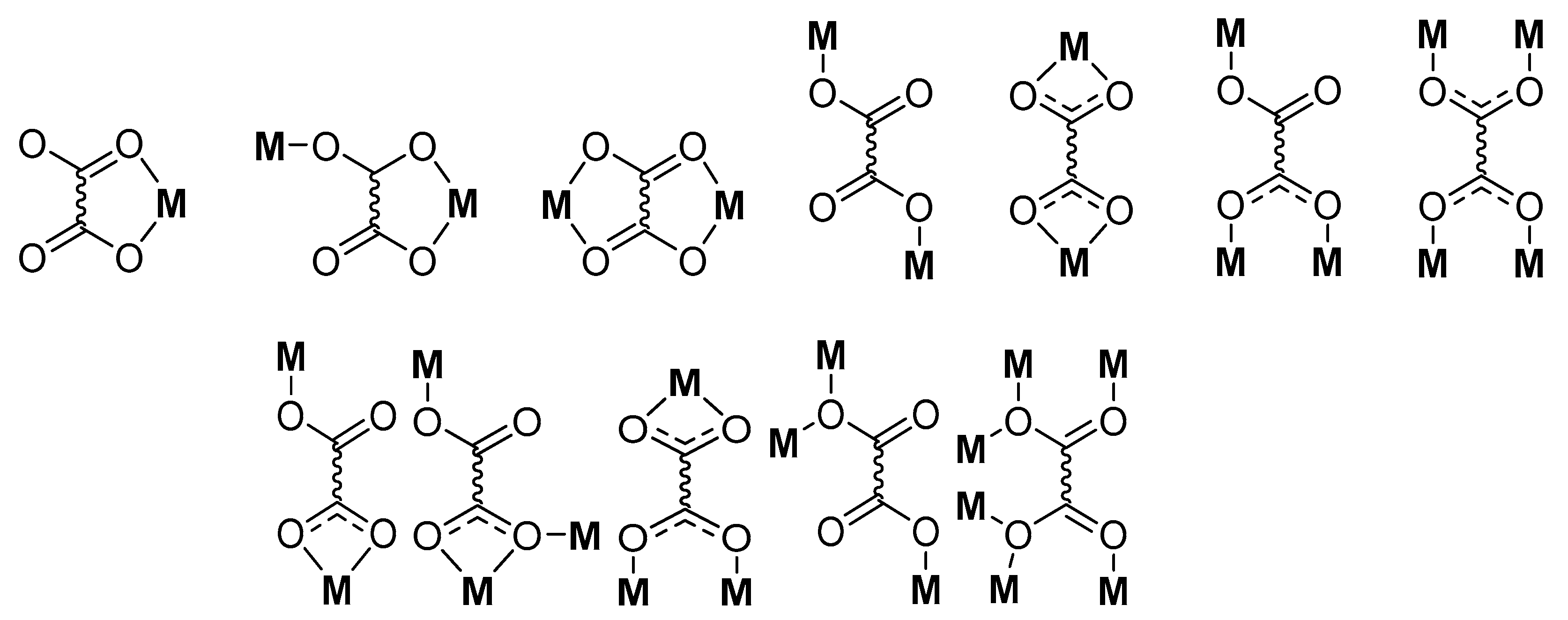
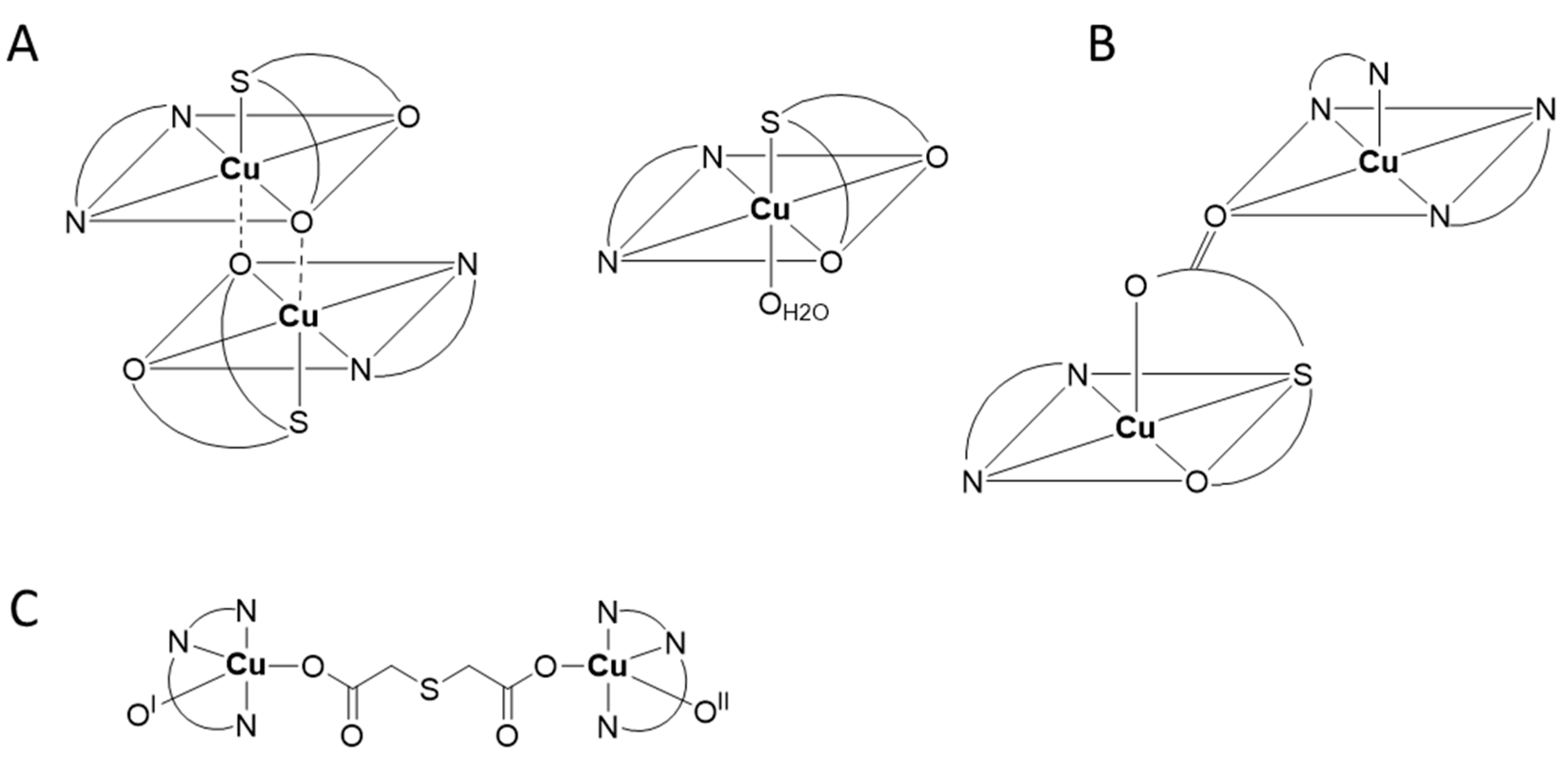





| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Cu(tda)(phen)]2·H2tda | Defor. OC-6 | Stda, Otda(i), OOtda(ii), NNphen | [74] |
| [Cu(tda)(im)2(H2O)] | Defor. OC-6 | Stda, Otda(i), OOtda(ii), NNim | [47] |
| [Cu(tda)(5Mphen)]·2H2O | SPY-5 | Stda, OOtda, NN5Mphen | [47] |
| [Cu(bipy)(tda)(H2O)]·4H2O | Defor. TBPY-5 | Stda, OOtda, NNbipy | [38] |
| [Cu(terpy)(tda)]n | Defor. TBPY-5 | Otda(i), Otda(ii), NNNterpy | [38] |
| [(phen)2Cu(μ-tda)Cu(phen)] (NO3)2 ·5H2O | Cu(1) Defor. SPY-5, Cu(2) TBPY-5—SPY-5 | Cu(1) Otda, NNphen(i), NNphen(ii), Cu(2) Stda, OOtda, NNphen(iii) | [8] |
| [(H2O)(pmdien)Cu(μ-tda)Cu(pmdien) (H2O)] (ClO4)2 | Defor. SPY-5 | Otda, OH2O, NNNpmdien | [8] |
| [Cu(tmen)(tda)]·(H2tmen) (NO3)2·H2O | SPY-5 | OOStda, NNtmen | [57] |
| [Cu4(tpbn)2(tda)2(H2O)4](ClO4)4 | Defor. SPY-5 | Otda, OH2O, NNNtpbn | [75] |
| [Cu4(tpbn)2(tda)2(H2O)4](ClO4)4·4H2 O | Defor. SPY-5 | Otda, OH2O, NNNtpbn | [75] |
| [(phen)2Cu(μ-tda)Cu(phen)] (ClO4)2·1.5H2O | Cu(1) TBPY-5, Cu(2) SPY-5 | Cu(1) OOStda, NNphen, Cu(2) Stda, Otda, NNphen(ii), NNphen(ii) | [73] |
| [Cu(phen)(tda)]·2H2O | SPY-5 | OOStda, NNphen | [73] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| {[Cu(μ3-tdp)(phen)]·2H2O}n | Defor. SPY-5 | Otdp(i), OOtdp(ii), NNphen | [78] |
| {[Cu(μ3-tdp)(bipy)]·H2O}n | SPY-5 | Otdp(i), Otdp(ii), Otdp(iii), NNbipy | [78] |
| [Cu2(μ2-tdp)(phen)4](NO3)2·2H2O | Defor. OC-6 | OOtdp, NNphen(i), NNphen(ii) | [78] |
| [Cu(tdp)(H2O)(bim)3]·4H2O | Defor. OC-6 | Otdp, OH2O, Nbim(i), Nbim(ii) Nbim(iii) | [79] |
| {[Cu(μ2-tdp)(bim)2]·4H2O}n | Defor. OC-6 | OOtdp(i), OOtdp(ii), Nbim(i), Nbim(ii) | [79] |
