Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis
Abstract
:1. Introduction
2. Methodology
3. Results
3.1. CO2 Adsorption on TiO2 Surfaces
3.2. CO2 Adsorption on SnO2, GeO2, PbO2 and IrO2 Rutile-Type Surfaces
3.3. Charge Redistribution and Effects over CO2
3.4. Water Molecules Dissociation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Quéré, C.; Takahashi, T.; Buitenhuis, E.T.; Rödenbeck, C.; Sutherland, S.C. Impact of climate change and variability on the global oceanic sink of CO2. Glob. Biogeochem. Cycles 2010, 24, 1–10. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, H.; Wei, Y.-M.; Li, Y.-M. The influence of climate change on CO2 (carbon dioxide) emissions: An empirical estimation based on Chinese provincial panel data. J. Clean. Prod. 2016, 131, 667–677. [Google Scholar] [CrossRef]
- Shine Keith, P.; Sturges William, T. CO2 Is Not the Only Gas. Science 2007, 315, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- Dellink, R.; Lanzi, E.; Chateau, J. The Sectoral and Regional Economic Consequences of Climate Change to 2060. Environ. Resour. Econ. 2019, 72, 309–363. [Google Scholar] [CrossRef]
- Socolow, R.; Hotinski, R.; Greenblatt, J.B.; Pacala, S. Solving the Climate Problem: Technologies Available to Curb CO2 Emissions. Environ. Sci. Policy Sustain. Dev. 2004, 46, 8–19. [Google Scholar] [CrossRef]
- Steinberg, M.; Cheng, H.C. Advanced technologies for reduced CO2 emissions. In Proceedings of the Annual Meeting of Air Pollution Control Association, Dallas, TX, USA, 19–24 June 1988. [Google Scholar]
- Ward, H.; Radebach, A.; Vierhaus, I.; Fügenschuh, A.; Steckel, J.C. Reducing global CO2 emissions with the technologies we have. Resour. Energy Econ. 2017, 49, 201–217. [Google Scholar] [CrossRef]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic Chemical Carbon Cycle for a Sustainable Future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef]
- Surdhar, P.S.; Mezyk, S.P.; Armstrong, D.A. Reduction potential of the carboxyl radical anion in aqueous solutions. J. Phys. Chem. 1989, 93, 3360–3363. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Deng, X.; Li, Z. Metal–organic frameworks (MOFs) for photocatalytic CO2 reduction. Catal. Sci. Technol. 2017, 7, 4893–4904. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Wu, J.; Zhao, R.; Lu, Y.; Xin, B. Controlled facile synthesis and photocatalytic activity of ultrafine high crystallinity TiO2 nanocrystals with tunable anatase/rutile ratios. Appl. Surf. Sci. 2014, 294, 36–41. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase–brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Handoko, A.D.; Li, K.; Tang, J. Recent progress in artificial photosynthesis: CO2 photoreduction to valuable chemicals in a heterogeneous system. Curr. Opin. Chem. Eng. 2013, 2, 200–206. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Omae, I. Aspects of carbon dioxide utilization. Catal. Today 2006, 115, 33–52. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, Y. Theoretical Study on the Mechanism of Photoreduction of CO2 to CH4 on the Anatase TiO2(101) Surface. ACS Catal. 2016, 6, 2018–2025. [Google Scholar] [CrossRef]
- Pipornpong, W.; Wanbayor, R.; Ruangpornvisuti, V. Adsorption CO2 on the perfect and oxygen vacancy defect surfaces of anatase TiO2 and its photocatalytic mechanism of conversion to CO. Appl. Surf. Sci. 2011, 257, 10322–10328. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Ehara, S. Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J. Electroanal. Chem. 1995, 396, 21–26. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Umezawa, N.; Kristoffersen, H.H.; Vilhelmsen, L.B.; Hammer, B. Reduction of CO2 with Water on Pt-Loaded Rutile TiO2(110) Modeled with Density Functional Theory. J. Phys. Chem. C 2016, 120, 9160–9164. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy Positions of Oxide Semiconductors and Photocatalysis with Iron Complex Oxides. J. Solid State Chem. 1996, 126, 227–234. [Google Scholar] [CrossRef]
- Peng, C.; Reid, G.; Wang, H.; Hu, P. Perspective: Photocatalytic reduction of CO2 to solar fuels over semiconductors. J. Chem. Phys. 2017, 147, 030901. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Wu, H.-L.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Semiconductor Quantum Dots: An Emerging Candidate for CO2 Photoreduction. Adv. Mater. 2019, 31, 1900709. [Google Scholar] [CrossRef]
