Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review
Abstract
1. Introduction
2. Methodology
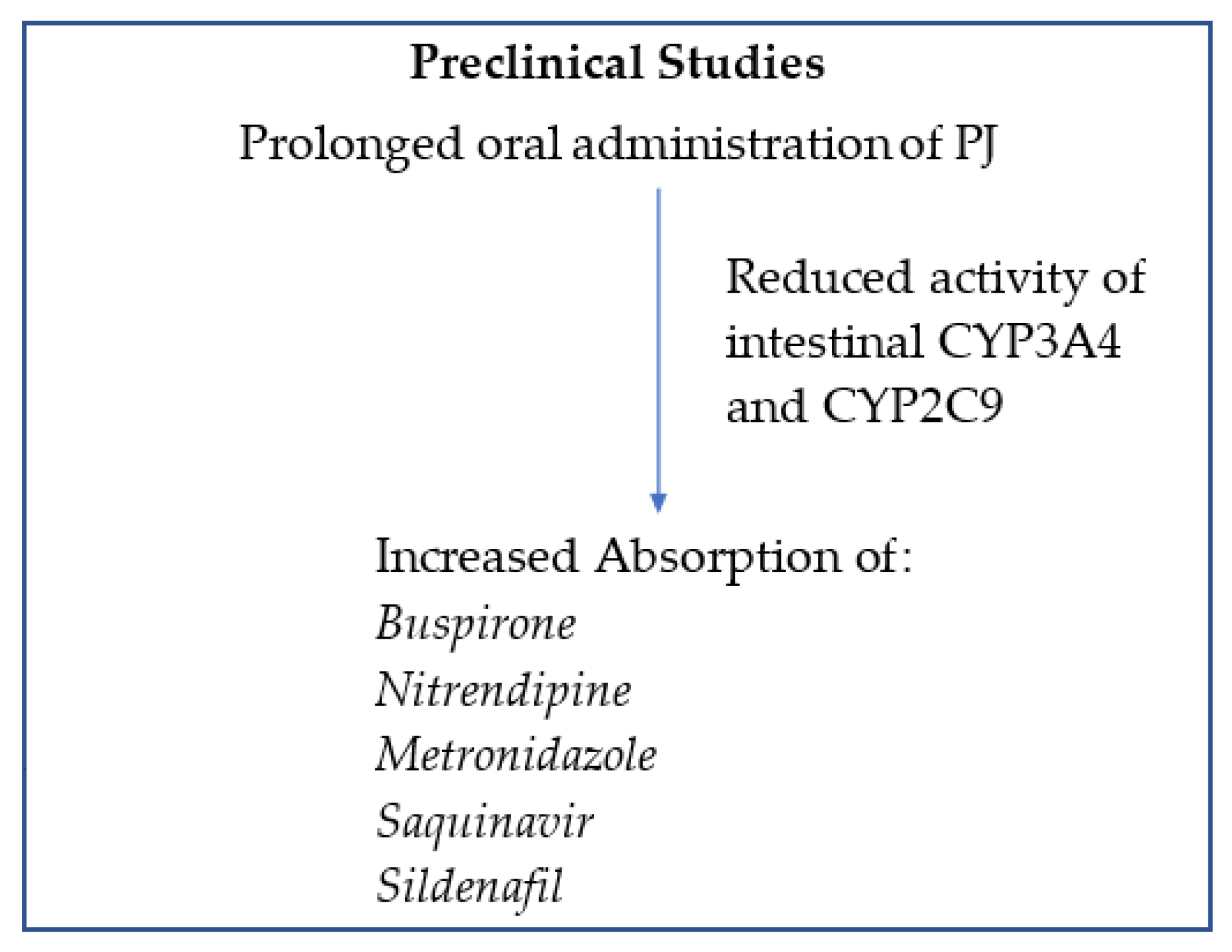
3. Preclinical Studies: PJ Impact on the Pharmacokinetics of CYP3A4- and CYP2C9- Mediated Drugs Metabolism
3.1. Pomegranate and Carbamazepine
3.2. Pomegranate and Tolbutamide
3.3. Pomegranate and Buspirone
3.4. Pomegranate and Nitrendipine
3.5. Pomegranate and Metronidazole
3.6. Pomegranate and Sildenafil
3.7. Pomegranate and Saquinavir
3.8. Pomegranate and Warfarin
4. Preclinical Studies: PJ Impact on Drugs Not Metabolized by CYP3A4 and CYP2C9
4.1. Pomegranate and Metformin
4.2. Pomegranate and Piracetam
4.3. Pomegranate and Theophylline
5. Clinical Studies: PJ Impact on CYP3A4- and CYP2C9-Mediated Drugs Metabolism
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Islamoglu, M.S.; Uysal, B.B.; Yavuzer, S.; Cengiz, M. Does the use of herbal medicine affect adherence to medication—A cross sectional study of outpatients with chronic disease? Eur. J. Integr. Med. 2021, 44, 101326. [Google Scholar] [CrossRef]
- Costache, I.-I.; Miron, A.; Hancianu, M.; Aursulesei, V.; Costache, A.D.; Aprotosoaie, A.C. Pharmacokinetic Interactions between Cardiovascular Medicines and Plant Products. Cardiovasc. Ther. 2019, 2019, 1–19. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.-Y.; Fabriaga, E.; Zhang, P.-H.; Zhou, Q. Food-drug interactions precipitated by fruit juices other than grapefruit juice: An update review. J. Food Drug Anal. 2018, 26, S61–S71. [Google Scholar] [CrossRef]
- Petric, Z.; Žuntar, I.; Putnik, P.; Bursać Kovačević, D. Food-Drug Interactions with Fruit Juices. Foods 2020, 10, 33. [Google Scholar] [CrossRef]
- Di Minno, A.; Frigerio, B.; Spadarella, G.; Ravani, A.; Sansaro, D.; Amato, M.; Kitzmiller, J.P.; Pepi, M.; Tremoli, E.; Baldassarre, D. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017, 31, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Paucar, A.; Vintimilla-Rojas, D. Interactions of clinical relevance associated with concurrent administration of prescription drug and food or medicinal plants: A systematic review protocol. Syst. Rev. 2020, 9, 1. [Google Scholar] [CrossRef]
- Asgary, S.; Javanmard, S.H.; Zarfeshany, A. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.; Abdel-Rahman, I.; Fouad, S.; Allemailem, K.; Istivan, T.; Ahmed, S.; Hasan, A.; Osman, H.; Elshabrawy, H. Pomegranate Peel Extract Is a Potential Alternative Therapeutic for Giardiasis. Antibiotics 2021, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, A.; Abu-Sini, M.; Amr, R.; Akasheh, R.T.; Zalloum, W.; Khdair, A.; Hamad, I.; Aburjai, T.; Darwish, R.M.; Abu-Qatouseh, L. Novel in vitro and in vivo anti-Helicobacter pylori effects of pomegranate peel ethanol extract. Veter. World 2021, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.E.; Al-Madboly, L.A.; Al-Ashmawy, G.M.; Saber-Ayad, M.; Abo-Saif, M.A. Unravelling the In Vitro and In Vivo Anti-Helicobacter pylori Effect of Delphinidin-3-O-Glucoside Rich Extract from Pomegranate Exocarp: Enhancing Autophagy and Downregulating TNF-α and COX2. Antioxidants 2022, 11, 1752. [Google Scholar] [CrossRef]
