A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress
Abstract
1. Introduction
2. Phytochemistry of Persimmon Leaves
2.1. Plant Characteristics and Spread
2.2. Chemical Composition of Persimmon Leaves
2.2.1. Flavonoids
2.2.2. Triterpenes
2.2.3. Other Natural Products in Persimmon Leaves
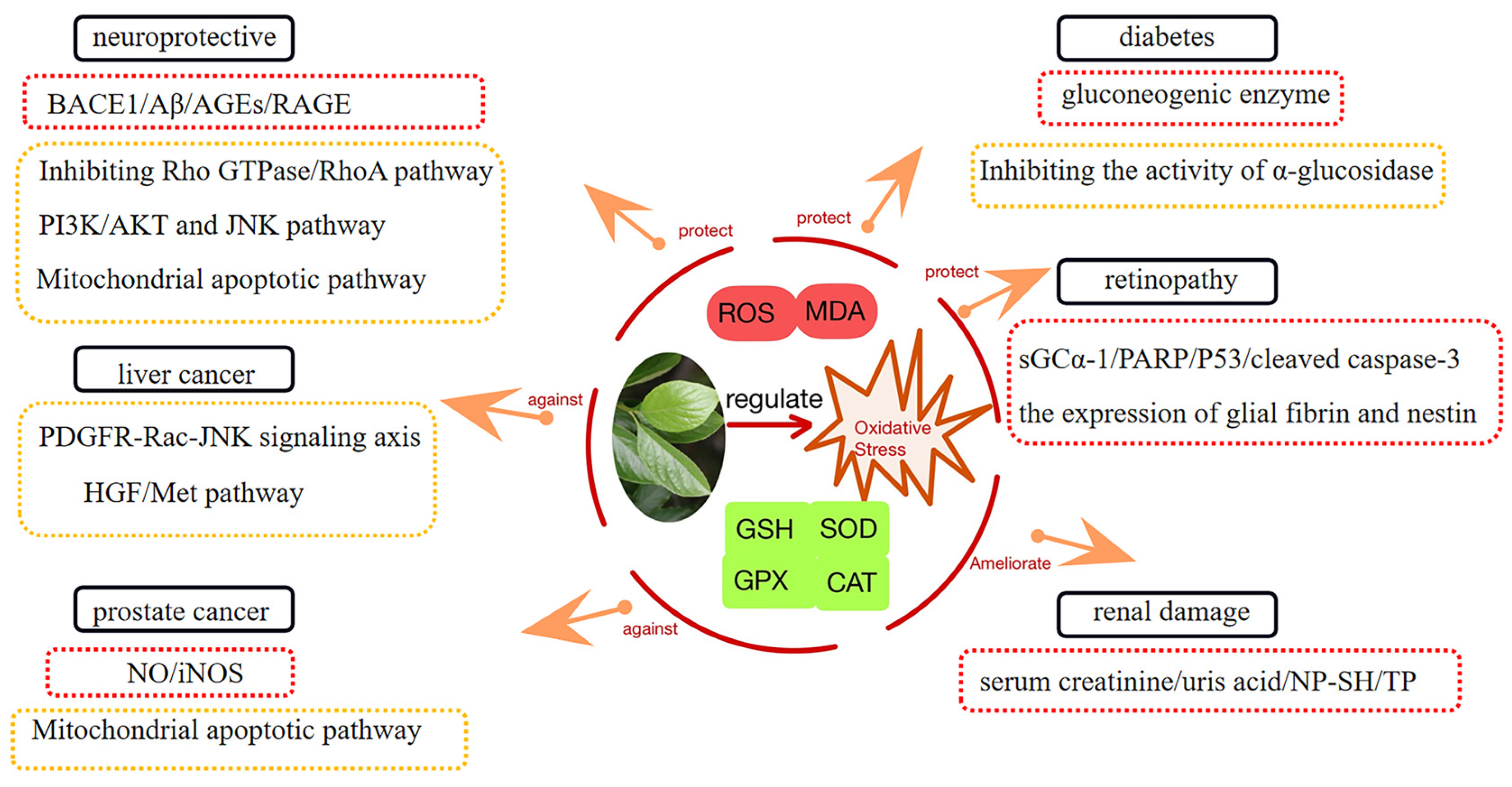
3. Diseases Related to Oxidative Stress
3.1. Diabetes and Its Complications
3.2. Neuroprotective Activity
3.3. Anti-Liver Cancer
3.4. Prostate Cancer
3.5. Cardio Cerebral Vascular and Myocardial Protection
4. Other Human Diseases
4.1. Anti-Lung Cancer
4.2. Acute Promyelocytic Leukemia
4.3. Anti-Inflammatory
5. Experimental and Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- WA, T.Y. What Is Oxidative Stress? Jpn Med. Assoc. 2000, 124, 1549–1553. [Google Scholar]
- Facchinetti, F.; Dawson, V.L.; Dawson, T.M. Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 1998, 18, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Barinaga, M. Stroke-damaged neurons may commit cellular suicide. Science 1998, 281, 1302–1303. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.Z.; Yang, C.X. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.-P.; Chuang, Y.-T.; Tang, J.-Y.; Yang, K.-H.; Chang, F.-R.; Hou, M.-F.; Yen, C.-Y.; Chang, H.-W. The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, R.; Fan, X. Targeting Oxidative Stress in Intracerebral Hemorrhage: Prospects of the Natural Products Approach. Antioxidants 2022, 11, 1811. [Google Scholar] [CrossRef]
- Jia, Z.; Babu, P.V.A.; Chen, W.; Sun, X. Natural Products Targeting on Oxidative Stress and Inflammation: Mechanisms, Therapies, and Safety Assessment. Oxidative Med. Cell. Longev. 2018, 2018, 6576093. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kushalan, S.; Paithankar, J.G.; D’souza, L.C.; Hegde, S.; Sharma, A. Environmental toxicants, oxidative stress and health adversities: Interventions of phytochemicals. J. Pharm. Pharmacol. 2022, 74, 516–536. [Google Scholar] [CrossRef]
- Agbor, G.A.; Dell’Agli, M.; Kuiate, J.-R.; Ojo, O. Editorial: The Role of Medicinal Plants and Natural Products in Modulating Oxidative Stress and Inflammatory Related Disorders. Front. Pharmacol. 2022, 13, 957296. [Google Scholar] [CrossRef]
- Editorial Committee of the State Administration of Traditional Chinese Medicine. Zhonghua Ben Cao; Shanghai Science and Technology Press: Shanghai, China, 1998. [Google Scholar]
- Deng, H.; Wen, Q.W.; Luo, Y.L.; Huang, Y.H.; Huang, R.B. Effect of different solvent extracts of persimmon leaf on antioxidant capacity in diabetic mice. J. Cent. South Univ. Sci. 2012, 37, 469–473. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.L. Chemical constituents and their pharmacological effects in persimmon leaves. Chin. J. Chem. Educ. 2016, 37, 5. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takabayashi, Y.; Nishi, M.; Suzuki, Y. Differences in the ascorbic acid, astragalin, and polyphenol contents, and the DPPH radical scavenging activity of 22 commercial persimmon leaf tea products. J. Home Econ. Jpn 2011, 62, 437–444. [Google Scholar] [CrossRef]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-Lightening Effect of a Polyphenol Extract from Acerola (Malpighia emarginata DC.) Fruit on UV-Induced Pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Lin, J.; Liu, M.; Wang, D.Q.; Li, C.Y. Chemical composition, pharmacological effects and clinical application research progress of Naoxinqing tablets. Cent. South Pharm. 2021, 19, 137–139. [Google Scholar]
- Kul, R. Integrated application of plant growth promoting rhizobacteria and biochar improves salt tolerance in eggplant seedlings. Turk. J. Agric. For. 2022, 46, 677–702. [Google Scholar] [CrossRef]
- Saleem, M.H.; Afzal, J.; Rizwan, M.; Shah, Z.-U.-H.; Depar, N.; Usman, K. Chromium toxicity in plants: Consequences on growth, chromosomal behavior andmineral nutrient status. Turk. J. Agric. For. 2022, 46, 371–389. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, H.T.; Xie, L.H.; Lin, Q.Y.; Chen, S.J.; Zhao, Y.F. Chemical composition, pharmacological effects, clinical application and development and utilization of persimmon leaf. Food Ferment. Ind. 2005, 31, 7. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal changes in the mineral nutrient content of persimmon leaves. Sci. Hortic. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- An, B.J.; Kwak, J.H.; Park, J.M.; Lee, J.Y.; Park, T.S.; Lee, J.T.; Son, J.H.; Jo, C.; Byun, M.W. Inhibition of enzyme activities and the antiwrinkle effect of polyphenol isolated from the persimmon leaf (Diospyros kaki folium) on human skin. Dermatol. Surg. 2005, 31, 848–854; discussion 854. [Google Scholar] [CrossRef]