- Yan, Y.-l.; Fang, Q.-J.; Pan, J.-k.; Yang, J.; Zhang, L.-l.; Zhang, W.; Zhuang, G.-l.; Zhong, X.; Deng, S.-w.; Wang, J.-g. Efficient photocatalytic reduction of CO2 using Fe-based covalent triazine frameworks decorated with in situ grown ZnFe2O4 nanoparticles. Chem. Eng. J. 2021, 408, 127358. [Google Scholar] [CrossRef]
- Krischok, S.; Höfft, O.; Kempter, V. The chemisorption of H2O and CO2 on TiO2 surfaces: Studies with MIES and UPS (HeI/II). Surf. Sci. 2002, 507–510, 69–73. [Google Scholar] [CrossRef]
- Liang, L.; Ling, P.; Li, Y.; Li, L.; Liu, J.; Luo, Q.; Zhang, H.; Xu, Q.; Pan, Y.; Zhu, J.; et al. Atmospheric CO2 capture and photofixation to near-unity CO by Ti3+-Vo-Ti3+ sites confined in TiO2 ultrathin layers. Sci. China Chem. 2021, 64, 953–958. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Al-Saidi, W.A.; Jordan, K.D. CO2 adsorption on TiO2(101) anatase: A dispersion-corrected density functional theory study. J. Chem. Phys. 2011, 135, 124701. [Google Scholar] [CrossRef] [PubMed]
- Mino, L.; Spoto, G.; Ferrari, A.M. CO2 Capture by TiO2 Anatase Surfaces: A Combined DFT and FTIR Study. J. Phys. Chem. C 2014, 118, 25016–25026. [Google Scholar] [CrossRef]
- Li, H.; Rameshan, C.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Rupprechter, G. CO2 activation on ultrathin ZrO2 film by H2O co-adsorption: In situ NAP-XPS and IRAS studies. Surf. Sci. 2019, 679, 139–146. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Cadi-Essadek, A.; Roldan, A.; de Leeuw, N.H. Density functional theory study of the interaction of H2O, CO2 and CO with the ZrO2 (111), Ni/ZrO2 (111), YSZ (111) and Ni/YSZ (111) surfaces. Surf. Sci. 2016, 653, 153–162. [Google Scholar] [CrossRef]
- Chen, H.-Y.T.; Tosoni, S.; Pacchioni, G. A DFT study of the acid–base properties of anatase TiO2 and tetragonal ZrO2 by adsorption of CO and CO2 probe molecules. Surf. Sci. 2016, 652, 163–171. [Google Scholar] [CrossRef]
- Bendavid, L.I.; Carter, E.A. CO2 Adsorption on Cu2O(111): A DFT+U and DFT-D Study. J. Phys. Chem. C 2013, 117, 26048–26059. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, L.; Li, P.; Zhang, B.; Zhao, G.; He, T. A computational study on linear and bent adsorption of CO2 on different surfaces for its photoreduction. Catal. Today 2019, 335, 278–285. [Google Scholar] [CrossRef]
- Tang, Q.-L.; Luo, Q.-H. Adsorption of CO2 at ZnO: A Surface Structure Effect from DFT+U Calculations. J. Phys. Chem. C 2013, 117, 22954–22966. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. A Theoretical Study of CO2 Anions on Anatase (101) Surface. J. Phys. Chem. C 2010, 114, 21474–21481. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Liang, Z.; Yu, Z.; Zhang, J.; Janik, M.J.; Guo, X.; Song, C. Comparative computational study of CO2 dissociation and hydrogenation over Fe-M (M = Pd, Ni, Co) bimetallic catalysts: The effect of surface metal content. J. CO2 Util. 2019, 29, 179–195. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Peng, X.; Liu, L.; Li, Y.; Andino, J.M. A Density Functional Theory and Experimental Study of CO2 Interaction with Brookite TiO2. J. Phys. Chem. C 2012, 116, 19755–19764. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Schobert, H.H.; Kubicki, J.D. Quantum Mechanical Modeling of CO2 Interactions with Irradiated Stoichiometric and Oxygen-Deficient Anatase TiO2 Surfaces: Implications for the Photocatalytic Reduction of CO2. Energy Fuels 2009, 23, 5247–5256. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jiang, C. Chapter 1—Principle and surface science of photocatalysis. In Interface Science and Technology; Yu, J., Jaroniec, M., Jiang, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 1–38. [Google Scholar]
- Zhang, L.; Jaroniec, M. Chapter 2—Fundamentals of adsorption for photocatalysis. In Interface Science and Technology; Yu, J., Jaroniec, M., Jiang, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 39–62. [Google Scholar]
- Huygh, S.; Bogaerts, A.; Neyts, E.C. How Oxygen Vacancies Activate CO2 Dissociation on TiO2 Anatase (001). J. Phys. Chem. C 2016, 120, 21659–21669. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; te Velde, G.; Baerends, E.J. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar] [CrossRef]
- ADF2014, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com/ (accessed on 2 May 2022).
