Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Biochar Co-Torrefaction
2.1.1. Properties of FB and PP
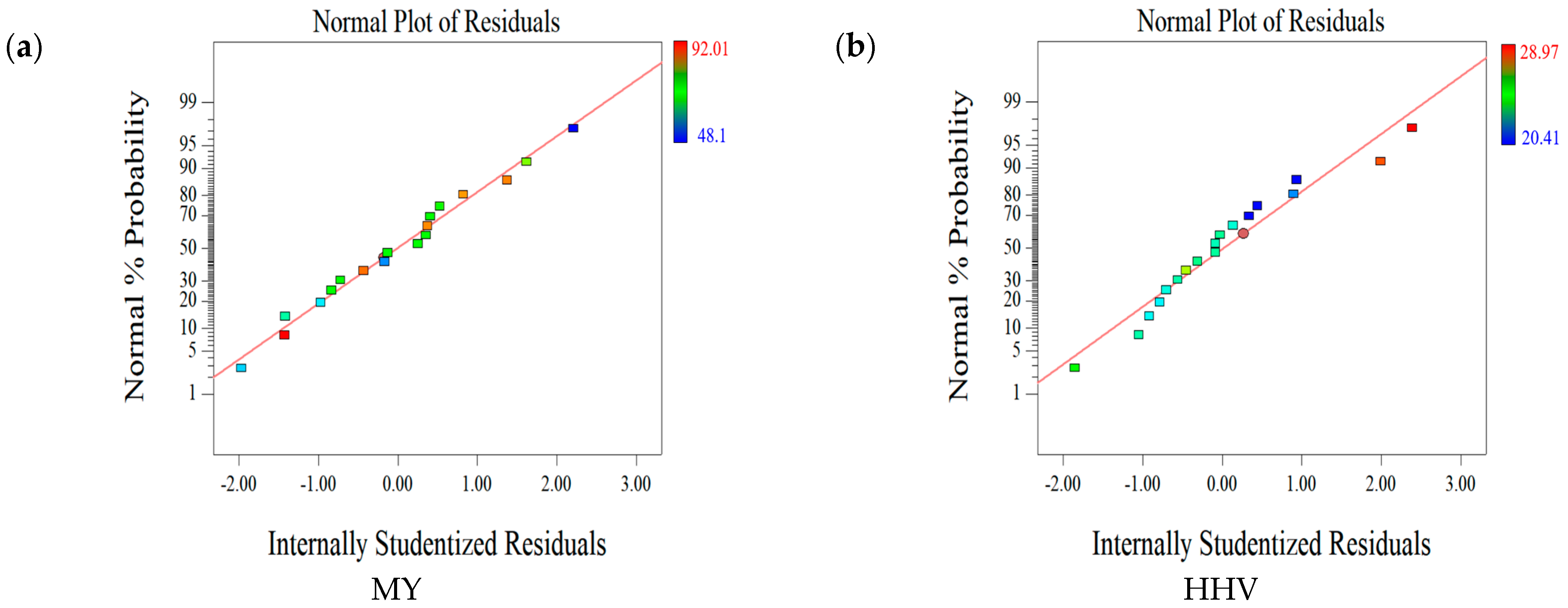
2.1.2. Statistical Evaluation of the Relationship between Factors and Responses
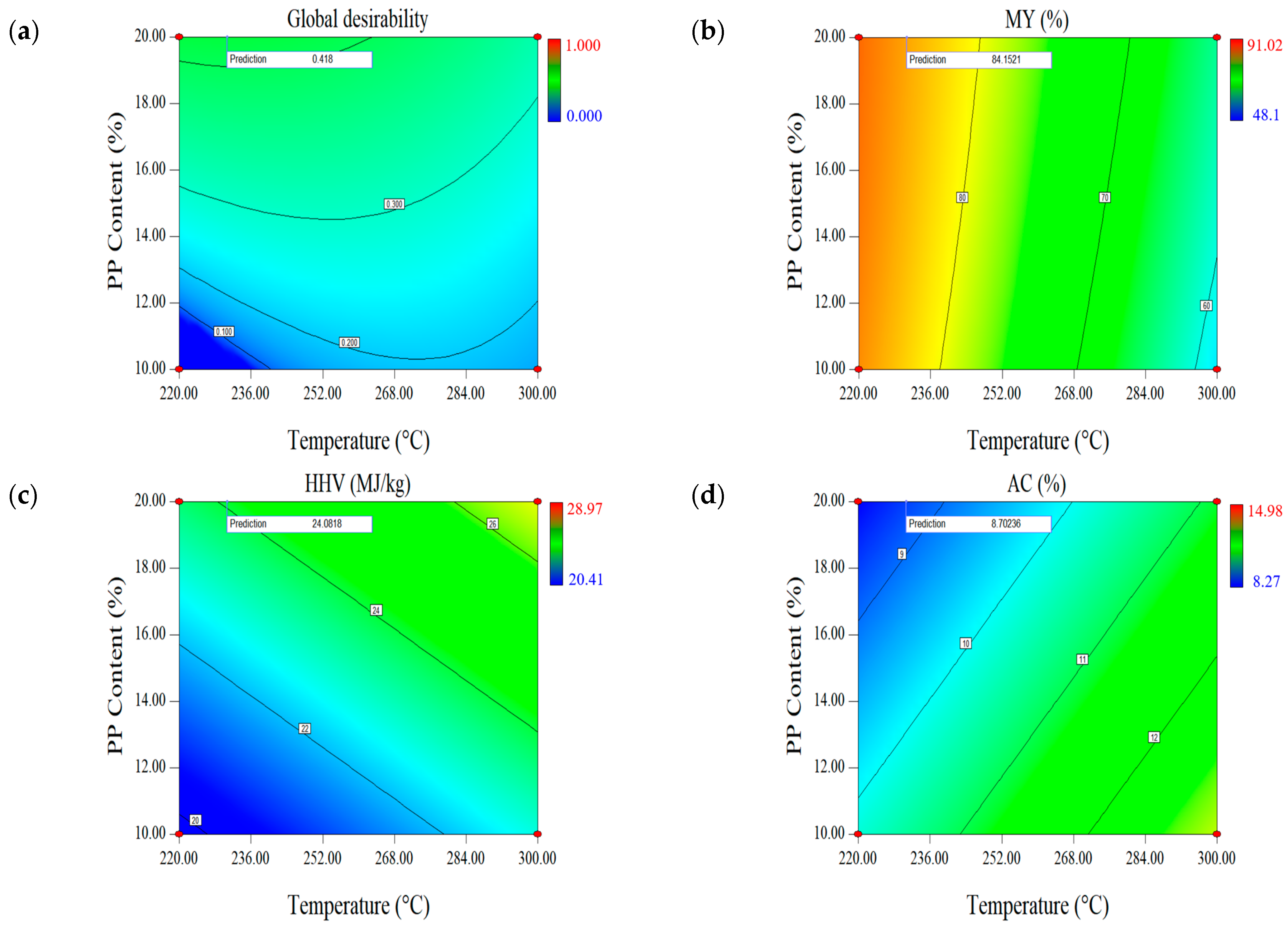
2.1.3. Effect of Parameters on the First Set of Responses (MY-HHV-AC)
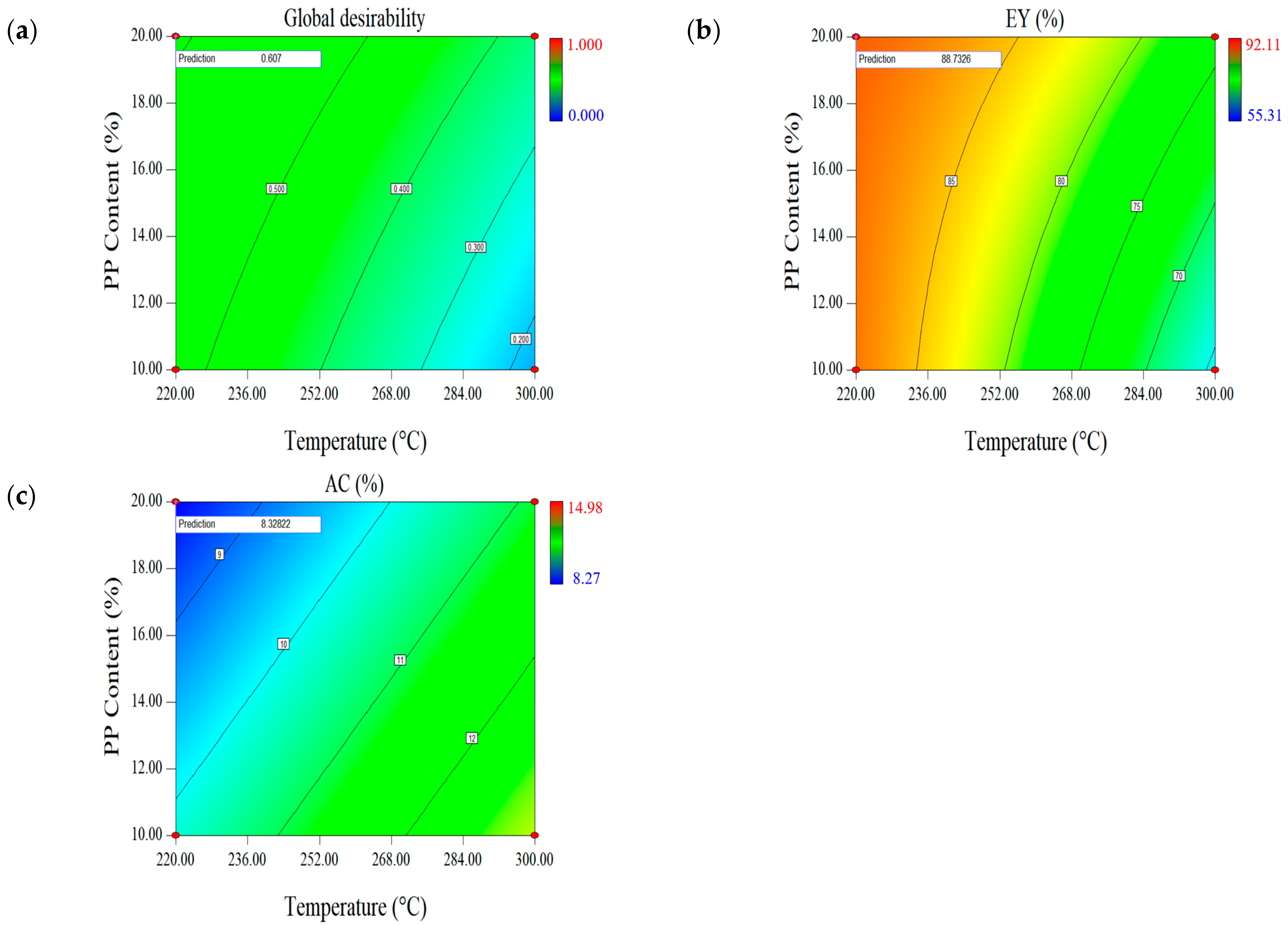
2.1.4. Effect of Parameters on the Second Set of Responses (EY-AC)
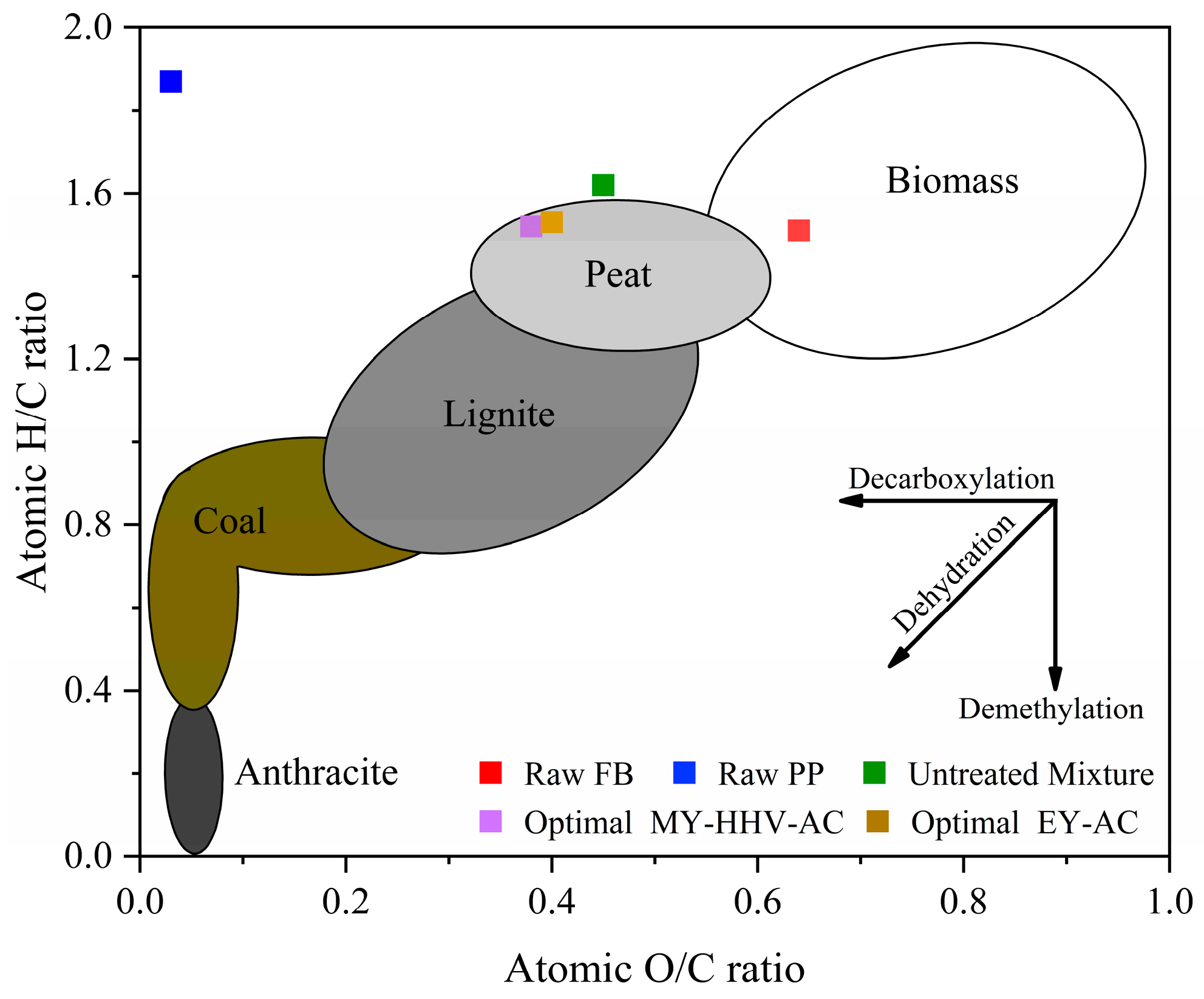
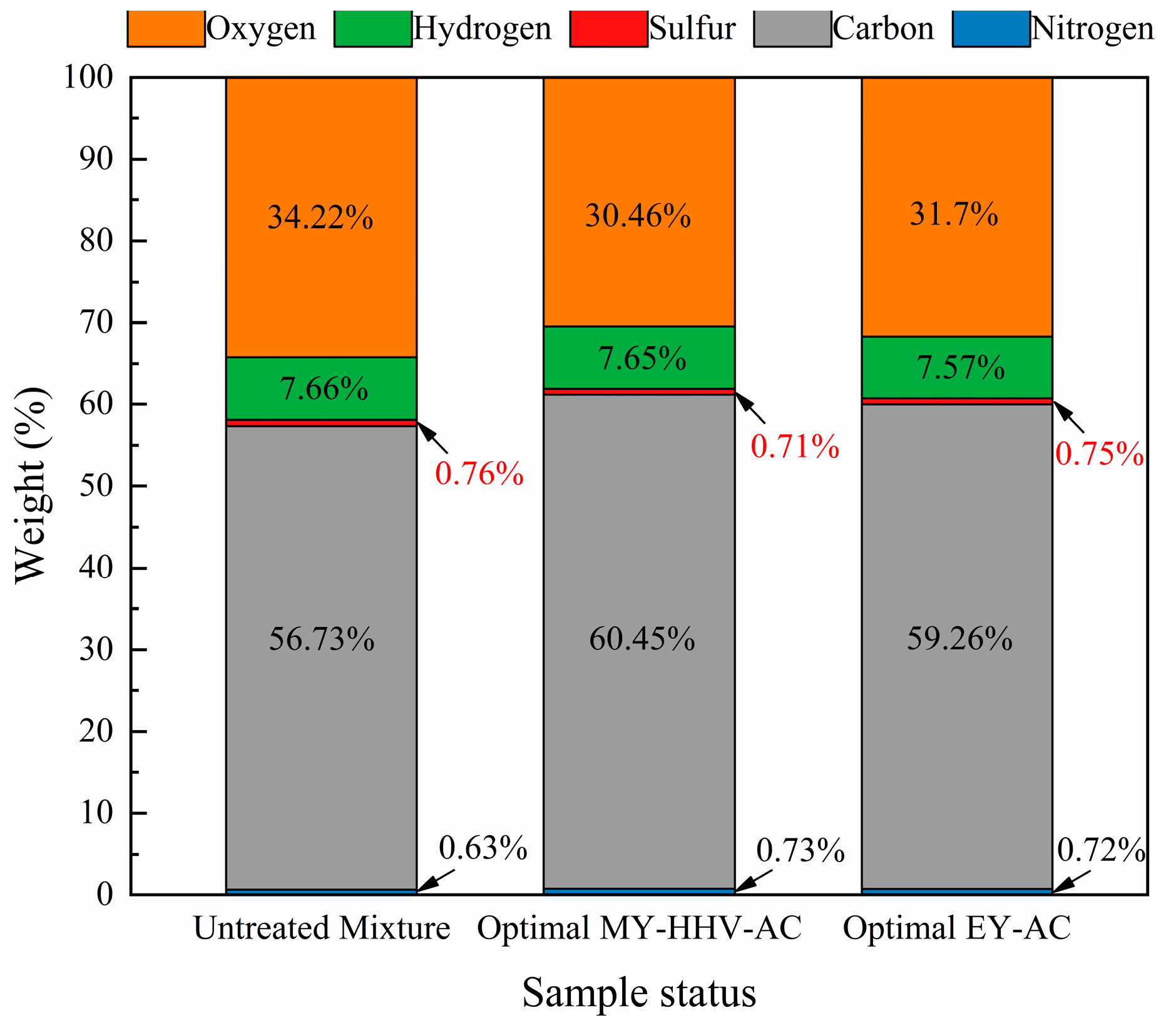
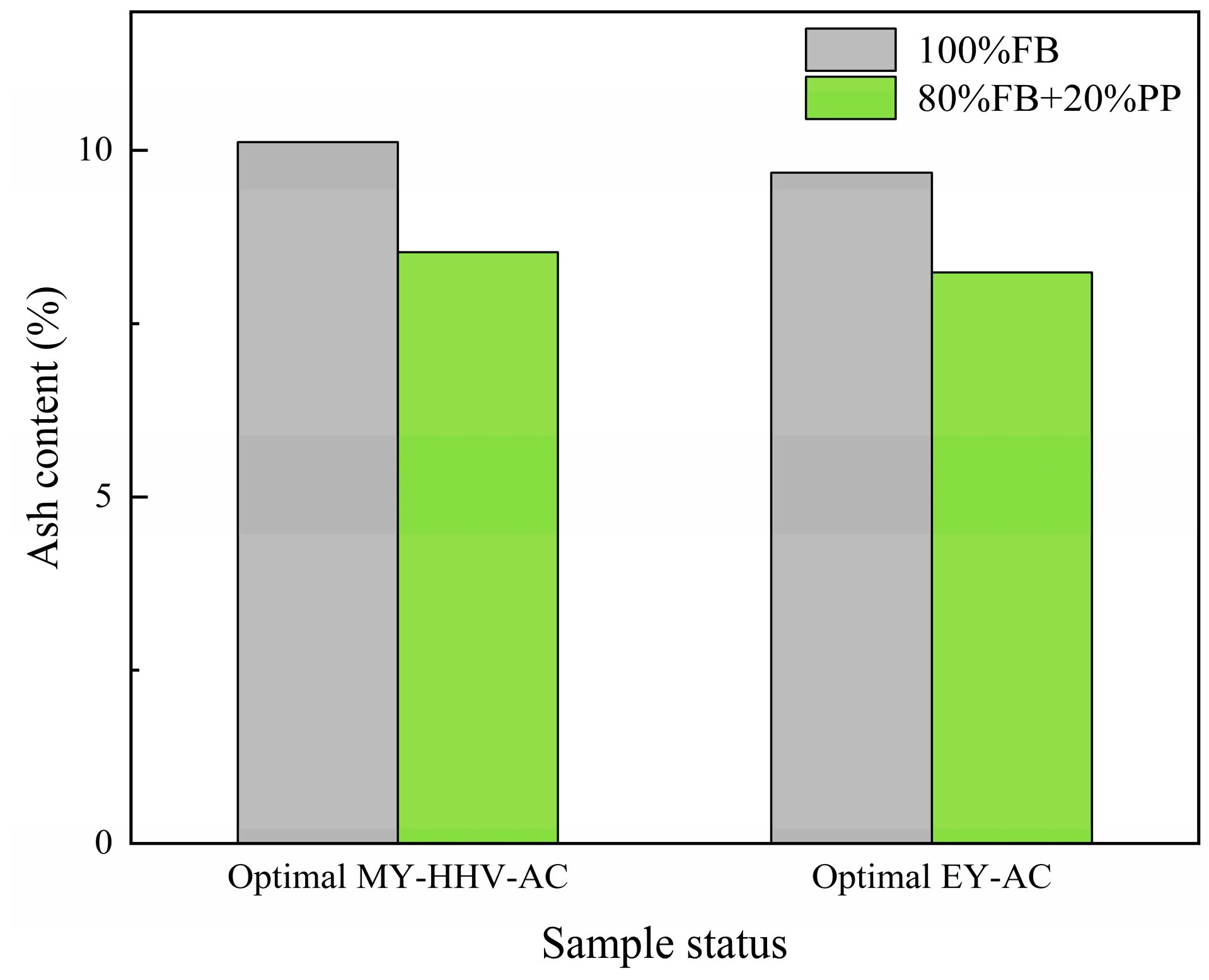
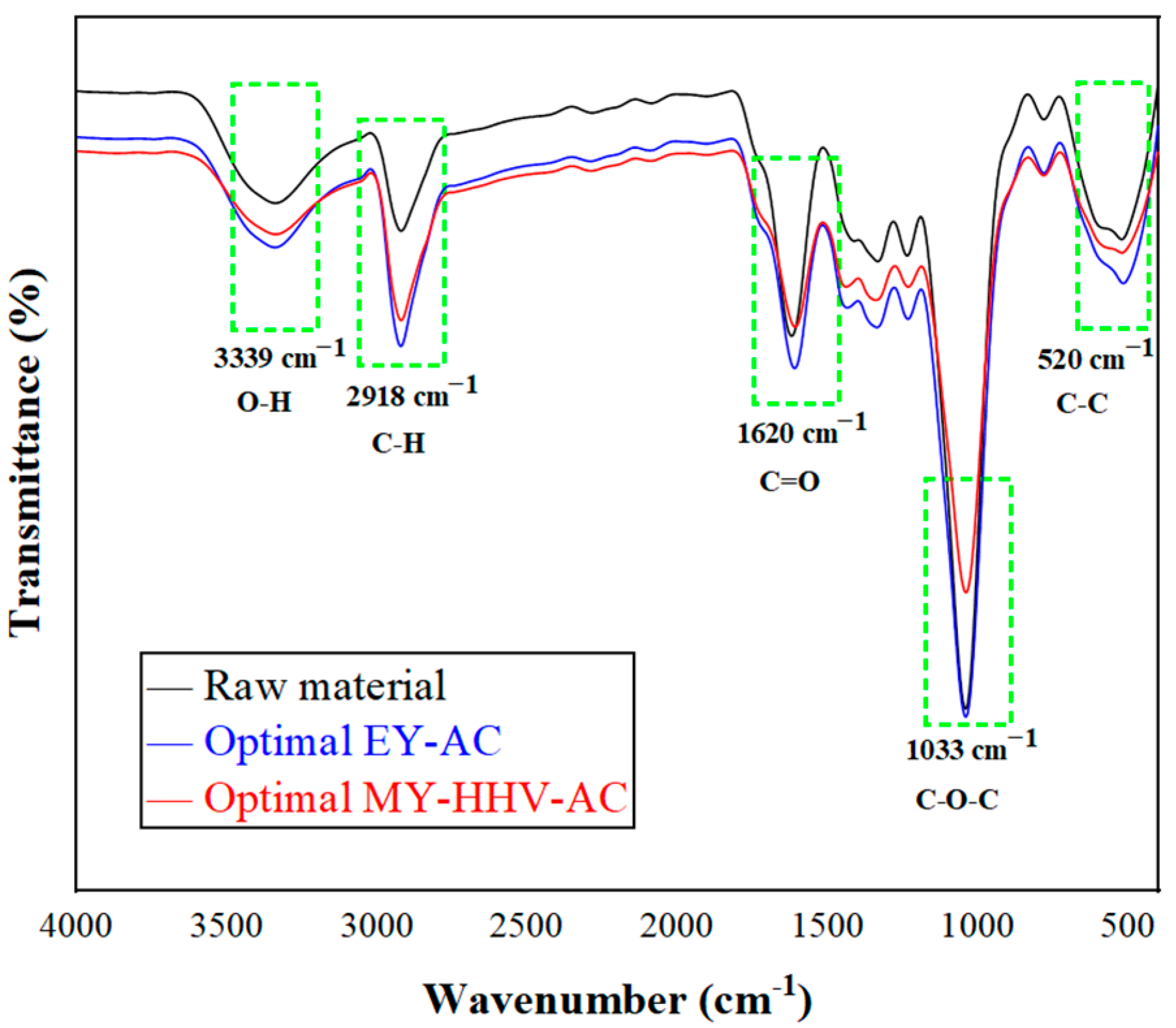
2.2. Characterization of the Optimal Co-Torrefaction Biochar
2.2.1. Fuel Characteristics
2.2.2. Fourier Transform Infrared (FTIR) Analysis
3. Applications and Discussion
3.1. Densification of Co-Torrefaction Biochar
3.2. Biofuel Recycling Economy
4. Materials and Methods
4.1. Materials
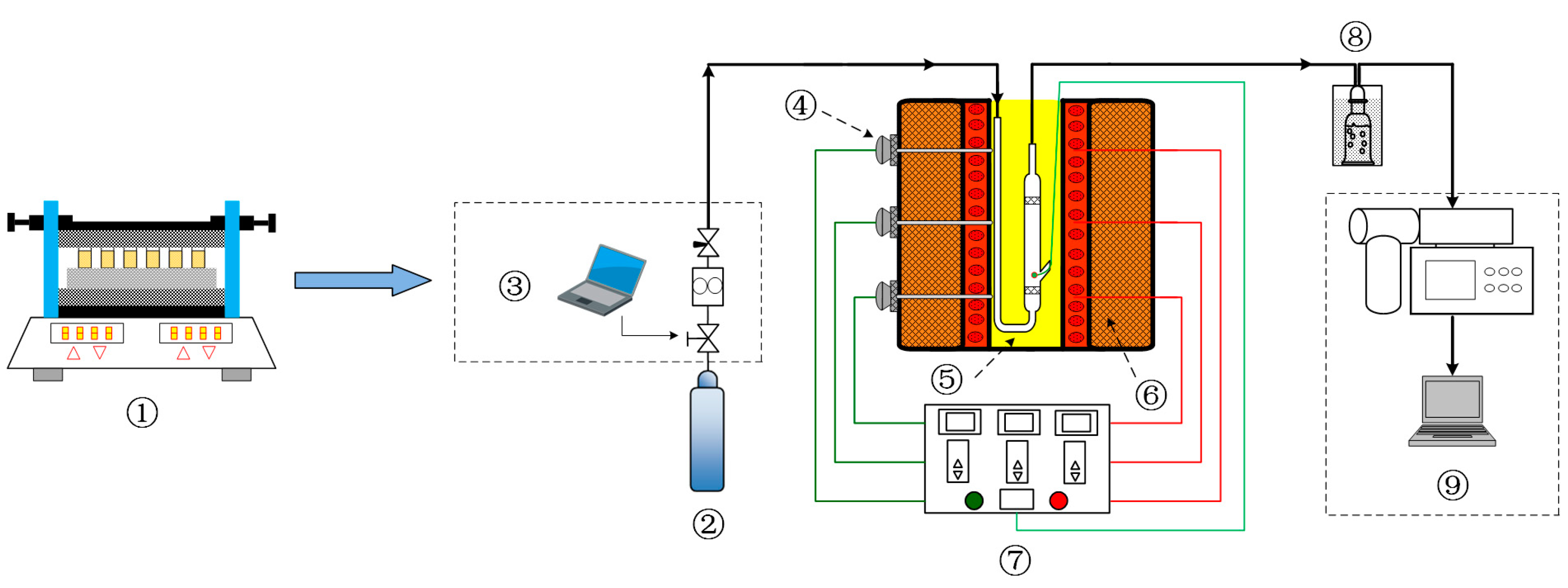
4.2. Torrefaction Experiment
4.3. Optimization of the Torrefaction Process
4.4. Characterization of the Optimal Co-Torrefaction of Biochar
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature
| FB | Fungus bran | HHV | Higher heating value |
| PP | Polypropylene | MY | Mass yield |
| AC | Ash content | EY | Energy yield |
| LDPE | Low-density polyethylene | A | Reaction temperature |
| HDPE | High-density polyethylene | B | Reaction time |
| RSM | Response surface methodology | C | PP blending ratio |
| CCD | Center composite design | α | Axis point |
References
- Saidur, R.; Abdelaziz, E.; Demirbas, A.; Hossain, M.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Saxena, R.; Adhikari, D.; Goyal, H. Biomass-based energy fuel through biochemical routes: A review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H.; Lei, L.; Zhao, W. Effects of inoculating with lignocellulose-degrading consortium on cellulose-degrading genes and fungal community during co-composting of spent mushroom substrate with swine manure. Bioresour. Technol. 2019, 291, 121876. [Google Scholar] [CrossRef]
- Wang, Q. Substituted Substrate Exploitation of Auricularia Auricular Cultivation and Spent Compost Recycling. Master’s Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Sharma, B.; Shekhar, S.; Sharma, S.; Jain, P. The paradigm in conversion of plastic waste into value added materials. Clean. Eng. Technol. 2021, 4, 100254. [Google Scholar] [CrossRef]