- Paller, C.J.; Pantuck, A.; A Carducci, M. A review of pomegranate in prostate cancer. Prostate Cancer Prostatic Dis. 2017, 20, 265–270. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.; Espín, J.C.; González-Sarrías, A. Evidence for health properties of pomegranate juices and extracts beyond nutrition: A critical systematic review of human studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Hidaka, M.; Okumura, M.; Fujita, K.-I.; Ogikubo, T.; Yamasaki, K.; Iwakiri, T.; Setoguchi, N.; Arimori, K. Effects of pomegranate juice on human cytochrome p450 3A (CYP3A) and carbamazepine pharmacokinetics in rats. Drug Metab. Dispos. 2005, 33, 644–648. [Google Scholar] [CrossRef]
- Farkas, D.; Oleson, L.E.; Zhao, Y.; Harmatz, J.S.; Zinny, M.A.; Court, M.H.; Greenblatt, D.J. Pomegranate Juice Does Not Impair Clearance of Oral or Intravenous Midazolam, a Probe for Cytochrome P450-3A Activity: Comparison with Grapefruit Juice. J. Clin. Pharmacol. 2007, 47, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Hidaka, M.; Sekiya, H.; Kawano, Y.; Yamasaki, K.; Okumura, M.; Arimori, K. Effects of Pomegranate Juice on Human Cytochrome P450 2C9 and Tolbutamide Pharmacokinetics in Rats. Drug Metab. Dispos. 2006, 35, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.J.; Masse, G.; Harmatz, J.S.; Court, M.H.; Greenblatt, D.J. Pomegranate Juice and Pomegranate Extract Do Not Impair Oral Clearance of Flurbiprofen in Human Volunteers: Divergence From In Vitro Results. Clin. Pharmacol. Ther. 2012, 92, 651–657. [Google Scholar] [CrossRef]
- Kumar, Y.S.; Adukondalu, D.; Latha, A.B.; Vishnu, Y.V.; Ramesh, G.; Kumar, R.S.; Rao, Y.M.; Sarangapani, M. Effect of pomegranate pretreatment on the oral bioavailability of buspirone in male albino rabbits. DARU J. Pharm. Sci. 2011, 19, 266–269. [Google Scholar]
- Voruganti, S.; Rapolu, K.; Tota, S.; Yamsani, S.K.; Yamsani, M.R. Effect of pomegranate juice on the pharmacokinetics of nitrendipine in rabbits. Eur. J. Drug Metab. Pharmacokinet. 2011, 37, 77–81. [Google Scholar] [CrossRef]
- Voruganti, S.; Yamsani, S.K.; Ravula, S.K.; Gannu, R.; Yamsani, M.R. Effect of Pomegranate Juice on Intestinal Transport and Pharmacokinetics of Nitrendipine in Rats. Phytotherapy Res. 2012, 26, 1240–1245. [Google Scholar] [CrossRef]
- Abu Tbeekh, H.T.; Abu Dayyih, W.A.; Mallah, E.M.; Qinna, N.A.; Awad, R.M.; Arafat, T.A. Pomegranate Juice Affects on Pharmacokinetic Parameters of Metronidazole By Using Hplc-Ms. World J. Pharm. Pharm. Sci. 2014, 3, 150–164. [Google Scholar]