- te Velde, G.; Baerends, E.J. Precise density-functional method for periodic structures. Phys. Rev. B 1991, 44, 7888–7903. [Google Scholar] [CrossRef]
- Wiesenekker, G.; Baerends, E.J. Quadratic integration over the three-dimensional Brillouin zone. J. Phys. Condens. Matter 1991, 3, 6721–6742. [Google Scholar] [CrossRef]
- Franchini, M.; Philipsen, P.H.T.; Visscher, L. The Becke Fuzzy Cells Integration Scheme in the Amsterdam Density Functional Program Suite. J. Comput. Chem. 2013, 34, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Philipsen, P.H.T.; van Lenthe, E.; Visscher, L. Accurate Coulomb Potentials for Periodic and Molecular Systems through Density Fitting. J. Chem. Theory Comput. 2014, 10, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Erratum: Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces [Phys. Rev. Lett. 100, 136406 (2008)]. Phys. Rev. Lett. 2009, 102, 039902. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic total energy using regular approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- van Lenthe, E.; van Leeuwen, R.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. Int. J. Quantum Chem. 1996, 57, 281–293. [Google Scholar] [CrossRef]
- van Lenthe, E.; Snijders, J.G.; Baerends, E.J. The zero-order regular approximation for relativistic effects: The effect of spin–orbit coupling in closed shell molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Kadish, K.M.; Boulas, P.; Chen, E.C.M. Relationship between the Electron Affinities and Half-Wave Reduction Potentials of Fullerenes, Aromatic Hydrocarbons, and Metal Complexes. J. Phys. Chem. 1995, 99, 8843–8850. [Google Scholar] [CrossRef]
- Conradie, J. A Frontier orbital energy approach to redox potentials. J. Phys. Conf. Ser. 2015, 633, 012045. [Google Scholar] [CrossRef]
- Bateni, S.B.; England, K.R.; Galatti, A.T.; Kaur, H.; Mendiola, V.A.; Mitchell, A.R.; Vu, M.H.; Gherman, B.F.; Miranda, J.A. Prediction of reduction potentials from calculated electron affinities for metal-salen compounds. Beilstein J. Org. Chem. 2009, 5, 82. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, R.Q.; Ng, A.M.C.; Djurišić, A.B.; Chan, H.T.; Chan, W.K.; Tong, S.Y. Splitting Water on Metal Oxide Surfaces. J. Phys. Chem. C 2011, 115, 19710–19715. [Google Scholar] [CrossRef]