- Wannapeera, J.; Worasuwannarak, N. Upgrading of woody biomass by torrefaction under pressure. J. Anal. Appl. Pyrolysis 2012, 96, 173–180. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Basu, P.; Sadhukhan, A.; Gupta, P.; Rao, S.; Dhungana, A.; Acharya, B. An experimental and theoretical investigation on torrefaction of a large wet wood particle. Bioresour. Technol. 2014, 159, 215–222. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, sulfur and nitrogen oxide emissions from combustion of pulverized raw and torrefified biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Kizuka, R.; Ishii, K.; Ochiai, S.; Sato, M.; Yamada, A.; Nishimiya, K. Improvement of Biomass Fuel Properties for Rice Straw Pellets Using Torrefaction and Mixing with Wood Chips. Waste Biomass Valorization 2021, 12, 3417–3429. [Google Scholar] [CrossRef]
- Iez-Rodrguez, I.; Maryn-Lara, M.; Blzquez, G.; Prez, A.; Calero, M. Effect of torrefaction conditions on greenhouse crop residue: Optimization of conditions to upgrade solid characteristics. Bioresour. Technol. 2017, 244, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Emadi, B.; Iroba, K.; Tabil, L. Effect of polymer plastic binder on mechanical, storage and combustion characteristics of torrefied and pelletized herbaceous biomass. Appl. Energy 2017, 198, 312–319. [Google Scholar] [CrossRef]
- Obiora, A.; Tabil, L.; Mupondwa, E.; Emadi, B. Torrefaction and Pelleting of Wheat and Barley Straw for Biofuel and Energy Applications. Front. Energy Res. 2021, 9, 699657. [Google Scholar]
- Chen, Y.; Chen, L. Pelleting spent coffee grounds by waste utensils as binders of biofuels. J. Environ. Chem. Eng. 2021, 9, 105006. [Google Scholar] [CrossRef]
- Leontiev, A.; Kiverin, A.; Medvetskaya, N.; Melnikova, K.; Kichatov, B.; Korshunov, A. Oxidative torrefaction of briquetted birch shavings in the bentonite. Energy 2018, 165, 303–313. [Google Scholar]
- Guo, S.; Guo, T.; Che, D.; Liu, H.; Sun, B. Response surface analysis of energy balance and optimum condition for torrefaction of corn straw. Korean J. Chem. Eng. 2022, 39, 1287–1298. [Google Scholar]
- Singh, R.; Chakraborty, J.; Sarkar, A. Optimizing the torrefaction of pigeon pea stalk (cajanus cajan) using response surface methodology (RSM) and characterization of solid, liquid and gaseous products. Renew. Energy 2020, 155, 677–690. [Google Scholar] [CrossRef]