- Mallah, E.M.; Rayyan, W.S.; Dayyih, W.A.; Elhajji, F.D.; Mansour, K.A.; Al-Majali, I.S.; Arafat, T.A. Dose-dependent synergistic effect of pomegranate juice on the bioavailability of sildenafil in rats by using HPLC method. Lat. Amercian J. Pharm. 2016, 35, 1277–1284. [Google Scholar]
- Vemulapalli, S. Influence of pomegranate juice on the CYP3A4-mediated metabolism and p-glycoprotein mediated transport of saquinavir in vivo and ex vivo models. Indones. J. Pharm. 2016, 27, 152. [Google Scholar] [CrossRef]
- Alnaqeeb, M.; Mansor, K.A.; Mallah, E.M.; Ghanim, B.Y.; Idkaidek, N.; Qinna, N.A. Critical pharmacokinetic and pharmacodynamic drug-herb interactions in rats between warfarin and pomegranate peel or guava leaves extracts. BMC Complement. Altern. Med. 2019, 19, 29. [Google Scholar] [CrossRef]
- Kerr, B.M.; Thummel, K.E.; Wurden, C.J.; Klein, S.M.; Kroetz, D.L.; Gonzalez, F.J.; Levy, R. Human liver carbamazepine metabolism: Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem. Pharmacol. 1994, 47, 1969–1979. [Google Scholar] [CrossRef]
- Katoh, M.; Nakajima, M.; Shimada, N.; Yamazaki, H.; Yokoi, T. Inhibition of human cytochrome P450 enzymes by 1, 4-dihydropyridine calcium antagonists: Prediction of in vivo drug–drug interactions. Eur. J. Clin. Pharmacol. 2000, 55, 843–852. [Google Scholar] [CrossRef]
- Bailey, D.; Spence, J.; Munoz, C.; Arnold, J. Interaction of citrus juices with felodipine and nifedipine. Lancet 1991, 337, 268–269. [Google Scholar] [CrossRef]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of met-ronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef]
- Pearce, R.E.; Cohen-Wolkowiez, M.; Sampson, M.R.; Kearns, G.L. The Role of Human Cytochrome P450 Enzymes in the Formation of 2-Hydroxymetronidazole: CYP2A6 is the High Affinity (Low Km) Catalyst. Drug Metab. Dispos. 2013, 41, 1686–1694. [Google Scholar] [CrossRef]
- Senthilkumaran, S.; Balamurugan, N.; Suresh, P.; Thirumalaikolundusubramanian, P. Priapism, pomegranate juice, and sildenafil: Is there a connection? Urol. Ann. 2012, 4, 108–110. [Google Scholar] [CrossRef]
- Warrington, J.S.; Shader, R.I.; von Moltke, L.L.; Greenblatt, D.J. In vitro biotransformation of sildenafil (Viagra): Identi-fication of human cytochromes and potential drug interactions. Drug Metab. Dispos. 2000, 28, 392–397. [Google Scholar]
- Eagling, V.A.; Back, D.J.; Barry, M.G. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br. J. Clin. Pharmacol. 1997, 44, 190–194. [Google Scholar] [CrossRef]
- Doherty, M.M.; Pang, K.S. First-Pass Effect: Significance of the Intestine for Absorption and Metabolism. Drug Chem. Toxicol. 1997, 20, 329–344. [Google Scholar] [CrossRef]
- Schmitt, C.; Hofmann, C.; Riek, M.; Patel, A.; Zwanziger, E. Effect of Saquinavir-Ritonavir on Cytochrome P450 3A4 Activity in Healthy Volunteers Using Midazolam as a Probe. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1175–1181. [Google Scholar] [CrossRef]
