Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Prepared Bioactive Nanocarriers
2.1.1. XRD Results
2.1.2. TGA Results
2.1.3. Antioxidant Activity (AOA)
2.1.4. Antimicrobial Activity
2.2. Characterization of LDPE Films Loaded with Bioactive Nanocarriers
2.2.1. XRD Results
2.2.2. TGA Results
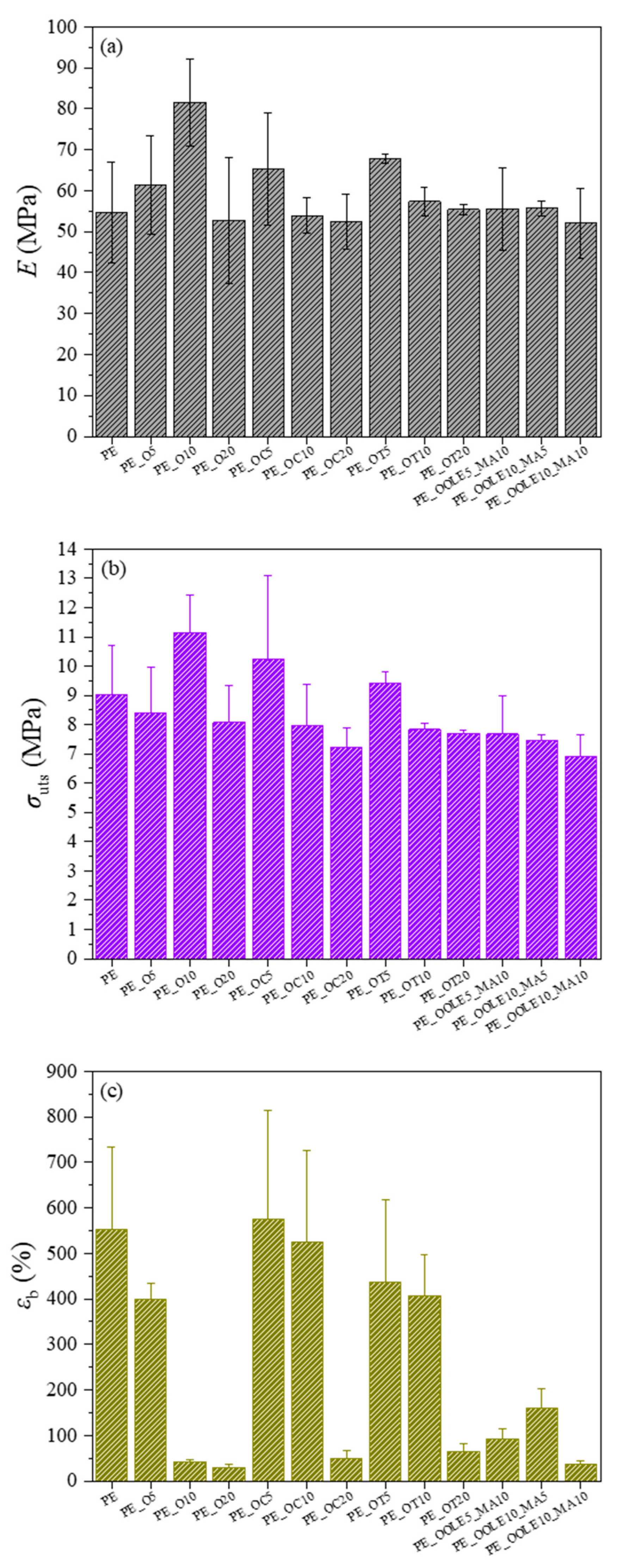
2.2.3. Mechanical Properties of Films
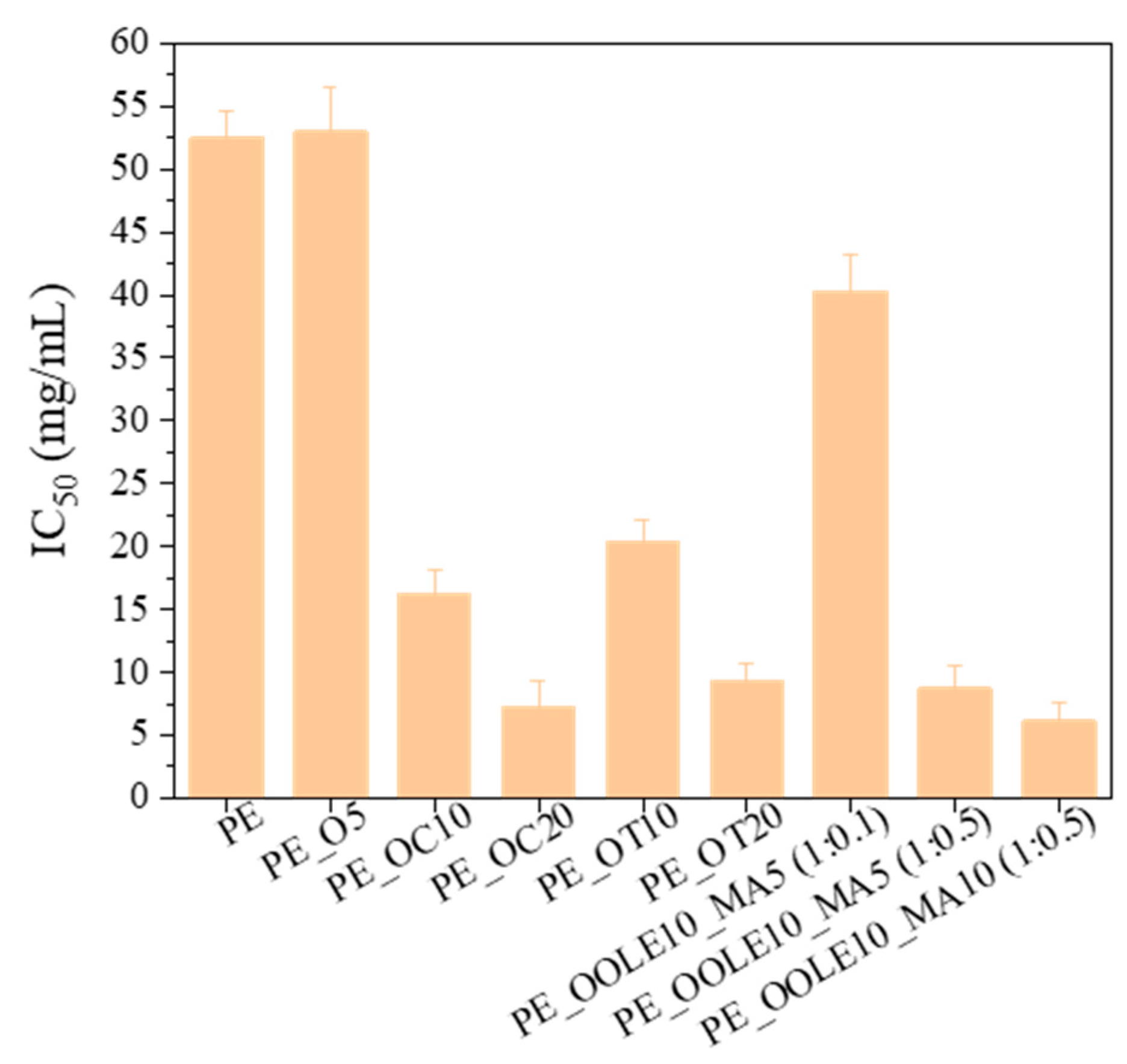
2.2.4. Antioxidant Activity (AOA)
2.2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Bioactive Nanocarriers
3.3. LDPE/Clay Bioactive Nanocomposite Film Preparation
3.4. Characterization Techniques
3.4.1. Structural Characterization Using X-ray Diffraction (XRD)
3.4.2. Thermogravimetric Analysis (TGA)
3.4.3. Mechanical Analysis
3.5. Determination of Antioxidant Activity Based on the Free Radical Binding Capacity of DPPH
3.6. Determination of Antimicrobial Activity
3.6.1. Bacterial Culture Preparation
3.6.2. Bacterial Reduction Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Baek, S.; Maruthupandy, M.; Lee, K.; Kim, D.; Seo, J. Freshness indicator for monitoring changes in quality of packaged kimchi during storage. Food Packag. Shelf Life 2020, 25, 100528. [Google Scholar] [CrossRef]
- Majid, I.; Ahmad Nayik, G.; Mohammad Dar, S.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. Saudi Soc. Agric. Sci. 2016, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.V.P.; Suneetha, J.; Kumari, B.A. Active packaging systems in food packaging for enhanced shelf life. J. Pharmacogn. Phytochem. 