- Nisar, M.; Shah, S.M.M.; Khan, I.; Sheema; Sadiq, A.; Khan, S.; Shah, S.M.H. Larvicidal, insecticidal, brine shrimp cytotoxicity and anti-oxidant activities of Diospyros kaki (L.) reported from Pakistan. Pak. J. Pharm. Sci. 2015, 28, 1239–1243. [Google Scholar] [PubMed]
- Fukai, S.; Tanimoto, S.; Maeda, A.; Fukuda, H.; Okada, Y.; Nomura, M. Pharmacological Activity of Compounds Extracted from Persimmon Peel (Diospyros kaki THUNB.). J. Oleo Sci. 2009, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Tran, B.Q.; Jang, Y.J.; Park, S.H.; Fondrie, W.E.; Chowdhury, K.; Yoon, S.H.; Goodlett, D.R.; Chae, S.W.; Chae, H.J.; et al. Assessment of the Therapeutic Potential of Persimmon Leaf Extract on Prediabetic Subjects. Mol. Cells 2017, 40, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.S.; Zhang, S.P.; Tang, W.H. Experimental study of persimmon leaf discrimination. Chin. Tradit. Herb. Drugs 1998, 9, 627–629. [Google Scholar]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Z.H. General situation of pharmaceutical research on persimmon. Chin. J. Ethnomedicine Ethnopharmacy 2015, 24, 40–43. [Google Scholar]
- Yang, H.; Zhao, H.; Liu, Y.H.; Li, Y.M.; Ren, S.F.; Lian, X.F.; Zhang, Z.H. Status of persimmon resource development and utilization. Biot. Resour. 2019, 41, 402–410. [Google Scholar] [CrossRef]
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef]
- Chen, G.; Xue, J.; Feng, X.Z. Inhibitory effect of water extract and its main contents of persimmon leaves on stimulus-induced superoxide generation in human neutrophils. J. Food Biochem. 2009, 33, 113–121. [Google Scholar] [CrossRef]
- Tian, Y.H.; Du, H.Z.; Wang, L.; Li, S.F.; Zhang, L.; Zhang, L.W. Nitrite Scavenging and Inhibition of N-Nitrosamines Formation by Phenolic Extracts From Diospyros lotus L. Leaves and Active Ingredients. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Sun, H.P. Optimization of persimmon resources in Hunan Province and research on the isolation and identification of flavonoids from persimmon leaves; Hunan Agricultural University: Changsha, China, 2010. [Google Scholar]
- Kawakami, K.; Shibukura, Y.; Kanno, T.; Furuki, T.; Aketa, S.; Hirayama, M. Identification of 2″-Galloylated Flavonol 3-O-Glycosides Accumulating in Developing Leaves of Persimmon. Phytochem. Anal. 2011, 22, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Meng, Z.; Xu, S.; Kodama, H. Effect of five flavonoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta Int. J. Clin. Chem. 2002, 326, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Park, J.E.; Lee, J.S.; Lee, J.H.; Hwang, H.; Jung, S.H.; Kwon, H.C.; Jang, D.S. Chemical Constituents of the Leaves of Diospyros kaki (Persimmon). Plants 2021, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wei, S.H.; Huang, J.; Sun, J. A novel C-glycosylflavone from the leaves of Diospyros kaki. J. Asian Nat. Prod. Res. 2009, 11, 503–507. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, N.; Zhang, H.F.; Wang, Q.Q.; Yu, Q.; Wang, F.; Dai, Y.H.; Wang, D.; Liu, D.C. Simultaneous quantitative analysis of 11 flavonoid derivatives with a single marker in persimmon leaf extraction and evaluation of their myocardium protection activity. J. Nat. Med. 2019, 73, 404–418. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.Z.; Meng, F.Y.; Li, Y.L.; Li, C.X.; Duan, F.P.; Wang, Q.; Zhang, X.T.; Zhang, C.N. Studies of the microbial metabolism of flavonoids extracted from the leaves of Diospyros kaki by intestinal bacteria. Arch. Pharmacal Res. 2015, 38, 614–619. [Google Scholar] [CrossRef]
- Ryu, R.; Jung, U.J.; Seo, Y.R.; Kim, H.J.; Moon, B.S.; Bae, J.S.; Lee, D.G.; Choi, M.S. Beneficial effect of persimmon leaves and bioactive compounds on thrombosis. Food Sci. Biotechnol. 2015, 24, 233–240. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Mohanta, B.C.; Harigaya, Y. Naturally Occurring Triterpenoid Saponins. Chem. Biodivers. 2010, 7, 2327–2580. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, S.; Sha, Y. Studies on the Constituents of Diospyros kaki Leaves (I). Chin. J. Med. Chem. 2000, 9, 347–353. [Google Scholar]
- Mallavadhani, U.V.; And, A.; Rao, Y.R. Diospyros melanoxylon Leaves: A Rich Source of Pentacyclic Triterpenes. Pharm. Biol. 2001, 39, 20–24. [Google Scholar] [CrossRef]