- E Komperda, K. Potential Interaction Between Pomegranate Juice and Warfarin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1002–1006. [Google Scholar] [CrossRef]
- Mima, M.; Mallah, E.; Abudayyih, W.; ElHaji, F.D.; Salih, H.; Zakarya, Z.; Othman, B.; Arafat, T. Pharmacokinetics and Pharmacodynamics Interaction of Warfarin in the Presence of Beverage Juices (Licorice and Pomegranate) in Rat Plasma by Using LC/MS. Lat. Am. J. Pharm. 2017, 36, 1181–1192. [Google Scholar]
- Kaminsky, L.S.; Zhang, Z.-Y. Human P450 metabolism of warfarin. Pharmacol. Ther. 1997, 73, 67–74. [Google Scholar] [CrossRef]
- Awad, R.; Mallah, E.; Al Khawaja, B.; Abu Dayyih, W.; El-Hajji, F.; Matalka, K.Z.; Arafat, T. Pomegranate and licorice juices modulate metformin pharmacokinetics in rats. Neuro Endocrinol. Lett. 2016, 37, 202–206. [Google Scholar] [PubMed]
- Hamad, M.A.-J.R.; Al Tamimi, L.; Abu Dayyih, A.; Mallah, E. Validation and Determination of Piracetam in Rat Plasma By Using High Performance Liquid Chromatography/Uv/Vis Spectrometry (Hplc/Uv/Vis) in Presence of Pomegranate and Liquorice Juices for Pharmacokinetic Study. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 2431–2449. [Google Scholar]
- Alanbaki, A.; Alani, I.; Mallah, E.; Zakareia, Z.; Arafat, T.; Abu Dayyih, W. The effect of pomegranate and licorice on pharma-cokinetics of theophylline in rat plasma. Fabad J. Pharm. Sci. 2019, 44, 9–15. [Google Scholar]
- Kim, H.G.; Hien, T.T.; Han, E.H.; Hwang, Y.P.; Choi, J.H.; Kang, K.W.; Kwon, K.-I.; Kim, B.-H.; Kim, S.K.; Song, G.Y.; et al. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharmacol. 2011, 162, 1096–1108. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, M.G. Effects of enzyme inducers and inhibitors on the pharmacokinetics of metformin in rats: Involvement of CYP2C11, 2D1 and 3A1/2 for the metabolism of metformin. Br. J. Pharmacol. 2006, 149, 424–430. [Google Scholar] [CrossRef]
- Nemati, S.; Tadibi, V.; Hoseini, R. Pomegranate juice intake enhances the effects of aerobic training on insulin resistance and liver enzymes in type 2 diabetic men: A single-blind controlled trial. BMC Nutr. 2022, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Sandoval, C.; Fabela-Illescas, H.E.; Fernández-Martínez, E.; Ortiz-Rodríguez, M.A.; Cariño-Cortés, R.; Ariza-Ortega, J.A.; Betanzos-Cabrera, G. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pom-egranate Juice. Antioxidants 2022, 11, 553. [Google Scholar] [CrossRef]
- Waegemans, T.; Wilsher, C.R.; Danniau, A.; Ferris, S.H.; Kurz, A.; Winblad, B. Clinical Efficacy of Piracetam in Cognitive Impairment: A Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2002, 13, 217–224. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef]