2018, 7, 2044–2046. [Google Scholar]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and applications of fungal mycelium-based advanced functional materials. J. Bioresour. Bioprod 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Almasi, H.; Oskouie, J.M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 26, 1–21. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Zhu, L.; Liu, G.; Wu, L. Food safety in post-COVID-19 pandemic: Challenges countermeasures (COVID-19 and food safety: Guidance for food businesses: Interim guidance). Biosensors 2021, 11, 71. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. Application of natural extracts as active ingredient in biopolymer based packaging systems. J. Food Sci. Technol. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Song, T.; Qian, S.; Lan, T.; Wu, Y.; Liu, J.; Zhang, H. Recent Advances in Bio-Based Smart Active Packaging Materials. Foods 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Kontominas, M. Advanced Bioactive Biopolymer-Based Materials in Food Packaging. In Bioactive Food Packaging: Strategies, Quality, Safety, 1st ed.; DEStech Publication, Inc.: Lancaster, PA, USA, 2016. [Google Scholar]
- Meeran, M.F.N.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Polymer/clay nanocomposite films as active packaging material: Modeling of antimicrobial release. Eur. Polym. J. 2015, 71, 461–475. [Google Scholar] [CrossRef]
- Giaconia, M.A.; Ramos, S.d.P.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Cavalcante Braga, A.R. Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Bioactive packaging: Combining nanotechnologies with packaging for improved food functionality. In Nanomaterials for Food Applications; Rubio, A.L., Sanz, M.M., Fabra Rovira, M.J., Gómez-Mascaraque, L.G., Eds.; Elsevier: North Andover, MA, USA, 