- Tadesse, Y.; Kassahun, S.; Kiflie, Z. Effects of operational parameters on torrefaction performance of coffee husk and cotton stalk mixed biomass: A surface response methodology approach. Biomass Convers. Biorefinery 2021, 1–16. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Z.; Fu, K.; Zeng, Z.; Wang, J.; Lu, M. Torrefaction of biomass stalk and its effect on the yieldand quality of pyrolysis products. Fuel 2015, 159, 27–32. [Google Scholar]
- Fuad, M.; Faizal, H.; Rahman, M.; Latiff, Z. Torrefaction of densified empty fruit bunches with addition of plastics waste. Biofuels 2018, 11, 491–501. [Google Scholar]
- Portilho, G.; Castro, V.; Carneiro, A.; Zanuncio, J. Potential of Briquette Produced with Torrefied Agroforestry Biomass to Generate Energy. Forests 2020, 11, 1272. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, A.; Song, K. Torrefaction of cultivation residue of Auricularia auricula-judae to obtain biochar with enhanced fuel properties. Bioresour. Technol. 2016, 206, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, W.; Ogunsuyi, H. Impact of torrefaction process temperature on the energy content and chemical composition of stool tree (Alstonia congenisis Engl) woody biomass. Curr. Opin. Green Sustain. 2021, 4, 100115. [Google Scholar] [CrossRef]
- Alam, M.; Dai, B.; Wu, X.; Hoadley, A.; Zhang, L. A critical review of ash slagging mechanisms and viscosity measurement for low-rank coal and bio-slags. Front. Energy 2021, 15, 46–67. [Google Scholar] [CrossRef]
- Gan, M.; Lim, W.; Ng, H.; Ong, M.; Gan, S.; Lee, L.; Gopakumar, S.T. Wet Torrefaction: Response Surface, Optimization, and Combustion Studies. Energy Fuels 2019, 33, 11009–11020. [Google Scholar] [CrossRef]
- Glyk, A.; Solle, D.; Scheper, T.; Beutel, S. Optimization of PEG-salt aqueous two-phase systems by design of experiments. Chemom. Intell. Lab. Syst. 2015, 149, 12–21. [Google Scholar] [CrossRef]
- Valdze, E.; Tabil, L.; Mupondwa, E.; Cree, D.; Moazed, H. Microwave Torrefaction of Oat Hull: Effect of Temperature and Residence Time. Energies 2021, 14, 4298. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Chen, M.; Cao, S.; Wang, X. Toughened Polypropylene with Balanced Rigidity (II): Morphology, Melt Flow Rate and Melting Point of Toughening Master Batch. Polym. Adv. Technol. 2000, 11, 342–348. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Meng, H.; Wu, Z.; Zhao, J. Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng. 2017, 111, 834–846. [Google Scholar] [CrossRef]
