Abstract
The Lewis-acidic character and robustness of NHC-Au(I) complexes enable them to catalyze a large number of reactions, and they are enthroned as the catalysts of choice for many transformations among polyunsaturated substrates. More recently, Au(I)/Au(III) catalysis has been explored either by utilizing external oxidants or by seeking oxidative addition processes with catalysts featuring pendant coordinating groups. Herein, we describe the synthesis and characterization of N-heterocyclic carbene (NHC)-based Au(I) complexes, with and without pendant coordinating groups, and their reactivity in the presence of different oxidants. We demonstrate that when using iodosylbenzene-type oxidants, the NHC ligand undergoes oxidation to afford the corresponding NHC=O azolone products concomitantly with quantitative gold recovery in the form of Au(0) nuggets ~0.5 mm in size. The latter were characterized by SEM and EDX-SEM showing purities above 90%. This study shows that NHC-Au complexes can follow decomposition pathways under certain experimental conditions, thus challenging the believed robustness of the NHC-Au bond and providing a novel methodology to produce Au(0) nuggets.
1. Introduction
Fundamental comprehension of the intrinsic reactivity of NHC-based gold complexes is needed to evaluate their success in different fields, such as in the biological sector [1,2,3,4,5] or in catalysis. NHC ligands are typically described as strongly σ-donating, which form very stable NHC-metal bonds throughout a catalytic cycle [6,7]. Complexes of transition metals with N-heterocyclic carbene (NHC) ligands are commonly applied as catalysts for a variety of C-C and C-heteroatom bond formations, C-H functionalizations, cross-couplings, atom-economic additions, olefin metathesis, and many other transformations [8,9,10,11,12]. Moreover, there is high variability of the steric and electronic parameters of NHC ligands, which allows effective tuning of the catalyst activity [13].
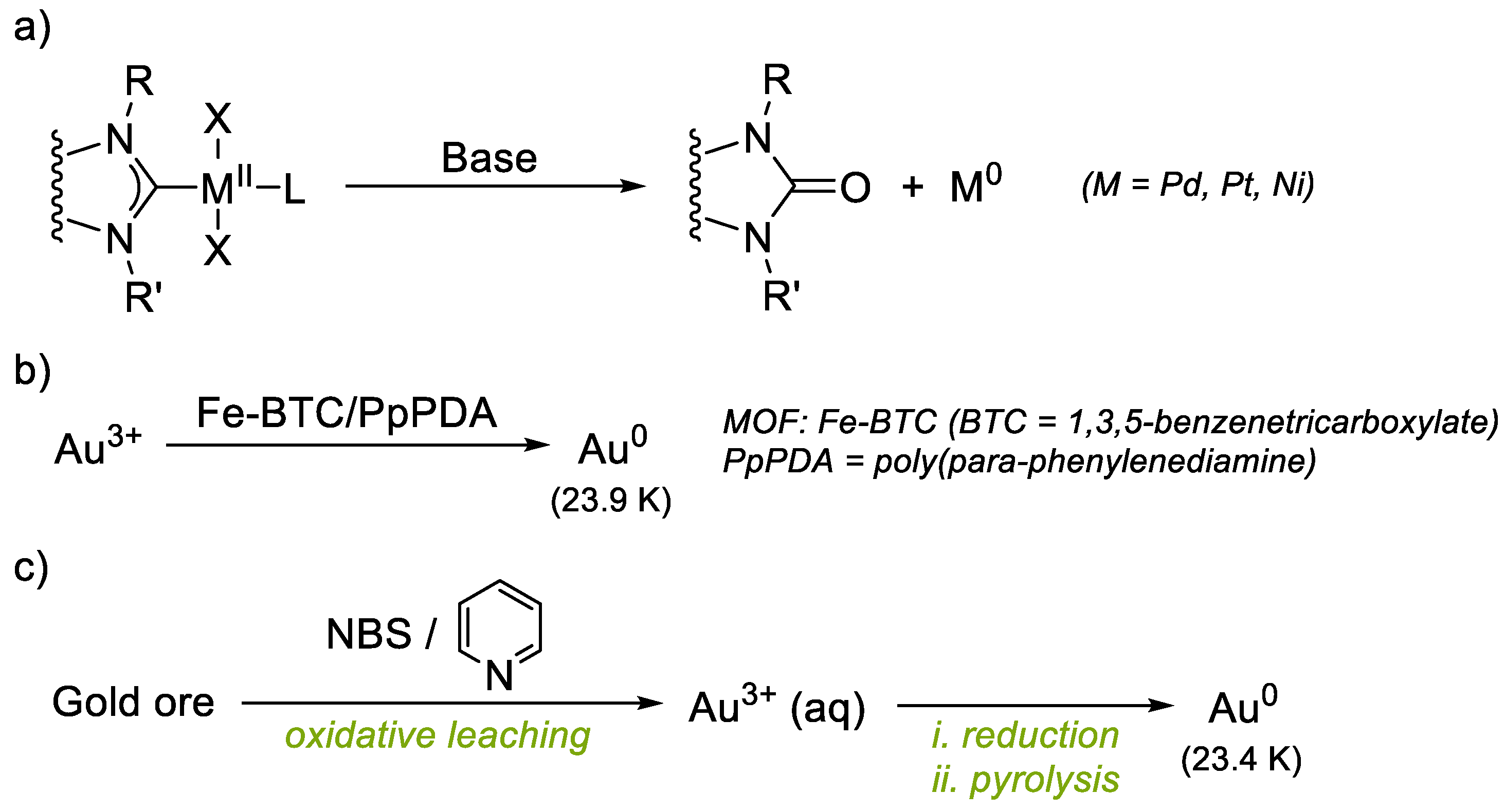
Focusing on gold catalysis, a large number of NHC-Au complexes have been successfully used in Lewis acid catalysis for the cyclization and rearrangements of polyunsaturated substrates [14,15]. However, in order to further expand the scope of applications of these complexes, it is necessary to explore experimental conditions where NHC-gold complexes might suffer from instability. For instance, the exploration of Au(I)/Au(III) catalysis using external oxidants or oxidant-free catalysis with ligands featuring pendant coordinating groups has become prominent in the last decade, but the intrinsic decomposition of gold complexes is underexplored. In a related work, Ananikov reported the unusual role of bases in the deactivation of NHC–metal catalysts (metal = Ni(II), Pd(II) and Pt(II)), undergoing metal reduction to M(0) and formation of NHC=O coupling azolone products (Figure 1a) [8]. In their study it is demonstrated that a base-mediated azolone NHC=O coupling reaction is integrated into the catalytic M/NHC systems and metal-free NHC derivatives are present in the catalytic mixture. A proposed mechanism of the revealed transformation includes NHC-OR reductive elimination, as implied by a series of mechanistic studies including 18OH− labeling experiments. Similar decomposition pathways were reported on triazolylidene Cu(I) complexes, which decompose into mesoionic oxides in the presence of CsOH [16]. Almost no reports exist for a detailed description of NHC-Au(I) decomposition pathways [17].
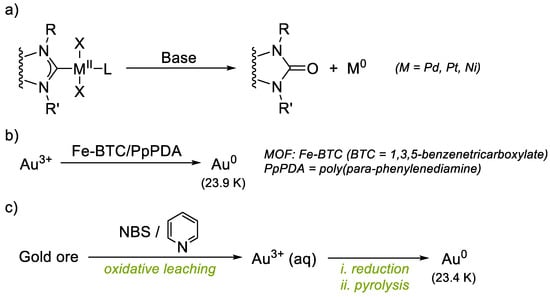
Figure 1.
(a) Examples of azolone decomposition pathways of NHC-M, and (b,c) strategies to recover highly pure Au(0) (>23 karats).