| [Cu(μ3-tdp)(im)2]n | Defor. SPY-5 | Otdp(i), Otdp(ii), Otdp(iii), Nim(i), Nim(ii) | [46] |
| {[Cu(μ3-tdp)(1-mim)2]·0.5H2O}n | SPY-5 | Otdp(i), Otdp(ii), Otdp(iii), N1-mim(i), N1-mim(ii) | [46] |
| {[Cu2(μ3-tdp)2(4-mim)4]·H2O}n | SPY-5 | Otdp(i), Otdp(ii), Otdp(iii), N4-mim(i), N4-mim(ii) | [46] |
| [Cu2(pmdien)2(H2O)2(μ-tdp)](ClO4)2·H2O | TBPY-5 | Otdp, OH2O, NNNpmdien | [53] |
| {[Cu2(tdp)4(QX)]n·DMF}n | OC-6 | Cu, Otdp(i), Otdp(ii), Otdp(iii), Otdp(iv), NQX | [80] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Cu(dpa)(dtdp)]n | Defor. OC-6 | OOdtdp(i), OOdtdp(ii), NNdpa | [59] |
| [{(phen)Cu}2(μ-dtdp)2]·2H2O | SP-4 | OOdtdp, NNphen | [81] |
| [Cu2(μ-dtdp)(pmdien)2(H2O)2](ClO4)2 | SPY-5 | Odtdp, OH2O, NNNpmdien | [82] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Cu2(dpypda)2(fu)2]n·8nH2O | Defor. OC-6 | Cu, Ofu(i), Ofu(ii), NNNdpypda | [84] |
| [Cu(im)2(H2O)(fu)]n | SPY-5 | Ofu(i), Ofu(ii), OH2O, Nim(i), Nim(ii) | [85] |
| [Cu4(ophen)4(fu)] | SPY-5 | Cu(1) Ofu, Oophen(i), NNophen(ii), Cu(2) | [56] |
| [Cu4(obipy)4(fu)]·6H2O | Cu(1) SPY-5, Cu(2) SP-4 | Cu(1) Ofu, Oobipy(i), NNobipy(ii), Cu(2), Cu(2) Oobipy(iii), NNobipy(iv), Cu(1) | [56] |
| [Cu2(fu)(phen)4](ClO4)2 ·2H2O | Defor. OC-6 | OOfu, NNphen(i), NNphen(ii) | [43] |
| [Cu(fu)(phen)(H2O)]n | Defor. OC-6 | Ofu(i), OOfu(iii), OH2O, NNphen | [43] |
| [Cu2(fu)(bipy)2(H2O)2]n(ClO4)2n | SPY-5 | OOfu(i), OH2O, NNbipy | [43] |
| {[Cu2(L4)2(fu)]·(H2O)·(MeOH)}n | Defor. SPY-5 | OONL4, OOfu | [86] |
| {[Cu(DMEDA)(μ-fu)(μ-H2O)]n | OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), NNDMEDA | [52] |
| [Cu2(pmdien)2(μ-fu)(H2O)2](ClO4)2 | SPY-5 | Ofu, OH2O, NNNpmdien | [45] |
| [Cu2(TPA)2(μ-fu)](ClO4)2·2H2O | TBPY-5 | Ofu, NNNNTPA | [45] |