- Chen, G.; Jia, P.Y.; Xu, S.X.; Zhang, R.Q. Studies on the Constituents of Diospyros kaki Leaves (II). Chin. Tradit. Herb. Drugs 2005, 36, 26–28. [Google Scholar]
- Zhang, Y.; Zhao, L.; Huang, S.W.; Wang, W.; Song, S.J. Triterpene saponins with neuroprotective effects from the leaves of Diospyros kaki Thunb. Fitoterapia 2018, 129, 138–144. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.Q.; Jia, J.M. Three Minor Novel Triterpenoids from the Leaves of Diospyros kaki. Chem. Pharm. Bull. 2009, 57, 532–535. [Google Scholar] [CrossRef]
- Chen, G.; Ren, H.M.; Yu, C.Y. A new 18,19-secoursane triterpene from the leaves of Diospyros kaki. Chem. Nat. Compd. 2012, 47, 918–920. [Google Scholar] [CrossRef]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the Leaves of Diospyros kaki (Persimmon) and Their Inhibitory Effects on Protein Tyrosine Phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef]
- Zhou, F.X.; Liang, P.Y.; Wen, J.; Ma, Y.; Zhang, K.S.; Wu, J.M.; Liang, C. Chemical composition of persimmon leaf. China J. Chin. Mater. Medica 1987, 12, 38. [Google Scholar]
- Higa, M.; Ogihara, K.; Yogi, S. Bioactive naphthoquinone derivatives from Diospyros maritima BLUME. Chem. Pharm. Bull. 1998, 46, 1189–1193. [Google Scholar] [CrossRef]
- Huang, S.W.; Qiao, J.W.; Sun, X.; Gao, P.Y.; Li, L.Z.; Liu, Q.B.; Sun, B.; Wu, D.L.; Song, S.J. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. J. Funct. Foods 2016, 24, 183–195. [Google Scholar] [CrossRef]
- Qiao, J.W.; Huang, S.W.; Song, S.J.; Xu, F.Q.; Huang, X.X.; Zhang, W.; Wu, D.L. Study on the Chemical Constituents of Persimmon Leaves. J. Chin. Med. Mater. 2016, 39, 2513–2517. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Rasmussen, S.K.; Wang, M.H. Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-d-glucopyranoside from the leaves of Diospyros kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011, 346, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Choi, J.W.; Song, N.E.; Cho, C.W.; Rhee, Y.K.; Hong, H.D. Polysaccharide isolated from persimmon leaves (Diospyros kaki Thunb.) suppresses TGF-β1-induced epithelial-to-mesenchymal transition in A549 cells. Int. J. Biol. Macromol. 2020, 164, 3835–3845. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ha, H.; Kim, R.; Cho, C.W.; Song, Y.R.; Hong, H.D.; Kim, T. Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition. Nutrients 2018, 10, 901. [Google Scholar] [CrossRef]
- Shin, M.S.; Lee, H.; Hong, H.D.; Shin, K.S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb. (Persimmon). J. Funct. Foods 2016, 26, 319–329. [Google Scholar] [CrossRef]
- Ue-Cachon, A.H.; Molina-Salinas, G.M.; Said-Fernandez, S.; Mendez-Gonzalez, M.; Caceres-Farfan, M.; Borges-Argaez, R. A new dimeric naphthoquinone from Diospyros anisandra. Nat. Prod. Res. 2013, 27, 1174–1178. [Google Scholar] [CrossRef]
- Row, L.R.; Rao, C.S. Chemical examination of diospyros species-part V: A novel aromatisation of ring B and other reactions of Bauerenol. Tetrahedron Lett. 1967, 8, 4845–4852. [Google Scholar] [CrossRef]
- ZHOU, X.-T. Research progress on chemical constituents and pharmacological effects of Diospyros kaki leaves. Chin. Tradit. Herb. Drugs 2014, 45, 3195–3203. [Google Scholar] [CrossRef]
- Fan, J.-P.; Zhang, R.-F.; Zhang, X.-H.; Zhu, J.-H.; Huang, J.-Z. Separation of three triterpene acids in leaves of Diospyros kaki by high performance liquid chromatography using hydroxypropyl-β-cyclodextrin as mobile phase modifier. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1340–1355. [Google Scholar] [CrossRef]
- Chen, G.; Xu, S.X.; Wang, H.Z.; Zhang, R.Q. Kakispyrol, a new biphenyl derivative from the leaves of Diospyros kaki. J. Asian Nat. Prod. Res. 2005, 7, 265–268. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.Q.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Alvarez, L.A.J.; Zhang, X.Z.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. Commun. Free Radic. Res. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. 2020, 25, 26–32. [Google Scholar] [CrossRef]
- Sun, L.J.; Zhang, J.B.; Lu, X.Y.; Zhang, L.Y.; Zhang, Y.L. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Ashry, O.M.; Hussein, E.M.; Abd El-Azime, A.S. Restorative role of persimmon leaf (Diospyros kaki) to gamma irradiation-induced oxidative stress and tissue injury in rats. Int. J. Radiat. Biol. 2017, 93, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Wang, Y.Q.; Xu, J.L.; Zhang, R.S. Effect of persimmon leaf extract on blood glucose and liver glycogen content in hyperglycemic rats. Chin. Arch. Tradit. Chin. Med. 2010, 2, 413–415. [Google Scholar]