Herein, we show how distinct NHC-Au(I) complexes undergo a controlled decomposition pathway towards the formation of the oxidized NHC=O azolone counterpart, together with the formation of naked eye-observable Au(0) nuggets. The NHC-Au(I) complexes studied under oxidative conditions cover various types, from typical IPrAuCl to novel (NHC-hemilabile)Au(I) complexes. Indeed, the ability of related triazolylidene ligands to bear pendant pyridine or pyrimidine groups for Au(I)/Au(III) redox processes has recently been studied [18]. We show that only typical NHC-Au(I) complexes such as IPr-Au-Cl and SIPr-Au-Cl are robust and remain intact after exposure to oxidants, and the gold complexes with NHC-hemilabile ligands or other NHC derivatives undergo the azolone/Au(0) nugget decomposition pathway. The origin of the O-atom in the azolone-products obtained is discussed. Remarkably, a straightforward and reliable chemical methodology to produce Au(0) nuggets from Au complexes is not available, and the main source of gold(0) nuggets are, like natural ores, hypogenic in origin.19 Native gold grains are usually small in size (10–500 mm), with a composition of 5 to <30% Ag, typical of hypogene gold, although Ag can be less than 1% depending on the goldfield of origin [19]. Supergene gold biomineralization has also been studied [20,21]. Recently, an Fe-based MOF/polymer composite (Fe-BTC/PpPDA) has been reported as a selective and efficient methodology to extract trace amounts of gold from water up to high purities (23.9 karats (K); >99%) (Figure 1b) [22]. Reduction of Au3+ to Au(0), mediated by the MOF-polymer composite, is proposed. On the other hand, recovery of Au(0) from ores or waste electronic materials entails stepwise tedious processes such as reduction with metallic Zn, HCl treatment, centrifugation and pyrolysis (Figure 1c) [23]. The decomposition to Au(0) observed in our systems could be used as a methodology to obtain metallic gold more easily, as we show that Au(0) nuggets of up to ~500 μm can be afforded in one step by treatment of NHC-Au(I) complexes with external oxidants.
2. Results and Discussion
2.1. Synthesis of Novel NHC-Au(I) Complexes (1–4)
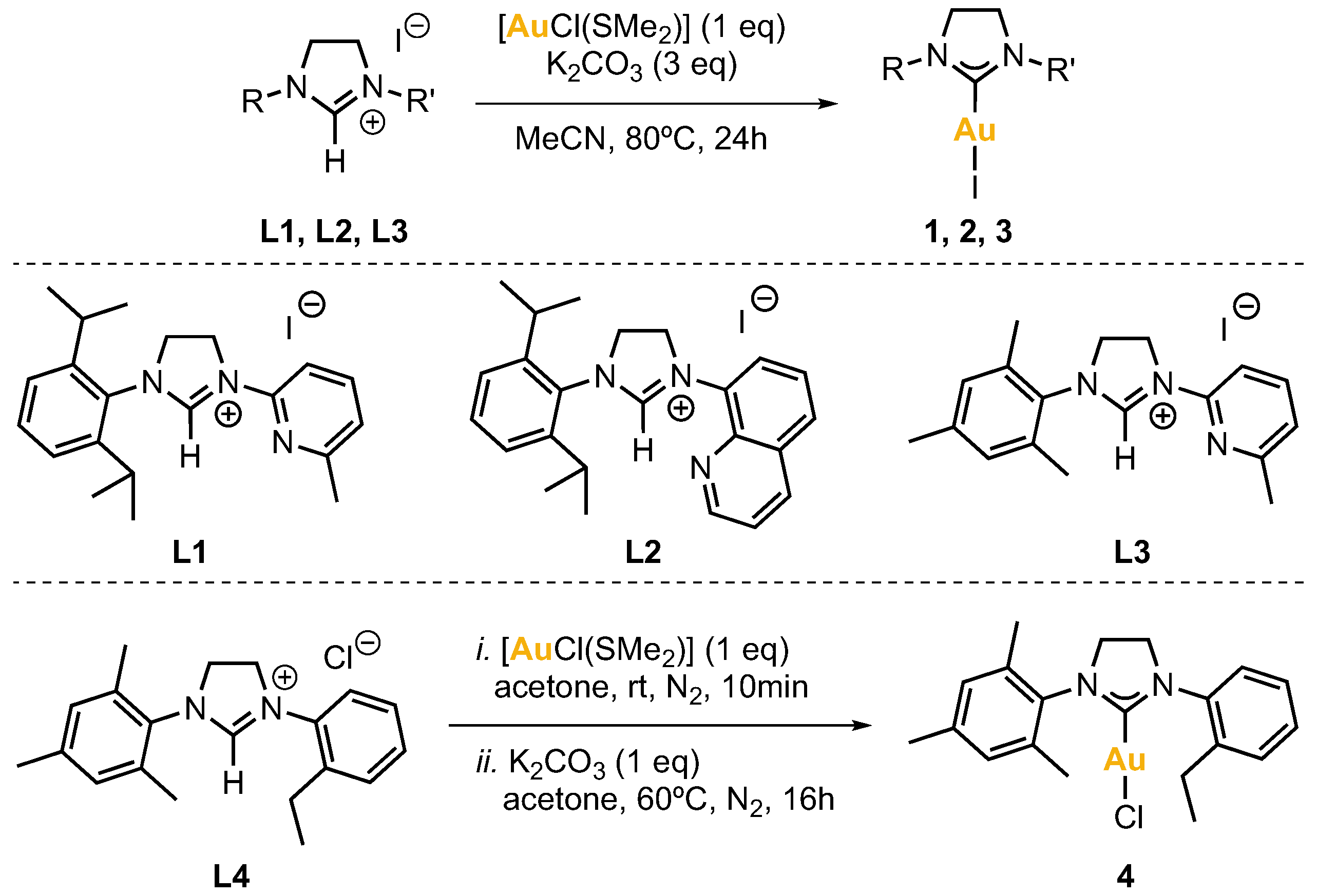
The synthesis of imidazolinium salts L1, L2, L3 and L4 was achieved by standard procedures [24,25]. L1–L3 contain a pendant pyridine or quinoline whereas L4 contains a non-coordinating arene moiety (Figure 2).
Figure 2.
Synthesis of complexes 1–4 from the respective imidazolinium salt ligand precursors L1–L4.
The synthesis of NHC-Au(I) complexes was based on previously reported works [26,27]. Complexes 1, 2 and 3 were obtained by reacting the corresponding imidazolinium salt L1, L2 or L3 (1 eq), chloro(dimethylsulfide)gold(I) (1 eq), and potassium carbonate (3 eq), in acetonitrile for 24 h at 80 °C. After this time, the reaction mixture was filtered over Celite® and all volatiles were removed under vacuum. The product was purified by column chromatography. The fractions that contained the product were combined, and the solvent was removed under vacuum to afford the gold(I) complexes as solids. Complex 4 was obtained analogously from imidazolinium salt L4 (Figure 2).