| [Cu(fu)(pyphen)]n | Defor. TBPY-5 | Ofu(i), Ofu(ii), Ofu(iii), NNpyphen | [87] |
| [Cu2(fu)2(bimb)2(H2O)2]n | SPY-5 | Ofu(i), Ofu(ii), OH2O, NNbimb | [44] |
| [Cu(dmbipy)(fu)(H2O)]n | OC-6 | OOfu(i), Ofu(ii), OH2O, NNdmbipy | [88] |
| [Cu2(fu)(μ-OH)(bipy)2(H2O)] | SPY-5 | Ofu, OH2O, OOH, NNbipy | [89] |
| [Cu(fu)(tmen)]·2H2O | SP-4 | Ofu(i), Ofu(ii), NNtmen | [89] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Zn(bipy)(tda)(H2O)]·4H2O | OC-6 | OOStda, OH2O, NNbipy | [55] |
| [Zn(tda)(phen)]2·5H2O | Defor.OC-6 | OOStda(i), Otda(ii), NNphen | [63] |
| [Zn(bib)(tda)]n | Defor. T-4 | Otda(i), Otda(ii), Nbib(i), Nbib(ii) | [90] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [{Zn(phen)(H2O)}2(μ-tdp)2]·3H2O | Defor. OC-6 | Otdp(i), OOtdp(ii), OH2O, NNphen | [92] |
| {[Zn(tdp)(bpe)]·2H2O}n | T-4 | Otdp(i), Otdp(ii), Nbpe(i), Nbpe(ii) | [61] |
| [Zn(tdp)(bpp)]n | T-4 | Otdp(i), Otdp(ii), Nbpp(i), Nbpp(ii) | [61] |
| [Zn(tdp)(bpypa)]n | T-4 | Otdp(i), Otdp(ii), Nbpypa(i), Nbpypa(ii) | [61] |
| [Zn(tdp)2(TMPy)2]n | SPY-5 | Otdp(i), OOtdp(ii), NTMPy(i), NTMPy(ii) | [91] |
| [Zn(tdp)(bipy)(H2O)]n | Defor. OC-6 | OOtdp(i), Otdp(ii), OH2O, NNbipy | [62] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Zn(fu)(datrz)2]n | T-4 | Ofu, Ndatrz(i), Ndatrz(ii), Ndatrz(iii) | [93] |
| [Zn(fu)(bib)(H2O)]n | Defor.OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), Nbib(i), Nbib(ii) | [94] |
| {[Zn(tptz)(fu)]·DMF}n | TBPY-5 | Ofu(i), Ofu(ii), NNNtptz | [95] |
| {[Zn2(azbipy)2(fu)2]·H2O}n | SPY-5 | Zn(1) OOfu(i), Ofu(ii), Nazbipy, Nazbipy(ii), Zn(2) OOfu(i), OOfu(ii), Nazbipy, Nazbipy(ii) | [96] |
| [Zn(tu)2(μ-fu)] | T-4 | Ofu(i), Ofu(ii), Stu(i), Stu(ii) | [97] |
| [Zn{tu(Me)2}2(fu)]n | T-4 | Ofu(i), Ofu(ii), Stu(i), Stu(ii) | [98] |