- Pan, C.W.; Wang, W.; Xie, Y.F.; Qi, X.Z.; Jiang, L.X. Effect of persimmon leaves on oxygen free radicals and lipid metabolizing enzymes in hyperlipidemic rats. Guid. J. Tradit. Chin. Med. Pharm. 2016, 22, 3. [Google Scholar]
- Jung, U.J.; Park, Y.B.; Kim, S.R.; Choi, M.S. Supplementation of Persimmon Leaf Ameliorates Hyperglycemia, Dyslipidemia and Hepatic Fat Accumulation in Type 2 Diabetic Mice. PLoS ONE 2012, 7, e49030. [Google Scholar] [CrossRef]
- Bae, U.-J.; Park, S.-H.; Jung, S.-Y.; Park, B.-H.; Chae, S.-W. Hypoglycemic effects of aqueous persimmon leaf extract in a murine model of diabetes. Mol. Med. Rep. 2015, 12, 2547–2554. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef]
- Yoshioka, H.; Usuda, H.; Fukuishi, N.; Nonogaki, T.; Onosaka, S. Carbon Tetrachloride-Induced Nephrotoxicity in Mice Is Prevented by Pretreatment with Zinc Sulfate. Biol. Pharm. Bull. 2016, 39, 1042–1046. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ullah, R.; Alqahtani, A.S.; Hassanein, H.M.; Husseiny, H.A.; Mohammed, N.M.; Herqash, R.N. Nephroprotective effect of persimmon leaves (Diospyros kaki L.f.) against CCl(4)-induced renal toxicity in Swiss Albino rats. Drug Chem. Toxicol. 2022, 45, 1578–1586. [Google Scholar] [CrossRef]
- Choi, M.-S.; Jeong, M.J.; Park, Y.B.; Kim, S.R.; Jung, U.J. The leaf of Diospyros kaki Thumb ameliorates renal oxidative damage in mice with type 2 diabetes. Prev. Nutr. Food Sci. 2016, 21, 378. [Google Scholar] [CrossRef][Green Version]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009, 88, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Oveson, B.C.; Lee, S.Y.; Jo, Y.J.; Yoshida, T.; Miki, A.; Miki, K.; Iwase, T.; Lu, L.L.; Campochiaro, P.A. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009, 110, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Prater, M.R.; Zimmerman, K.L.; Pinn, L.C.; Keay, J.M.; Laudermilch, C.L.; Holladay, S.D. Role of maternal dietary antioxidant supplementation in murine placental and fetal limb development. Placenta 2006, 27, 502–509. [Google Scholar] [CrossRef]
- Ahn, H.R.; Yang, J.W.; Kim, J.Y.; Lee, C.Y.; Kim, T.J.; Jung, S.H. The Intraocular Pressure-Lowering Effect of Persimmon leaves (Diospyros kaki) in a Mouse Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 5268. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Kim, K.A.; Kang, S.W.; Lee, J.Y.; Kim, T.J.; Jung, S.H. Persimmon Leaves (Diospyros kaki) Extract Protects Optic Nerve Crush-Induced Retinal Degeneration. Sci. Rep. 2017, 7, 46449. [Google Scholar] [CrossRef]
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X.; et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142. [Google Scholar] [CrossRef]
- Bei, W.; Peng, W.; Ma, Y.; Xu, A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005, 76, 1975–1988. [Google Scholar] [CrossRef]
- Ma, Y.J.; Ma, B.; Shang, Y.Y.; Yin, Q.Q.; Hong, Y.; Xu, S.; Shen, C.; Hou, X.Y.; Liu, X.P. Flavonoid-rich ethanol extract from the leaves of &ITDiospyros kaki &ITattenuates cognitive deficits, amyloid-beta production, oxidative stress, and neuroinflammation in APP/PS1 transgenic mice. Brain Res. 2018, 1678, 85–93. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Wang, D.; Xu, S.; Hong, Y.; Hou, X.; Liu, X. Flavonoid-Rich Ethanol Extract from the Leaves of Diospyros kaki Attenuates D-Galactose-Induced Oxidative Stress and Neuroinflammation-Mediated Brain Aging in Mice. Oxidative Med. Cell. Longev. 2018, 2018, 8938207. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, W.; Zhang, M.Y.; Liu, Q.B.; Luo, S.Y.; Peng, Y.; Sun, B.; Wu, D.L.; Song, S.J. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, A.W.; Luo, Y.; Feng, Y.P.; Liang, Y.H. Tumor suppressive effects of different polar parts of persimmon leaf in mice with H22 ascites tumor and H22, S180 solid tumor. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 167–173. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, U.; Park, S.K.; Kim, J.M.; Kim, M.J.; Moon, J.H.; Lee, H.L.; Jeong, H.R.; Park, H.W.; Kim, C.W.; et al. Persimmon Water Extract Suppresses Hepatic Lipotoxicity by Regulating Lipid Metabolites. J. Med. Food 2022, 25, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Alsaif, G.; Gao, Y. Total Flavonoids Isolated from Diospyros kaki L. f. Leaves Induced Apoptosis and Oxidative Stress in Human Cancer Cells. Anticancer Res. 2020, 40, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zhao, S.; Zhang, M.; Yan, X.; Gao, X.; Li, J.; Gao, Y.; Zhang, A.; Gao, Y. Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H-22 liver tumor-bearing mice. Sci. Rep. 2018, 8, 10523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, B.; Zhang, Y.; Zhang, A.W.; Luo, Y.; Ma, X.B. Effect of cyclophosphamide combined with ethyl acetate site of persimmon leaf on antioxidant capacity and protein expression of Bcl-2 and Bax in tumor tissues of H22 mice. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 120–123. [Google Scholar]