2019; pp. 233–270. [Google Scholar]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A novel method for the preparation of inorganic and organo-modified montmorillonite essential oil hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Oliveira-Pinto, P.R.; Mariz-Ponte, N.; Gil, R.L.; Cunha, E.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Fernandes-Ferreira, M.; Sousa, R.M.O.F.; Santos, C. Montmorillonite Nanoclay and Formulation with Satureja montana Essential Oil as a Tool to Alleviate Xanthomonas euvesicatoria Load on Solanum lycopersicum. Appl. Nano 2022, 3, 126–142. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Polymer Nanocomposites for Food Packaging Applications. In Functional and Physical Properties of Polymer Nanocomposites; Dasari, A., Njuguna, J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 29–55. [Google Scholar]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE based active nanocomposite films with nanoclays impregnated with volatile compounds. Food Res. Int. 2018, 107, 337–345. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Pack. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef]
- Ibarra-Alonso, M.C.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Fernandez-Tavizón, S.; Romero-Garcia, J.; Ledezma-Perez, A.S.; Ramos de Valle, L.F.; Rodriguez-Fernandez, O.S.; Espinoza-Martinez, A.B.; Martinez-Colunga, J.G.; et al. Preparation and characterization of Polyethylene/Clay/Silver nanocomposites using functionalized polyethylenes as an adhesion promoter. J. Adhes. Sci. Technol. 2015, 29, 1911–1923. [Google Scholar] [CrossRef]