- Verdaguer, A.; Sacha, G.M.; Bluhm, H.; Salmeron, M. Molecular Structure of Water at Interfaces: Wetting at the Nanometer Scale. Chem. Rev. 2006, 106, 1478–1510. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Thiel, P.A.; Madey, T.E. The interaction of water with solid surfaces: Fundamental aspects. Surf. Sci. Rep. 1987, 7, 211–385. [Google Scholar] [CrossRef]
- Zwicker, G.; Jacobi, K. Site-specific interaction of H2O with ZnO single-crystal surfaces studied by thermal desorption and UV photoelectron spectroscopy. Surf. Sci. 1983, 131, 179–194. [Google Scholar] [CrossRef]
- Henrich, V.E. Ultraviolet photoemission studies of molecular adsorption on oxide surfaces. Prog. Surf. Sci. 1979, 9, 143–164. [Google Scholar] [CrossRef]
- McKay, J.M.; Henrich, V.E. Surface electronic structure of NiO: Defect states, O2 and H2O interactions. Phys. Rev. B 1985, 32, 6764–6772. [Google Scholar] [CrossRef] [PubMed]
- Gercher, V.A.; Cox, D.F. Water adsorption on stoichiometric and defective SnO2(110) surfaces. Surf. Sci. 1995, 322, 177–184. [Google Scholar] [CrossRef]