- Kim, H.S.; Suh, J.S.; Jang, Y.K.; Ahn, S.H.; Raja, G.; Kim, J.C.; Jung, Y.; Jung, S.H.; Kim, T.J. Anti-cancer potential of persimmon (Diospyros kaki) leaves via the PDGFR-Rac-JNK pathway. Sci. Rep. 2020, 10, 18119. [Google Scholar] [CrossRef]
- Ko, H.; Huh, G.; Jung, S.H.; Kwon, H.; Jeon, Y.; Park, Y.N.; Kim, Y.J. Diospyros kaki leaves inhibit HGF/Met signaling-mediated EMT and stemness features in hepatocellular carcinoma. Food Chem. Toxicol. 2020, 142, 111475. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, K.; Dong, H.H.; Song, F.; Chen, J.; Guo, Y.T.; Liu, Y.S.; Tao, W.J.; Zhang, Y.L. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Naito, R.; Kano, H.; Shimada, T.; Makino, T.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Izumi, K.; Kadono, Y.; Nakata, H.; et al. A new flavonoid derivative exerts antitumor effects against androgen-sensitive to cabazitaxel-resistant prostate cancer cells. Prostate 2021, 81, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ma, Q.; Zhong, G.; He, J.; Sang, Z. Isolation and characterization of flavonoid derivatives with anti-prostate cancer and hepatoprotective activities from the flowers of Hosta plantaginea (Lam.) Aschers. J. Ethnopharmacol. 2020, 253, 112685. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med. Chem. 2013, 13, 995–1001. [Google Scholar] [CrossRef]
- Adeniyi, B.A.; Robert, M.F.; Chai, H.; Fong, H.H.S. In vitro cytotoxicity activity of diosquinone, a naphthoquinone epoxide. Phytother. Res. 2003, 17, 282–284. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Kim, H.J.; Ryu, R.; Han, H.J.; Han, Y.J.; Lee, M.K.; Choi, M.S.; Park, Y.B. A Mixture of Ethanol Extracts of Persimmon Leaf and Citrus junos Sieb Improves Blood Coagulation Parameters and Ameliorates Lipid Metabolism Disturbances Caused by Diet-Induced Obesity in C57BL/6J Mice. J. Microbiol. Biotechnol. 2016, 26, 295–308. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, M.; Gu, L.; Liu, Z.; Jia, J.; Chen, X. Effects of phospholipid complexes of total flavonoids from Persimmon (Diospyros kaki L.) leaves on experimental atherosclerosis rats. J. Ethnopharmacol. 2016, 191, 245–253. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef]
- Deng, R.C.; Zhang, W.S.; Yang, H.J.; Meng, F.Y.; Du, S.S.; Hua, Y.; Sun, L.P.; Wang, Y.Y. Anti ischemic effect of ethanolic extract of persimmon leaf in rats. Chin. J. Inf. Tradit. Chin. Med. 2004, 11, 2. [Google Scholar] [CrossRef]
- Ou, Y.P.; Bei, W.J.; Lai, W.Y.; Xu, D.L.; Peng, W.L. Effect of persimmon leaf flavone on hypoxia reoxygenation as well as advanced glycation end products induced apoptosis in neonatal rat cardiomyocytes. J. South. Med. Univ. 2003, 23, 680–682. [Google Scholar] [CrossRef]
- Miao, M.S.; Zhang, X.X.; Wang, L.A. Persimmon leaf flavonoid induces brain ischemic tolerance in mice. Neural Regen. Res. 2013, 8, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Eguchi-Kasai, K.; Hirayama, M. Persimmon Leaf Flavonols Enhance the Anti-Cancer Effect of Heavy Ion Radiotherapy on Murine Xenograft Tumors. J. Cancer Ther. 2013, 04, 1150–1157. [Google Scholar] [CrossRef][Green Version]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Nakajima, Y.; Konishi, T.; Hirayama, M. Persimmon Leaf Extract Inhibits the ATM Activity during DNA Damage Response Induced by Doxorubicin in A549 Lung Adenocarcinoma Cells. Biosci. Biotechnol. Biochem. 2011, 75, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, S.S.; Simkhada, J.R.; Park, S.J.; Lee, H.J.; Kim, T.S.; Yoo, J.C. Effects and action mechanism of Diospyros kaki on the differentiation of human leukemia HL-60 cells. Oncol. Rep. 2010, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Park, J.M.; Jeon, I.H.; Kim, H.S.; Jang, S.I. Effect of Persimmon Leaf Extract on Utraviolet B-induced Inflammation in HaCaT Keratinocytes and Mice. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 583–590. [Google Scholar] [CrossRef]