- Sánchez-Valdes, S.; Ramírez-Vargas, E.; Ibarra-Alonso, M.C.; Ramos de Valle, L.F.; Mendez-Nonell, J.; Medellın-Rodrıguez, F.J.; Martınez-Colunga, J.G.; Vazquez-Rodriguez, S.; Betancourt-Galindo, R. Itaconic acid and amino alcohol functionalized polyethylene as compatibilizers for polyethylene nanocomposites. Compos. B Eng. 2012, 43, 497–502. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2018, 121, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries—In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Luzi, F.; Dominici, F.; Armentano, I.; Fortunati, E.; Burgos, N.; Fiori, S.; Jiménez, A.; Kenny, J.M.; Torre, L. Combined effect of cellulose nanocrystals, carvacrol and oligomeric lactic acid in PLA_PHB polymeric films. Carbohydr. Polym. 2019, 223, 115131. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-βcyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B 2019, 176, 176. [Google Scholar] [CrossRef]
- Nguemtchouin, M.M.G.; Ngassoum, M.B.; Ngamo, L.S.T.; Gaudu, X.; Cretin, M. Insecticidal formulation based on Xylopia aethiopica essential oil and kaolinite clay for maize protection. Crop Prot. 2010, 29, 985–991. [Google Scholar] [CrossRef]
- Kinninmonth, M.A.; Liauw, C.M.; Verran, J.; Taylor, R.; Edwards-Jones, V.; Shaw, D.; Webb, M. Investigation into the suitability of layered silicates as adsorption media for essential oils using FTIR and GC–MS. Appl. Clay Sci. 2013, 83, 415–425. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/halloysite nanotube films with sustained carvacrol release for broad-spectrum antimicrobial activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Abdul Hamid, A.R.; Azlin Fazlina, O.; Zaleha, M.; Rajakumar, A. Pre-swelling process of the surface modified montmorillonite (o-mmt) as a strategy to enhance exfoliation and dispersion. Int. J. Adv. Manuf. Technol. 2020, 14, 29–42. [Google Scholar]