- Kim, S.; Jang, H.; Cha, W. production properties of pyrolytic matter of PP and PS plastics in a low temperature pyrolysis condition. J. Korean Phys. Soc. 2007, 8, 105295. [Google Scholar]
- Chen, C.; Ji, G.; Mu, L.; Zhang, Y.; Li, A. Comprehensive research on the solid, liquid, and gaseous products of rice husk and rice straw torrefaction. Energy Fuels 2021, 5, 687–697. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- Cardona, S.; Gallego, L.; Valencia, V.; Martínez, E.; Rios, L. Torrefaction of eucalyptus-tree residues: A new method for energy and mass balances of the process with the best torrefaction conditions. Sustain. Energy Technol. 2019, 31, 17–24. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.; Domingos, I.; Pereira, H. Heat-induced colour changes of pine (Pinus pinaster) and eucalypt (Eucalyptus globulus) wood. Wood Sci. Technol. 2008, 42, 369–384. [Google Scholar] [CrossRef]
- González-Pea, M.; Hale, M. Colour in thermally modified wood of beech, Norway spruce and Scots pine, Part 1: Colour evolution and colour changes. Holzforschung 2009, 63, 385–393. [Google Scholar] [CrossRef]
- Melkior, T.; Barthomeuf, C.; Bardet, M. Inputs of solid-state NMR to evaluate and compare thermal reactivity of pine and beech woods under torrefaction conditions and modified atmosphere. Fuel 2017, 187, 250–260. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A review of wood thermal pretreatments to improve wood composite properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Elder, T.; Soltes, E. Pyrolysis of Lignocellulosic Materials. Phenolic Constituents of A Wood Pyrolytic Oil. Wood Fiber Sci. 1981, 12, 217–226. [Google Scholar]
- Sheng, C.; Azevedo, J. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2006, 28, 499–507. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Sun, G.; Qiu, L.; Gu, Y.; Zhang, T.; Zhu, M.; Sun, R. Microwave-assisted hydrothermal carbonization of corn stalk for solid biofuel production: Optimization of process parameters and characterization of hydrochar. Energy 2019, 186, 115795. [Google Scholar] [CrossRef]
- Tag, A.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344. [Google Scholar]