- Mok, J.Y.; Jeon, I.H.; Cho, J.-K.; Park, J.M.; Kim, H.S.; Kang, H.J.; Kim, H.S.; Jang, S.I. Effect of Persimmon Leaf Extract on Phthalic Anhydride-induced Allergic Response in Mice. Prev. Nutr. Food Sci. 2012, 17, 14–21. [Google Scholar] [CrossRef][Green Version]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci. Rep. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.S.; Kim, D.C.; Yoon, C.S.; Ko, W.; Oh, H.; Kim, Y.C. Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2016, 21, 1206. [Google Scholar] [CrossRef]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Cho, J.K.; Jeon, I.H.; Park, J.M.; Kim, H.S.; Jang, S.I. Inhibitory Effect of Persimmon Leaf Extract on Development of Atopic Dermatitis-Like Skin Lesions. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 653–657. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.N.; Kim, G.R.; Jeong, G.S. Persimmon leaf extract protects mice from atopic dermatitis by inhibiting T cell activation via regulation of the JNK pathway. Phytother. Res. 2021, 35, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.H.; Lin, J.; Guo, H.B.; Li, C.Y. Anti inflammatory and anti apoptotic effects of S1-12 Naoxinqing Tablet on BV-2 cells induced by lipopolysaccharide. Acta Neuropharmacol. 2018, 8, 35. [Google Scholar]




| No. | Type | Name | R | References |
|---|---|---|---|---|
| 1 | A | quercetin | H | [31] |
| 2 | A | rutin | glc (6→1) rha | [36] |
| 3 | A | isoquercitrin | glc | [31] |
| 4 | A | quercetin-3-O-β-galactoside(hyperoside) | gal | [31] |
| 5 | A | quercetin-3-O-β-2″-galloylglucoside | 2″-galloyl-glc | [31] |
| 6 | A | quercetin-3-O-β-2″-galloylgalactoside | 2″-galloyl-gal | [35] |
| 7 | A | quercetin-3-O-β-2″-coumaroylglucoside | 2″-coumaroyl-glc | [37] |
| 8 | B | kampferol | H | [31] |
| 9 | B | kaempferol-3-O-glucoside(astragalin) | glc | [31] |
| 10 | B | trifolin | gal | [31] |
| 11 | B | kaempferol-3-O-α-l-rhamnopyranoside | rha | [5] |
| 12 | B | kampferol-3-O-β-2″-galloylglucoside | 2″-galloyl-glc | [35] |
| 13 | B | kampferol-3-O-β-2″-galloylgalactoside | 2″-galloyl-gal | [35] |
| 14 | B | kaempferol-3-O-β-2″-coumaroylgalactoside | 2″-coumaroyl-gal | [37] |
| 15 | B | kaempferol-3-O-β-2″-coumaroylglucoside | 2″-coumaroyl-glc | [37] |
| 16 | B | kaempferol-3-O-β-2″-feruloylglucoside | 2″-feruloyl-glc | [37] |
| 17 | B | kaempferol-3-O-α-arabinoside | ara | [37] |
| 18 | C | myricetin | H | [32] |
| 19 | C | annulatin | CH3 | [38] |
| 20 | C | myricetin-3-O-α-l-rhamnopyranoside | rha | [39] |
| 21 | C | myricetin-3-O-β-d-glucopyranoside | glc | [39] |
| 22 | C | myricetin-3-O-β-d-galactoside | gal | [40] |
| 23 | D | vitexin | glc | [40] |
| 24 | D | 2″-O-rhamnosyl vitexin | glc (2→1) rha | [40] |
| 25 | D | 8-C-[α-l-rhamnopyranosyl-(1→4)]-α-d-glucopyranosylapigenin | glc (4→1) rha | [39] |
| 26 | isorhamnetin | [41] | ||
| 27 | isorhamnetin-3-O-β-d-glucopyranoside | [36] | ||
| 28 | catechin | [42] | ||
| 29 | isocatechin | [42] | ||
| 30 | epicatechin gallate | [42] | ||
| 31 | chrysontemin | [31] |
| No. | Name | References |
|---|---|---|
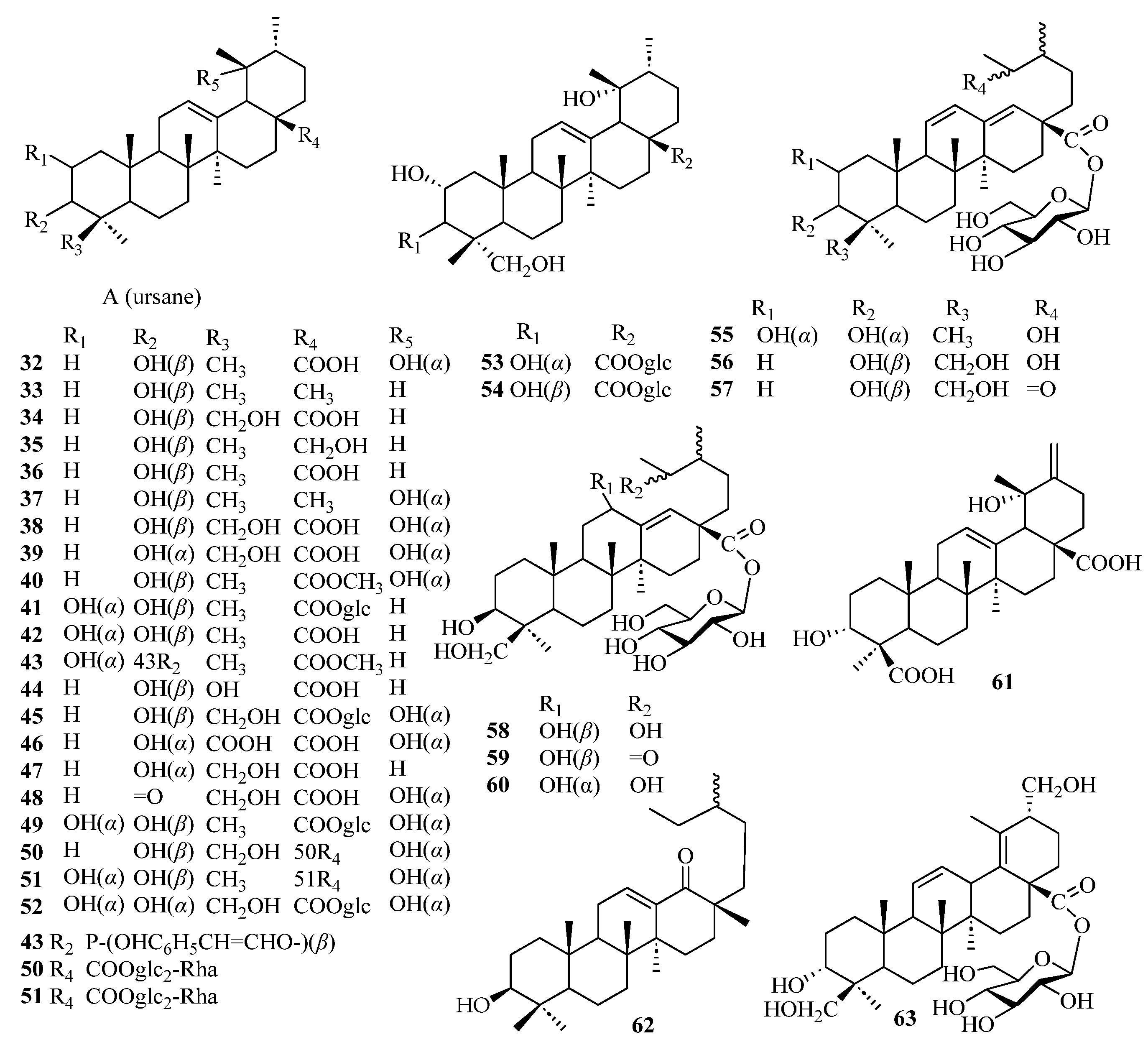
| 32 | 19α-hydroxy ursolic acid | [36] |
| 33 | α-amyrin | [36] |
| 34 | 24-hydroxyursolic acid | [45] |
| 35 | uvaol | [36] |
| 36 | ursolic acid | [36] |
| 37 | pomolic acid | [46] |
| 38 | rotungenic acid | [46] |
| 39 | barbinervic acid | [46] |
| 40 | pomolic acid methyl ester | [47] |
| 41 | rosamutin | [48] |
| 42 | corsolic acid | [46] |
| 43 | jacoumaric acid methyl ester | [46] |
| 44 | 24-hydroxy ursolic acid | [45] |
| 45 | kakisaponin A | [49] |
| 46 | 3α, 19α-dihydroxyurs-12-en-24, 28-dioic acid | [46] |
| 47 | 24-hydroxy-3-epi-ursolic acid | [46] |
| 48 | 19, 24-dihydroxyurs-12-en-3-on-28-oic acid | [46] |
| 49 | rosamultin | [50] |
| 50 | rotungenicacid-28-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | [49] |
| 51 | 28-O-α-l-rhamnopyranosyl (1→2)-β-d-glucopyranoside tormentic acid ester | [49] |
| 52 | 2α, 3α, 19α, 24-tetrahydroxyurs-12-en-28-oic acid-28-O-β-d-glucopyranosyl ester | [49] |
| 53 | 2α, 3α, 19α, 23-tetrahydroxyurs-12-en-28-oic acid-O-β-d-glucopyranosyl ester | [49] |
| 54 | niga-ichigoside F1 | [49] |
| 55 | kakisaponin C | [50] |
| 56 | 28-O-β-d-glucopyranosyl-3α, 24-dihydroxy-19-oxo-18, 19-seco-urs-11, 13 (18)-dien-28-oic acid | [49] |
| 57 | kakisaponin B | [49] |
| 58 | 28-O-β-d-glucopyranosyl-3β, 12β, 19, 24-tetrahydroxy-18, 19-seco-urs-13 (18)-en-28-oic acid | [49] |
| 59 | 28-O-β-d-glucopyranosyl-3β, 12β, 24-trihydroxy-19-oxo-18, 19-secours-13 (18)-en-28-oic acid | [49] |