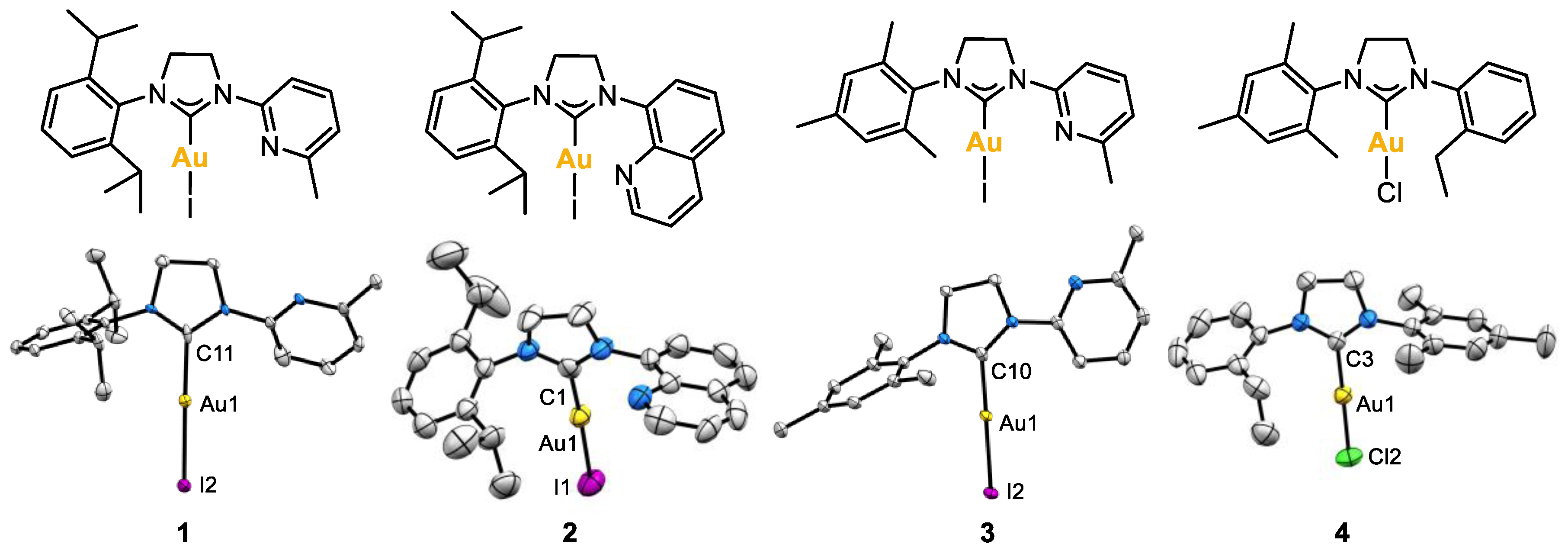
X-ray molecular structures of complexes 1, 2, 3 and 4 were obtained (Figure 3). In complexes 1–3, the pendant coordinating arm did not show any interaction with the metal center, and the linear coordination for the Au(I) center was confirmed in all cases, since the angle formed between the carbenic carbon, the gold center and the halide atom was close to 180° (for 1, 177.4(4)°; for 2, 175.4(2)°; for 3, 178.38(8)°; for 4, 179.12(16)°). No H-bonding network was observed with the non-coordinating pendant groups.
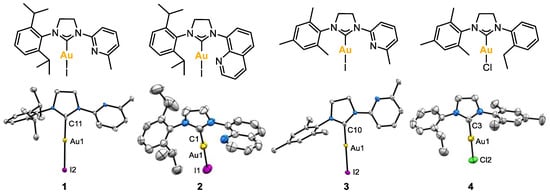
Figure 3.
X-ray molecular structures of complexes 1, 2, 3 and 4 (ellipsoids set at 50% probability and H atoms removed for clarity). Selected bond distances (Å): for 1, Au1-C11 2.007(13), Au1-I2 2.5580(10); for 2, Au1-C1 1.981(9), Au1-I1 2.5467(8); for 3, Au1-C10 2.000(3), Au1-I2 2.5516(2); for 4, Au1-C3 1.966(6), Au1-Cl2 2.262(3). Selected angles (°): for 1, C11-Au1-I2 177.4(4); for 2, C1-Au1-I1 175.4(2); for 3, C10-Au1-I2 178.38(8); for 4, C3-Au1-Cl2 179.12(16).
Complexes 1–4 were asymmetrical; therefore, the two methylenes of the imidazolidine ring appeared at different 1H NMR chemical shifts: for complexes 1 and 3, the two -NCH2- appeared as multiplets at 4.54 and 3.90 ppm, respectively, and for complex 2, at 4.68 and 4.08 ppm. For complex 4, the two -NCH2- appeared at 4.22 and 3.96 ppm. On the other hand, the characteristic 13C NMR peak of the Ccarbene-Au appeared at 201.0, 203.7, 192.2 and 195.5 ppm for 1, 2, 3 and 4, respectively.
2.2. Reactivity of Novel NHC-Au(I) Complexes (1–4) with External Oxidants
Complexes 1–3 were reacted with external oxidants in order to stabilize the NHC-Au(III) species, taking advantage of the pendant coordinating arms (pyridine-type for 1 and 3, and quinoline-type for 2). The external oxidants used were PhI(OAc)2, PhI(Cl)2, H2O2, XeF2, CH3CO3H under different solvents and temperatures (Table 1). However, the expected NHC-Au(III) species could not be stabilized in any case, and the mixture underwent a decomposition pathway involving the formation of a) the corresponding NHC=O azolones and b) Au(0) nuggets.

Table 1.
Reactivity of gold(I) complexes towards the formation of gold(0) nuggets and azolones (Lxox).
The formation of the NHC=O azolone products L1ox, L2ox, L3ox, L4ox was monitored by HRMS, and L2ox and L3ox were fully characterized by 1D and 2D NMR. In some reactions, the NHC-Au(I) complexes were treated with silver salts (as halide scavengers) and the oxidant. The aim of using a silver salt as additive was to promote the halide abstraction from the gold(I) starting complex and generate a reactive species that, hopefully, would evolve to a gold(III) complex in the presence of an oxidant, expecting the hemilabile N-donor arm to be coordinated to gold in the case of using complexes 1–3. In addition, the halide abstraction could likely induce the formation of head-to-tail dimeric Au(I) species with the C^N ligand bridging two metals [18,28,29]. However, the synthesis of such dimeric species was not the focus of our interest.
When complex 1 was reacted with PhI(OAc)2, regardless whether AgOAc was added or not, Au(0) nuggets and azolones L1ox and L1ox-I were formed (entries 1,2). The azolones were detected by ESI-MS and, in the case of the reaction in entry 3, the L1ox-I product was isolated in 53% yield. When the oxidant was changed to H2O2, the outcome of the reaction in presence of AgOAc was mainly the starting complex 1, whereas in the absence of AgOAc, L1ox-I could be detected by ESI-MS as well. L1ox-I was also detected in the reaction of 1 with PhI(Cl)2 at room temperature. Intriguingly, the formation of azolones that underwent a C-H iodination on the most activated position of the heteroaromatic system of the ligand was also observed. In this manner, products L1ox-I and L2ox-I were isolated and characterized. Indeed, the 2D NMR experiments revealed that the iodination occurred at the para position to the imidazolinone substituent (see Supplementary Material, Sections S5.7 and S5.9). The selective C-H halogenation at the para position to N-substituted positions and C5-halogenation of 8-aminoquinolines is a reactivity pattern that has already been reported [30,31,32,33,34]. Neither the reactions with H2O2 nor that with PhI(Cl)2 afforded Au(0) nuggets (entries 4–6). A blank experiment was conducted with complex 2; without oxidant and additives, the complex was recovered after being heated at 100 °C overnight (entry 7). When using PhI(OAc)2, Au(0) nuggets were obtained in high yields (56–91%), and L2ox and L2ox-I products were detected by ESI-MS in all cases (entries 8–11). The isolation of both L2ox and L2ox-I azolones allowed their full characterization. When complex 2 was reacted with CH3CO3H as oxidant (entry 12), Au(0) nuggets were formed in moderate yield (34%) and NMR revealed the presence of complex 2 and L2ox.
The reaction of complex 3 with PhI(OAc)2 and AgOAc yielded Au(0) quantitatively, and azolone L3ox was isolated in 60% yield and characterized (entry 13). On the contrary, when the oxidant XeF2 was tested with complex 3 at room temperature, no Au(0) nuggets were formed (entry 14). However, the formation of azolone L3ox was detected by ESI-MS.
Complex 4 was employed to search for contrast with the complexes that contained a hemilabile pendant arm. The reaction with PhI(OAc)2 in DCM at room temperature did not provide Au(0) nuggets nor azolone, as the starting complex was recovered (entry 15). However, by heating at 100 °C for 5 h, the mixture decomposed, affording Au(0) nuggets in a 97% yield, and the corresponding L4ox azolone was detected by ESI-MS (entry 16). When 4 was reacted with PhI(OAc)2 in 1,2-DCE at 90 °C, Au(0) nuggets were obtained in a 32% yield (entry 17).