- Zhang, L.; Wang, Z.; Ma, J.; Kong, W.; Yuan, P.; Sun, R. Analysis of functionality distribution and microstructural characteristics of upgraded rice husk after undergoing non-oxidative and oxidative torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of corn straw in oxygen and carbon dioxide containing gases: Mass/energy yields and evolution of gaseous species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Sun, Y.; Yu, Y.; Liu, J.; Wang, D.; Wang, Z. Effects of combined pretreatement with rod-milled and torrefaction on physicochemical and fuel characteristics of wheat straw. Bioresour. Technol. 2018, 267, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Buyukada, M.; Yan, Y.; Li, L. Combustions of torrefaction-pretreated bamboo forest residues: Physicochemical properties, evolved gases, and kinetic mechanisms. Bioresour. Technol. 2020, 304, 122960. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.; Wirth, B.; Lder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Li, H.; Shi, Z.; Kan, H. Preparation and properties of hydrocharsfrom macadamia nut shell via hydrothermal carbonization. R. Soc. Open Sci. 2018, 5, 181126. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Q.; Wang, Z.; Liu, H.; Wang, B.; Yu, Y. Valorization of Food Waste via Torrefaction: Effect of Food Waste Type on the Characteristics of Torrefaction Products. Energy Fuels 2020, 34, 6041–6051. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lin, Y.; Tsai, C.H.; Chung, M.H.; Chu, M.H.; Huang, H.J.; Jao, Y.H.; Yeh, S.I. Conversion of water caltrop husk into torrefied biomass by torrefaction. Energy 2020, 195, 116967. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Liu, J.; Evrendilek, F.; He, Y.; Xie, W. Co-combustion, life-cycle circularity, and artificial intelligence-based multi-objective optimization of two plastics and textile dyeing sludge. J. Hazard. Mater. 2022, 426, 128069. [Google Scholar] [CrossRef] [PubMed]
- Stevanic, J.; Salmén, S. Characterizing Wood Polymers in the Primary Cell Wall of Norway Spruce (Picea Abies (L.) Karst.) Using Dynamic FT-IR Spectroscopy. Cellulose 2008, 15, 285–295. [Google Scholar] [CrossRef]
- Pushkin, S.; Kozlova, L.; Makarov, A.; Grachev, A.; Gorshkova, T. Cell wall components in torrefied softwood and hardwood samples. J. Anal. Appl. Pyrolysis 2015, 116, 102–113. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Shao, Q.; Wang, G. Physicochemical Properties Evolution of Chars from Palm Kernel Shell Pyrolysis. J. Therm. Anal. Calorim. 2018, 133, 1271–1280. [Google Scholar] [CrossRef]