| 60 | 28-O-β-d-glucopyranosyl-3β, 12α,19, 24-tetrahydroxy-18, 19-seco-urs-13 (18)-en-28-oic acid | [49] |
| 61 | 3α, 19α-dihydroxyurs-12, 20 (30)-dien-24,28-dioic acid | [46] |
| 62 | 18, 19-seco-3β-hydroxy-urs-12-en-18-one | [51] |
| 63 | 28-O-β-d-glucopyranosyl-3α, 24, 30-trihydroxyurs-12, 18-diene-28-oic acid | [49] |
| No. | Name | References |
|---|---|---|
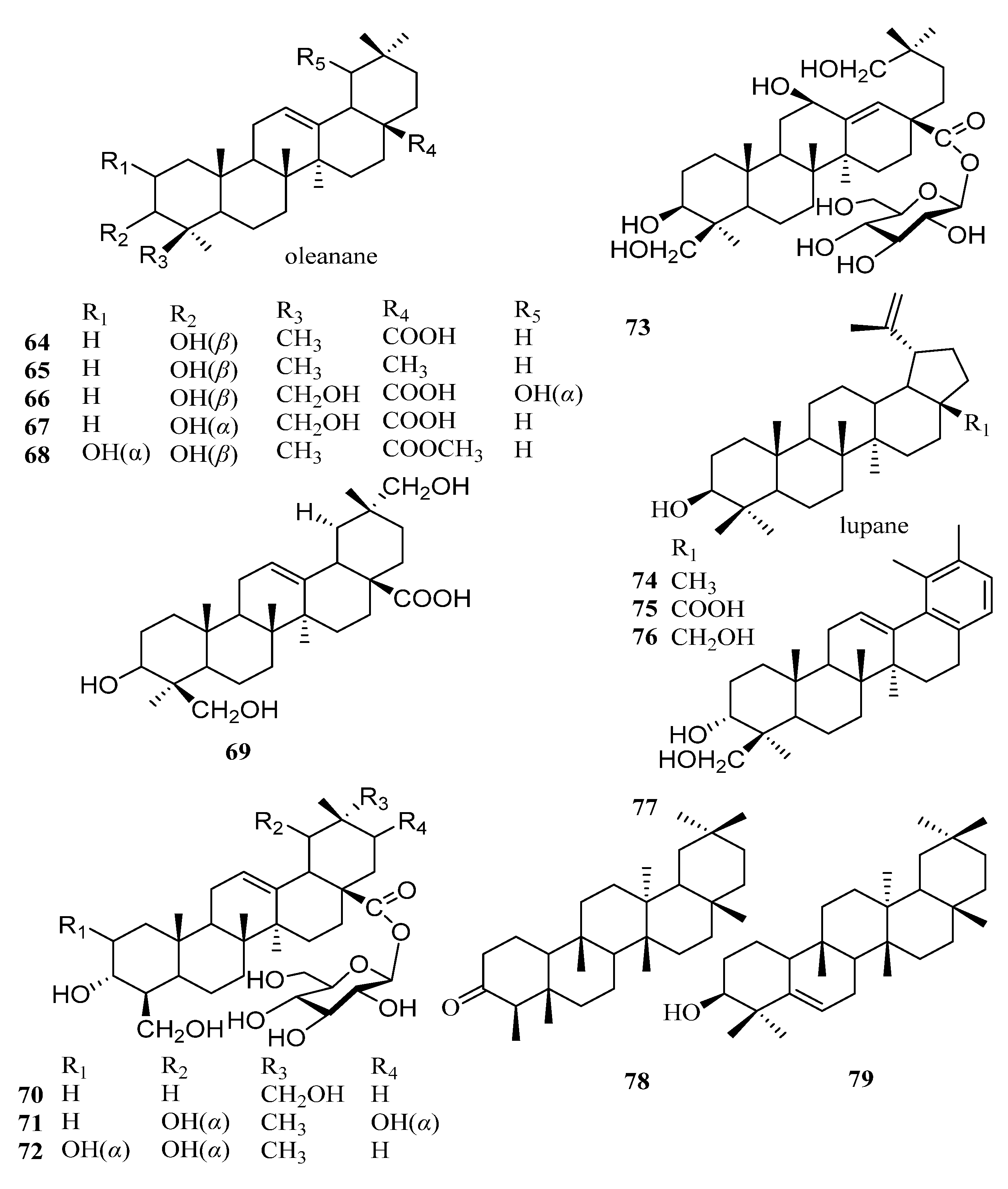
| 64 | oleanolic acid | [46] |
| 65 | β-amyrin | [48] |
| 66 | spathodic acid | [52] |
| 67 | 24-hydroxy-3-epi-oleanolic acid | [52] |
| 68 | maslinic acid methyl ester | [47] |
| 69 | 3R, 24, 29-trihydroxyolean-12-en-28-oic acid | [53] |
| 70 | 3α, 24, 29-trihydroxyolean-12(13)-en-28-oic acid-O-β-d-glucopyranoside | [49] |
| 71 | ryobunin C | [49] |
| 72 | 2α, 3α, 19α, 24 tetrahydroxyolea-12-en-28-oic acid-β-d-glucopyranosyl ester | [49] |
| 73 | 28-O-β-d-glucopyranosyl-3β, 1 2β, 19, 24-tetrahydroxy-18, 19-seco-ole-13 (18)-en-28-oic acid | [49] |
| 74 | lupeol | [48] |
| 75 | betulinic acid | [46] |
| 76 | betulin | [54] |
| 77 | kakidiol | [50] |
| 78 | friedelin | [54] |
| 79 | glutinol | [54] |
| No. | Name | References |
|---|---|---|
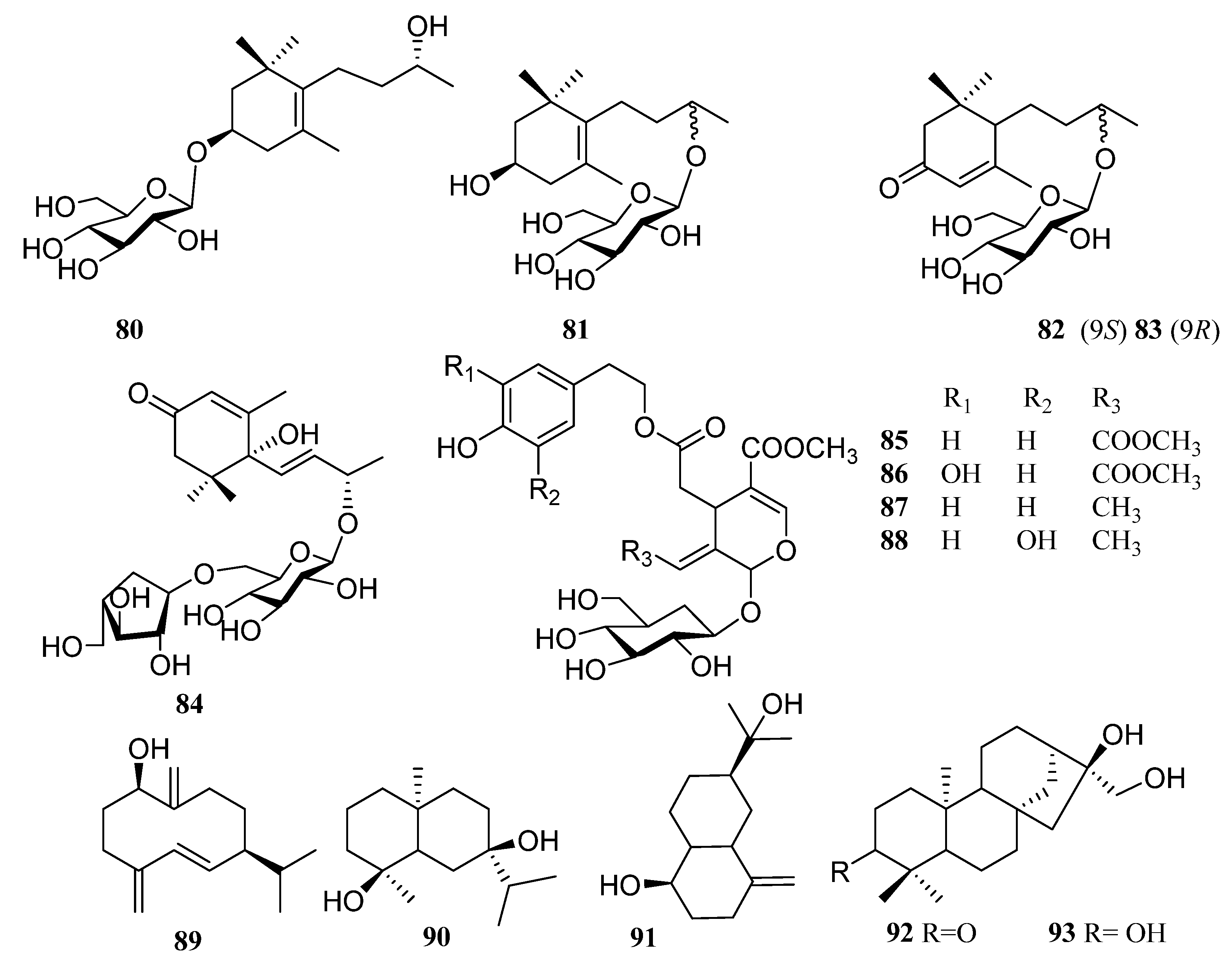
| 80 | Linarionoside A | [48] |
| 81 | Linarionoside B | [48] |
| 82 | blumeol C glucoside | [48] |
| 83 | byzantionoside B | [48] |
| 84 | vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-d-glucopyranoside | [57] |
| 85 | persimmonoid A | [55] |
| 86 | persimmonoid B | [55] |
| 87 | ligustroside | [55] |
| 88 | oleuropein | [55] |
| 89 | 1β-hydroxy-4 (15), 5E, 10 (14)-germacratriene | [61] |
| 90 | teucdiol A | [61] |
| 91 | selin-4 (15)-en-1β, 11-diol | [61] |
| 92 | Abbeokutone | [54] |
| 93 | trihydroxykaurine 3α, 6α, 17-trihydorxykaurane | [54] |
| No. | Name | References |
|---|---|---|
| 94 | (+)-medioresinol | [55] |
| 95 | (+)-syringaresinol | [55] |
| 96 | (+)-pinoresinol | [55] |
| 97 | (+)-medioresinol monoglucoside | [55] |
| 98 | (+)-syringaresinol-β-d-glucoside | [55] |
| 99 | (+)-pinoresinol-β-d-glucoside | [55] |
| 100 | (−)(7′S, 8S, 8′R)-4,4′-dihydroxy-3, 3′, 5, 5′-tetramethoxy-7′, 9-epoxylignan-9′-ol-7-one | [55] |
| 101 | (+)-isolariiresinol | [55] |
| 102 | 4, 4-dihydroxy intercoca acid | [55] |
| 103 | diospyrin | [62] |
| 104 | diosprol | [62] |
| 105 | 6-hydroxy-7-methoxycoumarin | [53] |
| 106 | scopolamine (6-methoxy-7-hydroxycoumarin) | [53] |
| 107 | daucosterol | [63] |
| 108 | β-sitosterol | [63] |
| 109 | tatarine C | [38] |
| No. | Name | References |
|---|---|---|
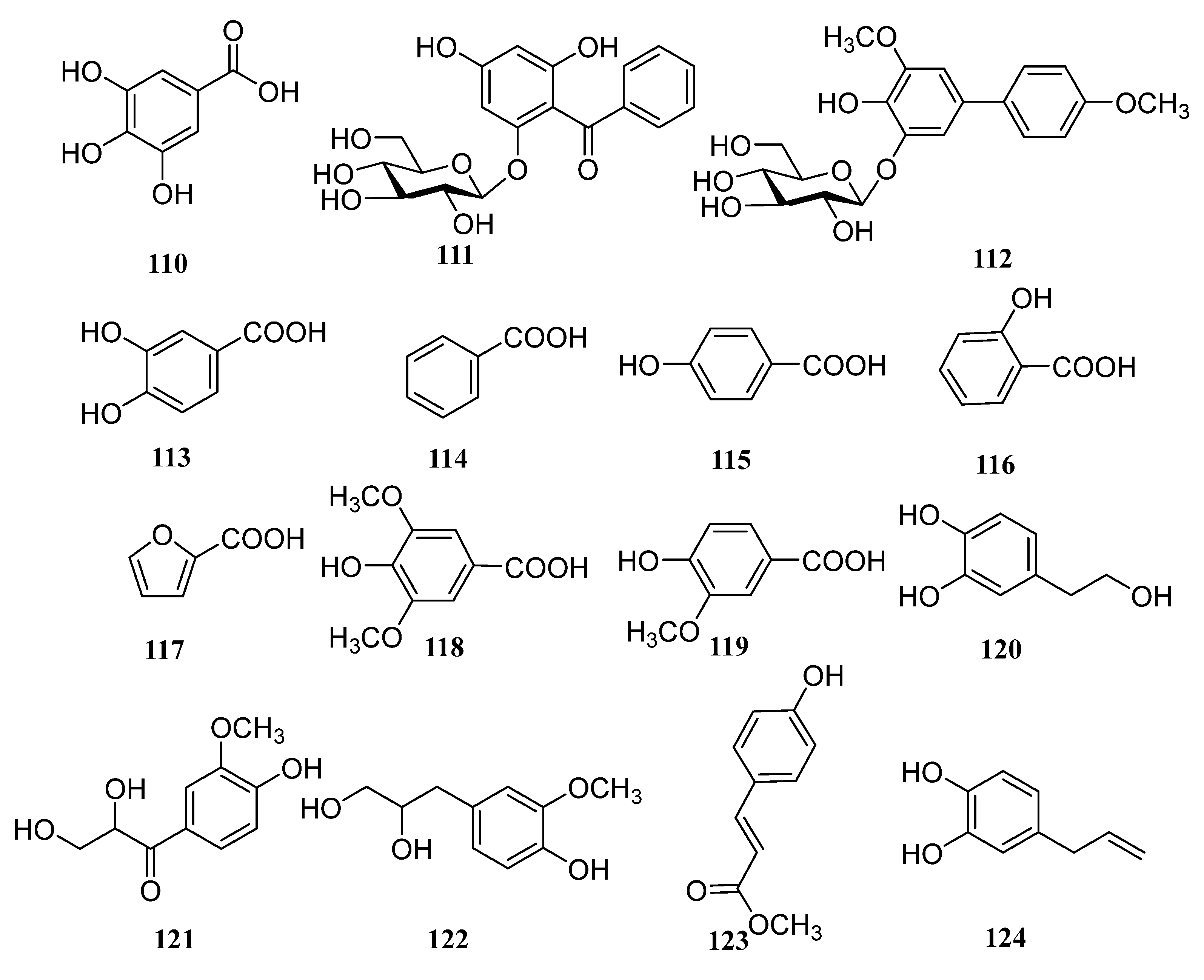
| 110 | gallic acid | [64] |
| 111 | kakispyrone | [38] |
| 112 | kakispyrol | [65] |
| 113 | protocatechuic acid | [46] |
| 114 | benzoic acid | [46] |
| 115 | p-hydroxybenzoic acid | [46] |
| 116 | salicylic acid | [53] |
| 117 | furoic acid | [53] |
| 118 | syringic acid | [53] |
| 119 | vanillic acid | [53] |
| 120 | hydroxytyrosol | [56] |
| 121 | C-veratroylglycol | [56] |
| 122 | 3-(4-hydroxyl-3-methoxyphenyl) propane-1, 2-diol | [56] |
| 123 | methyl coumarate | [56] |
| 124 | 4-allyl pyrocatechol | [56] |
| Medicine | PLF-PC | CTX | CTX | DOX | Heavy Ion Radiotherapy |
|---|---|---|---|---|---|
| Persimmon leaf | + | +(PLF) | +(PE) | +(PLE) | +(PLF) |
| Diseases | atherosclerosis | liver cancer (H22) | liver cancer (H22) | lung cancer (A549) | lung cancer (A549) |
| Effect | 1. Improve oral bioavailability | 1. Reduce side effects 2. Develop immunity from disease | 1. Regulation of oxidative stress 2. Increased tumor suppression rate | 1. Increased toxicity to cancer cells | 1. Increased toxicity to cancer cells |
| Main Objective | Conclusion | References |
|---|---|---|
| Anti-diabetics | ||
| To investigate the effects of different solvent extracts from persimmon leaves on the antioxidant capacity of streptozotocin (STZ) diabetic model mice | Improving the antioxidant capacity of diabetic mice may be one of the mechanisms of the hypoglycemic effect of ethyl acetate extract and alcohol precipitation extract from persimmon leaf leaves | [12] |
| To evaluate the hypoglycemic effect of aqueous extract of persimmon leaves on a mouse model of diabetes | Persimmon leaf extract exhibits considerable anti-diabetic effects by inhibiting α-glucosidase and maintaining the function of β-cells | [78] |
| To study the efficacy of persimmon leaf extract in patients with prediabetes | Based on proteomic changes in different body fluids obtained by prediabetic patients after controlling PLE intake, it has been shown that persimmon leaf extract can improve blood sugar levels | [26] |