- Lindan, P.J.D. Water chemistry at the SnO2(110) surface: The role of inter-molecular interactions and surface geometry. Chem. Phys. Lett. 2000, 328, 325–329. [Google Scholar] [CrossRef]
- Slater, B.; Richard, C.; Catlow, A.; Williams, D.E.; Stoneham, A.M. Competitive Adsorption of O2 and H2O at the Neutral and Defective SnO2 (110) Surface. MRS Online Proc. Libr. 2001, 658, 933. [Google Scholar] [CrossRef]


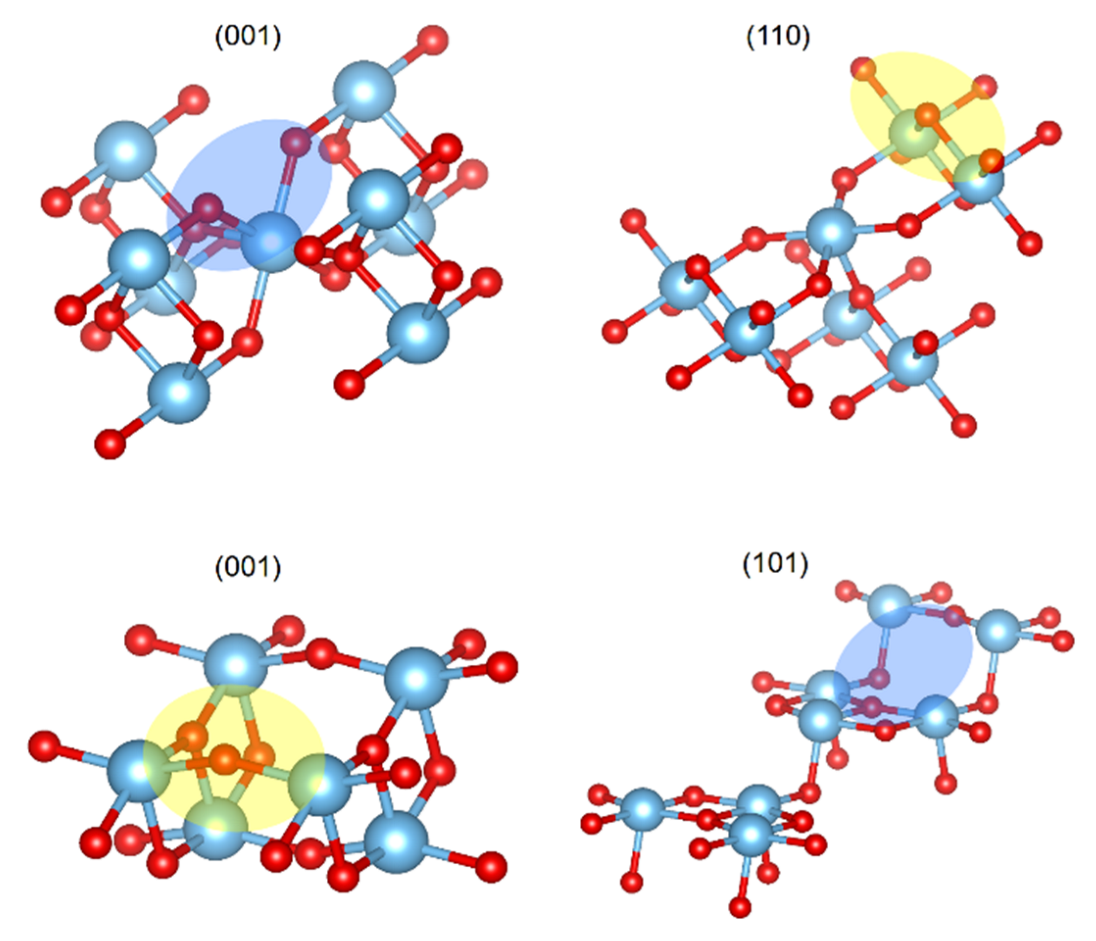
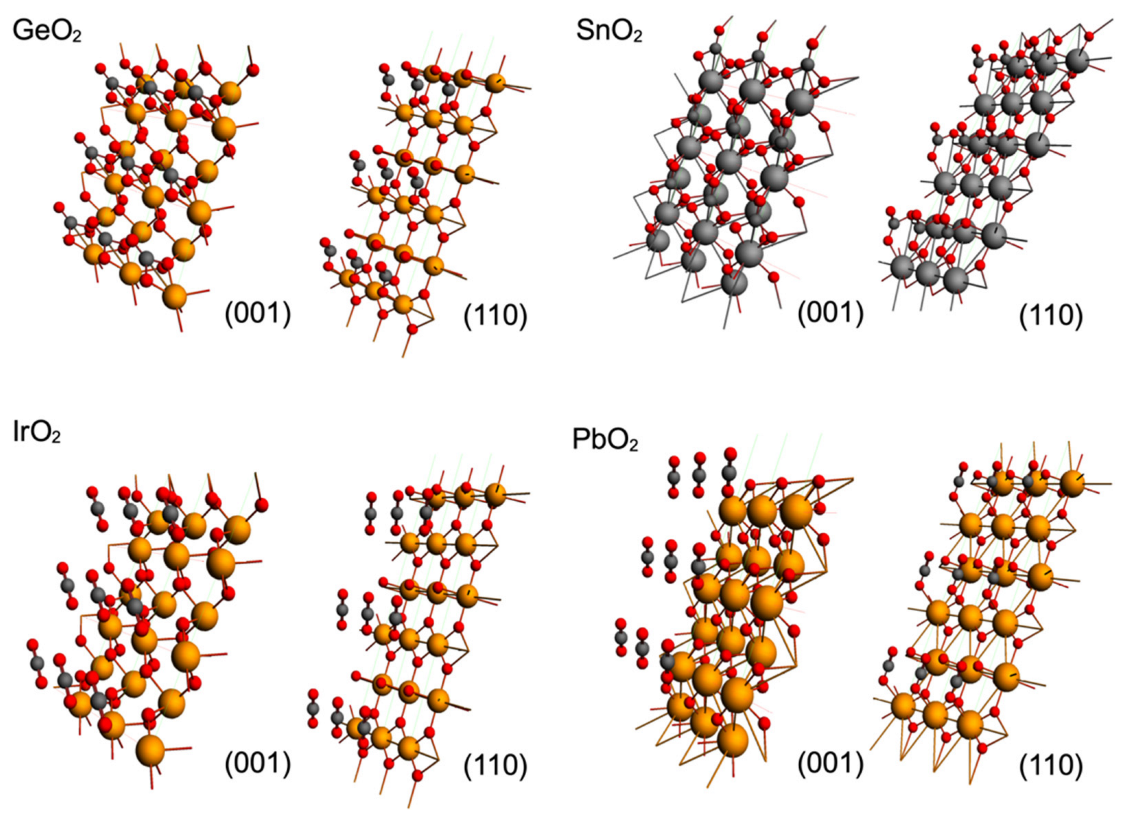
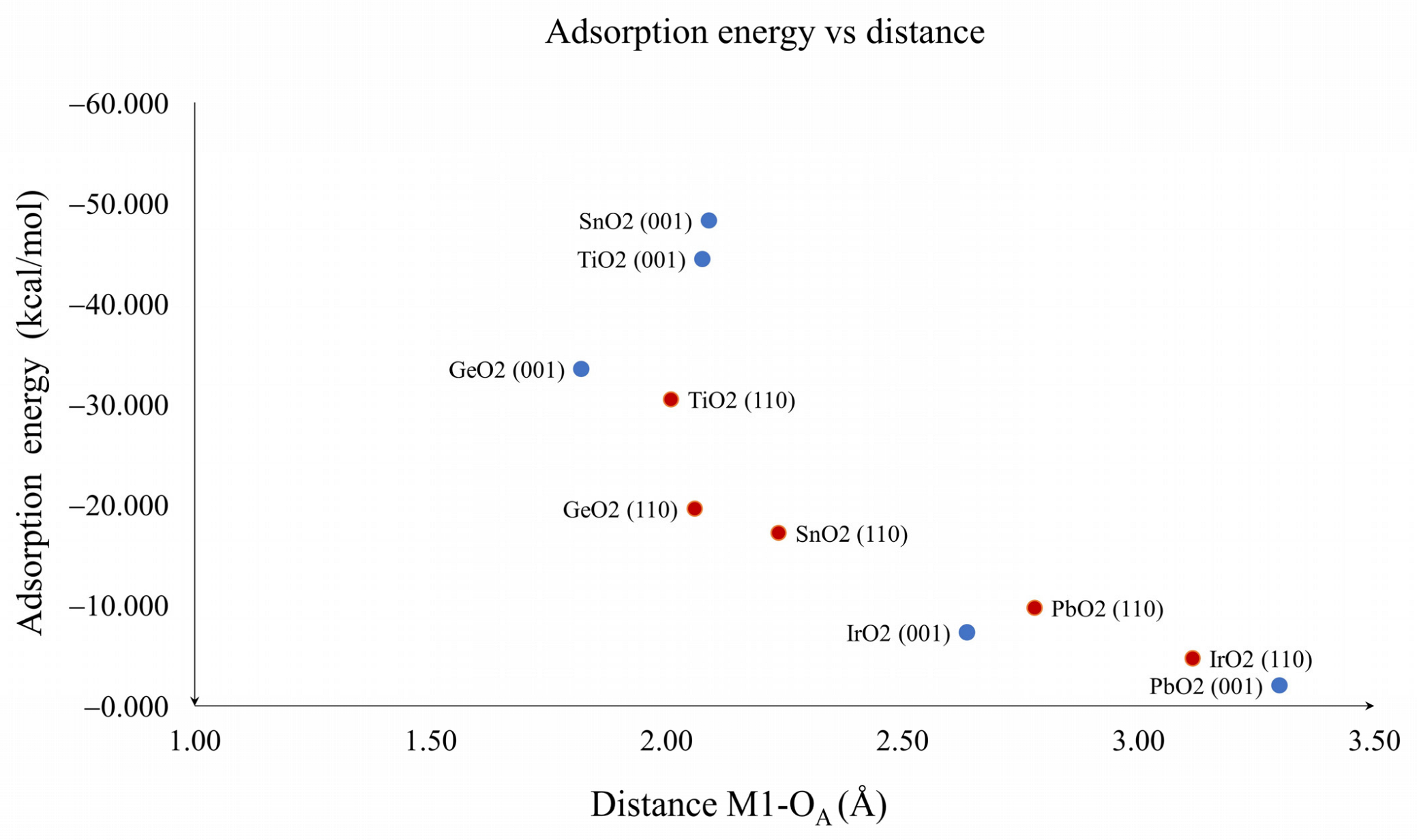
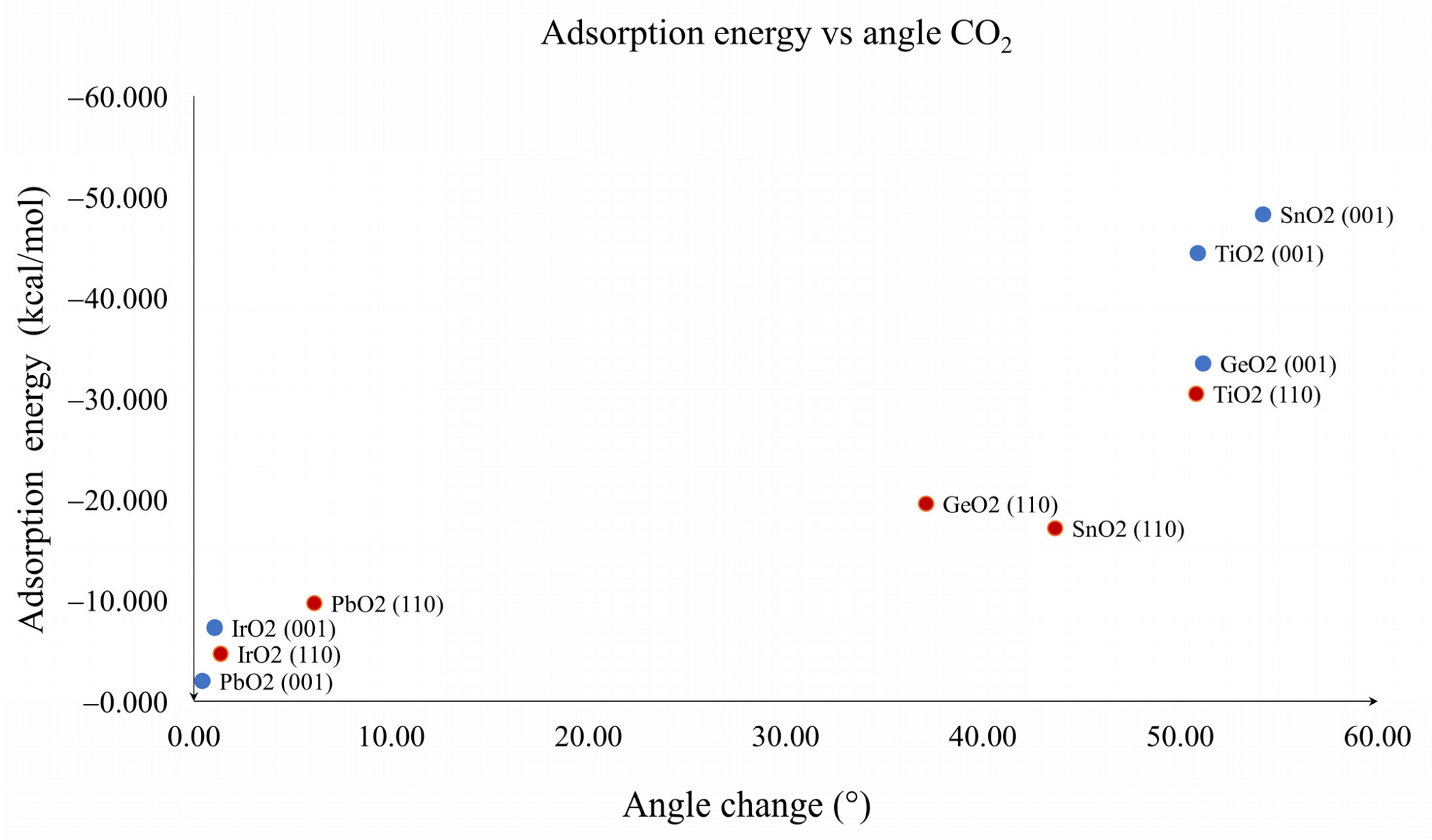
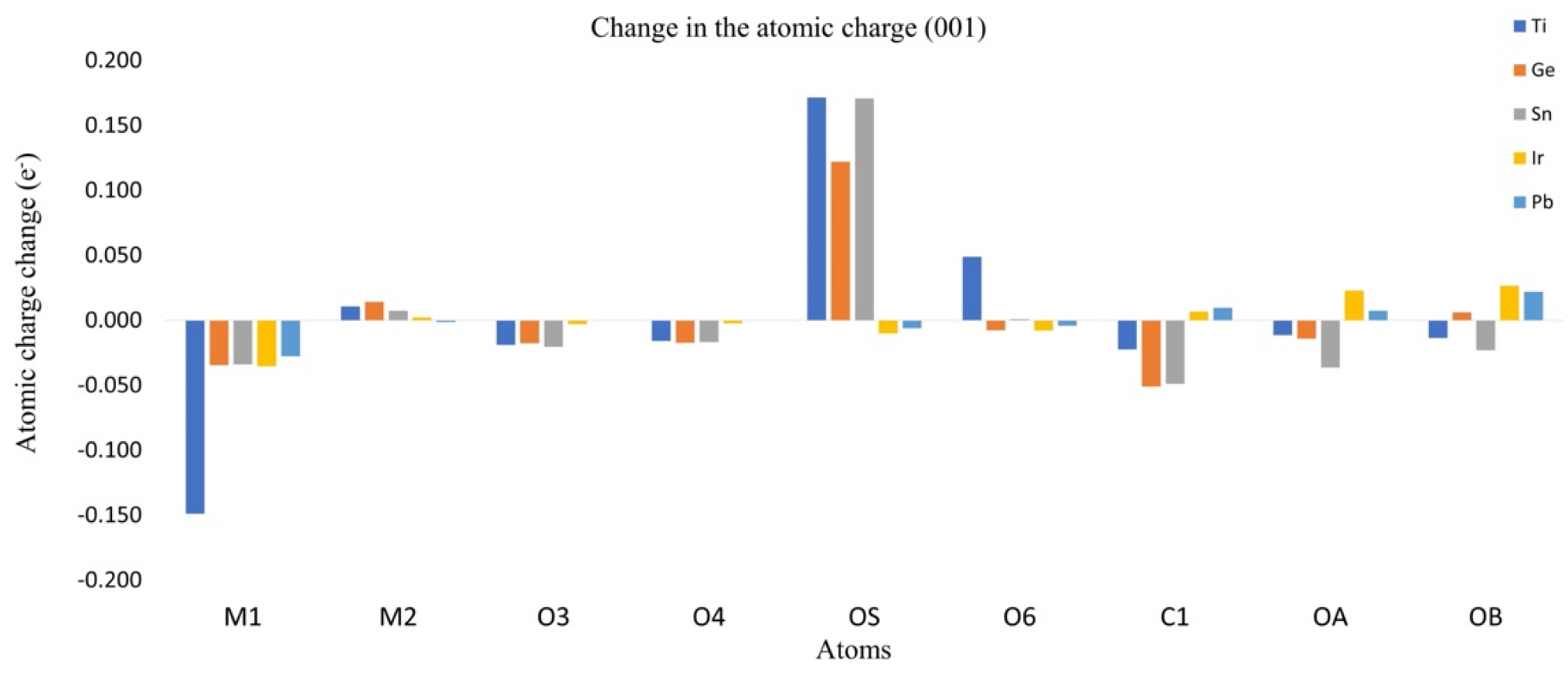

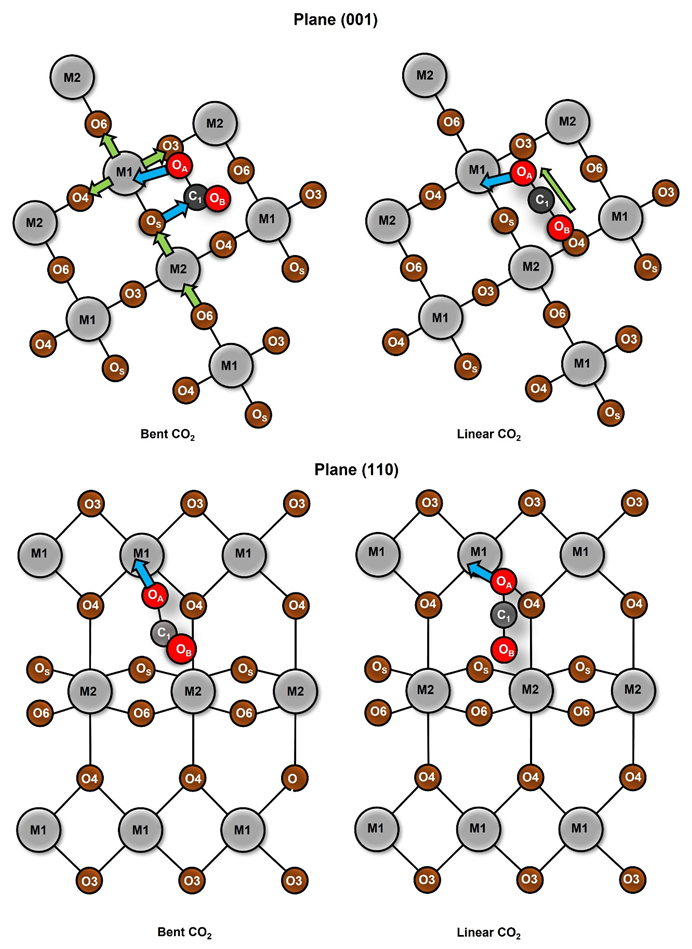

| Oxide | Plane (001) (kcal/mol) | Plane (110) (kcal/mol) |
|---|---|---|
| TiO2 | −44.420 | −30.450 |
| GeO2 | −33.447 | −19.540 |
| SnO2 | −48.242 | −17.113 |
| IrO2 | −7.249 | −4.671 |
| PbO2 | −1.982 | −9.663 |
| Oxide | Angle O-C-O | Distance C-OA (Å) | Distance C-OB (Å) | Distance C-OS (Å) | Distance M-OA (Å) |
|---|---|---|---|---|---|
| TiO2 (001) | 129.1 | 1.27 | 1.24 | 1.39 | 2.08 |
| TiO2 (110) | 129.2 | 1.31 | 1.19 | 1.60 | 2.01 |