- Lim, E.; Chong, K.; Chok, M.; Lock, C.; Chong, Y.; Gopakumar, S.T.; Lee, L.; Gan, S. Investigation of eggshell as catalyst on the torrefaction of empty fruit bunch. Mater. Sci. Energy Technol. 2021, 4, 189–201. [Google Scholar] [CrossRef]
- Mendes, J.; Martins, J.; Manrich, A.; Neto, A.; Pinheiro, A.; Mattoso, J.; Martins, M. Development and physical-chemical properties of pectin film reinforced with spent coffee grounds by continuous casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef]
- Ru, B.; Wang, S.; Dai, G.; Li, Z. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.; Zuber, M.; Kamal, S.; Aslam, N. Starch based polyurethanes: A critical review updating recent literature. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef]
- Acma, H.H.; Yaman, S. Thermogravimetric investigation on the thermal reactivity of biomass during slow pyrolysis. Int. J. Green Energy 2009, 6, 333–342. [Google Scholar] [CrossRef]
- Park, J.; Meng, J.; Lim, K.; Rojas, O.; Park, S. Transformation of lignocellulosic biomass during torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 199–206. [Google Scholar] [CrossRef]
- Stelte, W.; Clemons, C.; Holm, J.; Sanadi, J.; Ahrenfeldt, J.; Shang, L.; Henriksen, U. Pelletizing Properties of Torrefied Spruce. Biomass Bioenergy 2011, 35, 4690–4698. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterisation of renewable fuels torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R. Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn stover and switchgrass. Bioresour. Technol. 2010, 101, 1082–1090. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, T.; Zhu, Y.; Peng, C.; Wang, B.; Li, X.; Li, C.; Zeng, G. Production of fuel pellets via hydrothermal carbonization of food waste using molasses as a binder. Waste Manag. 2018, 77, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Panigrahi, S.; Dubey, B. Food waste hydrothermal carbonization: Study on the effects of reaction severities, pelletization and framework development using approaches of the circular economy. Bioresour. Technol. 2021, 333, 125187. [Google Scholar] [CrossRef]
- Peng, J.; Bi, X.; Jim, L.; Peng, H.; Soo, K.; Jia, D.; Zuo, H. Sawdust as an effective binder for making torrefied pellets. Appl. Energy 2015, 157, 491–498. [Google Scholar] [CrossRef]
- Bergman, P.; Boersma, A.; Zwart, R.; Kiel, J. Torrefaction for Biomass Co-Firing in Existing Coal -Fired Power Stations; Energy Research Centre of the Netherlands ECN: Petten, The Netherlands, 2015. [Google Scholar]