- Misaka, S.; Nakamura, R.; Uchida, S.; Takeuchi, K.; Takahashi, N.; Inui, N.; Kosuge, K.; Yamada, S.; Watanabe, H. Effect of 2 Weeks’ Consumption of Pomegranate Juice on the Pharmacokinetics of a Single Dose of Midazolam: An Open-Label, Randomized, Single-Center, 2-Period Crossover Study in Healthy Japanese Volunteers. Clin. Ther. 2011, 33, 246–252. [Google Scholar] [CrossRef]
- Park, S.-J.; Yeo, C.-W.; Shim, E.-J.; Kim, H.; Liu, K.-H.; Shin, J.-G.; Shon, J.-H. Pomegranate juice does not affect the disposition of simvastatin in healthy subjects. Eur. J. Drug Metab. Pharmacokinet. 2015, 41, 339–344. [Google Scholar] [CrossRef]
- Abdlekawy, K.S.; Donia, A.M.; Elbarbry, F. Effects of Grapefruit and Pomegranate Juices on the Pharmacokinetic Properties of Dapoxetine and Midazolam in Healthy Subjects. Eur. J. Drug Metab. Pharmacokinet. 2016, 42, 397–405. [Google Scholar] [CrossRef]
- Anlamlert, W.; Sermsappasuk, P. Pomegranate Juice does not Affect the Bioavailability of Cyclosporine in Healthy Thai Volunteers. Curr. Clin. Pharmacol. 2020, 15, 145–151. [Google Scholar] [CrossRef]
- Khuda, F.; Iqbal, Z.; Khan, A.; Sahibzada, M.U.K.; Alam, M.; Khusro, A. Effect of fresh pomegranate juice on the pharmacokinetic profile of artemether: An open-label, randomized, 2-period crossover study in healthy human volunteers. J. Pharm. Biomed. Anal. 2021, 203, 114179. [Google Scholar] [CrossRef]
- Ali, S.; Najmi, M.H.; Tarning, J.; Lindegardh, N. Pharmacokinetics of artemether and dihydroartemisinin in healthy Pakistani male volunteers treated with artemether-lumefantrine. Malar. J. 2010, 9, 275. [Google Scholar] [CrossRef]
- Lefèvre, G.; Carpenter, P.; Souppart, C.; Schmidli, H.; McClean, M.; Stypinski, D. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet® ) with concomitant administration of ketoconazole in healthy subjects. Br. J. Clin. Pharmacol. 2002, 54, 485–492. [Google Scholar] [CrossRef]
- Khuu, T.; Hickey, A.; Deng, M.C. Pomegranate-containing products and tacrolimus: A potential interaction. J. Hear. Lung Transplant. 2013, 32, 272–274. [Google Scholar] [CrossRef]
- Staatz, C.E.; Goodman, L.K.; Tett, S.E. Effect of CYP3A and ABCB1 Single Nucleotide Polymorphisms on the Pharmacokinetics and Pharmacodynamics of Calcineurin Inhibitors: Part II. Clin. Pharmacokinet. 2010, 49, 207–221. [Google Scholar] [CrossRef]
- Srinivas, N.R. Is pomegranate juice a potential perpetrator of clinical drug–drug interactions? Review of the in vitro, preclinical and clinical evidence. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 223–229. [Google Scholar] [CrossRef]
- Andrade, C. Potentially Significant Versus Clinically Significant Drug Interactions: Pomegranate Juice as a Case in Point. J. Clin. Psychiatry 2014, 75, e292–e293. [Google Scholar] [CrossRef]
- Guttman, Y.; Nudel, A.; Kerem, Z. Polymorphism in Cytochrome P450 3A4 Is Ethnicity Related. Front. Genet. 2019, 10, 224. [Google Scholar] [CrossRef]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef]