| GeO2 (001) | 128.8 | 1.35 | 1.19 | 1.44 | 1.82 |
| GeO2 (110) | 142.9 | 1.25 | 1.17 | 1.94 | 2.06 |
| SnO2 (001) | 125.8 | 1.29 | 1.24 | 1.40 | 2.09 |
| SnO2 (110) | 136.3 | 1.27 | 1.19 | 1.74 | 2.24 |
| IrO2 (001) | 178.9 | 1.18 | 1.17 | 2.98 | 2.64 |
| IrO2 (110) | 178.6 | 1.17 | 1.17 | 2.89 | 3.12 |
| PbO2 (001) | 179.6 | 1.17 | 1.17 | 4.25 | 3.30 |
| PbO2 (110) | 173.9 | 1.18 | 1.17 | 2.69 | 2.78 |
| Oxides | EB (001) kcal /mol | EB (110) kcal /mol |
|---|---|---|
| TiO2 | −182.42 | −186.33 |
| GeO2 | −128.66 | −139.57 |
| SnO2 | −121.73 | −131.80 |
| IrO2 | −149.97 | −162.54 |
| PbO2 | −106.52 | −112.86 |
| Oxide | (001) D (kcal/mol) | (110) D (kcal/mol) | (001) EA (eV) | (110) EA (eV) |
|---|---|---|---|---|
| TiO2 | 57.433 | 59.433 | 1.28 | 1.20 |
| GeO2 | 66.058 | 29.763 | 1.33 | −0.16 |
| SnO2 | 66.182 | 41.964 | 1.57 | 0.55 |
| IrO2 | 0.596 | 0.607 | −3.89 | −3.88 |
| PbO2 | 0.232 | 1.177 | −4.00 | −3.51 |
| Oxide | ED (001) (kcal/mol) | ED (110) (kcal/mol) |
|---|---|---|
| TiO2 | −31.508 | −71.164 |
| SnO2 | −60.094 | −56.541 |