Interestingly, the commercial IPrAuCl was also reacted with PhI(OAc)2 under the same experimental conditions as 1–4, and in this case, neither azolone product nor Au(0) nuggets were formed, and the complex was recovered after the reaction time (entries 18–19). Additionally, when the commercial saturated SIPrAuCl analog complex was reacted with PhI(OAc)2, a very low 11% conversion to Au(0) nuggets was obtained along with a 6% conversion to the corresponding azolone, as determined by NMR, according to the reported description of the azolone (entries 20,21) [35]. The stability of these well-known complexes is remarkable compared to that of 1–4, showing that when a structural modification of the most typical NHC ligands, such as IPr and SIPr, is performed, a sharp change in reactivity occurs. Additionally, it is worth mentioning that there are examples of unsaturated NHC-Au(I) complexes bearing a hemilabile pyridine moiety that could be oxidized with PhI(Cl)2 to the corresponding NHC-Au(III) complexes without observing decomposition [36,37].
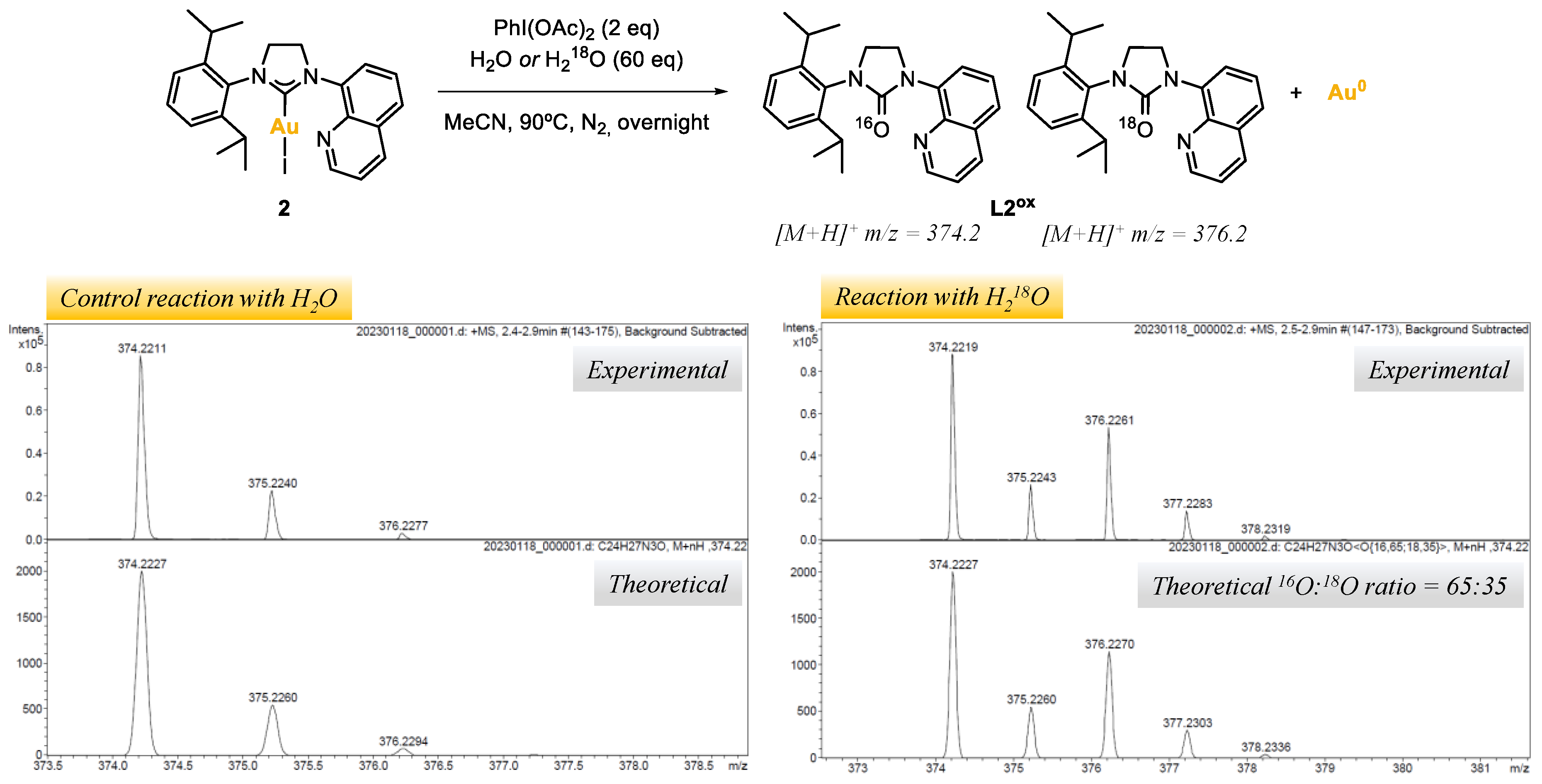
The origin of the O-atom in azolones was investigated by reacting complex 2, with PhI(OAc)2 as the oxidant and water as the additive, under nitrogen atmosphere (Table 1, entries 22,23, and Figure 4). In order to track the origin of the O-atom, a control reaction was carried out using distilled H2O, and another using 97% labeled 18O-water (see Supporting Material, Section S6). The reaction crudes were analyzed by ESI-HRMS. For the control experiment, a major peak at m/z = 374.2 was obtained, corresponding to the NHC=16O azolone L2ox, whereas for the reaction with H218O two major peaks were obtained at m/z = 374.2 and m/z = 376.2, corresponding to the NHC=16O and NHC=18O azolones L2ox, respectively. According to the isotopic pattern of such peaks, a 35% of 18O incorporation was obtained, suggesting that the O-atom in the azolones obtained in the reaction outcomes may come from adventitious water. The low 18O incorporation from water suggests that an oxygen-transferring mechanism may exist, and that at some point of the mechanism, water might react with the oxidant to deliver a 18O-labeled reactive intermediate. Further investigations would be needed to elucidate the mechanism; at this stage, the alternative possibility of considering acetate to be the O-atom source cannot be ruled out [38].
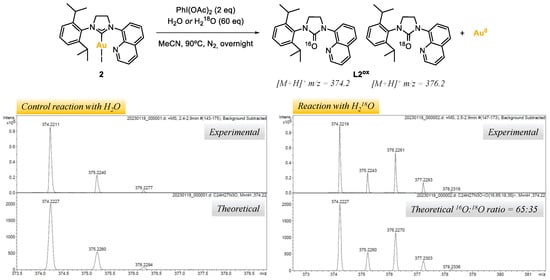
Figure 4.
Reaction of complex 2 with PhI(OAc)2 (2 eq) and water or 18O-labeled water (60 eq), and peaks of L2ox obtained by ESI-HRMS(+).