| [Zn(fu)(bpmp)(H2O)2] | OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), Nbpmp(i), Nbpmp(ii) | [51] |
| [Zn(μ-fu)(L5)]n | SPY-5 | Ofu(i), Ofu(ii), NNNL5 | [64] |
| [Zn(fu)(pyphen)]n | Defor. TBPY-5 | Ofu(i), Ofu(ii), Ofu(iii), NNpyphen | [99] |
| [Zn(tpb)(fu)]·2H2O | SP-4 | Ofu(i), Ofu(ii), Ntpb(i), Ntpb(ii) | [67] |
| [Zn(fu)(bbmi)0,5(H2O)] | SPY-5 | Ofu(i), Ofu(ii), Ofu(iii), OH2O, Nbbmi | [100] |
| [Zn4(OH)2(fu)3(4,4′-bipy)] | Zn(1) OC-6, Zn(2) T-4 | Zn(1) Ofu(i), OOfu(ii), OOH(i), OOH(ii), N4,4′-bipy, Zn(2) Ofu(i), Ofu(ii), OOH, N4,4′-bipy | [101] |
| [Zn(H2O)2(dbipy)(fu)] | Defor. TPR-6 | OOfu(i), OH2O(i), OH2O(ii), NNdbipy | [49] |
| {[Zn(fu)(3-pina)]·1.5H2O}n | T-4 | Ofu(i), Ofu(ii), N3-pina(i), N3-pina(ii) | [102] |
| [Zn(fu)(4-pna)]n | Defor. SPY-5 | OOfu(i), Ofu(ii), N4-pna(i), N4-pna(ii) | [102] |
| [Zn(fu)(4-pina)]n | Defor. TBPY-5 | Ofu(i), Ofu(ii), Ofu(iii), N4-pina(i), N4-pina(ii) | [102] |
| [Zn5(pytpy)8(fu)4(H2O)4(OH)2]n·n(CH3OH)·2n(H2O) | Zn(1) and Zn(3) Defor. OC-6, Zn(2) Defor. TBPY-5 | Zn(1) a Zn(3) Ofu(i), Ofu(ii), Ofu(iii), Ofu(iv), Npytpy(i), Npytpy(ii), Zn(2) Ofu(i), Ofu(ii), Ofu(iii), Npytpy(i), Npytpy(ii) | [103] |
| [Zn(bpe)(fu)]n | T-4 | Ofu(i), Ofu(ii), Nbpe(i), Nbpe(ii) | [68] |
| {[Zn(bib)(fu)]·CH3OH}n | T-4 | Ofu(i), Ofu(ii), Nbib(i), Nbib(ii) | [104] |
| [Zn(phen(fu)]n | T-4 | Ofu(i), Ofu(ii), NNphen | [105] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Ni(tda)(dabt)(H2O)]·H2O | Defor. OC-6 | OOStda, OH2O, NNdabt | [54] |
| [Ni(tda))(1H-im)3]·H2O | Defor. OC-6 | OOStda, N1H-im(i), N1H-im(ii), N1H-im(iii) | [72] |
| [Ni(bipy)(tda)(H2O)]·4H2O | Defor. OC-6 | OOStda, OH2O, NNbipy | [7] |
| [(en)Ni(μ-tda)2Ni(en)]·4H2O | Defor. OC-6 | OOStda, NNen | [7] |