- Marouf, R.; Dali, N.; Boudouara, N.; Ouadjenia, F.; Zahaf, F. Study of Adsorption Properties of Bentonite Clay. In Montmorillonite Clay; Uddin, F., Ed.; Intech Open Limited: London, UK, 2021. [Google Scholar]
- Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Baer, E.; Segal, E. Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers 2018, 10, 864. [Google Scholar] [CrossRef] [Green Version]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled release of essential oils using laminar nanoclay and porous halloysite/essential oil composites in a multilayer film reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innovat. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Cutter, C.N. Microbial control by packaging: A review. Crit. Rev. Food Sci. Nutr. 2002, 42, 151–161. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Memiş, S.; Tornuk, F.; Bozkurt, F.; Durak, M.Z. Production and characterization of a new biodegradable fenugreek seed gum based active nanocomposite film reinforced with nanoclays. Int. J. Biol. Macromol. 2017, 103, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Hassani, F.; Mohammadi Nafchi, A. Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int. J. Biol. Macromol. 2014, 67, 458–462. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A.; Armada, M.; García, M.G.; Ochoa, N.A. Preparation and characterization of montmorillonite/brea gum nanocomposites films. Food Hydrocoll. 2014, 35, 270–278. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Sun, X.S.; Shi, Y.-c.; Wang, D. Effects of glycerol and nanoclay on physiochemical properties of camelina gum-based films. Carbohydr. Polym. 2016, 152, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-A.; Lim, G.-O.; Song, K.B. Use of nano-clay (Cloisite Na+) improves tensile strength and vapour permeability in agar rich red algae (Gelidium corneum)—gelatin composite films. Int. J. Food Sci. Technol. 2010, 45, 1883–1888. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Active edible packaging. Encyclopedia 2021, 1, 30. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Giannakopoulou, A.; Spyrou, S.; Simos, Y.V.; Kontogianni, V.G.; Peschos, D.; Katapodis, P.; Polydera, A.C.; Stamatis, H. Production of hydroxytyrosol rich extract from Olea europaea leaf with enhanced biological activity using immobilized enzyme reactors. Environ. Sci Pollut. Res Int. 2022, 29, 29624–29637. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–2000. [Google Scholar] [CrossRef]
- Payum, T.; Das, A.K.; Shankar, R.; Tamuly, C.; Hazarika, M. Antioxidant Potential of Solanum spirale Shoot and Berry: A Medicinal Food Plant Used in Arunachal Pradesh. Am. J. Pharmtech Res. 2015, 5, 307–314. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH• Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
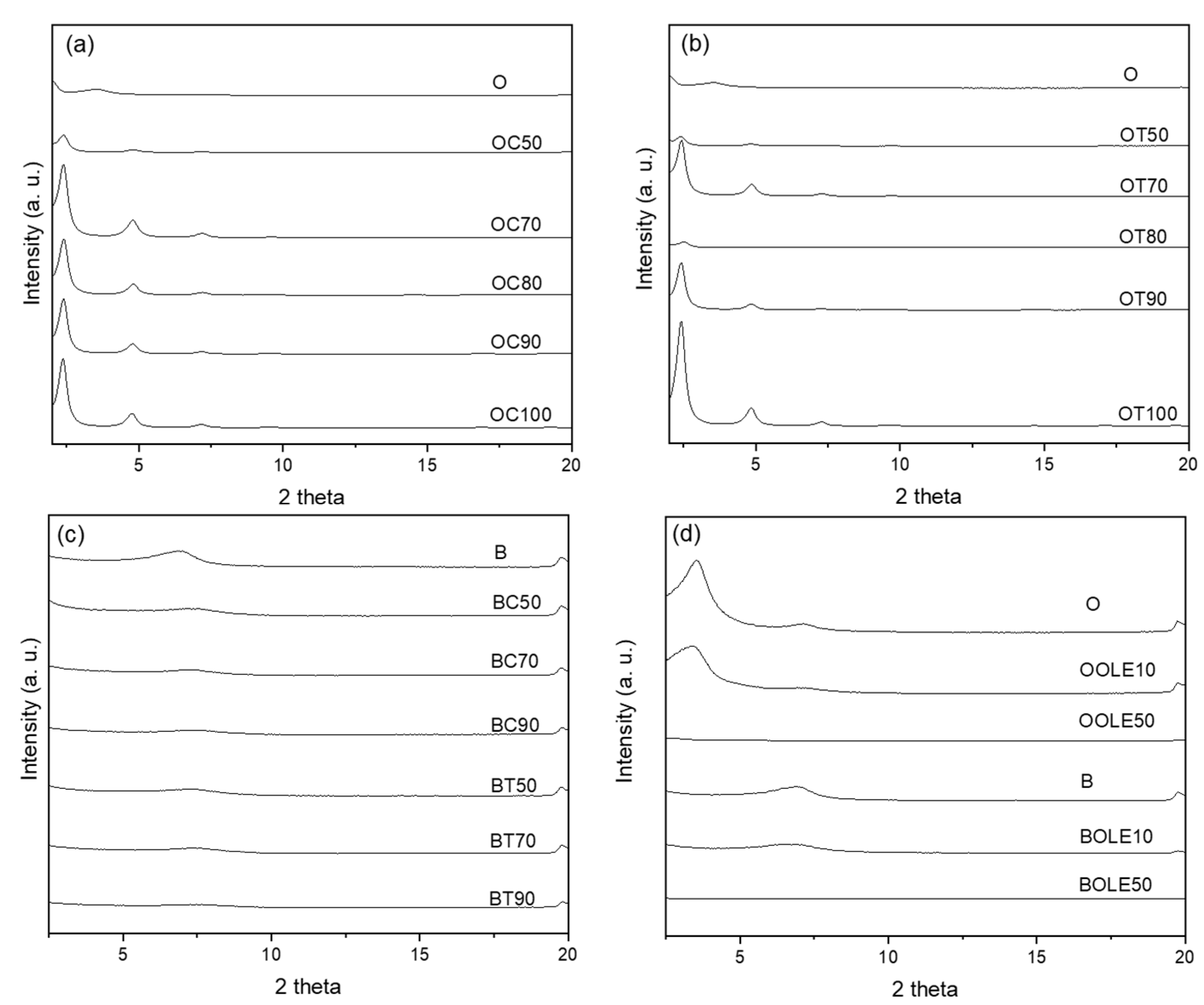
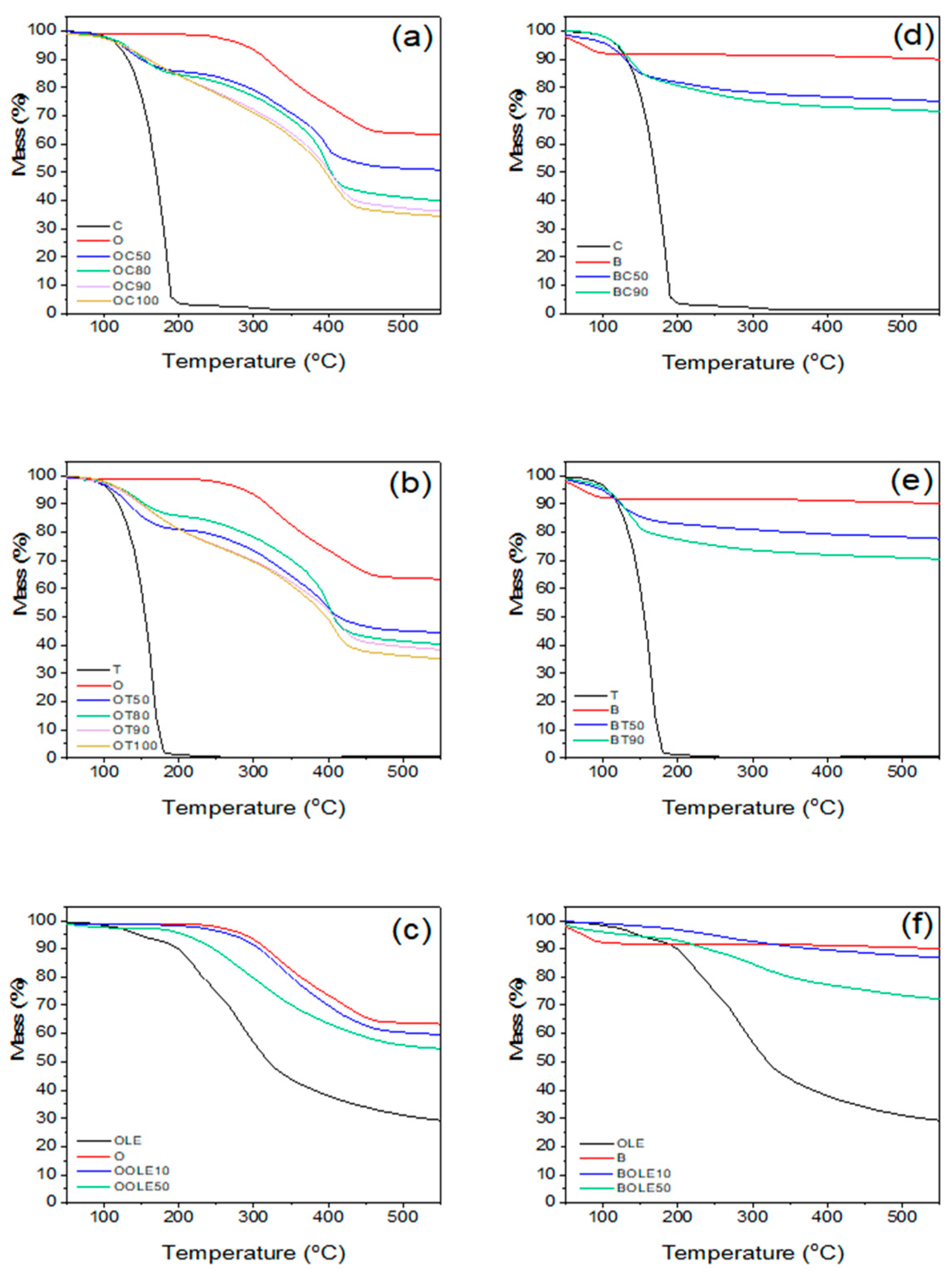

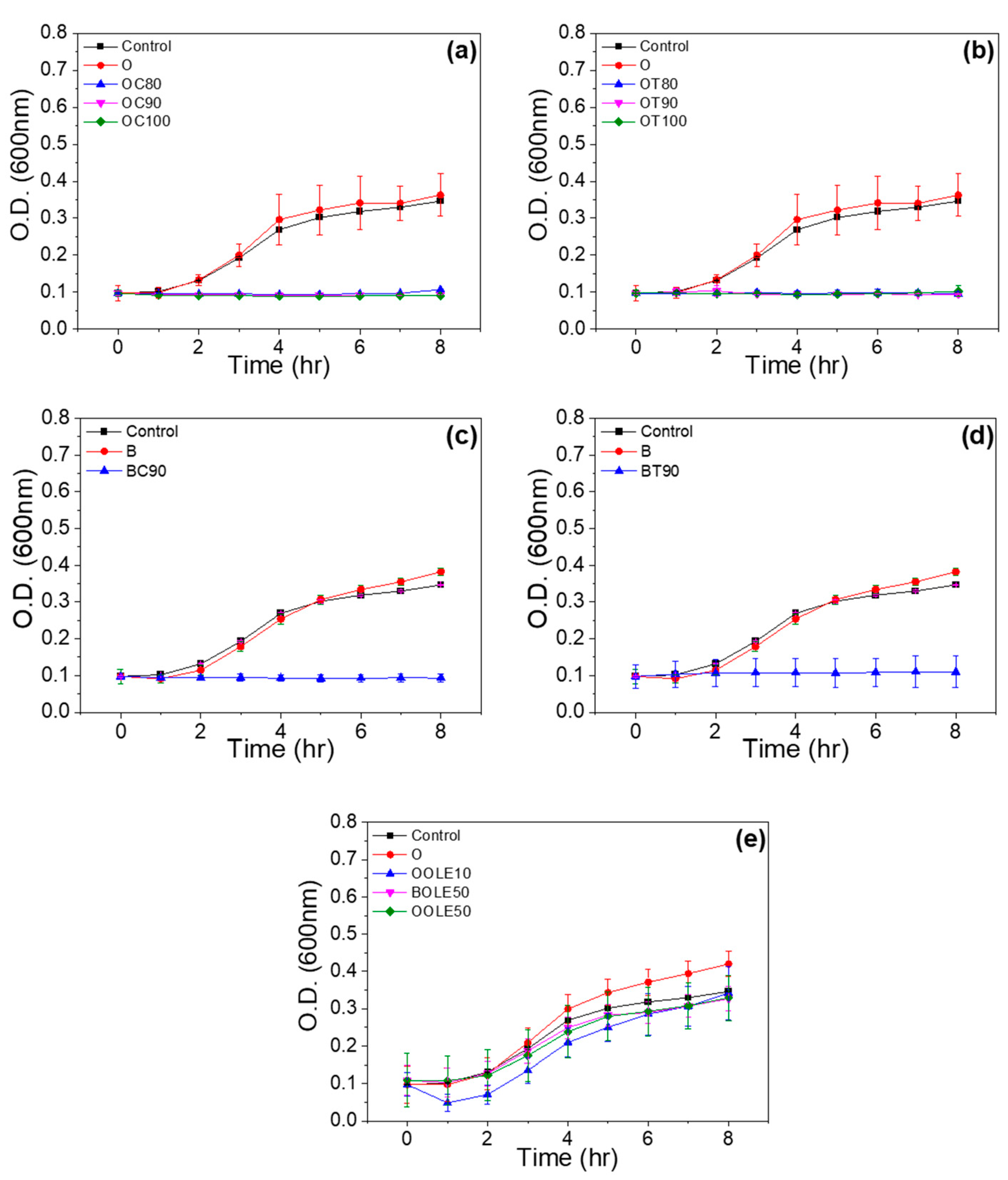


| Material’s Code Name | Clay:Bioactive Substance Ratio (r) | 2θ (ο) | d001 (Å) | TGA Results | |
|---|---|---|---|---|---|
| T20 (°C) | Βioactive Substance Content (% wt.) 2 | ||||
| O | 3.54 | 25 | 362 | - | |
| O/C hybrids | - | ||||
| OC1 | 1:0.01 | 3.44 | 25.7 | - | - |