2.3. Characterization and Reactivity of the Au(0) Nuggets
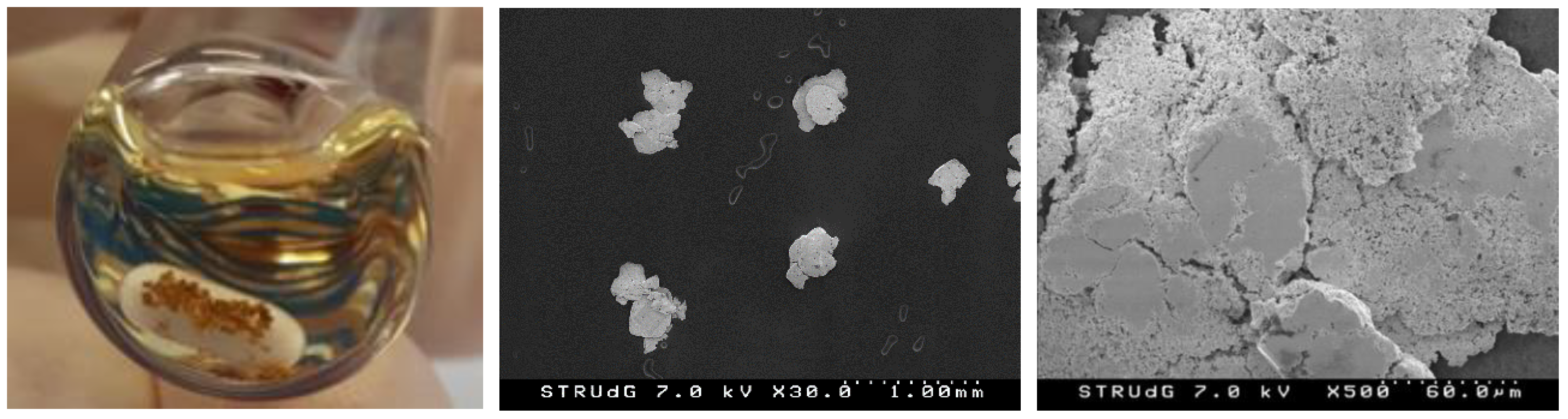
On the other hand, the formation of large Au(0) nuggets was naked-eye evident (Figure 5). The metallic gold grains were easily filtered/decanted and represented quantitative recovery (up to 90%) of all the Au(I) into Au(0) nuggets. The Au(0) nuggets were characterized by SEM and SEM-EDX, showing grains of 0.4–0.5 mm diameter mean size and purity up to 90% by EDX (only C and N impurities from the carbon tape support).

Figure 5.
Reaction outcome with Au(0) precipitate (left), and SEM images of the isolated Au(0) nuggets at ×30 and ×500 (middle and right), from the reaction employing complex 1 (1 eq), AgOAc (1 eq) and PhI(OAc)2 (2 eq) in 1,2-DCE, at 90 °C overnight, under N2 atmosphere in the absence of light (Table 1, entry 1).
The Au(0) nuggets were tested as a heterogeneous catalyst for transformations typically catalyzed by gold complexes, such as Sonogashira, A3 and Glaser couplings [39,40,41,42,43]. None of these attempts resulted in effective coupling, which is in line with the macroscopic size of the Au(0) nuggets and the absence of more reactive Au(0) nanoparticles.
3. Materials and Methods
All reagents and solvents were purchased from Sigma Aldrich-Merck (Madrid, Spain), Fischer Scientific (Madrid, Spain), TCI (Windrush, Belgium) or Fluorophen (Glossip, United Kingdom) and were used without further purification. NMR spectra were recorded at 298 K unless otherwise specified, on Bruker spectrometers (Billerica, MA, USA)) operating at 400 MHz (1H NMR) and 101 MHz (13C{1H} NMR), or on a Varian Mercury 200 MHz spectrometer (Palo Alto, CA, USA), and referenced to residual solvent (δ in ppm and J in hertz). High resolution mass spectra (HRMS) were recorded on a Bruker Microsoft-Q IITM or a QTOF maxis Impact (Bruker) spectrometer using ESI source at Servais Tècnics de Recerca, University of Girona, or at the National and Kapodistrian University of Athens. For reactions carried out under inert atmosphere, a N2 drybox with O2 and H2O concentrations <1 ppm was employed, or standard Schlenk techniques were followed. SEM images of the Au(0) nuggets were carried out with a scanning electron microscope FE-SEM Hitachi S-4100 (Hitachi, Chiyoda City, Japan). Digital images were collected and processed by Quarz PCI program. SEM-EDX analysis was performed with a scanning electron microscope Zeiss DSM 960 Germany (EDX Bruker, Quantax Esprit Spectrometer SVE III, Billerica, MA, USA).
Synthesis of complexes 1–3. For the synthesis of NHC-Au(I) complexes 1, 2 and 3, the corresponding imidazolinium salt L1, L2 or L3 (1 eq), chloro(dimethylsulfide)gold(I) (1 eq), and potassium carbonate (3 eq), were reacted in acetonitrile for 24 h at 80 °C. After this time, the reaction mixture was filtered over Celite® and all volatiles were removed under vacuum. The product was purified by column chromatography. The fractions that contained the product were combined, and the solvent was removed under vacuum to afford the gold(I) complexes as solids.
Complex 1. The imidazolinium iodide salt L1 (102.4 mg, 0.23 mmol, 1.0 eq.), K2CO3 (126.7 mg, 0.92 mmol, 4.0 eq.) and [AuCl(SMe2)] (87.2 mg, 0.30 mmol, 1.3 eq.) were reacted in acetonitrile (1 mL). The product was purified by column chromatography using DCM:hexane (4:1). By slow diffusion of pentane into a concentrated solution of complex 1 in chloroform, pale yellow crystals suitable for X-ray diffraction analysis were obtained (59.3 mg, 40% yield). 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 8.3 Hz, 1H, CHpy), 7.63 (t, J = 7.8 Hz, 1H, CHpy), 7.43 (t, J = 7.8 Hz, 1H, CHAr), 7.24 (d, J = 7.8 Hz, 2H, CHAr), 7.02 (d, J = 7.5 Hz, 1H, CHpy), 4.59–4.52 (m, 2H, NCH2), 3.95–3.87 (m, 2H, NCH2), 2.97 (hept, J = 6.8 Hz, 2H, CH(CH3)2), 2.53 (s, 3H, CH3), 1.39 (d, J = 6.8 Hz, 6H, CH(CH3)2), 1.26 (d, J = 6.9 Hz, 6H, CH(CH3)2). 13C{1H} NMR (101 MHz, CDCl3) δ 201.0 (Ccarbene-Au), 157.3 (Cpy), 151.9 (Cpy), 146.2 (CAr, 2C), 138.3 (CHpy), 134.7 (CAr), 130.3 (CHAr), 124.8 (CHAr, 2C), 120.5 (CHpy), 111.4 (CHpy), 53.1 (NCH2), 48.7 (NCH2), 28.7 (CH(CH3)2, 2C), 25.0 (CH(CH3)2, 2C), 24.6 (CH(CH3)2, 2C), 24.4 (CH3). HRMS (ESI+): calcd. for C21H27AuIN3 [M + H]+: m/z 646.0988; found: m/z 646.0984; [M + Na]+: m/z 668.0807; found: 668.0840; [2M − I]+: m/z 1163.2780; found: 1163.2804.
Complex 2. The imidazolinium iodide salt L2 (180.0 mg, 0.37 mmol, 1.0 eq.), K2CO3 (261.2 mg, 1.89 mmol, 5.1 eq.) and [AuCl(SMe2)] (139.1 mg, 0.47 mmol, 1.3 eq.) were reacted in acetonitrile (2 mL). After the filtration over Celite®, the product was purified by filtering the residue over a pad of silica using DCM. The solvent was removed to obtain a yellow solid. It was washed with hexane and diethyl ether to afford complex 2 as a white solid (105.8 mg, 42% yield). 1H NMR (400 MHz, CDCl3) δ 8.96 (dd, J = 4.2, 1.7 Hz, 1H, CHQuin), 8.23 (dd, J = 8.3, 1.7 Hz, 1H, CHQuin), 8.10 (dd, J = 7.3, 1.4 Hz, 1H, CHQuin), 7.87 (dd, J = 8.2, 1.4 Hz, 1H, CHQuin), 7.60 (dd, J = 8.3, 7.4 Hz, 1H, CHQuin), 7.50 (dd, J = 8.3, 4.2 Hz, 1H, CHQuin), 7.42 (t, J = 7.8 Hz, 1H, CHAr), 7.25 (d, J = 7.8 Hz, 2H, CHAr), 4.72–4.64 (m, 2H, NCH2), 4.12–4.04 (m, 2H, NCH2), 3.23 (hept, J = 6.9 Hz, 2H, CH(CH3)2), 1.43 (d, J = 6.8 Hz, 6H, CH(CH3)2), 1.39 (d, J = 6.8 Hz, 6H, CH(CH3)2). 13C{1H} NMR (101 MHz, CDCl3) δ 203.7 (Ccarbene-Au), 150.4 (CHQuin), 146.8 (CAr, 2C), 144.1 (CQuin), 137.6 (CQuin), 136.6 (CHQuin), 134.6 (CAr), 130.0 (CHAr), 129.6 (CQuin), 128.8 (CHQuin), 128.3 (CHQuin), 126.4 (CHQuin), 124.7 (CHAr, 2C), 122.0 (CHQuin), 54.4 (NCH2), 53.0 (NCH2), 28.7 (CH(CH3)2, 2C), 25.3 (CH(CH3)2, 2C), 24.6 (CH(CH3)2, 2C). HRMS (ESI+): calcd. for C24H27AuIN3 [M + H]+: m/z 682.0988; found: m/z 682.0974; [2M − I]+: m/z 1235.2780; found: 1235.2746.