| To study the effects of persimmon leaf supplementation on mice with type 2 diabetes | Persimmon leaves ameliorate hyperglycemia by altering the activity and mRNA expression of liver enzymes involved in glucose utilization and glucose production, and also ameliorate dyslipidemia and hepatic steatosis by combining a decrease in hepatic lipogenesis and an increase in fecal fat excretion | [77] |
| Anti-tumor | ||
| The crude polysaccharides in persimmon leaves were used as the research objects, and their anti-tumor and anti-metastatic activities were evaluated by oral administration in mice. | Crude polysaccharides in persimmon leaves induce natural killer (NK) cells-mediated tumoricidal activity and inhibit tumor metastasis in mice in a dose-dependent manner. | [77] |
| The purpose of this study was to investigate the anti-cancer properties of flavonoids isolated from persimmon leaves. | Flavonoids isolated from persimmon leaves (PLF) can induce apoptosis of HCT116 (colorectal cancer) and HepG2 (liver cancer) cells, and the intracellular ROS level is increased. In addition, PLF has a strong ability to scavenge free radicals. The anti-proliferative activity of PLF on cancer cells is related to the induction of apoptosis and oxidative stress. | [96] |
| This study investigated the effect of persimmon leaf extract on cellular DNA damage checkpoint signaling on cancer chemotherapy sensitivity. | Persimmon leaf extract inhibits ATM activity during DNA damage response in A549 lung adenocarcinoma cells induced by doxorubicin. | [97] |
| To study the anti-tumor and immunomodulatory activities of total flavonoids extract from persimmon leaves on H22 hepatoma mice. | The total flavonoids extract of persimmon leaf can effectively inhibit the growth of liver tumors in vivo by enhancing the immune function of mice, showing the potential of a safe and effective anti-cancer drug or functional immune enhancer. | [117] |
| Neuroprotective activity | ||
| The effects of ethanol extract of flavonoid-rich persimmon leaf on APP/PS1 transgenic mice were studied by oral administration. | Alleviate cognitive deficits, amyloid production, oxidative stress, and neuroinflammation in APP/PS1 transgenic mice. | [98] |
| The protective effects and mechanisms of flavonoid-rich ethanol extracts on the cortex and hippocampus of D-galactose aged mice were studied. | Flavonoid-rich ethanol extract of persimmon leaf attenuates D-galactose-induced oxidative stress and neuroinflammation-mediated brain aging in mice. | [92] |
| APP/PS1 mice were used as AD models to investigate whether the protective effect of flavonoids extracted from persimmon leaves on the synapses of AD mice was related to Rho GTPases activity. | It significantly inhibited RhoA-GTP activity, improved learning and memory function, and antagonized the downregulated expression of synaptophysin and synapse-associated proteins. | [91] |
| To investigate the neuroprotective effect of persimmon leaf flavonoid extracts in an in vivo model of focal ischemia/reperfusion (I/R) injury induced by middle cerebral artery occlusion (MCAO) and transient global cerebral ischemia (4-VO) due to quadruple vascular occlusion. | Significantly protects rats from MCAO and 4-VO ischemic injury, protects hippocampal neurons from glutamate-induced excitotoxic damage, and protects cortical neurons from hypoxia-induced damage in vivo. Useful in the prevention and treatment of related neurodegenerative diseases such as ischemia/reperfusion injury. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.; Wang, X.; Xu, J.; Guo, J.; Peng, H.; Zhang, Y. A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress. Molecules 2024, 29, 215. https://doi.org/10.3390/molecules29010215
Hong C, Wang X, Xu J, Guo J, Peng H, Zhang Y. A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress. Molecules. 2024; 29(1):215. https://doi.org/10.3390/molecules29010215
Chicago/Turabian StyleHong, Chong, Xu Wang, Jianjian Xu, Jianxing Guo, Houlin Peng, and Yan Zhang. 2024. "A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress" Molecules 29, no. 1: 215. https://doi.org/10.3390/molecules29010215
APA StyleHong, C., Wang, X., Xu, J., Guo, J., Peng, H., & Zhang, Y. (2024). A Review: Pharmacological Effect of Natural Compounds in Diospyros kaki Leaves from the Perspective of Oxidative Stress. Molecules, 29(1), 215. https://doi.org/10.3390/molecules29010215