| OC10 | 1:0.1 | 2.58 | 34.2 | - | - |
| OC50 | 1:0.5 | 2.40 | 36.8 | 297 | 14 |
| OC70 | 1:0.7 | 2.40 | 36.8 | - | - |
| OC80 | 1:0.8 | 2.48 | 35.6 | 280 | 24 |
| OC90 | 1:0.9 | 2.40 | 36.8 | 244 | 27 |
| OC100 | 1:1 | 2.37 | 37.3 | 240 | 29 |
| O/T hybrids | |||||
| OT1 | 1:0.01 | 3.43 | 25.8 | - | - |
| OT10 | 1:0.1 | 2.65 | 33.3 | - | - |
| OT50 | 1:0.5 | 2.40 | 36.8 | 238 | 19 |
| OT70 | 1:0.7 | 2.43 | 36.4 | - | - |
| OT80 | 1:0.8 | 2.52 | 35.1 | 288 | 23 |
| OT90 | 1:0.9 | 2.40 | 36.8 | 203 | 25 |
| OT100 | 1:1 | 2.41 | 36.7 | 202 | 26 |
| O/OLE hybrids | |||||
| OOLE10 | 1:0.1 | 3.35 | 26.4 | 349 | 4 |
| OOLE50 | 1:0.5 | n.d. | n.d. | 297 | 9 |
| B | 6.80 | 13 | n.d.1 | - | |
| B/C hybrids | |||||
| BC50 | 1:0.5 | 7.20 | 12.3 | 223 | 15 |
| BC70 | 1:0.7 | 7.28 | 12.1 | - | - |
| BC90 | 1:0.9 | 7.34 | 12 | 198 | 17 |
| B/T hybrids | |||||
| BT50 | 1:0.5 | 7.10 | 12.5 | 331 | 13 |
| BT70 | 1:0.7 | 7.20 | 12.3 | - | - |
| BT90 | 1:0.9 | 7.25 | 12.2 | 153 | 20 |
| B/OLE hybrids | |||||
| BOLE10 | 1:0.1 | 6.40 | 13.8 | n.d.1 | 3 |
| BOLE50 | 1:0.5 | n.d. | n.d. | 346 | 18 |
| Film’s Code Name | Blends | Clay:Bioactive Substance Ratio (r) | Composition (% wt.) |
|---|---|---|---|
| PE_O5 | LDPE/O | - | 95/5 |
| PE_O10 | LDPE/O | - | 90/10 |
| PE_O20 | LDPE/O | - | 80/20 |
| PE_OC5 | LDPE/OC | 1:1 | 95/5 |
| PE_OC10 | LDPE/OC | 1:1 | 90/10 |
| PE_OC20 | LDPE/OC | 1:1 | 80/20 |
| PE_OΤ5 | LDPE/OT | 1:1 | 95/5 |
| PE_OΤ10 | LDPE/OT | 1:1 | 90/10 |
| PE_T10 | LDPE/T | - | 90/10 |
| PE_OΤ20 | LDPE/OT | 1:1 | 80/20 |
| PE_OOLE5_MA5 | LDPE/OOLE/PE-g-MA | 1:0.1 | 90/5/5 |
| PE_OOLE5_MA10 | LDPE/OOLE/PE-g-MA | 1:0.5 | 85/5/10 |