Complex 3. The imidazolinium iodide salt L3 (67.5 mg, 0.17 mmol, 1.0 eq.), K2CO3 (91.1 mg, 0.66 mmol, 4.0 eq.) and [AuCl(SMe2)] (63.8 mg, 0.22 mmol, 1.3 eq.) were reacted in acetonitrile (1 mL). The product was purified by column chromatography using DCM. By slow diffusion of pentane into a concentrated solution of complex 3 in chloroform, crystals suitable for X-ray diffraction analysis were obtained (32.9 mg, 33% yield). 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 8.2 Hz, 1H, CHpy), 7.62 (t, J = 7.9 Hz, 1H, CHpy), 7.02 (d, J = 7.5 Hz, 1H, CHpy), 6.94 (s, 2H, CHAr), 4.57–4.48 (m, 2H, NCH2), 3.94–3.85 (m, 2H, NCH2), 2.52 (s, 3H, CH3py), 2.30 (s, 3H, CH3Ar), 2.27 (s, 6H, CH3Ar). 13C{1H} NMR (101 MHz, CDCl3) δ 192.2 (Ccarbene-Au), 157.3 (Cpy), 151.9 (Cpy), 139.3 (CAr), 138.3 (CHpy), 135.5 (CAr), 135.1 (CAr, 2C), 130.0 (CHAr, 2C), 120.6 (CHpy), 112.0 (CHpy), 50.3 (NCH2), 48.8 (NCH2), 24.3 (CH3py), 21.2 (CH3Ar), 18.2 (CH3Ar, 2C). HRMS (ESI+): calcd. for C18H21AuIN3 [M + Na]+: m/z 626.0338; found: m/z 626.0336; [(C18H21N3)2Au]+: m/z 755.3131; found: 755.3150; [2M − I]+: m/z 1079.1841; found: 1079.1830.
Complex 4. For the synthesis of NHC-Au(I) complex 4, imidazolinium salt L4 (60.1 mg, 0.18 mmol, 1.0 eq) and chloro(dimethylsulfide)gold(I) (54.8 mg, 0.19 mmol, 1.0 eq) were mixed in acetone (0.6 mL), under nitrogen atmosphere, and stirred at room temperature for 10 min. Then, potassium carbonate (27.6 mg, 0.20 mmol, 1.1 eq) was added and the mixture was stirred and heated at 60 °C for 16 h. After this time, the reaction mixture was cooled down to room temperature, then the solvent was removed under reduced pressure, and DCM was added. The mixture was filtered over a pad of silica, which was washed with more DCM, and the resulting solution was concentrated to the minimal volume. Next, pentane was added to precipitate the desired product. It was washed with more pentane, and it was dried under vacuum. Complex 4 was obtained as a white solid (75.5 mg, 67% yield). 1H NMR (400 MHz, CDCl3) δ 7.40–7.24 (m, 4H, CHAr), 6.94 (d, J = 0.7 Hz, 2H, CHAr), 4.11 (t, J = 9.6 Hz, 2H, NCH2), 3.96 (td, J = 10.4, 1.7 Hz, 2H, NCH2), 2.76 (q, J = 7.6 Hz, 2H, CH2CH3), 2.31 (s, 6H, CH3), 2.30 (s, 3H, CH3), 1.35 (t, J = 7.6 Hz, 3H, CH2CH3). 13C{1H} NMR (101 MHz, CDCl3) δ 195.0 (Ccarbene-Au), 141.2 (CAr), 139.1 (CAr), 138.4 (CAr), 135.6 (CAr, 2C), 134.8 (CAr), 129.9 (CHAr, 2C), 129.9 (CHAr), 129.5 (CHAr), 128.4 (CHAr), 127.5 (CHAr), 53.3 (NCH2), 51.0 (NCH2), 24.4 (CH2CH3), 21.2 (CH3), 18.1 (CH3, 2C), 15.0 (CH2CH3). HRMS (ESI+): calcd. for C20H24AuClN2 [M + Na]+: m/z 547.1186; found: m/z 547.1181; [2M + Na]+: m/z 1071.2479; found: 1071.2438; [2M − Cl]+: m/z 1013.2899; found: 1013.2865.
Compound L1ox-I.1H NMR (400 MHz, 228K, CDCl3) δ 7.91–7.83 (m, 2H, CHpy), 7.38 (t, J = 7.7 Hz, 1H, CHAr), 7.23 (d, J = 7.6 Hz, 2H, CHAr), 4.23 (t, J = 8.2 Hz, 2H, NCH2), 3.71 (t, J = 8.2 Hz, 2H, NCH2), 2.98 (hept, J = 6.8 Hz, 2H, CH(CH3)2), 2.63 (s, 3H, CH3), 1.23 (d, J = 6.9 Hz, 6H, CH(CH3)2), 1.20 (d, J = 6.6 Hz, 6H, CH(CH3)2). 13C{1H} NMR (101 MHz, CDCl3) δ 157.7 (Cpy), 156.2 (C=O), 152.1 (Cpy), 148.0 (CAr, 2C), 147.3 (CHpy), 132.8 (CAr), 129.2 (CHAr), 124.3 (CHAr, 2C), 112.2 (CHpy), 86.2 (Cpy-I), 45.7 (NCH2), 41.7 (NCH2), 28.9 (CH(CH3)2, 2C), 28.8 (CH3), 24.6 (CH(CH3)2, 2C), 24.4 (CH(CH3)2, 2C). HRMS (ESI+): calcd for C21H26IN3O [M + H]+: m/z 464.1193; found: m/z 464.1193; [M + Na]+: m/z 486.1013; found: m/z 486.1012; [2M + Na]+: m/z 949.2133; found: m/z 949.2141.