| [Ni2(pmdien)2(H2O)2(μ-tda)](ClO4)2·H2O | Defor. OC-6 | OOtda, OH2O, NNNpmdien | [41] |
| [Ni(tda)(1,3-pn)(H2O)]·H2O | Defor. OC-6 | OOStda, OH2O, NN1,3-pn | [82] |
| [Ni2(μ-tda)2(1,2-pn)2] | Defor. OC-6 | OOStda, NN1,2-pn | [82] |
| Compound | Coordinating Polyhedron | Donor Set | Literature |
|---|---|---|---|
| [Ni(μ-fu)(4-Cnpy)2(H2O)2]n | OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), N4-Cnpy(i), N4-Cnpy(ii) | [40] |
| [Ni(fu)(nam)2(H2O)2]n | Defor. OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), Nnam(i), Nnam(ii) | [66] |
| [Ni(fu)(bpe)] | Defor. OC-6 | OOfu(i), OOfu(ii), Nbpe(i), Nbpe(ii) | [107] |
| [Ni(fu)(bpp)(H2O)] | Defor. OC-6 | OOfu(i), Ofu(ii), OH2O, Nbpp(i), Nbpp(ii) | [107] |
| [Ni(fu)(bim)(H2O)2] | OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), Nbim(i), Nbim(ii) | [108] |
| [Ni2(μ-fu)(phen)4(H2O)2] ·fu·16H2O | OC-6 | Ofu, OH2O, NNphen(i), NNphenii) | [71] |
| [Ni2(ntb)2(μ-fu)(H2O)(CH3OH)] (NO3)2·6CH3OH·H2O | OC-6 | Ni(1) Ofu, OCH3OH, NNNNntb(i), Ni(2) Ofu, OH2O, NNNNntb(ii) | [65] |
| [Ni(μ-fu)(py)3]·py·2H2O | OC-6 | OOfu(i), Ofu(ii), Npy(i), Npy(ii), Npy(iii) | [109] |
| [Ni(fu)(dpa)(H2O)2·4H2O]n | Defor. OC-6 | Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), NNdpa | [39] |
| [Ni2(μ-bpym)(fu)2(H2O)6]·5H2O | Defor. OC-6 | Ofu, OH2O(i), OH2O(ii), OH2O(iii), NNdpa | [69] |
| [Ni(fu)(phen)(H2O)]n | Defor. OC-6 | OOfu(i), Ofu(ii), OH2O(i), NNphen | [70] |
| [Ni3(Hdatrz)6(fu)2(H2O)4]fu·11H2O | Ni(1), Ni(2) and Ni(3) Defor.OC-6 | Ni(1) a (3) Ofu(i), Ofu(ii), OH2O(i), OH2O(ii), NNNHdatrz, Ni(2) NHdatrz(i), NHdatrz(ii), NHdatrz(iii), NHdatrz(iv), NHdatrz(v), NHdatrz(vi) | [110] |
| [Ni2(fu)(OH)2(dpa)2]·2H2O]n | Defor. SPY-5 | Ofu, OOH(i), OOH(ii), NNdpa | [42] |