- Murillo, H.; Robles, L.; Santander, R.; Cubillos, F. Conversion of residual biomass into valuable biofuels by co-hydrothermal carbonization for utilization in household pellet stoves. Biomass Bioenergy 2021, 151, 106153. [Google Scholar] [CrossRef]




| Sample | Ultimate Analysis (wt.%, daf.) | HHV | V | FC | AC | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O a | N | S | (MJ/kg) | (wt.%, db.) | (wt.%, db.) | (wt.%, db.) | |
| FB | 49.81 | 6.25 | 42.41 | 0.79 | 0.74 | 17.32 | 72.81 | 19.7 | 7.49 |
| PP | 82.88 | 12.9 | 3.33 | - | 0.89 | 44.11 | 100 | - | - |
| Std. Order | Run Order | Temperature (°C) | Time (min) | PP (%) | HHV (MJ/kg) | MY (%) | EY (%) | AC (wt.%, db.) |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 220 | 30 | 10 | 20.49 | 85.28 | 87.4 | 9.05 |
| 2 | 12 | 300 | 30 | 10 | 22.9 | 57.02 | 65.3 | 12.94 |
| 3 | 11 | 220 | 60 | 10 | 20.51 | 84.56 | 86.71 | 10.9 |
| 4 | 9 | 300 | 60 | 10 | 23.13 | 54.06 | 62.53 | 14.45 |
| 5 | 15 | 220 | 30 | 20 | 23.24 | 86.38 | 88.53 | 8.27 |
| 6 | 6 | 300 | 30 | 20 | 28.26 | 62.54 | 77.95 | 9.8 |
| 7 | 7 | 220 | 60 | 20 | 23.26 | 85.52 | 87.77 | 9.42 |
| 8 | 18 | 300 | 60 | 20 | 28.97 | 58.1 | 74.23 | 10.97 |
| 9 | 2 | 192.73 | 45 | 15 | 21.59 | 91.02 | 92.11 | 9.86 |
| 10 | 3 | 327.27 | 45 | 15 | 24.53 | 48.1 | 55.31 | 14.98 |
| 11 | 14 | 260 | 19.77 | 15 | 22.48 | 76.85 | 80.98 | 10.56 |
| 12 | 17 | 260 | 70.23 | 15 | 23.53 | 70.82 | 78.1 | 12.96 |
| 13 | 13 | 260 | 45 | 6.59 | 20.41 | 70.44 | 75.34 | 13.18 |
| 14 | 10 | 260 | 45 | 23.41 | 26.37 | 75.58 | 84.47 | 10.84 |
| 15 | 1 | 260 | 45 | 15 | 22.81 | 72.95 | 77.99 | 11.27 |
| 16 | 4 | 260 | 45 | 15 | 23.36 | 73.75 | 80.74 | 11.98 |
| 17 | 5 | 260 | 45 | 15 | 22.62 | 73.39 | 80.35 | 11.35 |
| 18 | 16 | 260 | 45 | 15 | 23.41 | 73.79 | 80.96 | 12.04 |
| Parameter | Model | Equation | p-Value | Adjusted R2 | Predicted R2 | C.V.% | Adequate Precision |
|---|---|---|---|---|---|---|---|
| HHV | Linear | 23.44 + 1.52 × A + 0.2 × B + 1.96 × C | <0.0001 | 0.852 | 0.779 | 3.9 | 17.043 |
| MY | Quadratic | 73.49 − 13.34 × A − 1.4 × B + 1.48 × C − 0.73 × AB + 0.94 × AC − 0.2 × BC − 1.46 × A2 + 0.049 × B2 − 0.24 × C2 | <0.0001 | 0.995 | 0.983 | 1.19 | 70.149 |
| EY | Quadratic | 79.95 − 9.69 × A − 0.94 × B + 3.07 × C − 0.63 × AB + 2.77 × AC − 0.13 × BC − 1.96 × A2 + 0.099 × B2 + 0.23 × C2 | <0.0001 | 0.964 | 0.891 | 2.33 | 23.815 |