| Pomegranate Dose | Drug | Animal Species | PK/PD Effects | Drug’s Analytical Technique- Biological Sample * | Reference |
|---|---|---|---|---|---|
| Pomegranate juice (2 mL) 1 h before the drug | Carbamazepine (50 mg/kg) | Rats | AUC increased, but no effect on the elimination half-life | HPLC-UV Plasma | [13] |
| Pomegranate juice (3 mL) administered 1 h before the drug | Tolbutamide (20 mg/kg) | Rats | AUC increased, but no effect on the elimination half-life | HPLC-UV Serum | [15] |
| Pomegranate juice (10 mL/kg) for 7 days | Buspirone (10 mg/kg) | Rabbits | AUC, Cmax, and elimination half-life increased | HPLC-UV Serum | [17] |
| Pomegranate juice, single dose Pomegranate juice, multiple doses for 1 week | Nitrendipine (10 mg/kg) | Rabbits | No PK changes AUC and Cmax increased, but no effect on the elimination half-life | HPLC-UV Plasma | [18] |
| Pomegranate juice, single dose (3 mL/rat) Pomegranate juice, multiple doses (3 mL/rat/day for 7 days) | Nitrendipine (10 mg/kg) | Rats | AUC and Cmax increased, but no effect on the elimination half-life AUC and Cmax increased, but no effect on the elimination half-life | HPLC-UV Plasma | [19] |
| Pomegranate juice was administered at 5 mL/kg dose 30 min before drug administration or multiple doses twice a day for 2 days | Metronidazole (14 mg/kg) | Rats | No PK changes from a single dose. Multiple doses of Pomegranate increased AUC and Cmax | HPLC-MS Serum | [20] |
| Pomegranate juice was added to the drinking water 16 h before sildenafil administration, and then each group of rats received 2, 4, 6, and 8 mL of pomegranate juice when sildenafil was administered. | Sildenafil (5 mL/kg) | Rats | AUC increased, and Tmax delayed | HPLC-UV Plasma | [21] |
| Pomegranate juice One dose (0.5 mL, 1 mL, and 2 mL/200 g) Dose for 15 days (0.5 mL, 1 mL, and 2 mL/200 g/day) | Saquinavir (100 mg/kg) Saquinavir (100 mg/kg) for 15 days | Rats Rats | AUC, Cmax, and elimination half-life increased AUC, Cmax, and elimination half-life decreased. | HPLC-UV Plasma | [22] |
| Pomegranate juice was administered as a single dose of 100 mg/kg for 5 days. | Warfarin (0.5 mg/kg) | Rats | Pomegranate did not change PK, but Prothrombin time and INR increased | HPLC-UV Plasma | [23] |
| Pomegranate Dose | Drug | Animal Species | PK Effects | Drug Analytical Technique- Biological Sample * | Reference |
|---|---|---|---|---|---|
| Pomegranate juice was added to drinking water 12 h before the experiment and a dose of (5 mL) 30 min before the metformin dose. | Metformin (20 mg/kg) | Rats | Pomegranate juice reduced Cmax (and showed a pattern of AUC reduction) | HPLC-UV Plasma | [37] |
| Pomegranate juice was administered orally at a dose of 12 mL/kg before the administration of piracetam. | Piracetam (50 mg/kg) | Rats | No change | HPLC-UV Plasma | [38] |