Compound L2ox.1H NMR (400 MHz, CDCl3) δ 8.93 (dd, J = 4.2, 1.8 Hz, 1H, CHAr), 8.17 (dd, J = 8.3, 1.8 Hz, 1H, CHAr), 7.93 (dd, J = 7.5, 1.4 Hz, 1H, CHAr), 7.71 (dd, J = 8.2, 1.4 Hz, 1H, CHAr), 7.56 (dd, J = 8.2, 7.5 Hz, 1H, CHAr), 7.42 (dd, J = 8.3, 4.1 Hz, 1H, CHAr), 7.35 (dd, J = 8.3, 7.1 Hz, 1H, CHAr), 7.23 (d, J = 7.7 Hz, 2H, CHAr), 4.45–4.40 (m, 2H, NCH2), 3.90–3.84 (m, 2H, NCH2), 3.32 (hept, J = 6.9 Hz, 2H, CH(CH3)2), 1.32 (d, J = 6.9 Hz, 6H, CH(CH3)2), 1.30 (d, J = 6.8 Hz, 6H, CH(CH3)2). 13C{1H} NMR (101 MHz, CDCl3) δ 159.5 (C=O), 149.3 (CHQuin), 148.5 (CAr, 2C), 144.3 (CQuin), 138.3 (CQuin), 136.5 (CHQuin), 133.8 (CAr), 129.7 (CQuin), 128.8 (CHAr), 127.5 (CHQuin), 126.7 (CHQuin), 126.1 (CHQuin), 124.2 (CHAr, 2C), 121.3 (CHQuin), 47.4 (NCH2), 47.0 (NCH2), 28.8 (CH(CH3)2, 2C), 24.8 (CH(CH3)2, 2C), 24.5 (CH(CH3)2, 2C). HRMS (ESI+): calcd. for C24H27N3O [M + H]+: m/z 374.2227; found: m/z 374.2211; [M + Na]+: m/z 396.2046; found: 396.2036; [2M + Na]+: m/z 769.4200; found: m/z 769.4164.
Compound L2ox-I.1H NMR (400 MHz, CDCl3) δ 8.88 (dd, J = 4.1, 1.6 Hz, 1H, CHQuin), 8.39 (dd, J = 8.5, 1.6 Hz, 1H, CHQuin), 8.13 (d, J = 8.1 Hz, 1H, CHQuin), 7.69 (d, J = 8.0 Hz, 1H, CHQuin), 7.49 (dd, J = 8.6, 4.1 Hz, 1H, CHQuin), 7.35 (dd, J = 8.3, 7.1 Hz, 1H, CHAr), 7.23 (d, J = 7.4 Hz, 2H, CHAr), 4.46–4.39 (m, 2H, NCH2), 3.89–3.83 (m, 2H, NCH2), 3.28 (hept, J = 6.9 Hz, 2H, CH(CH3)2), 1.32 (d, J = 6.9 Hz, 6H, CH(CH3)2), 1.29 (d, J = 6.8 Hz, 6H, CH(CH3)2). 13C{1H} NMR (101 MHz, CDCl3) δ 159.2 (C=O), 149.9 (CHQuin), 148.4 (CAr, 2C), 144.8 (CQuin), 140.9 (CHQuin), 139.4 (CQuin), 137.7 (CHQuin), 133.5 (CAr), 131.2 (CQuin), 129.0 (CHAr), 128.5 (CHQuin), 124.2 (CHAr, 2C), 122.9 (CHQuin), 95.3 (CQuin-I), 47.4 (NCH2), 47.0 (NCH2), 28.8 (CH(CH3)2, 2C), 24.8 (CH(CH3)2, 2C), 24.5 (CH(CH3)2, 2C). HRMS (ESI+): calcd. for C24H26IN3O [M + H]+: m/z 500.1193; found: m/z 500.1184; [M + Na]+: m/z 522.1013; found: 522.0999.
Compound L3ox.1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 8.4 Hz, 1H, CHpy), 7.51 (t, J = 7.9 Hz, 1H, CHpy), 6.93 (s, 2H, CHAr), 6.78 (d, J = 7.3 Hz, 1H, CHpy), 4.31–4.23 (m, 2H, NCH2), 3.74–3.67 (m, 2H, NCH2), 2.47 (s, 3H, CH3py), 2.29 (s, 3H, CH3Ar), 2.25 (s, 6H, CH3Ar). 13C{1H} NMR (101 MHz, CDCl3) δ 156.3 (Cpy), 155.9 (C=O), 152.4 (Cpy), 138.0 (CAr), 137.7 (CHpy), 137.0 (CAr, 2C), 133.1 (CAr), 129.5 (CHAr, 2C), 116.8 (CHpy), 109.9 (CHpy), 43.2 (NCH2), 42.0 (NCH2), 24.5 (CH3py), 21.1 (CH3Ar), 18.0 (CH3Ar, 2C). HRMS (ESI+): calcd. for C18H21N3O [M + H]+: m/z 296.1757; found: m/z 296.1774; [M + Na]+: m/z 318.1577; found: 318.1595; [2M + Na]+: m/z 613.3261; found: m/z 613.3278.
4. Conclusions
In summary, novel NHC-Au(I) complexes (1–4) were synthesized and thoroughly characterized, and their reactivity in front external oxidants was analyzed. In contrast to the stability shown by commercial IPr- and SIPr-Au(I) complexes, complexes 1–4 underwent a controlled decomposition pathway to form oxidized NHC=O azolones as the main organic product, and quantitative conversion of all the Au(I) contained in complexes into macroscopic Au(0) nuggets (~0.4–0.5 mm). Azolones and M(0) have previously been described as decomposition products of NHC-metal complexes (M = Pd(II), Pt(II), Ni(II)), and we show herein a singular case of gold transforming this decomposition pathway, representing a good strategy to recover gold nuggets of high purity from soluble gold species (as NHC-Au(I)).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052302/s1, Figure S1–S80, Table S1–S6, Schemes S1–S8. CCDC 2238757 (1), 2238754 (2), 2238755 (3), 2238756, (4) contain the supplementary crystallographic data for this paper [44].
Author Contributions
Conceptualization, G.C.V. and X.R; methodology and formal analysis, P.F., A.T.P. and N.V.T.; writing—original draft preparation and editing, P.F, N.V.T., G.C.V. and X.R.; funding acquisition, G.C.V. and X.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research project was supported by MINECO-Spain, PID2019-104498GB-I00 to X.R., as well as by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the procurement of high-cost research equipment grant” (Project Number: 16).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
X.R. is grateful for an ICREA-Acadèmia award. Athanasios Zarkadoulas is acknowledged for carrying out the catalytic activity evaluation experiments. We also thank L. Capdevila and STR-UdG for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1–4 are available from the authors.