| AC | Linear | 11.38 + 1.4 × A + 0.71 × B − 0.94 × C | <0.0001 | 0.769 | 0.654 | 7.7 | 14.779 |
| Optimal Factors | Units | Data | Responses | MY (%) | HHV (MJ/kg) | AC (wt.%, db.) |
|---|---|---|---|---|---|---|
| Temperature | °C | 230.68 | Prediction | 84.15 | 24.08 | 8.70 |
| PP | % | 20 | ||||
| Time | min | 30 | Validation | 84.28 (0.13) | 24.13 (0.09) | 8.53 (0.02) |
| Optimal Factors | Units | Data | Responses | EY (%) | AC (wt.%, db.) |
|---|---|---|---|---|---|
| Temperature | °C | 220 | Prediction | 88.73 | 8.33 |
| PP | % | 20 | |||
| Time | min | 30 | Validation | 88.17 (0.01) | 8.22 (0.04) |
| Biomass | Torrefaction Parameter | HHV (MJ/kg) | Energy Yield (%) | Ash Content (db%) | Reference |
|---|---|---|---|---|---|
| Rice husk | Raw 220–300 °C, 30–60 min | 12.27 13.48–17.88 | - 64.82–78.96 | 15.98 19.71–78.19 | Zhang [44] |
| Corn straw | Raw 275–375 °C, 60–120 min | 16.8 16.90–19.80 | - 35.4–66.2 | 6.49 8.36–12.87 | Liu [45] |
| Wheat straw | Raw 250–300 °C, 30 min | 17.29 19.17–24.32 | - 64.91–80.8 | 8.14 11.96–13.09 | Bai [46] |
| Bamboo residues | Raw 200–300 °C, 60 min | 16.79 17.57–21.96 | - 64.72–90.25 | 12.4 14.98–27.03 | Hu [47] |
| FB-PP | Raw MY-HHV-AC EY-AC | 21.89 24.13 23.18 | - 92.9 88.17 | 6.08 8.53 8.22 | This study |
| Range and Levels | ||||||
|---|---|---|---|---|---|---|
| Factor | Units | −α | −1 | 0 | 1 | +α |
| Temperature | °C | 192.73 | 220 | 260 | 300 | 327.27 |
| Time | Min | 19.77 | 30 | 45 | 60 | 70.23 |
| PP | % | 6.59 | 10 | 15 | 20 | 23.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Liu, L.; Zhao, D.; Zhao, C.; Li, X.; Li, G. Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology. Molecules 2023, 28, 2568. https://doi.org/10.3390/molecules28062568
Guo S, Liu L, Zhao D, Zhao C, Li X, Li G. Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology. Molecules. 2023; 28(6):2568. https://doi.org/10.3390/molecules28062568
Chicago/Turabian StyleGuo, Shuai, Lidong Liu, Deng Zhao, Chenchen Zhao, Xingcan Li, and Guangyu Li. 2023. "Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology" Molecules 28, no. 6: 2568. https://doi.org/10.3390/molecules28062568
APA StyleGuo, S., Liu, L., Zhao, D., Zhao, C., Li, X., & Li, G. (2023). Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology. Molecules, 28(6), 2568. https://doi.org/10.3390/molecules28062568