| Compound | Biological Activity Studied | Stability in Solution | Literature |
|---|---|---|---|
| [(phen)2Cu(μ-tda)Cu(phen)](ClO4)2·1.5H2O | Inhibition zone, B. subtilis, S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa | UV-vis, CV | [73] |
| [Cu(phen)(tda)]·2H2O | Inhibition zone, B. subtilis, S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa | UV-vis, CV | [73] |
| [Cu2(pmdien)2(H2O)2(μ-tdp)](ClO4)2·H2O | E. coli, S. aureus, MRSA, MDA-MB-231, HBL-100, p53, DNA binding, ROS | UV-vis | [53] |
| [{(phen)Cu}2(μ-dtdp)2]·2H2O | photoinduced DNA cleavage activity, ROS, CT-DNA binding | UV-vis | [81] |
| [Cu2(μ-dtdp)(pmdien)2(H2O)2](ClO4)2 | E. faecalis, S. aureus, P. aeruginosa, E. coli | UV-vis | [82] |
| {[Cu2(L4)2(fu)]·(H2O)·(MeOH)}n | BSA, HSA, CT-DNA binding | UV-vis, fluorescence spectroscopy | [86] |
| [Ni(tda)(1,3-pn)(H2O)]·H2O | E. faecalis, S. aureus, P. aeruginosa, E. coli | UV-vis | [82] |
| [Ni2(μ-tda)2(1,2-pn)2] | E. faecalis, S. aureus, P. aeruginosa, E. coli | UV-vis | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loubalová, I.; Kopel, P. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules 2023, 28, 1445. https://doi.org/10.3390/molecules28031445
Loubalová I, Kopel P. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules. 2023; 28(3):1445. https://doi.org/10.3390/molecules28031445
Chicago/Turabian StyleLoubalová, Ivana, and Pavel Kopel. 2023. "Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review" Molecules 28, no. 3: 1445. https://doi.org/10.3390/molecules28031445
APA StyleLoubalová, I., & Kopel, P. (2023). Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules, 28(3), 1445. https://doi.org/10.3390/molecules28031445