| Pomegranate juice was added to drinking water 12 h before the experiment and a dose of (5 mL) 30 min before the theophylline dose. | Theophylline (5 mg/kg) | Rats | No change | HPLC-UV Plasma | [39] |
| Pomegranate Dose | Objective Drug | Type of Study | PK Effect | Drug Analytical Technique-Biological Sample(s) * | Reference |
|---|---|---|---|---|---|
| Pomegranate juice (200 mL) for 2 weeks | Midazolam and metabolite 1-hydroxymidazolam and 4-hydroxhymidozolam | Open-label, randomized, 2-period, crossover n = 16 | No effect on AUC and Cmax | LC-MS/MS Plasma Urine | [46] |
| Pomegranate juice, one dose of 240 mL or 1 g extract | Flurbiprofen (100 mg) | Open-label, randomized n = 12 | No effect on PK | HPLC-FLD Plasma | [16] |
| Pomegranate juice (237 mL) as a single dose | Midazolam oral (6 mg) Midazolam intravenous (2 mg) | Randomized controlled trial n = 13 | No effect on Cmax, AUC, or clearance after oral administration No effect on elimination half-life, volume of distribution, or clearance | LC-MS Plasma | [14] |
| Pomegranate juice, 3 doses per day (900 mL/day) for 3 days | Simvastatin 40 mg | Open-label, 3-way crossover design n = 12 | No significant change in AUC or Cmax. | LC-MS/MS Plasma | [47] |
| Pomegranate juice (250 mL) for 3 days | Dapoxetine (60 mg) and Midazolam (7.5 mg) | Open-label, 3-way crossover n = 12 | Slight but not significant effect on AUC or Cmax | HPLC-UV Plasma | [48] |
| Pomegranate juice (500 mL) | Cyclosporine (200 mg) | Open-label, randomized, crossover n = 18 | No significant change in AUC or Cmax | Chemiluminescent microparticle immunoassay | [49] |
| Pomegranate juice (250 mL) twice daily doses for 14 days | Artemether (80 mg) | Open-label, randomized, crossover n = 26 | No significant change in AUC, Cmax, Tmax, or elimination half-life for both artemether and dihydroartemisinin (metabolite) | LC-MS/MS Plasma | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, K.; Bardees, R.; Alkhawaja, B.; Mallah, E.; AbuQatouseh, L.; Schmidt, M.; Matalka, K. Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review. Molecules 2023, 28, 2117. https://doi.org/10.3390/molecules28052117
Mansoor K, Bardees R, Alkhawaja B, Mallah E, AbuQatouseh L, Schmidt M, Matalka K. Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review. Molecules. 2023; 28(5):2117. https://doi.org/10.3390/molecules28052117
Chicago/Turabian StyleMansoor, Kenza, Razan Bardees, Bayan Alkhawaja, Eyad Mallah, Luay AbuQatouseh, Mathias Schmidt, and Khalid Matalka. 2023. "Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review" Molecules 28, no. 5: 2117. https://doi.org/10.3390/molecules28052117
APA StyleMansoor, K., Bardees, R., Alkhawaja, B., Mallah, E., AbuQatouseh, L., Schmidt, M., & Matalka, K. (2023). Impact of Pomegranate Juice on the Pharmacokinetics of CYP3A4- and CYP2C9-Mediated Drugs Metabolism: A Preclinical and Clinical Review. Molecules, 28(5), 2117. https://doi.org/10.3390/molecules28052117