References
- Hemmert, C.; Gornitzka, H. Luminescent bioactive NHC–metal complexes to bring light into cells. Dalton Trans. 2016, 45, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.G.; Aktaş, A.; Gürses, C.; Gök, Y.; Ateş, B. Chemistry, structure, and biological roles of Au-NHC complexes as TrxR inhibitors. Bioorganic Chem. 2020, 95, 103552. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, B.; Xiong, X.; Chan, A.S.C.; Sun, R.W.; Zou, T. Bioorthogonal Activation of Dual Catalytic and Anti-Cancer Activities of Organogold(I) Complexes in Living Systems. Angew. Chem. Int. Ed. 2021, 60, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Nelson, D.J.; Nolan, S.P. Optimizing Catalyst and Reaction Conditions in Gold(I) Catalysis–Ligand Development. Chem. Rev. 2021, 121, 8559–8612. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.M.; Khazipov, O.V.; Shevchenko, M.A.; Chernenko, A.Y.; Astakhov, A.V.; Eremin, D.B.; Pasyukov, D.V.; Kashin, A.S.; Ananikov, V.P. Revealing the unusual role of bases in activation/deactivation of catalytic systems: O–NHC coupling in M/NHC catalysis. Chem. Sci. 2018, 9, 5564–5577. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.M.; Denisova, E.A.; Eremin, D.B.; Ananikov, V.P. The key role of R–NHC coupling (R = C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species. Chem. Sci. 2020, 11, 6957–6977. [Google Scholar] [CrossRef] [PubMed]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef]
- Petronilho, A.; Müller-Bunz, H.; Albrecht, M. Mesoionic oxides: Facile access from triazolium salts or triazolylidene copper precursors, and catalytic relevance. Chem. Commun. 2012, 48, 6499–6501. [Google Scholar] [CrossRef]
- Nandy, A.; Samanta, T.; Mallick, S.; Mitra, P.; Seth, S.K.; Saha, K.D.; Al-Deyab, S.S.; Dinda, J. Synthesis of gold(iii) ← gold(i)–NHC through disproportionation: The role of gold(i)–NHC in the induction of apoptosis in HepG2 cells. New J. Chem. 2016, 40, 6289–6298. [Google Scholar] [CrossRef]
- Font, P.; Valdés, H.; Guisado-Barrios, G.; Ribas, X. Hemilabile MIC^N ligands allow oxidant-free Au(i)/Au(iii) arylation-lactonization of γ-alkenoic acids. Chem. Sci. 2022, 13, 9351–9360. [Google Scholar] [CrossRef]
- Hough, R.M.; Butt, C.R.M.; Reddy, S.M.; Verrall, M. Gold nuggets: Supergene or hypogene? Aust. J. Earth. Sci. 2007, 54, 959–964. [Google Scholar] [CrossRef]
- Bütof, L.; Wiesemann, N.; Herzberg, M.; Altzschner, M.; Holleitner, A.; Reith, F.; Nies, D.H. Synergistic gold–copper detoxification at the core of gold biomineralisation in Cupriavidus metallidurans. Metallomics 2018, 10, 278–286. [Google Scholar] [CrossRef]
- Reith, F.; Rogers, S.L.; McPhail, D.C.; Webb, D. Biomineralization of Gold: Biofilms on Bacterioform Gold. Science 2006, 313, 233–236. [Google Scholar] [CrossRef]
- Sun, D.T.; Gasilova, N.; Yang, S.; Oveisi, E.; Queen, W.L. Rapid, Selective Extraction of Trace Amounts of Gold from Complex Water Mixtures with a Metal–Organic Framework (MOF)/Polymer Composite. J. Am. Chem. Soc. 2018, 140, 16697–16703. [Google Scholar] [CrossRef]
- Yue, C.; Sun, H.; Liu, W.-J.; Guan, B.; Deng, X.; Zhang, X.; Yang, P. Environmentally Benign, Rapid, and Selective Extraction of Gold from Ores and Waste Electronic Materials. Angew. Chem. Int. Ed. 2017, 56, 9331–9335. [Google Scholar] [CrossRef]
- Papastavrou, A.T.; Pauze, M.; Gómez-Bengoa, E.; Vougioukalakis, G.C. Unprecedented Multicomponent Organocatalytic Synthesis of Propargylic Esters via CO2 Activation. ChemCatChem 2019, 11, 5379–5386. [Google Scholar] [CrossRef]
- Liori, A.A.; Stamatopoulos, I.K.; Papastavrou, A.T.; Pinaka, A.; Vougioukalakis, G.C. A Sustainable, User-Friendly Protocol for the Pd-Free Sonogashira Coupling Reaction. Eur. J. Org. Chem. 2018, 2018, 6134–6139. [Google Scholar] [CrossRef]
- Collado, A.; Gómez-Suárez, A.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes. Chem. Commun. 2013, 49, 5541–5543. [Google Scholar] [CrossRef]
- Visbal, R.; Laguna, A.; Gimeno, M.C. Simple and efficient synthesis of [MCI(NHC)] (M = Au, Ag) complexes. Chem. Commun. 2013, 49, 5642–5644. [Google Scholar] [CrossRef]
- Catalano, V.J.; Moore, A.L. Mono-, Di-, and Trinuclear Luminescent Silver(I) and Gold(I) N-Heterocyclic Carbene Complexes Derived from the Picolyl-Substituted Methylimidazolium Salt: 1-Methyl-3-(2-pyridinylmethyl)-1H-imidazolium Tetrafluoroborate. Inorg. Chem. 2005, 44, 6558–6566. [Google Scholar] [CrossRef]
- Navarro, M.; Tabey, A.; Szalóki, G.; Mallet-Ladeira, S.; Bourissou, D. Stable Au(III) Complexes Bearing Hemilabile P∧N and C∧N Ligands: Coordination of the Pendant Nitrogen upon Oxidation of Gold. Organometallics 2021, 40, 1571–1576. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Narender, N.; Rohitha, C.N.; Kulkarni, S.J. Iodination of Aromatic Compounds Using Potassium Iodide and Hydrogen Peroxide. Synth. Commun. 2008, 38, 3894–3902. [Google Scholar] [CrossRef]
- Manke, D.R.; Golen, J.A.; Stennett, C.R.; Naeem, M.; Javier-Jimenez, D.R.; Power, P.P. Reusing meta-terphenyl ligands: Synthesis, metalation and recycling of 5-pyrrolidino-m-terphenyl. Polyhedron 2022, 222, 115947. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Choudhary, P. Ni-Catalyzed Regioselective C-5 Halogenation of 8-Aminoquinoline and Co-Catalyzed Chelation Assisted C−H Iodination of Aromatic Sulfonamides with Molecular Iodine. Chem. Asian J. 2022, 17, e202200874. [Google Scholar] [CrossRef]
- Motati, D.R.; Uredi, D.; Watkins, E.B. A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines. Chem. Sci. 2018, 9, 1782–1788. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Liu, Y.; Wang, T.; Lin, A.; Yao, H.; Xu, J. Metal-free C5-selective halogenation of quinolines under aqueous conditions. Org. Chem. Front. 2017, 4, 622–626. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, E.; Qiu, R.; Sohail, M.; Wu, S.; Chen, F.-X. Oxygen-atom insertion of NHC–copper complex: The source of oxygen from N,N-dimethylformamide. J. Organomet. Chem. 2013, 743, 44–48. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Toullec, P.Y.; Borge, J.; Conejero, S.; Michelet, V.; Cadierno, V. Water-Soluble Gold(I) and Gold(III) Complexes with Sulfonated N-Heterocyclic Carbene Ligands: Synthesis, Characterization, and Application in the Catalytic Cycloisomerization of γ-Alkynoic Acids into Enol-Lactones. ACS Catal. 2013, 3, 3086–3098. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Toullec, P.Y.; Díez, J.; Conejero, S.; Michelet, V.; Cadierno, V. Cycloisomerization versus Hydration Reactions in Aqueous Media: A Au(III)-NHC Catalyst That Makes the Difference. Org. Lett. 2012, 14, 2520–2523. [Google Scholar] [CrossRef]
- Ghavami, Z.S.; Anneser, M.R.; Kaiser, F.; Altmann, P.J.; Hofmann, B.J.; Schlagintweit, J.F.; Grivani, G.; Kühn, F.E. A bench stable formal Cu(iii) N-heterocyclic carbene accessible from simple copper(ii) acetate. Chem. Sci. 2018, 9, 8307–8314. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterials 2019, 9, 838. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, R.; Chen, C.; Li, J.; Li, Y. Synergistic effect of bimetallic PdAu nanocrystals on oxidative alkyne homocoupling. Chem. Commun. 2018, 54, 13155–13158. [Google Scholar] [CrossRef]
- Kidwai, M.; Bansal, V.; Kumar, A.; Mozumdar, S. The first Au-nanoparticles catalyzed green synthesis of propargylamines via a three-component coupling reaction of aldehyde, alkyne and amine. Green Chem. 2007, 9, 742–745. [Google Scholar] [CrossRef]
- Li, Q.; Das, A.; Wang, S.; Chen, Y.; Jin, R. Highly efficient three-component coupling reaction catalysed by atomically precise ligand-protected Au38(SC2H4Ph)24 nanoclusters. Chem. Commun. 2016, 52, 14298–14301. [Google Scholar] [CrossRef]
- Kyriakou, G.; Beaumont, S.K.; Humphrey, S.M.; Antonetti, C.; Lambert, R.M. Sonogashira Coupling Catalyzed by Gold Nanoparticles: Does Homogeneous or Heterogeneous Catalysis Dominate? ChemCatChem 2010, 2, 1444–1449. [Google Scholar] [CrossRef]
- Prasad, B.; Gilbertson, S. One-Pot Synthesis of N-Heterocyclic Carbene Ligands From a N-(2-iodoethyl)arylamine salts. Org. Lett. 2009, 11, 3710–3713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).