| PE_OOLE10_MA5 | LDPE/OOLE/PE-g-MA | 1:0.1 | 85/10/5 |
| PE_OOLE10_MA10 | LDPE/OOLE/PE-g-MA | 1:0.5 | 80/10/10 |
| PE_BC5_MA10 | LDPE/BC/PE-g-MA | 1:0.9 | 85/5/10 |
| PE_BC10_MA10 | LDPE/BC/PE-g-MA | 1:0.9 | 80/10/10 |
| PE_BT5_MA10 | LDPE/BT/PE-g-MA | 1:0.9 | 85/5/10 |
| PE_BT10_MA10 | LDPE/BT/PE-g-MA | 1:0.9 | 80/10/10 |
| PE_BOLE5_MA5 | LDPE/BOLE/PE-g-MA | 1:0.1 | 90/5/5 |
| PE_BOLE10_MA5 | LDPE/BOLE/PE-g-MA | 1:0.1 | 85/10/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safakas, K.; Giotopoulou, I.; Giannakopoulou, A.; Katerinopoulou, K.; Lainioti, G.C.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications. Molecules 2023, 28, 2945. https://doi.org/10.3390/molecules28072945
Safakas K, Giotopoulou I, Giannakopoulou A, Katerinopoulou K, Lainioti GC, Stamatis H, Barkoula N-M, Ladavos A. Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications. Molecules. 2023; 28(7):2945. https://doi.org/10.3390/molecules28072945
Chicago/Turabian StyleSafakas, Konstantinos, Iro Giotopoulou, Archontoula Giannakopoulou, Katerina Katerinopoulou, Georgia C. Lainioti, Haralambos Stamatis, Nektaria-Marianthi Barkoula, and Athanasios Ladavos. 2023. "Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications" Molecules 28, no. 7: 2945. https://doi.org/10.3390/molecules28072945
APA StyleSafakas, K., Giotopoulou, I., Giannakopoulou, A., Katerinopoulou, K., Lainioti, G. C., Stamatis, H., Barkoula, N.-M., & Ladavos, A. (2023). Designing Antioxidant and Antimicrobial Polyethylene Films with Bioactive Compounds/Clay Nanohybrids for Potential Packaging Applications. Molecules, 28(7), 2945. https://doi.org/10.3390/molecules28072945






_Stamatis.png)