| GeO2 | −62.578 | −57.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Rocha, R.; Mercado-Sánchez, I.; Vargas-Rodriguez, I.; Hernández-Lima, J.; Bazán-Jiménez, A.; Robles, J.; García-Revilla, M.A. Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis. Molecules 2023, 28, 1776. https://doi.org/10.3390/molecules28041776
Chávez-Rocha R, Mercado-Sánchez I, Vargas-Rodriguez I, Hernández-Lima J, Bazán-Jiménez A, Robles J, García-Revilla MA. Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis. Molecules. 2023; 28(4):1776. https://doi.org/10.3390/molecules28041776
Chicago/Turabian StyleChávez-Rocha, Rogelio, Itzel Mercado-Sánchez, Ismael Vargas-Rodriguez, Joseelyne Hernández-Lima, Adán Bazán-Jiménez, Juvencio Robles, and Marco A. García-Revilla. 2023. "Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis" Molecules 28, no. 4: 1776. https://doi.org/10.3390/molecules28041776
APA StyleChávez-Rocha, R., Mercado-Sánchez, I., Vargas-Rodriguez, I., Hernández-Lima, J., Bazán-Jiménez, A., Robles, J., & García-Revilla, M. A. (2023). Modeling Adsorption of CO2 in Rutile Metallic Oxide Surfaces: Implications in CO2 Catalysis. Molecules, 28(4), 1776. https://doi.org/10.3390/molecules28041776






